Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug?
Abstract
:1. Introduction
2. Results and Discussion
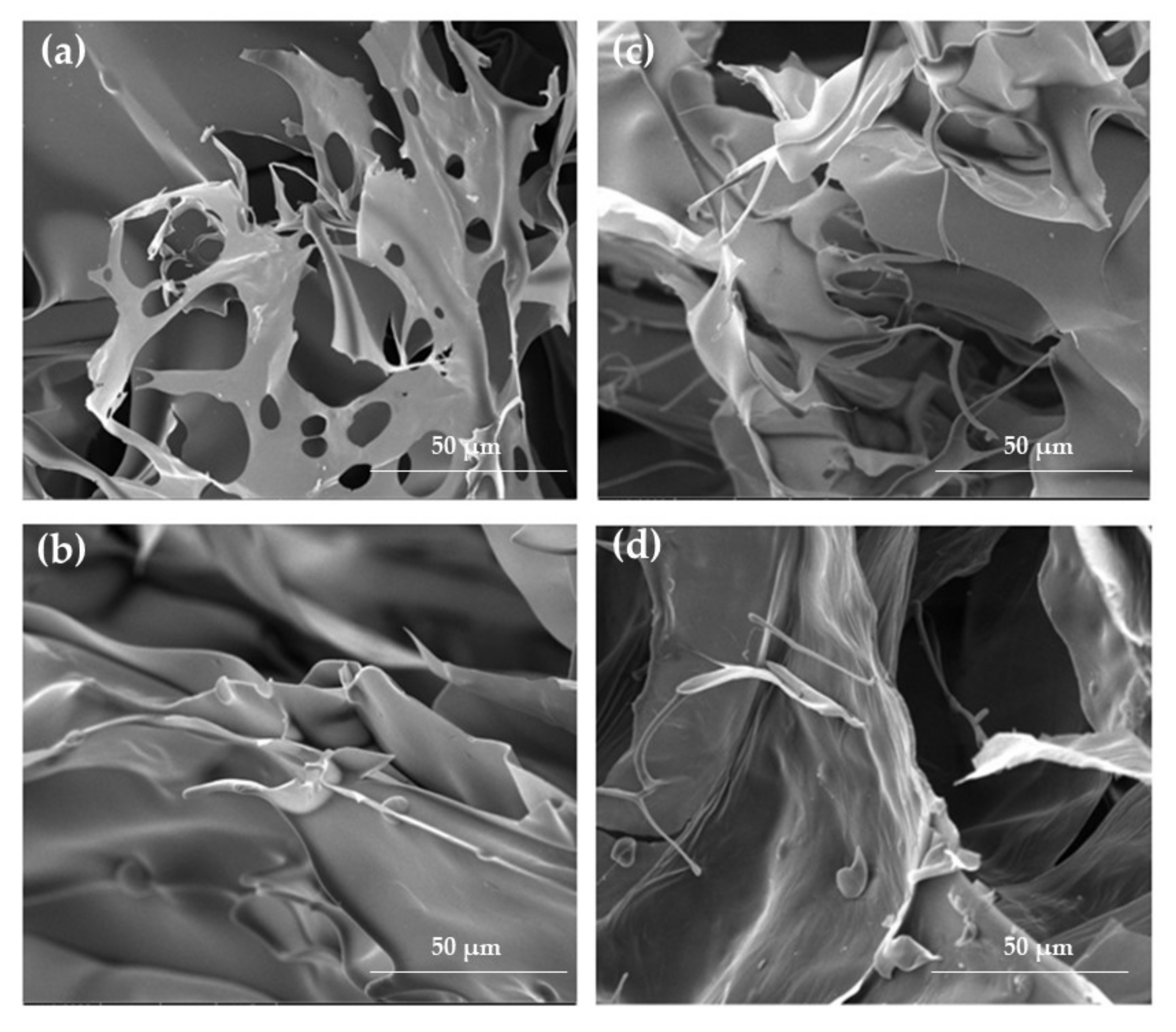
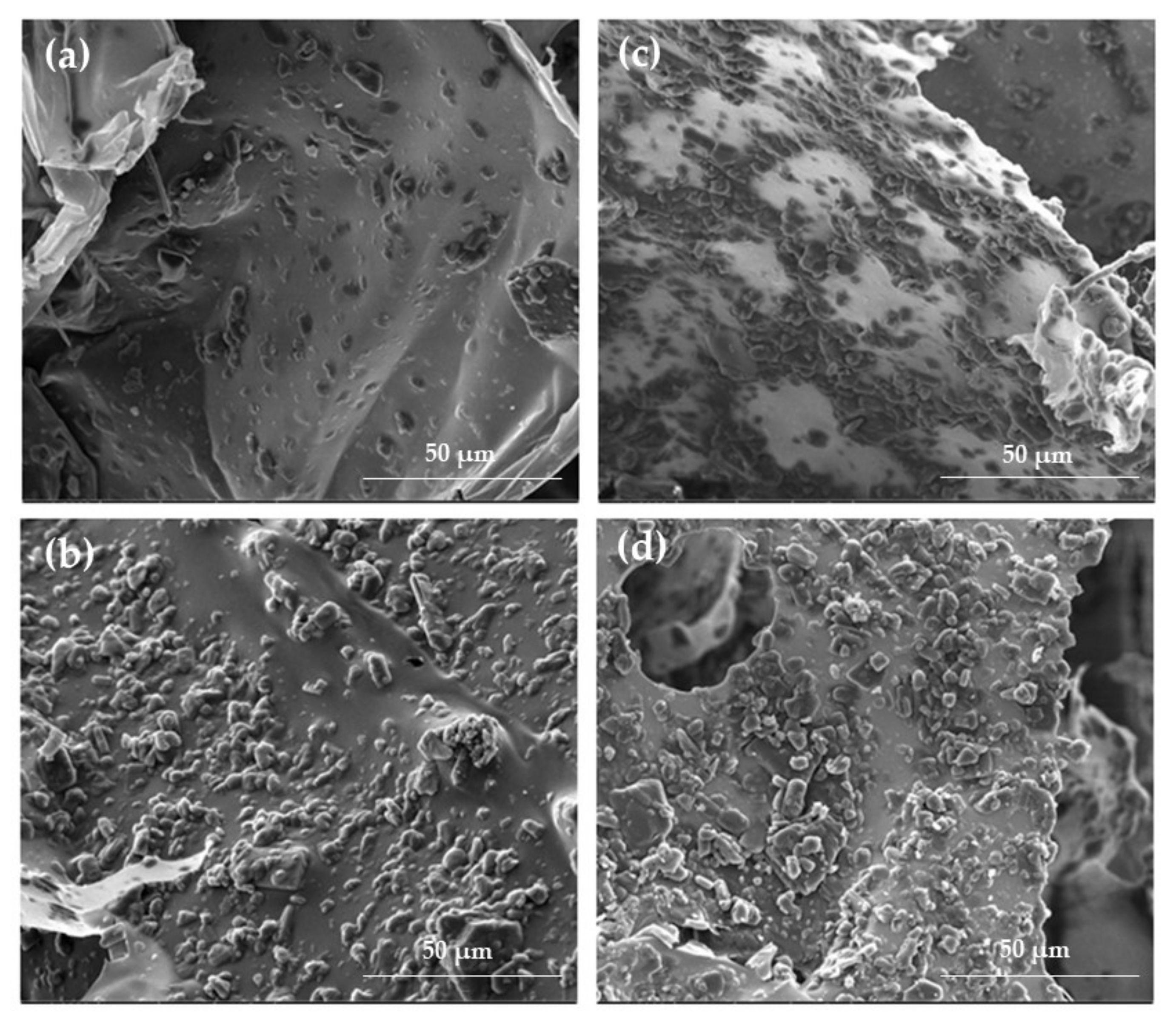
2.1. Hydrogels and Cryogels Characteristics
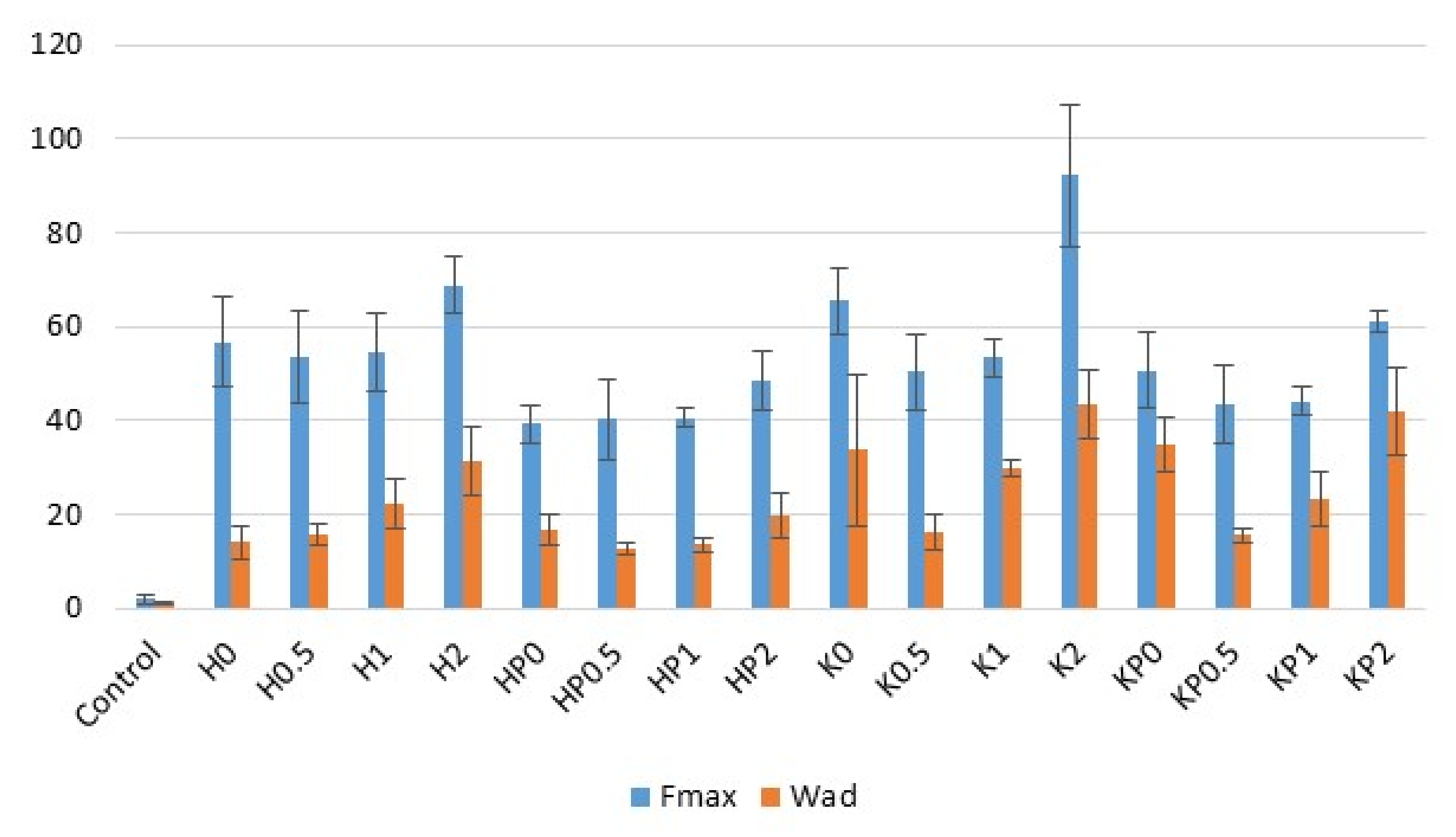
2.2. Bioadhesion
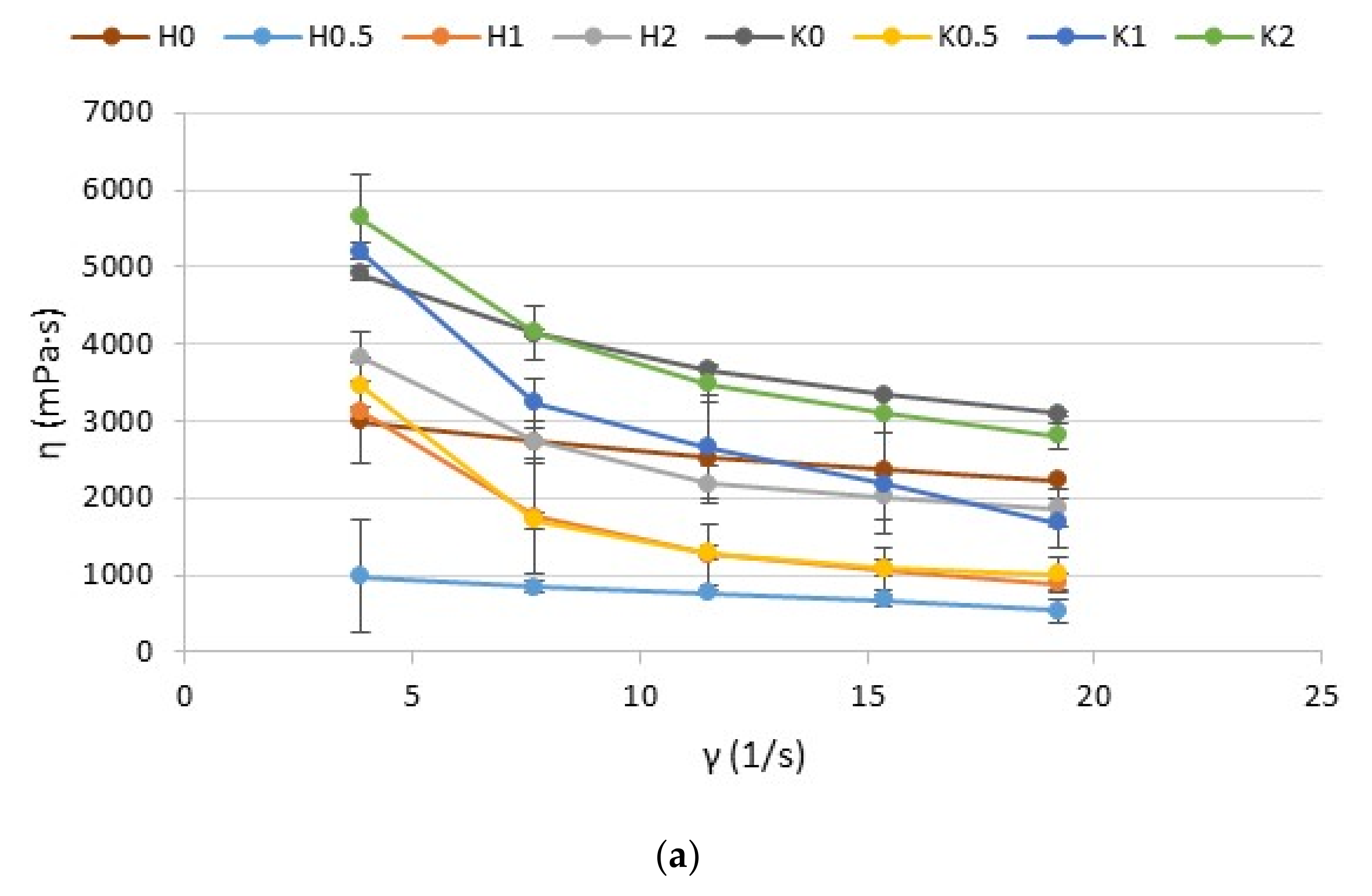
2.3. Rheological and Textural Properties
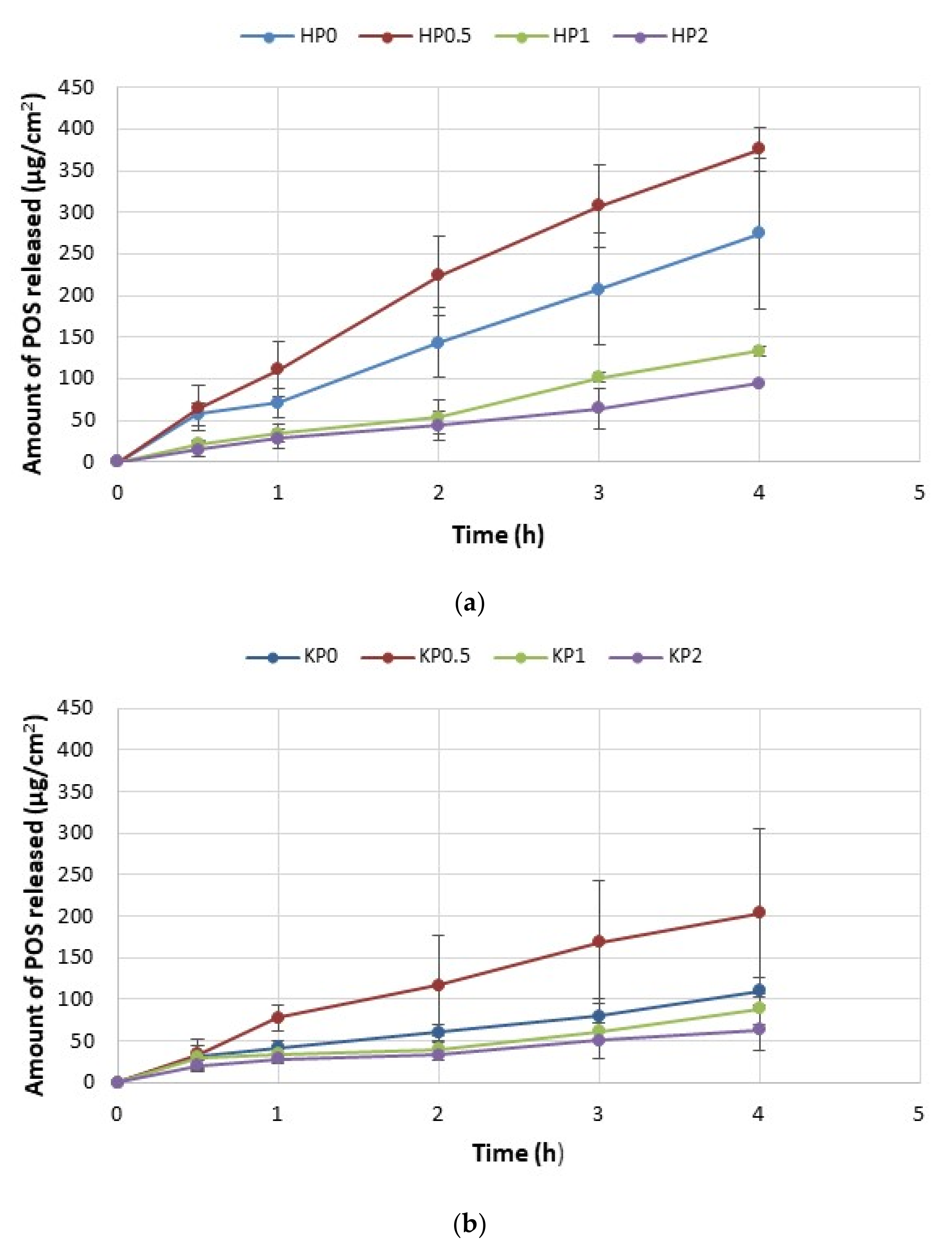
2.4. In Vitro POS Release
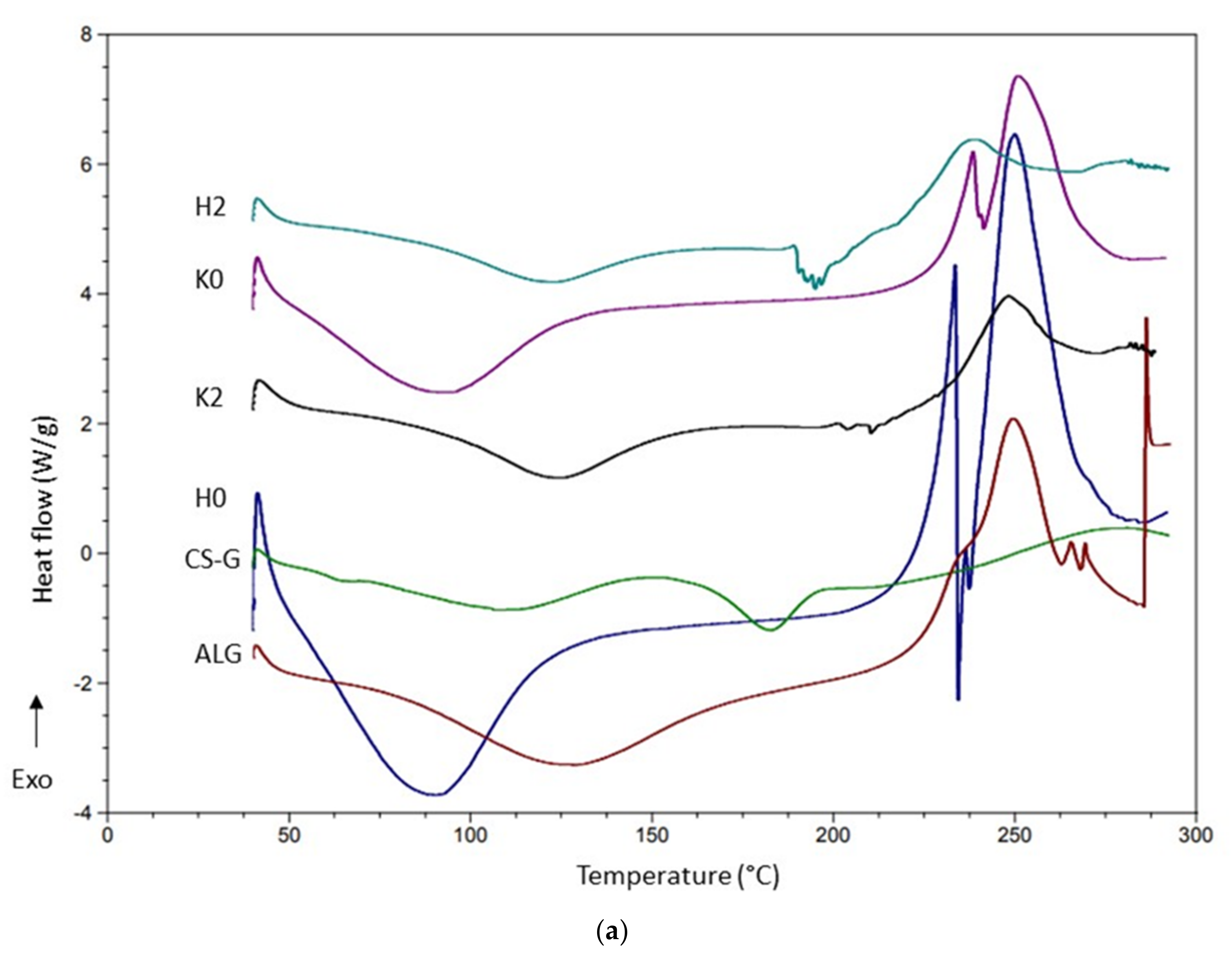
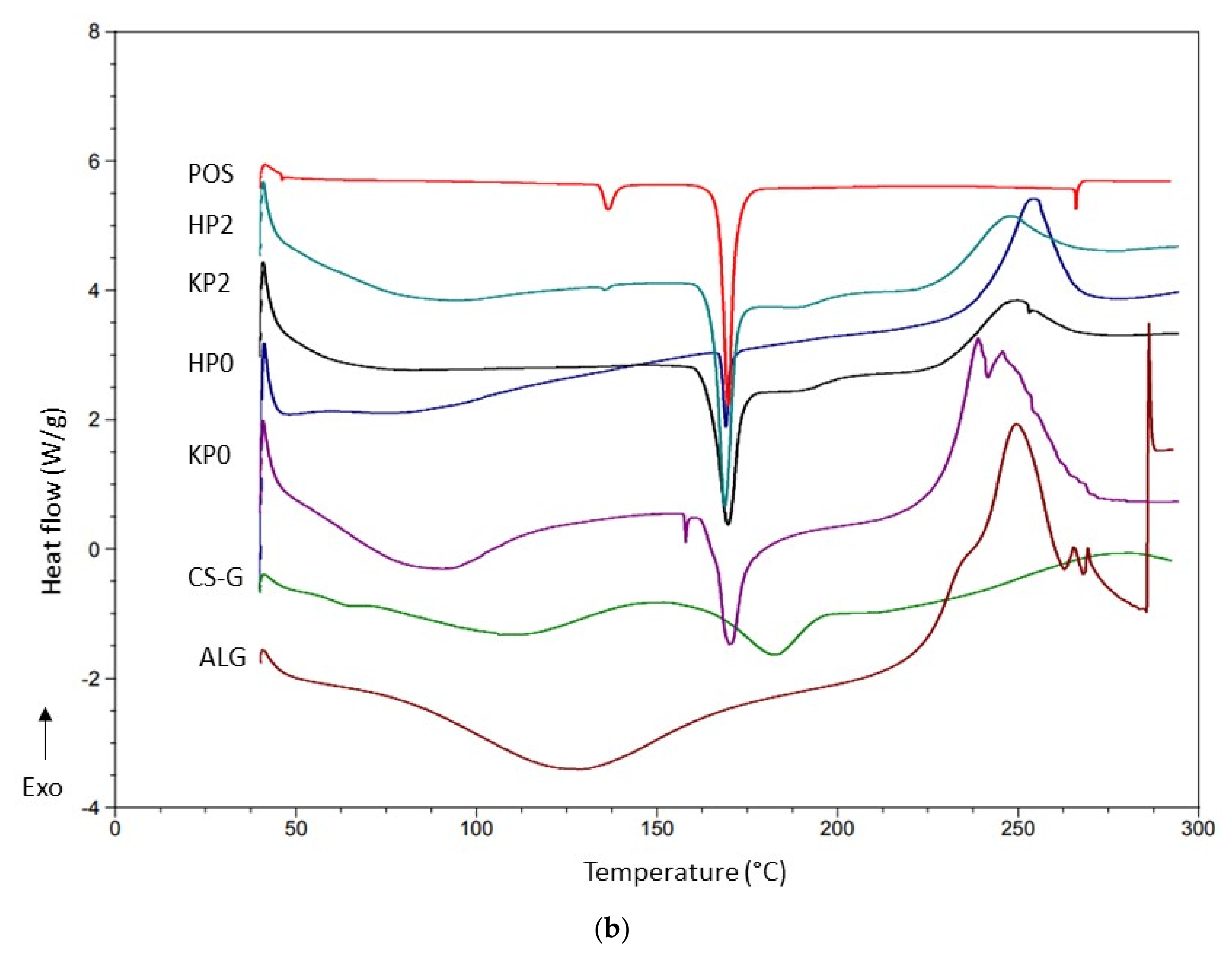
2.5. DSC Analysis
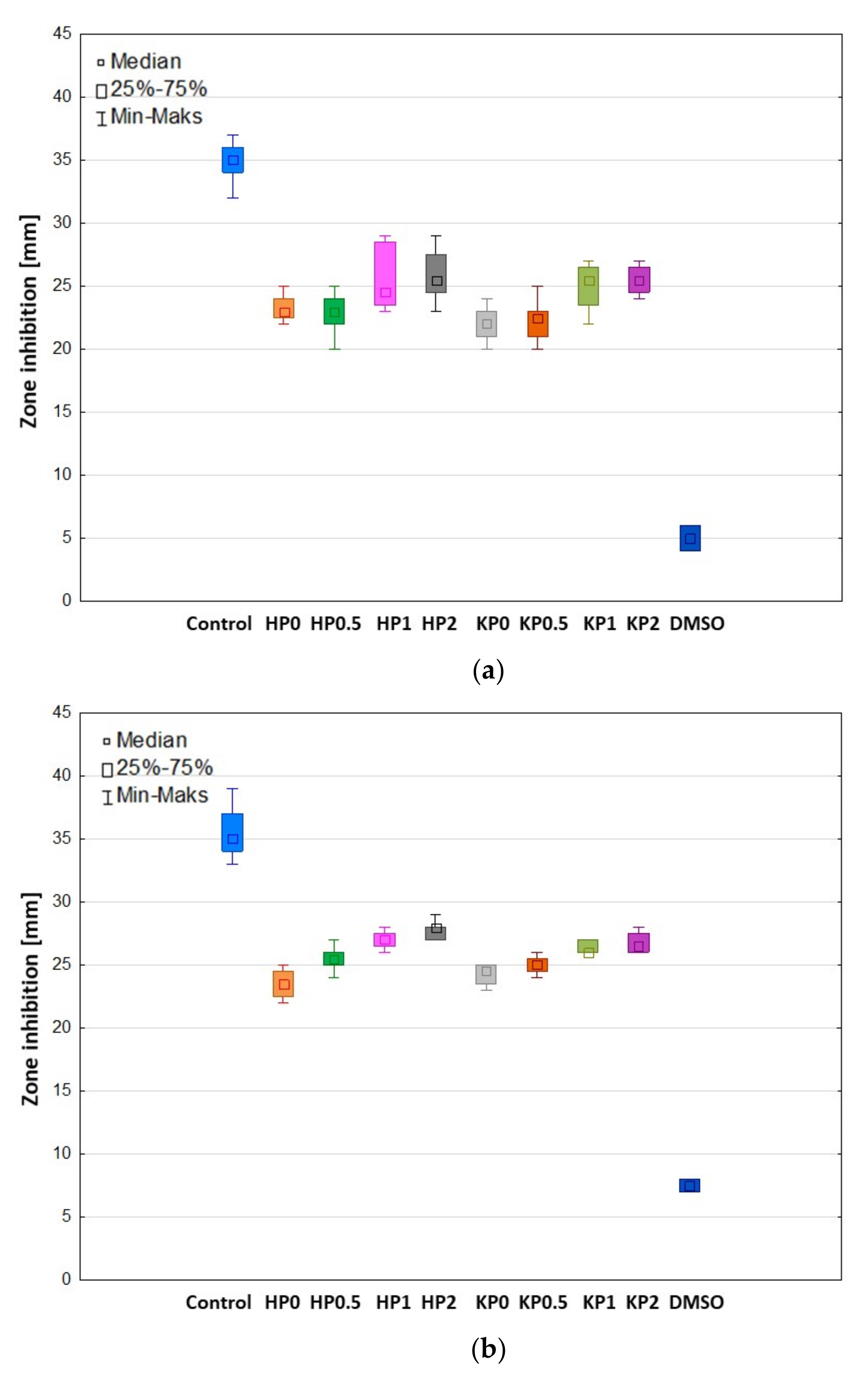
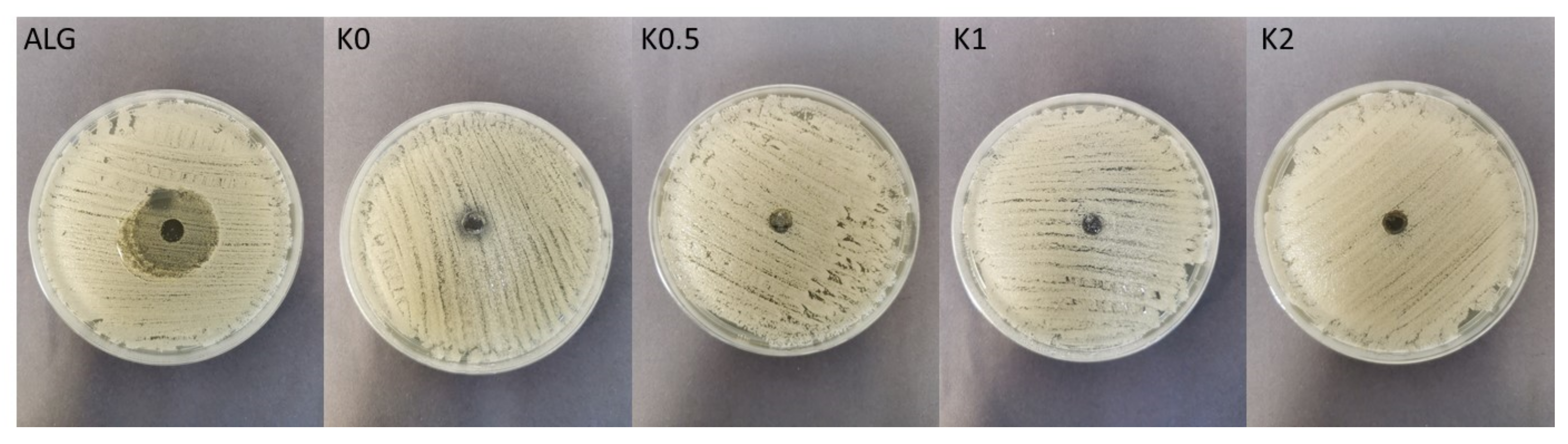
2.6. Antifungal Activity
3. Materials and Methods
3.1. Preparation of Hydrogels
3.2. Preparation of Cryogels by Freeze–Thaw Method
3.3. pH Determination
3.4. Scanning Electron Microscope (SEM) Analysis
3.5. Particles Size Analysis
3.6. Drug Content
3.7. Ex Vivo Bioadhesiveness
3.8. Viscosity and Rheological Properties Analysis
3.9. Texture Analysis
3.10. In Vitro POS Release
3.11. High-Performance Liquid Chromatography (HPLC) Assay
3.12. Mathematical Modeling of POS Release Profile
3.13. Index of Similarity and Dissimilarity
3.14. Differential Scanning Calorimetry (DSC)
3.15. Antifungal Activity
3.16. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J. Funct. Biomater. 2018, 1, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Chan, L.K.N.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.T.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan glutamate-coated niosomes: A proposal for nose-to-brain delivery. Pharmaceutics 2018, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 14, 7449. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 4, 462. [Google Scholar] [CrossRef] [Green Version]
- Taudorf, E.H.; Jemec, G.B.E.; Hay, R.J.; Saunte, D.M.L. Cutaneous candidiasis-an evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Dermatol. Venereol. 2019, 10, 1863–1873. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Bukhalo, M.; Blauvelt, A. A clinician’s guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am. J. Clin. Dermatol. 2016, 4, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Loo, Y.S.; Veettil, S.K.; Wong, P.S.; Divya, G.; Ching, S.M.; Menon, R.K. Efficacy and safety of posaconazole for the prevention of invasive fungal infections in immunocompromised patients: A systematic review with meta-analysis and trial sequential analysis. Sci. Rep. 2020, 1, 14575. [Google Scholar] [CrossRef]
- Chen, L.; Krekels, E.H.J.; Verweij, P.E.; Buil, J.B.; Knibbe, C.A.J.; Brüggemann, R.J.M. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 2020, 80, 671–695. [Google Scholar] [CrossRef] [Green Version]
- Drugbank Online. Available online: https://go.drugbank.com/drugs/DB01263 (accessed on 24 April 2022).
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-thaw induced gelation of alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan–based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Hermanto, D.; Mudasir, M.; Siswanta, D.; Kuswandi, B.; Ismillayli, N. Polyelectrolyte complex (PEC) of the alginate-chitosan membrane for immobilizing urease. J. Math. Fund. Sci. 2019, 51, 309–319. [Google Scholar] [CrossRef]
- Mei, J.Y.; Huang, T.; Bai, C.H.; Fu, Z. Influences of chitosan on freeze–thaw stability of Arenga pinnata starch. Int. J. Food Sci. Technol. 2021, 56, 692–699. [Google Scholar] [CrossRef]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 5, 511–518. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. The United States Pharmacopeial Convention; USP 41–NF 36; The United States Pharmacopeia: Rockville, MD, USA, 2016. [Google Scholar]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 3, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin barriers in dermal drug delivery: Which barriers have to be overcome and how can we measure them? Pharmaceutics 2020, 7, 684. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifications of chitosan: Syntheses and applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 2, 91–181. [Google Scholar] [CrossRef]
- Ways, M.T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 3, 267. [Google Scholar] [CrossRef] [Green Version]
- Kopplin, G.; Lervik, A.; Draget, K.I.; Aachmann, F.L. Alginate gels crosslinked with chitosan oligomers—A systematic investigation into alginate block structure and chitosan oligomer interaction. RSC Adv. 2021, 11, 13780–13798. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.P.; Spada, F. Hydrogels for atopic dermatitis and wound management: A superior drug delivery vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavda, H.; Modhia, I.; Mehta, A.; Patel, R.; Patel, C. Development of bioadhesive chitosan superporous hydrogel composite particles based intestinal drug delivery system. Biomed. Res. Int. 2013, 2013, 563651. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different types of gel carriers as metronidazole delivery systems to the oral mucosa. Polymers 2020, 3, 680. [Google Scholar] [CrossRef] [Green Version]
- Blanco-López, M.; González-Garcinuño, Á.; Tabernero, A.; Del Valle, M.E.M. Steady and oscillatory shear flow behavior of different polysaccharides with Laponite®. Polymers 2021, 6, 966. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik. Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Pandit, A. Cohesion mechanisms for bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Ilić, T.; Pantelić, I.; Savić, S. The implications of regulatory framework for topical semisolid drug products: From critical quality and performance attributes towards establishing bioequivalence. Pharmaceutics 2021, 13, 710. [Google Scholar] [CrossRef]
- Rençber, S.; Cheaburu-Yilmaz, C.N.; Köse, F.A.; Karavana, S.Y.; Yilmaz, O. Preparation and characterization of alginate and chitosan IPC based gel formulation for mucosal application. Cellulose Chem. Technol. 2019, 53, 655–665. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 12, 2326–2333. [Google Scholar] [CrossRef] [Green Version]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Bhutani, U.; Laha, A.; Mitra, K.; Majumdar, S. Sodium alginate and gelatin hydrogels: Viscosity effect on hydrophobic drug release. Mater. Lett. 2016, 164, 76–79. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. Available online: https://www.ptfarm.pl/pub/File/Acta_Poloniae/2010/3/217.pdf (accessed on 24 April 2022).
- Nikolova, D.; Simeonov, M.; Tzachev, C.; Apostolov, A.; Christov, L.; Vassileva, E. Polyelectrolyte complexes of chitosan and sodium alginate as a drug delivery system for diclofenac sodium. Polym. Int. 2021, 71, 668–678. [Google Scholar] [CrossRef]
- European Medicines Agency. Investigation of Bioequivalence; European Medicines Agency: London, UK, 29 January 2010. [Google Scholar]
- Shirkhorshidi, A.S.; Aghabozorgi, S.; Wah, T.Y. A comparison study on similarity and dissimilarity measures in clustering continuous data. PLoS ONE 2015, 10, e0144059. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elghany, M.; Klapötke, T.M. A review on differential scanning calorimetry technique and its importance in the field of energetic materials. Phys. Sci. Rev. 2018, 3, 20170103. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Mucha, M.; Pawlak, A. Thermal analysis of chitosan and its blends. Thermochim. Acta 2005, 427, 69–76. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R. New type of alginate/chitosan microparticle membranes for highly efficient pervaporative dehydration of ethanol. RSC Adv. 2018, 8, 39567–39578. [Google Scholar] [CrossRef] [Green Version]
- Gubanova, G.N.; Petrova, V.A.; Kononova, S.V.; Popova, E.N.; Smirnova, V.E.; Bugrov, A.N.; Klechkovskaya, V.V.; Skorik, Y.A. Thermal properties and structural features of multilayer films based on chitosan and anionic polysaccharides. Biomolecules 2021, 11, 762. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Profire, L.; Nita, L.E.; Dragostin, O.M.; Ghetu, N.; Pieptu, D.; Vasile, C. Sulfadiazine-chitosan conjugates and their polyelectrolyte complexes with hyaluronate destined to the management of burn wounds. Materials 2015, 8, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalho, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Pendekal, M.S.; Tegginamat, P.K. Hybrid drug delivery system for oropharyngeal, cervical and colorectal cancer-in vitro and in vivo evaluation. Saudi Pharm. J. 2013, 21, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Spadari, C.C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, M.F.; Powell, L.C.; Jack, A.A.; Powell, K.; Beck, K.; Florance, H.; Forton, J.; Rye, P.D.; Dessen, A.; Hill, K.E.; et al. A low-molecular-weight alginate oligosaccharide disrupts Pseudomonal microcolony formation and enhances antibiotic effectiveness. Antimicrob. Agents Chemother. 2017, 9, e00762-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 11, e112518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan loaded into a hydrogel delivery system as a strategy to treat vaginal co-infection. Pharmaceutics 2018, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate oligosaccharides affect mechanical properties and antifungal activity of alginate buccal films with posaconazole. Mar. Drugs. 2019, 17, 692. [Google Scholar] [CrossRef] [Green Version]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. BioMed Res. Int. 2013, 2013, 15. [Google Scholar] [CrossRef] [Green Version]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic cross-linking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Lee, J.A.; Chee, H.Y. In vitro antifungal activity of equol against Candida albicans. Mycobiology 2010, 38, 328–330. [Google Scholar] [CrossRef] [Green Version]





| Formulation | pH | Mean Particle Size (μm) | Drug Content (%) | Viscosity * (mPa∙s) |
|---|---|---|---|---|
| H0 | 7.17 ± 0.02 | – | – | 2986 ± 29 |
| H0.5 | 5.05 ± 0.06 | – | – | 984 ± 73 |
| H1 | 4.93 ± 0.03 | – | – | 3135 ± 675 |
| H2 | 4.94 ± 0.02 | – | – | 3831 ± 942 |
| HP0 | 6.78 ± 0.02 | 4.93 ± 2.45 | 100.93 ± 2.97 | 2916 ± 28 |
| HP0.5 | 5.06 ± 0.01 | 4.75 ± 1.95 | 105.54 ± 2.10 | 794 ± 108 |
| HP1 | 5.02 ± 0.03 | 4.99 ± 2.05 | 103.89 ± 1.20 | 2784 ± 290 |
| HP2 | 5.04 ± 0.04 | 4.61 ± 2.73 | 98.11 ± 2.56 | 3107 ± 315 |
| K0 | 6.98 ± 0.04 | – | – | 4919 ± 89 |
| K0.5 | 5.14 ± 0.04 | – | – | 3469 ± 256 |
| K1 | 5.12 ± 0.01 | – | – | 5204 ± 110 |
| K2 | 5.00 ± 0.03 | – | – | 5644 ± 545 |
| KP0 | 6.80 ± 0.05 | 4.45 ± 2.09 | 100.58 ± 3.07 | 4591 ± 392 |
| KP0.5 | 5.18 ± 0.02 | 4.28 ± 1.45 | 97.69 ± 8.45 | 2097 ± 915 |
| KP1 | 5.15 ± 0.01 | 4.60 ± 1.71 | 100.65 ± 4.06 | 3418 ± 659 |
| KP2 | 5.07 ± 0.02 | 4.06 ± 1.82 | 98.29 ± 2.36 | 4798 ± 108 |
| Formulation | Zero Order Kinetics | First Order Kinetics | Higuchi Model | Korsmeyer–Peppas Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | n | |
| HP0 | 0.995 | 0.608 | 0.968 | 0.466 | 0.966 | 1.631 | 0.988 | 0.555 | 0.422 |
| HP0.5 | 0.991 | 0.860 | 0.915 | 0.492 | 0.995 | 2.347 | 0.973 | 0.600 | 0.499 |
| HP1 | 0.980 | 0.308 | 0.975 | 0.519 | 0.937 | 0.824 | 0.992 | 0.629 | 0.652 |
| HP2 | 0.987 | 0.205 | 0.955 | 0.488 | 0.951 | 0.549 | 0.988 | 0.587 | 0.645 |
| KP0 | 0.988 | 0.207 | 0.955 | 0.488 | 0.952 | 0.554 | 0.997 | 0.414 | 0.341 |
| KP0.5 | 0.984 | 0.448 | 0.859 | 0.466 | 0.994 | 1.229 | 0.937 | 0.573 | 0.425 |
| KP1 | 0.975 | 0.155 | 0.927 | 0.309 | 0.849 | 0.405 | 0.923 | 0.356 | 0.293 |
| KP2 | 0.970 | 0.115 | 0.971 | 0.313 | 0.929 | 0.306 | 0.969 | 0.369 | 0.358 |
| Formulation | ALG (%) | CS-G (%) | POS (%) |
|---|---|---|---|
| H0 | 2 | – | – |
| H0.5 | 2 | 0.5 | – |
| H1 | 2 | 1 | – |
| H2 | 2 | 2 | – |
| HP0 | 2 | – | 2 |
| HP0.5 | 2 | 0.5 | 2 |
| HP1 | 2 | 1 | 2 |
| HP2 | 2 | 2 | 2 |
| K0 | 2 | – | – |
| K0.5 | 2 | 0.5 | – |
| K1 | 2 | 1 | – |
| K2 | 2 | 2 | – |
| KP0 | 2 | – | 2 |
| KP0.5 | 2 | 0.5 | 2 |
| KP1 | 2 | 1 | 2 |
| KP2 | 2 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug? Int. J. Mol. Sci. 2022, 23, 6775. https://doi.org/10.3390/ijms23126775
Szekalska M, Sosnowska K, Wróblewska M, Basa A, Winnicka K. Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug? International Journal of Molecular Sciences. 2022; 23(12):6775. https://doi.org/10.3390/ijms23126775
Chicago/Turabian StyleSzekalska, Marta, Katarzyna Sosnowska, Magdalena Wróblewska, Anna Basa, and Katarzyna Winnicka. 2022. "Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug?" International Journal of Molecular Sciences 23, no. 12: 6775. https://doi.org/10.3390/ijms23126775
APA StyleSzekalska, M., Sosnowska, K., Wróblewska, M., Basa, A., & Winnicka, K. (2022). Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug? International Journal of Molecular Sciences, 23(12), 6775. https://doi.org/10.3390/ijms23126775










