IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis
Abstract
:1. Introduction
2. Results
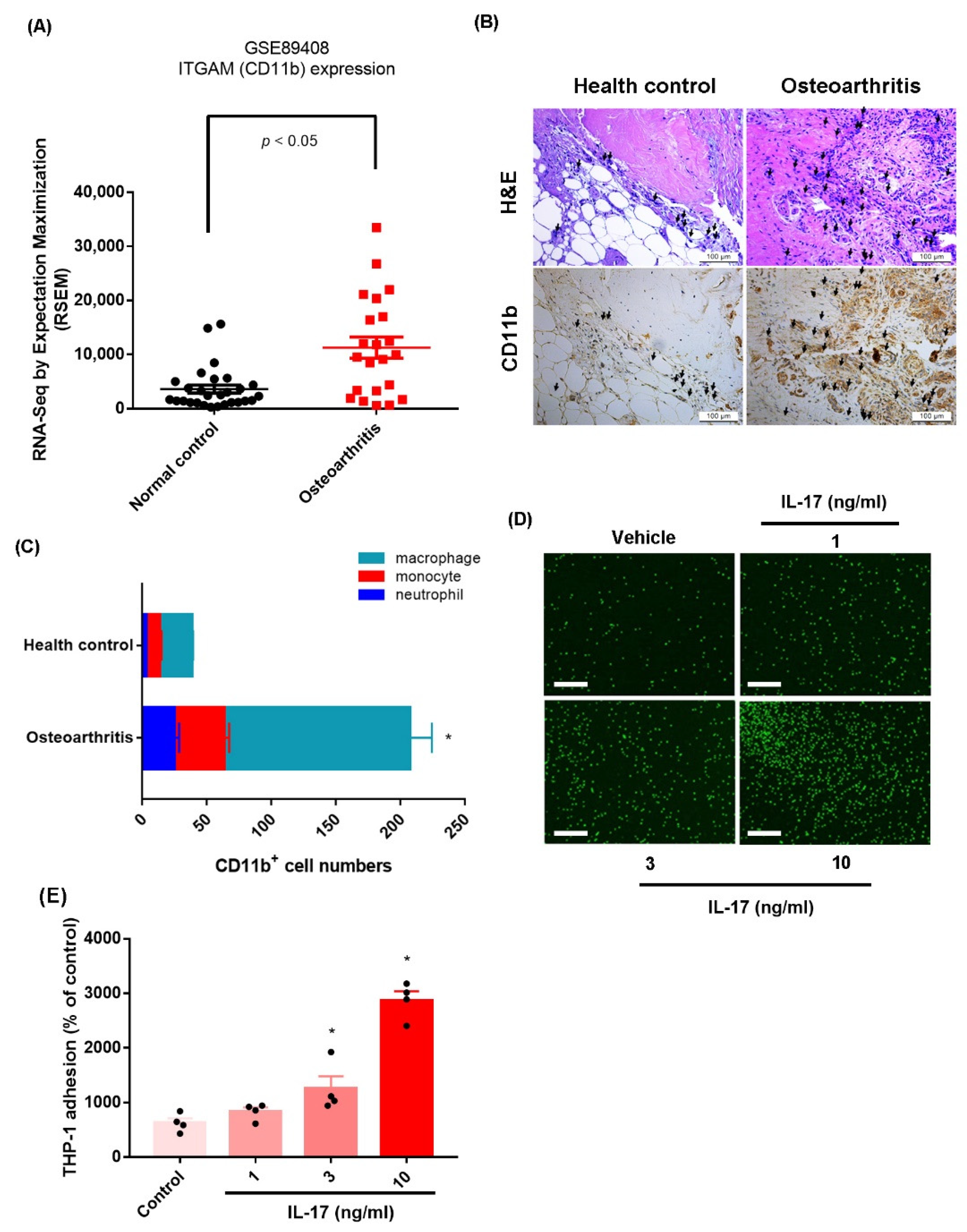
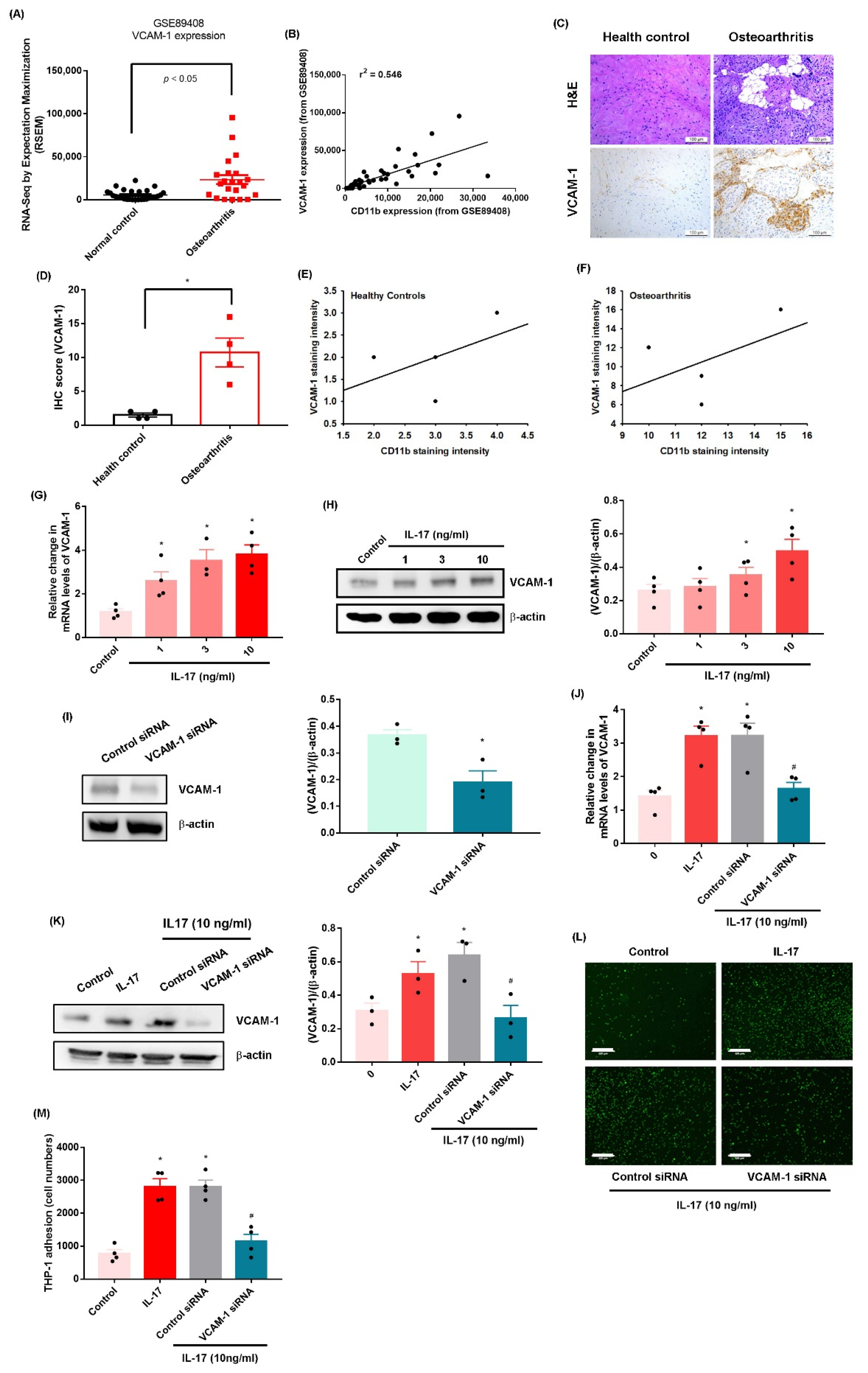
2.1. IL-17 Promotes Monocyte Adhesion in Human OASFs by Enhancing VCAM-1 Production
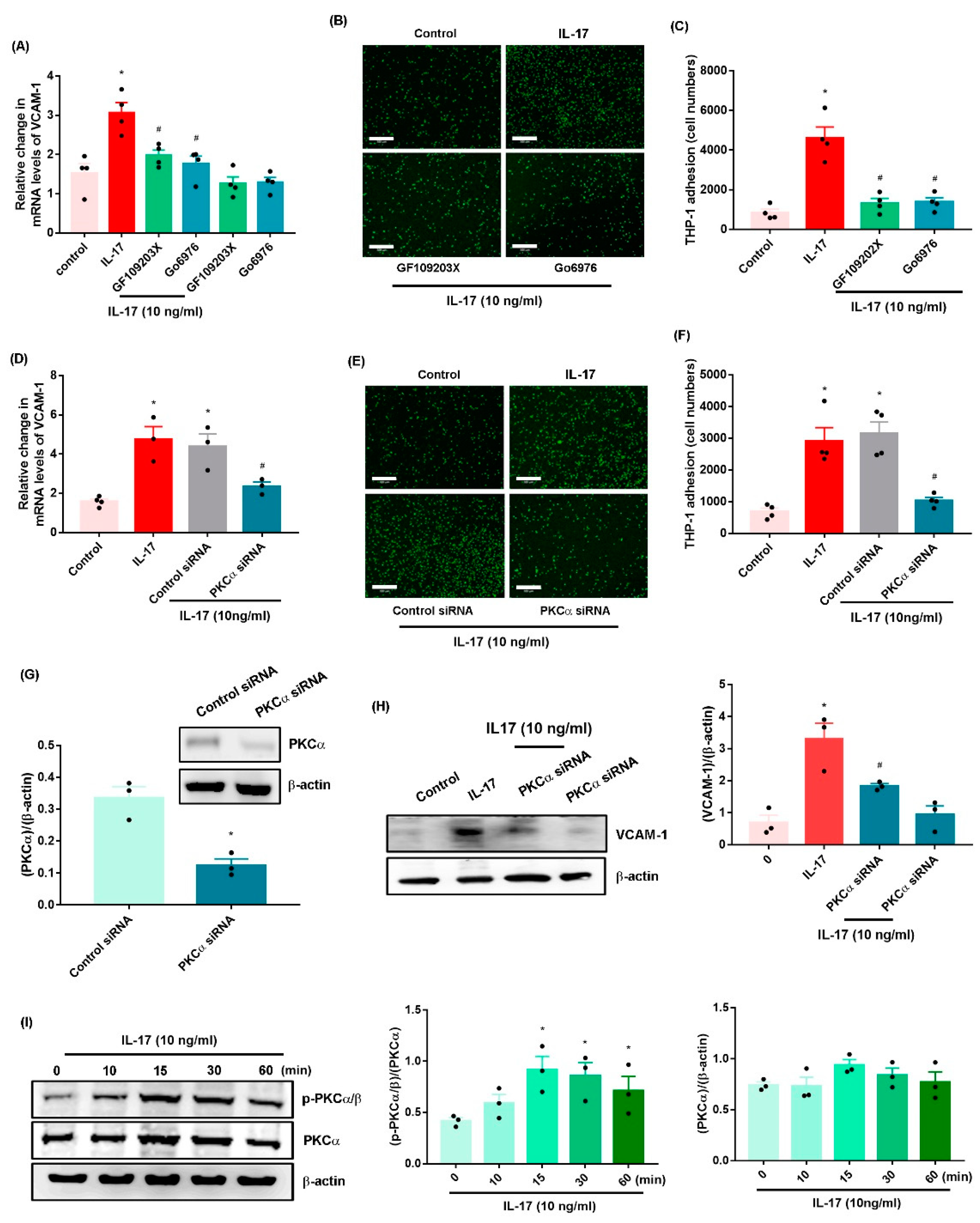
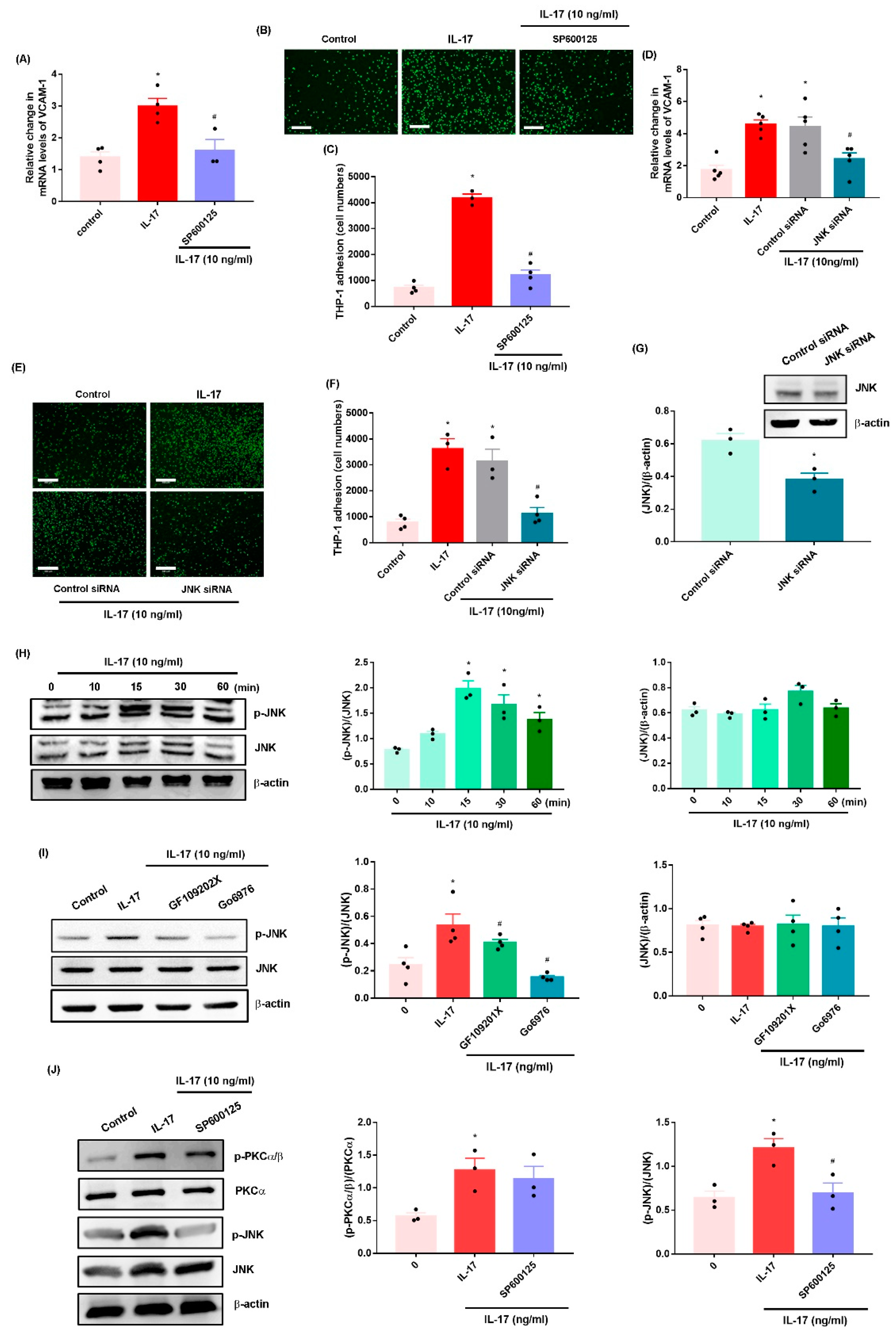
2.2. IL-17 Promotes VCAM-1-Dependent Monocyte Adhesion in OASFs via the PKC-α and JNK Signaling Pathways
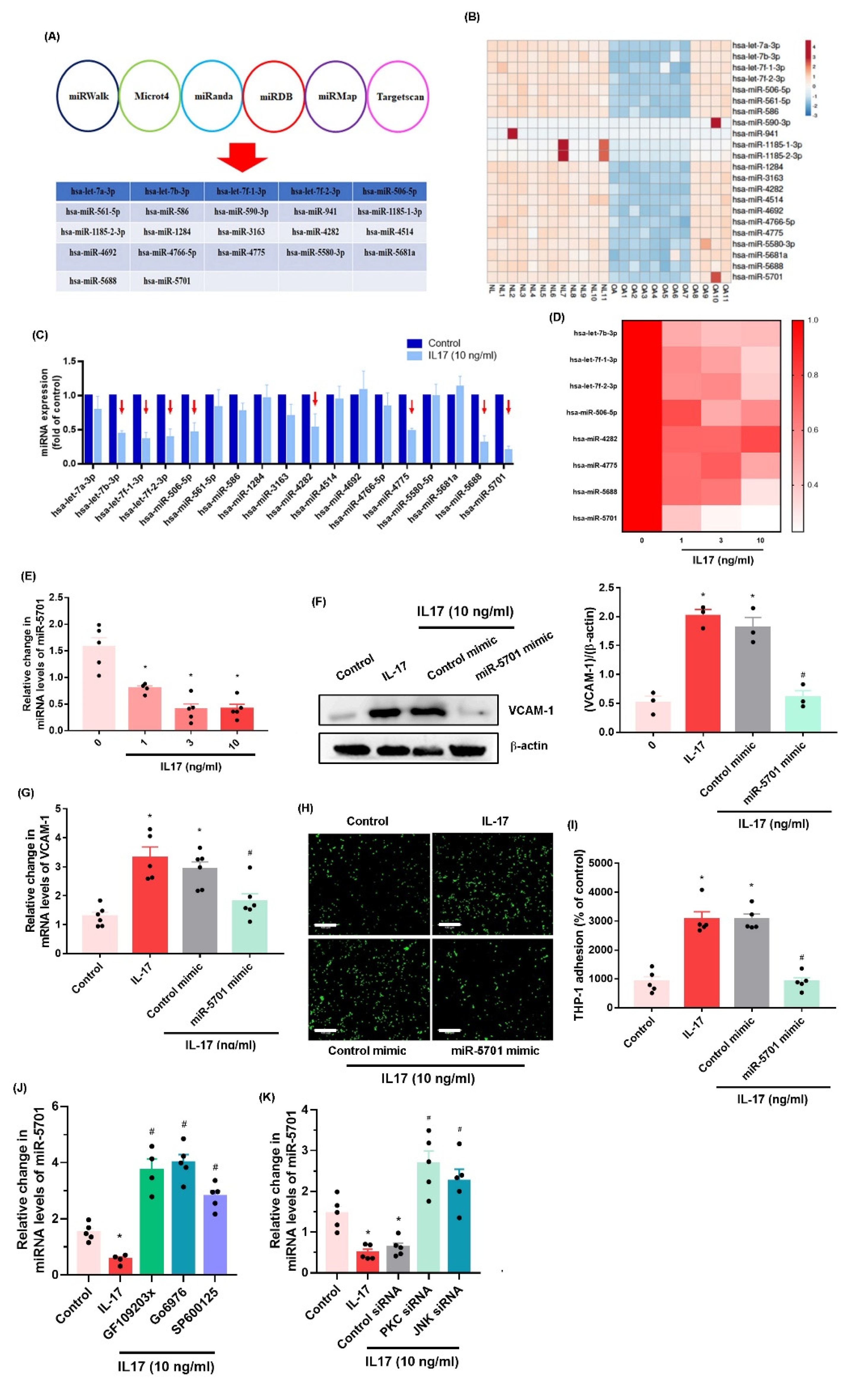
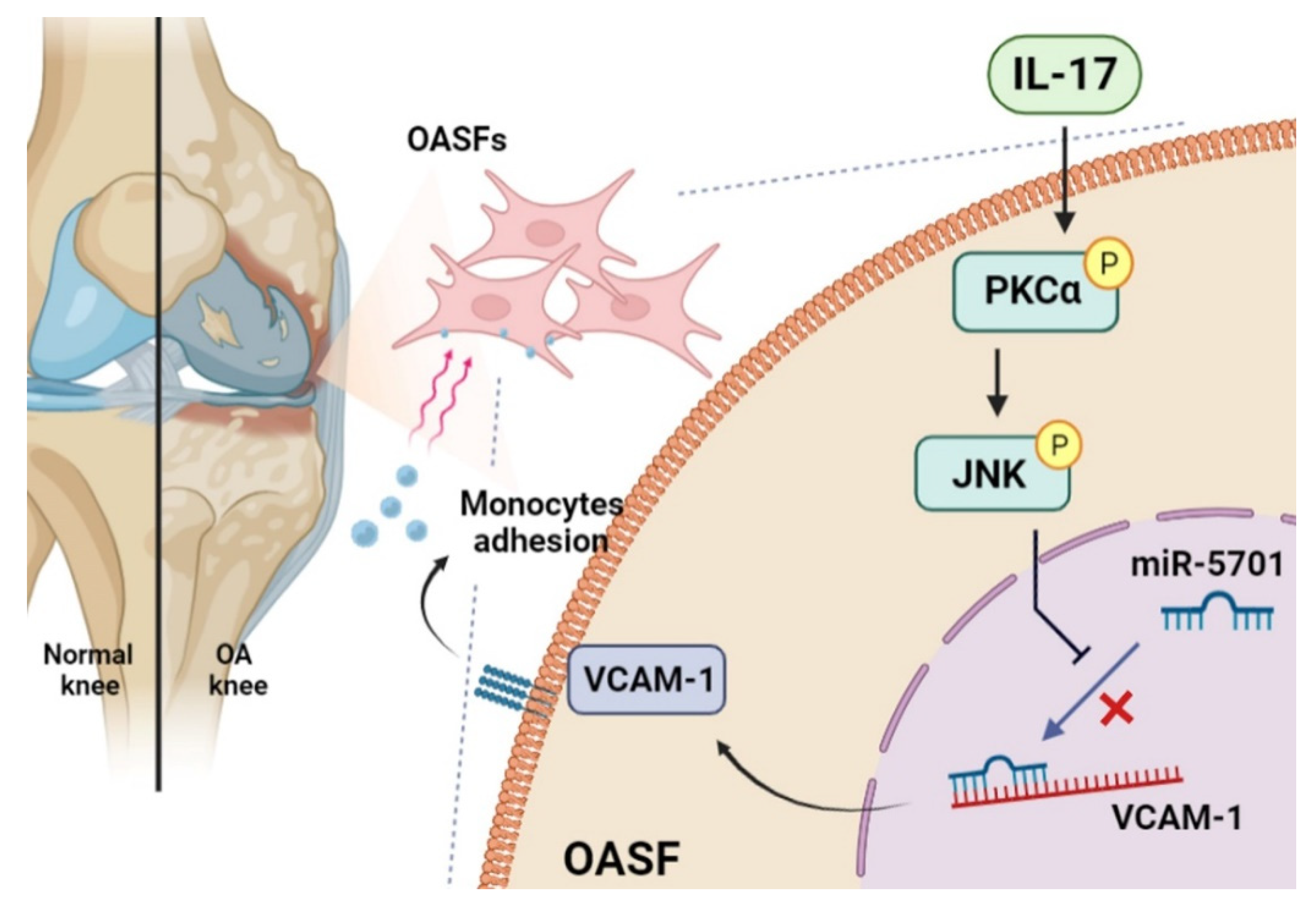
2.3. IL-17 Enhances VCAM-1 Synthesis and Monocyte Adhesion by Suppressing miR-5701 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Monocyte Adhesion Analysis
4.3. Human Clinical Samples
4.4. Real-Time Quantitative PCR Analysis of mRNA and miRNA
4.5. Western Blot
4.6. Measurement of Data from the Gene Expression Omnibus (GEO) Database
4.7. Immunohistochemistry (IHC)
4.8. Small Interfering RNA (siRNA) Transfection
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Meng, H.; Wang, Y.; Peng, J.; Guo, Q.; Wang, A.; Lu, S. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, I.J.; Liu, S.C.; Su, C.M.; Wang, Y.H.; Tsai, C.H.; Tang, C.H. Implications of angiogenesis involvement in arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.J.; Yang, W.H.; Liu, S.C.; Tsai, C.H.; Hsu, H.C.; Tang, C.H. Transforming growth factor beta1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microrna 519b synthesis. PLoS ONE 2017, 12, e0176052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.S.; Tang, C.H.; Chie, M.J.; Tsai, C.H.; Fong, Y.C.; Lu, Y.C.; Chen, W.C.; Lai, C.T.; Wei, C.Y.; Tai, H.C.; et al. Resistin facilitates vegf-a-dependent angiogenesis by inhibiting mir-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, M.; Asgharian, S.; Khalesi, E.; Ahmadi, A.; Lorigooini, Z. Comparative study of the effect of thymus daenensis gel 5% and diclofenac in patients with knee osteoarthritis. Biomedicine 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Liu, Y.L.; Lin, J.B.; Cheng, K.L.; Wang, Y.G.; Yao, H.L.; Wei, P.; Wu, H.Y.; Su, W.W.; Shaw, P.C.; et al. Preparative expression and purification of a nacreous protein n16 and testing its effect on osteoporosis rat model. Int. J. Biol. Macromol. 2018, 111, 440–445. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Schett, G.; Kiechl, S.; Bonora, E.; Zwerina, J.; Mayr, A.; Axmann, R.; Weger, S.; Oberhollenzer, F.; Lorenzini, R.; Willeit, J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009, 60, 2381–2389. [Google Scholar] [CrossRef]
- Lee, H.-P.; Liu, S.-C.; Wang, Y.-H.; Chen, B.-C.; Chen, H.-T.; Li, T.-M.; Huang, W.-C.; Hsu, C.-J.; Wu, Y.-C.; Tang, C.-H. Cordycerebroside a suppresses vcam-dependent monocyte adhesion in osteoarthritis synovial fibroblasts by inhibiting mek/erk/ap-1 signaling. J. Funct. Foods 2021, 86, 104712. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, C.Y.; Kuo, S.J.; Liu, S.C.; Lu, Y.C.; Chen, Y.L.; Wang, S.W.; Tang, C.H. Resistin enhances vcam-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by inhibiting mir-381 expression through the pkc, p38, and jnk signaling pathways. Cells 2020, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, H.; Meng, Z.; Wei, M. Correlation of il-17 level in synovia and severity of knee osteoarthritis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1732–1736. [Google Scholar]
- Jin, Y.; Chen, X.; Gao, Z.; Liu, K.; Hou, Y.; Zheng, J. Expression levels of il-15 and il-17 in synovial fluid of rheumatoid arthritis animal model. Exp. Ther. Med. 2018, 16, 3377–3382. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, S.; Liu, Y.; Jin, S.; Zhang, H.; Su, K. Correlation between interleukin-17 gene polymorphism and osteoarthritis susceptibility in han chinese population. BMC Med. Genet. 2019, 20, 20. [Google Scholar] [CrossRef] [Green Version]
- Eftedal, R.; Vrgoc, G.; Jotanovic, Z.; Dembic, Z. Alternative interleukin 17a/f locus haplotypes are associated with increased risk to hip and knee osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 1972–1978. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, C.; Zhong, Y.; He, J.; Cao, X.; Xia, H.; Ba, H.; Li, P.; Wu, S.; Peng, C. Interleukin-17 can induce osteoarthritis in rabbit knee joints similar to hulth’s method. BioMed Res. Int. 2017, 2017, 2091325. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.M.; Yoon, B.Y.; Her, Y.M.; Oh, H.J.; Lee, J.S.; Kim, K.W.; Lee, S.Y.; Woo, Y.J.; Park, K.S.; Park, S.H.; et al. Il-32 and il-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R246. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The c. Elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Tehrani, S.S.; Zaboli, E.; Sadeghi, F.; Khafri, S.; Karimian, A.; Rafie, M.; Parsian, H. Microrna-26a-5p as a potential predictive factor for determining the effectiveness of trastuzumab therapy in her-2 positive breast cancer patients. BioMedicine 2021, 11, 30–39. [Google Scholar]
- Asahara, H. Current status and strategy of microrna research for cartilage development and osteoarthritis pathogenesis. J. Bone Metab. 2016, 23, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Ko, C.Y.; Liu, S.C.; Wang, Y.H.; Hsu, C.J.; Tsai, C.H.; Wu, T.J.; Tang, C.H. Mir-144-3p ameliorates the progression of osteoarthritis by targeting il-1beta: Potential therapeutic implications. J. Cell. Physiol. 2021, 236, 6988–7000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage m1 polarisation exacerbates experimental osteoarthritis partially through r-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, S.M.; Tsai, C.H.; Huang, C.Y.; Hsu, C.J.; Tang, C.H. Ccn4 induces vascular cell adhesion molecule-1 expression in human synovial fibroblasts and promotes monocyte adhesion. Biochim. Biophys. Acta 2013, 1833, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.J.; Liu, S.C.; Huang, Y.L.; Tsai, C.H.; Fong, Y.C.; Hsu, H.C.; Tang, C.H. Tgf-beta1 enhances foxo3 expression in human synovial fibroblasts by inhibiting mir-92a through ampk and p38 pathways. Aging 2019, 11, 4075–4089. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses il-1beta-induced mmp-1 expression through the fak, mek, erk, and ap-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef]
- Taipaleenmaki, H. Regulation of bone metabolism by micrornas. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Gao, B.; Gao, W.; Wu, Z.; Zhou, T.; Qiu, X.; Wang, X.; Lian, C.; Peng, Y.; Liang, A.; Qiu, J.; et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Lee, C.W.; Xu, H.; Wang, Y.F.; Yung, P.S.H.; Jiang, Y.; Lee, O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021, 23, 110. [Google Scholar] [CrossRef]
- Wasilewska, A.; Winiarska, M.; Olszewska, M.; Rudnicka, L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol. Alergol. 2016, 33, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.M.; Hsu, C.J.; Liao, Y.Y.; Chou, M.C.; Tang, C.H. The ccl2/ccr2 axis enhances vascular cell adhesion molecule-1 expression in human synovial fibroblasts. PLoS ONE 2012, 7, e49999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Su, C.M.; Hsu, C.J.; Huang, C.C.; Wang, S.W.; Liu, S.C.; Chen, W.C.; Fuh, L.J.; Tang, C.H. Ccn1 promotes vegf production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting mir-126 expression in rheumatoid arthritis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horng, C.T.; Shieh, P.C.; Tan, T.W.; Yang, W.H.; Tang, C.H. Paeonol suppresses chondrosarcoma metastasis through up-regulation of mir-141 by modulating pkcdelta and c-src signaling pathway. Int. J. Mol. Sci. 2014, 15, 11760–11772. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Chi, M.C.; Lin, C.Y.; Lee, C.W.; Chang, T.M.; Han, C.K.; Huang, Y.L.; Fong, Y.C.; Chen, H.T.; Tang, C.H. PM2.5 facilitates il-6 production in human osteoarthritis synovial fibroblasts via ask1 activation. J. Cell. Physiol. 2021, 236, 2205–2213. [Google Scholar] [CrossRef]
- Wu, M.H.; Tsai, C.H.; Huang, Y.L.; Fong, Y.C.; Tang, C.H. Visfatin promotes il-6 and tnf-alpha production in human synovial fibroblasts by repressing mir-199a-5p through erk, p38 and jnk signaling pathways. Int. J. Mol. Sci. 2018, 19, 190. [Google Scholar] [CrossRef] [Green Version]
- Nugent, M. Micrornas: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Law, Y.Y.; Lin, Y.M.; Liu, S.C.; Wu, M.H.; Chung, W.H.; Tsai, C.H.; Fong, Y.C.; Tang, C.H.; Wang, C.K. Visfatin increases icam-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing mir-320a expression. Aging 2020, 12, 18635–18648. [Google Scholar] [CrossRef]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway protective effects of micrornas on human chondrocytes in an in vitro model of osteoarthritis. Mol. Ther.-Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-P.; Wu, Y.-C.; Chen, B.-C.; Liu, S.-C.; Li, T.-M.; Huang, W.-C.; Hsu, C.-J.; Tang, C.-H. Soya-cerebroside reduces interleukin production in human rheumatoid arthritis synovial fibroblasts by inhibiting the erk, nf-κb and ap-1 signalling pathways. Food Agric. Immunol. 2020, 31, 740–750. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Lin, L.W.; Tsai, F.J.; Yang, J.S.; Li, T.M.; Tang, C.H. Soya-cerebroside inhibits vegf-facilitated angiogenesis in endothelial progenitor cells. Food Agric. Immunol. 2020, 31, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y.; et al. Hesperidin is a potential inhibitor against sars-cov-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Su, C.M.; Tang, C.H.; Chi, M.J.; Lin, C.Y.; Fong, Y.C.; Liu, Y.C.; Chen, W.C.; Wang, S.W. Resistin facilitates vegf-c-associated lymphangiogenesis by inhibiting mir-186 in human chondrosarcoma cells. Biochem. Pharmacol. 2018, 154, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C.; Tsai, F.J.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Cheng, L.Y.; Su, P.H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in h9c2 cardiomyoblast cells via ros suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef]
- Liu, S.C.; Tsai, C.H.; Wu, T.Y.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Soya-cerebroside reduces il-1 beta-induced mmp-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agric. Immunol. 2019, 30, 620–632. [Google Scholar] [CrossRef]
- Achudhan, D.; Liu, S.C.; Lin, Y.Y.; Lee, H.P.; Wang, S.W.; Huang, W.C.; Wu, Y.C.; Kuo, Y.H.; Tang, C.H. Antcin k inhibits vegf-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022, 46, e14022. [Google Scholar] [CrossRef]
- Su, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Lee, H.-P.; Lo, L.-C.; Huang, W.-C.; Wu, Y.-C.; Hsieh, C.-L.; Tang, C.-H. Betulin suppresses tnf-α and il-1β production in osteoarthritis synovial fibroblasts by inhibiting the mek/erk/nf-κb pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Lee, K.T.; Su, C.H.; Liu, S.C.; Chen, B.C.; Chang, J.W.; Tsai, C.H.; Huang, W.C.; Hsu, C.J.; Chen, W.C.; Wu, Y.C.; et al. Cordycerebroside a inhibits icam-1-dependent m1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022, e14108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-J.; Chang, S.L.-Y.; Lin, C.-Y.; Lai, C.-Y.; He, X.-Y.; Tsai, C.-H.; Ko, C.-Y.; Fong, Y.-C.; Su, C.-M.; Tang, C.-H. IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis. Int. J. Mol. Sci. 2022, 23, 6804. https://doi.org/10.3390/ijms23126804
Wu T-J, Chang SL-Y, Lin C-Y, Lai C-Y, He X-Y, Tsai C-H, Ko C-Y, Fong Y-C, Su C-M, Tang C-H. IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis. International Journal of Molecular Sciences. 2022; 23(12):6804. https://doi.org/10.3390/ijms23126804
Chicago/Turabian StyleWu, Tsung-Ju, Sunny Li-Yun Chang, Chih-Yang Lin, Chao-Yang Lai, Xiu-Yuan He, Chun-Hao Tsai, Chih-Yuan Ko, Yi-Chin Fong, Chen-Ming Su, and Chih-Hsin Tang. 2022. "IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis" International Journal of Molecular Sciences 23, no. 12: 6804. https://doi.org/10.3390/ijms23126804
APA StyleWu, T.-J., Chang, S. L.-Y., Lin, C.-Y., Lai, C.-Y., He, X.-Y., Tsai, C.-H., Ko, C.-Y., Fong, Y.-C., Su, C.-M., & Tang, C.-H. (2022). IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis. International Journal of Molecular Sciences, 23(12), 6804. https://doi.org/10.3390/ijms23126804






