Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Self-Adhesive Properties of a Transdermal Patch Containing Various Active Substances
2.2. Microscopy and Stability Assessment of Acrylate Transdermal Patches with Structurally Modified Ibuprofen
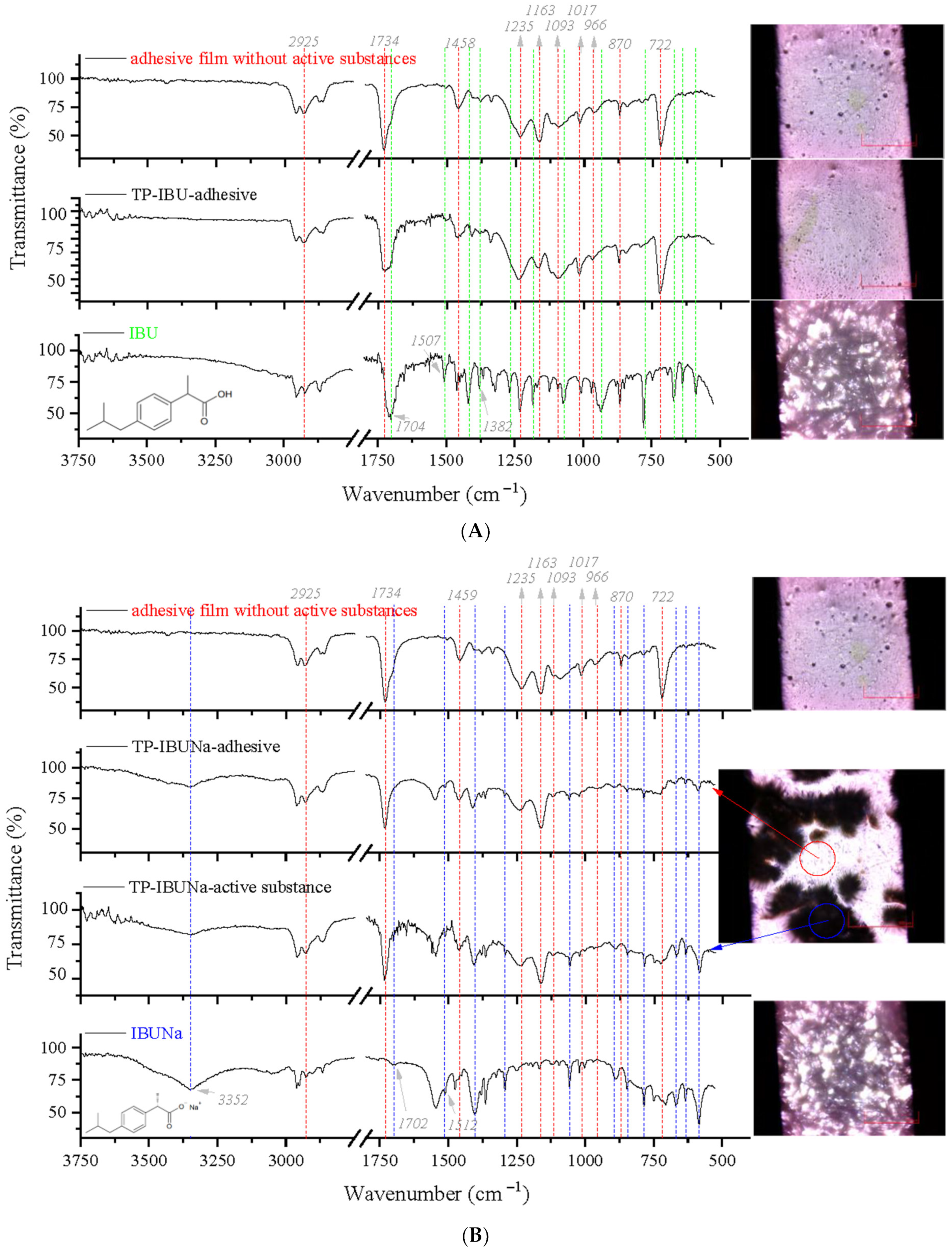
2.3. Microspectroscopy Analysis
2.4. TG and DSC
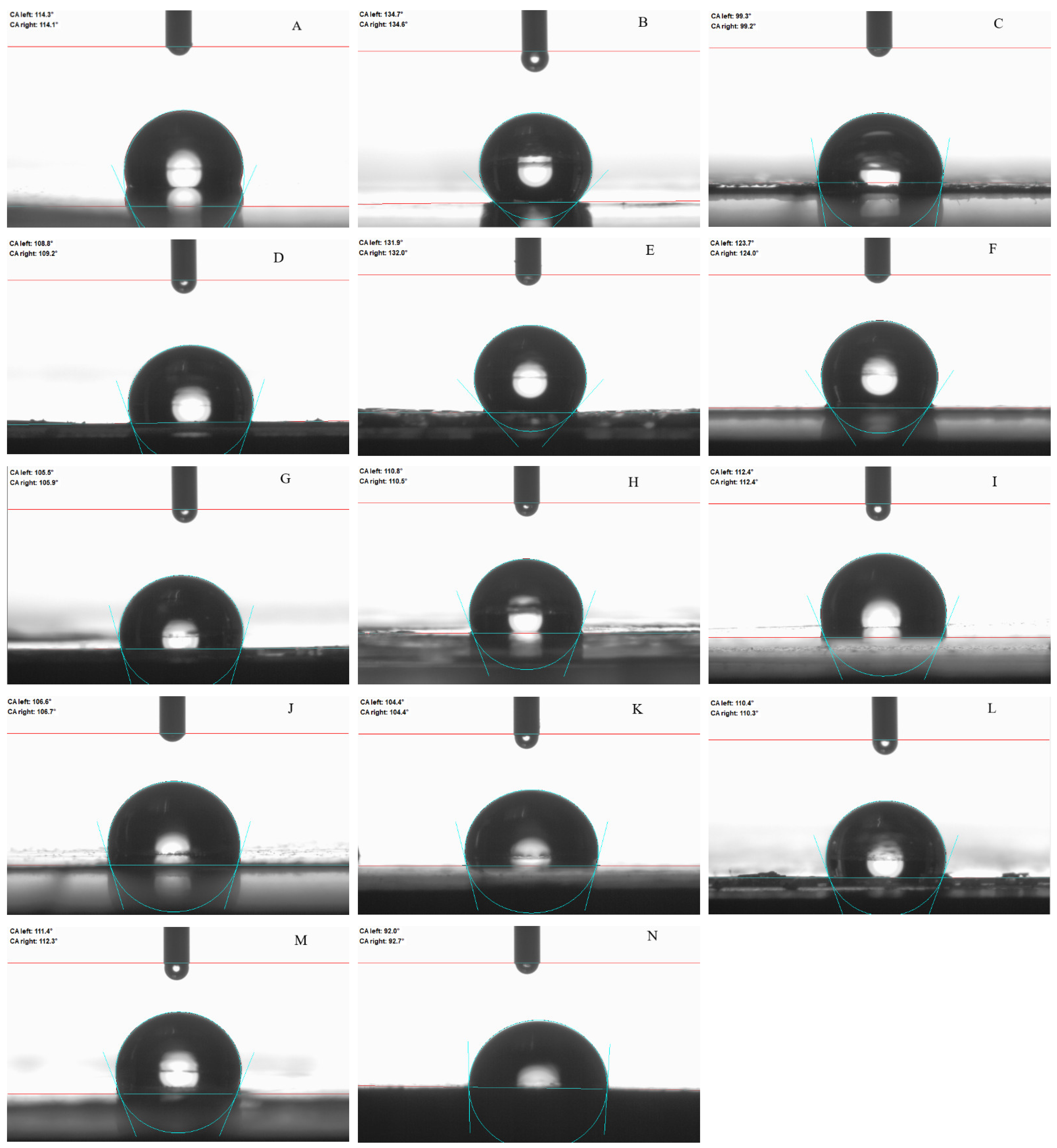
2.5. Contact Angle
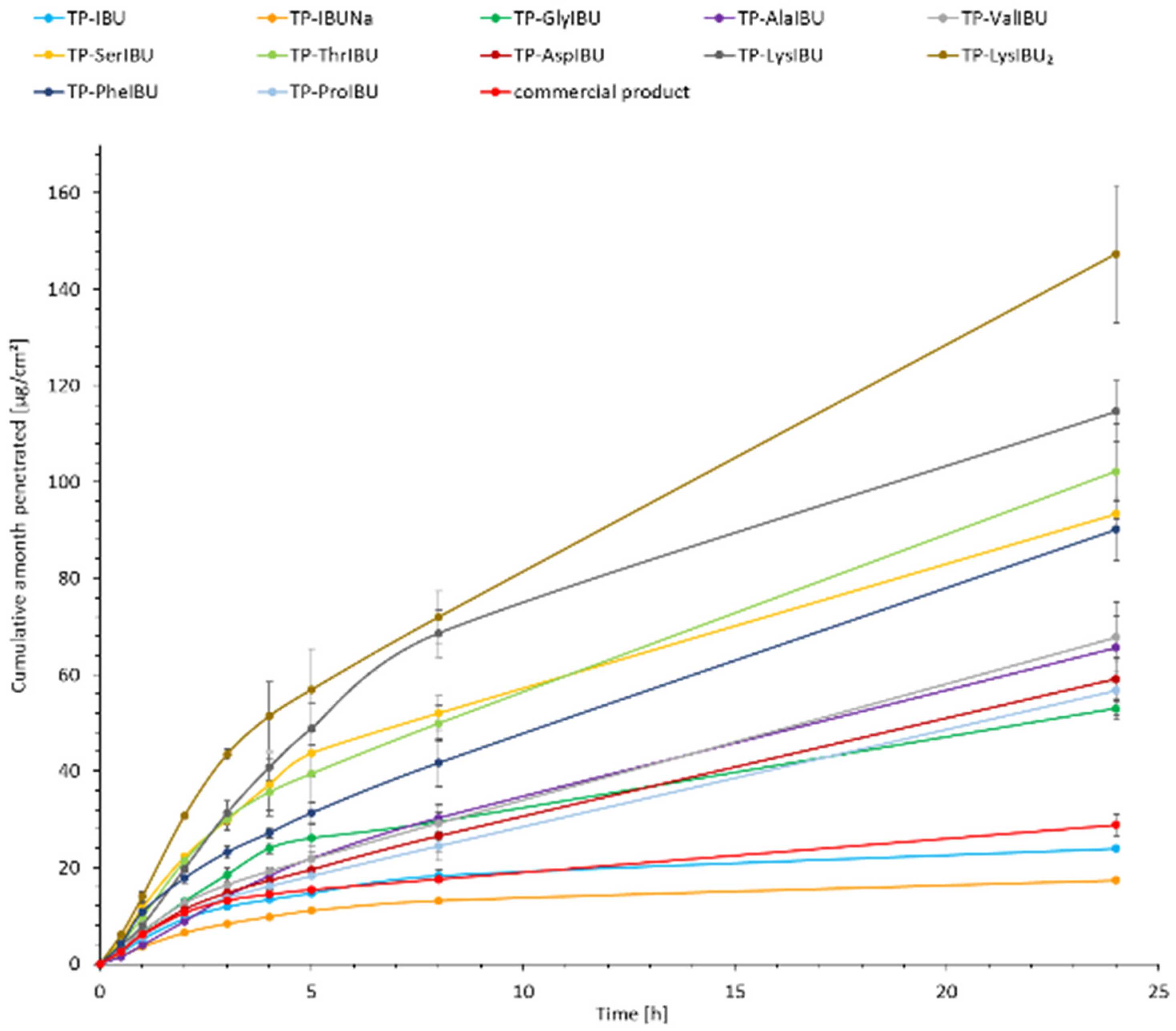
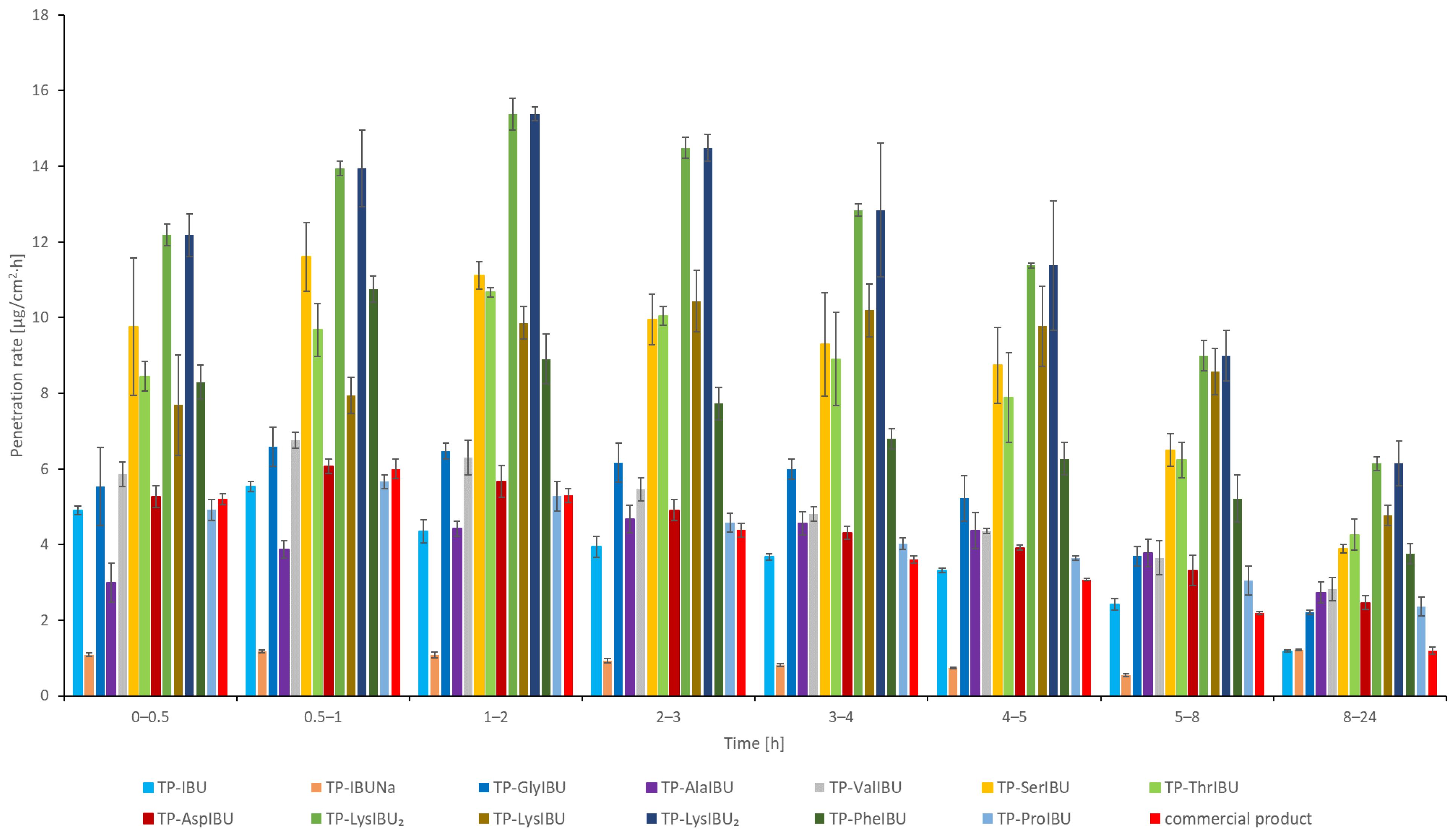
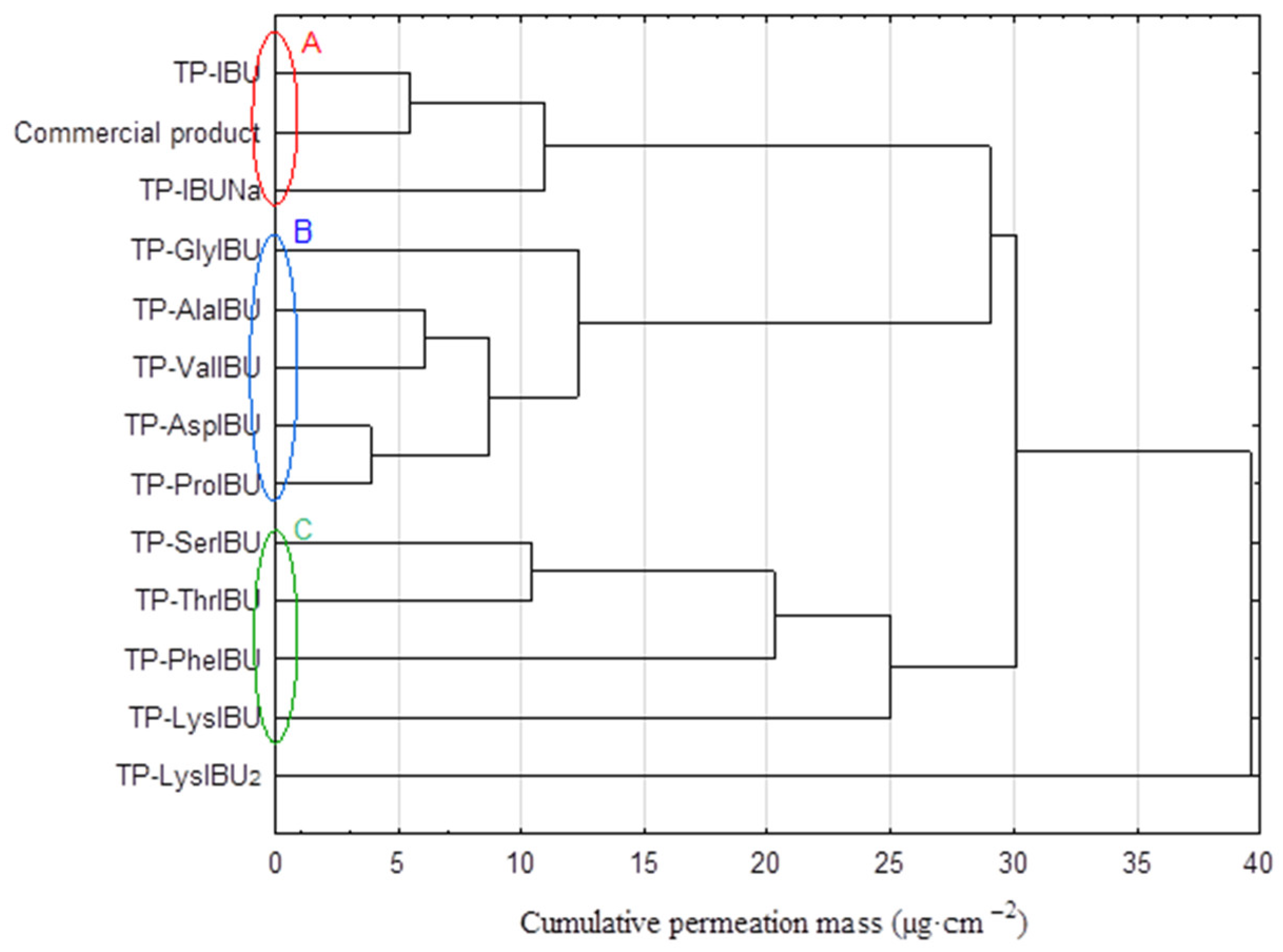
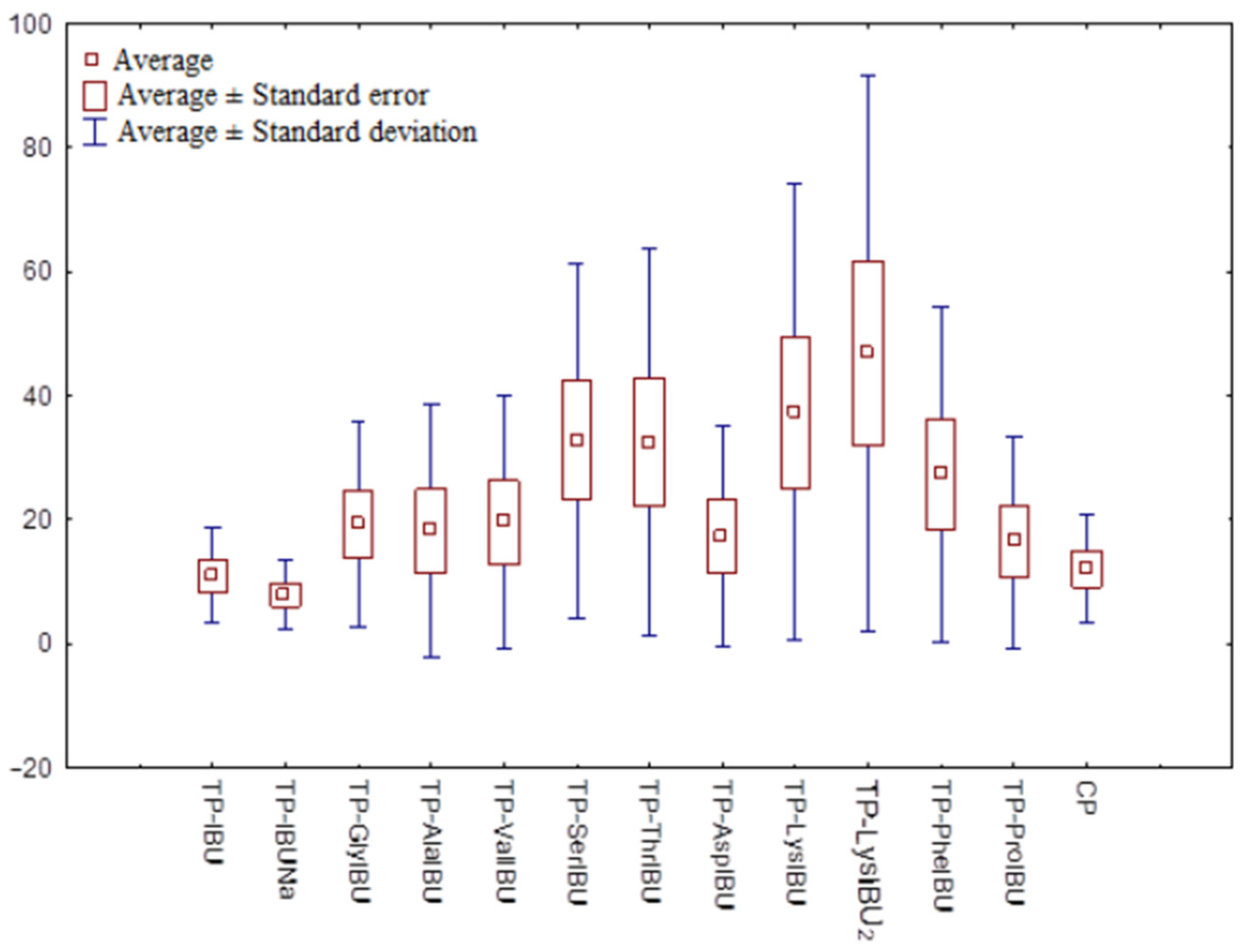
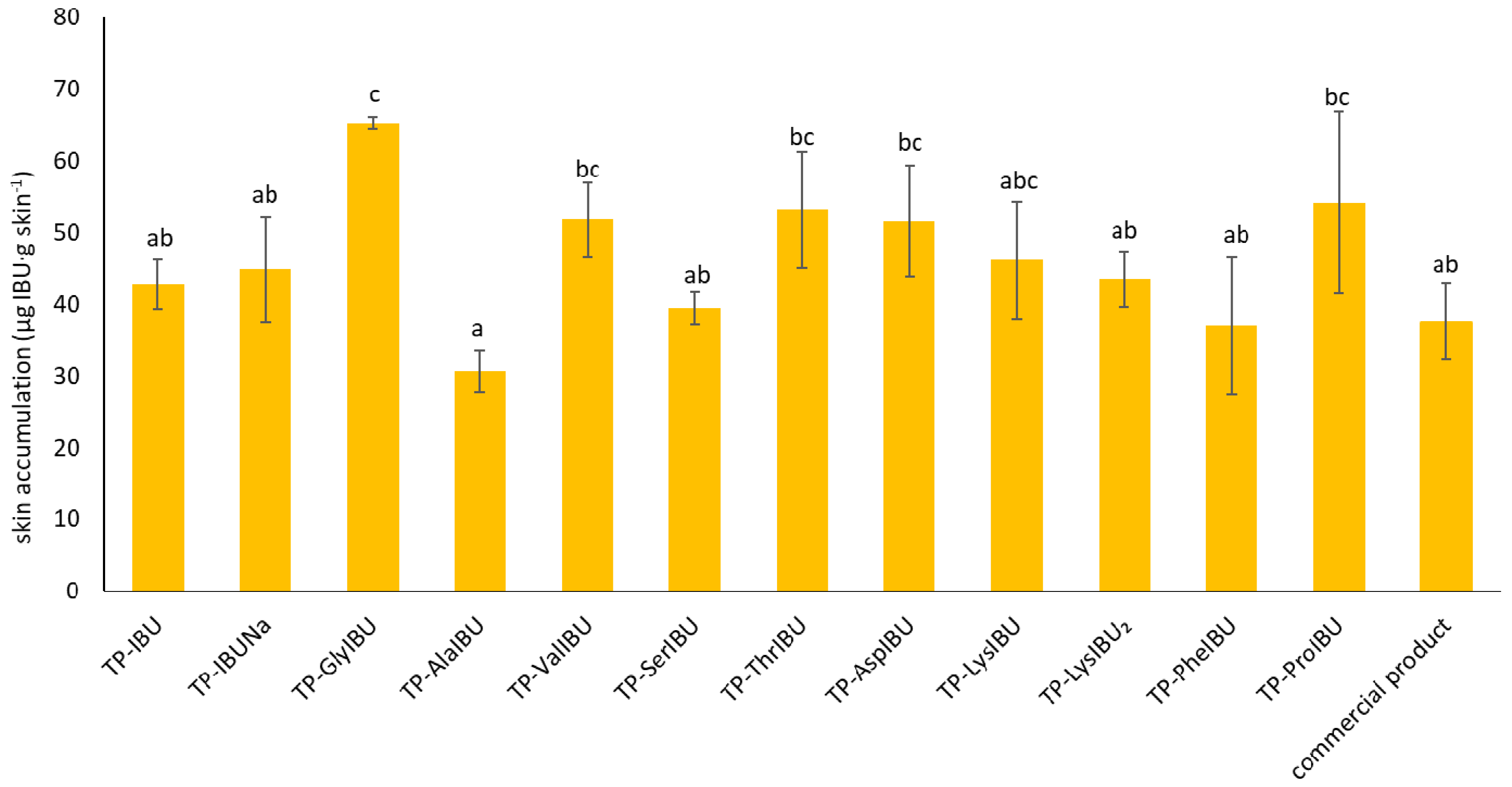
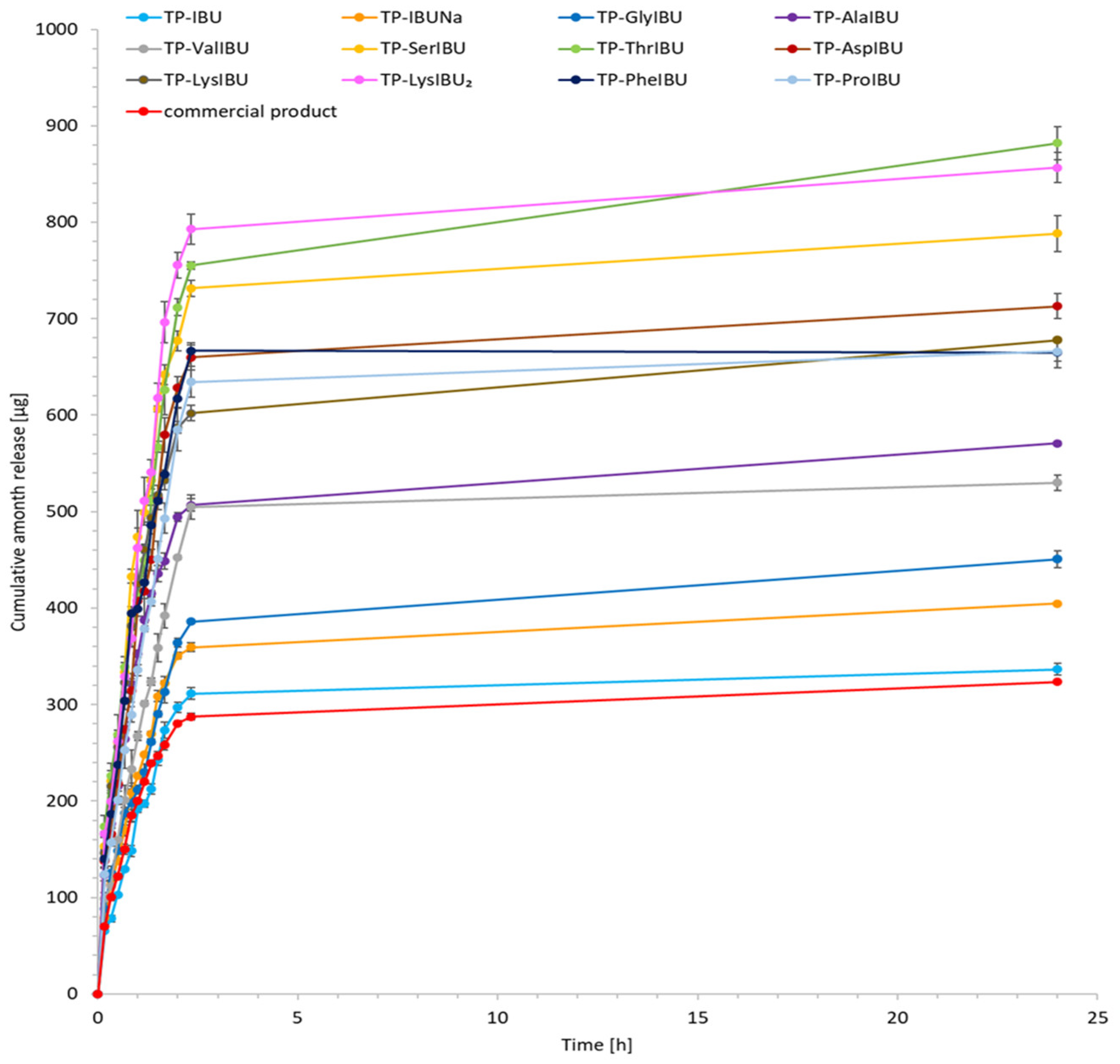
2.6. Permeability, Release, and Accumulation in Skin Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of Adhesive Films
3.3. Characterization and Performance Evaluation
3.4. Self-Adhesive Properties
3.5. Microscopy and Stability Assessment of Acrylate Transdermal Patches
3.6. Infrared Microspectroscopy
3.7. Contact Angle
3.8. In Vitro Skin Permeation Studies
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irvine, J.; Afrose, A.; Islam, N. Formulation and delivery strategies of ibuprofen: Challenges and opportunities. Drug Dev. Ind. Pharm. 2017, 44, 173–183. [Google Scholar] [CrossRef]
- Pratiwi, G.; Susanti, S.; Shiyan, S.; Selatan, S. Application of Factorial Design for Optimization of PVC-HPMC Polymers in Matrix Film Ibuprofen Patch-Transdermal Drug Delivery System. Indones. J. Chemom. Pharm. Anal. 2021, 1, 11–21. [Google Scholar] [CrossRef]
- Tombs, E.L.; Nikolaou, V.; Nurumbetov, G.; Haddleton, D.M. Transdermal Delivery of Ibuprofen Utilizing a Novel Solvent-Free Pressure-sensitive Adhesive (PSA): TEPI® Technology. J. Pharm. Innov. 2018, 13, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz-rupniewska, P.; Rakoczy, R.; Nowak, A.; Konopacki, M.; Klebeko, J.; Świątek, E.; Janus, E.; Duchnik, W.; Wenelska, K.; Kucharski, Ł.; et al. Transdermal Delivery Systems for Ibuprofen and Ibuprofen Modified with Amino Acids Alkyl Esters Based on Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 6252. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Dhiman, S.; Gurjeet Singh, T.; Kumar Rehni, A. Transdermal patches: A recent approch to new drug delivery system. Int. J. Pharm. Pharm. Sci. 2011, 3, 26–34. [Google Scholar]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Zakari, Z.; Adwan, S.; Al-Remawi, M. Preparation, characterization and ex-vivo human skin permeation of ibuprofen-soluplus polymeric nanomicelles. Int. J. Pharm. Sci. Res. 2020, 12, 2863–2869. [Google Scholar]
- Hadgraft, J.; Whitefield, M.; Rosher, P.H. Skin Penetration of Topical Formulations of Ibuprofen 5%: An in vitro Comparative Study. Skin Pharmacol. Physiol. 2003, 16, 137–142. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with L-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Klebeko, J.; Ossowicz-Rupniewska, P.; Nowak, A.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N.; et al. Permeability of Ibuprofen in the Form of Free Acid and Salts of L-Valine Alkyl Esters from a Hydrogel Formulation through Strat-MTM Membrane and Human Skin. Materials 2021, 14, 6678. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Nowak, A.; Klebeko, J.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N.; et al. Assessment of the Effect of Structural Modification of Ibuprofen on the Penetration of Ibuprofen from Pentravan® (Semisolid) Formulation Using Human Skin and a Transdermal Diffusion Test Model. Materials 2021, 14, 6808. [Google Scholar] [CrossRef]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The effect of alcohols as vehicles on the percutaneous absorption and skin retention of ibuprofen modified with L-valine alkyl esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, D.H. Quality by design approach to the development of transdermal patch systems and regulatory perspective. J. Pharm. Investig. 2021, 51, 669–690. [Google Scholar] [CrossRef]
- Yang, D.; Liu, C.; Piao, H.; Quan, P.; Fang, L. Enhanced Drug Loading in the Drug-in-Adhesive Transdermal Patch Utilizing a Drug-Ionic Liquid Strategy: Insight into the Role of Ionic Hydrogen Bonding. Mol. Pharm. 2021, 18, 1157–1166. [Google Scholar] [CrossRef]
- Yang, D.; Liu, C.; Quan, P.; Fang, L. A systematic approach to determination of permeation enhancer action efficacy and sites: Molecular mechanism investigated by quantitative structure−activity relationship. J. Control. Release 2020, 322, 1–12. [Google Scholar] [CrossRef]
- Choi, H.K.; Acharya, G.; Lee, Y.; Lee, C.H. A Data-Mining Approach for the Quantitative Assessment of Physicochemical Properties of Molecular Compounds in the Skin Flux. AAPS PharmSciTech 2021, 22, 117. [Google Scholar] [CrossRef]
- Liu, J.; Fang, L.; Liu, C. Investigating the influences of intermolecular interactions on viscoelastic performance of pressure-sensitive adhesive by FT–IR spectroscopy and molecular modeling. Drug Dev. Ind. Pharm. 2020, 46, 1005–1014. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Mozelewska, K.; Czech, Z. Influence of the UV crosslinking method on the properties of acrylic adhesive. Int. J. Adhes. Adhes. 2020, 102, 102652. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Quaroni, G.M.G.; Creton, C.; Minghetti, P.; Cilurzo, F. SEBS block copolymers as novel materials to design transdermal patches. Int. J. Pharm. 2020, 575, 118975. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Rokicka, J.; Klimowicz, A.; Czech, Z. Sustainable UV-Crosslinkable Acrylic Pressure-Sensitive Adhesives for Medical Application. Int. J. Mol. Sci. 2021, 22, 11840. [Google Scholar] [CrossRef]
- Banga, A.K.; Puri, A.; Bhattaccharjee, S.A.; Zhang, W.; Clark, M.; Singh, O.N.; Doncel, G.F. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics 2019, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Droesbeke, M.A.; Simula, A.; Asua, J.M.; Du Prez, F.E. Biosourced terpenoids for the development of sustainable acrylic pressure-sensitive adhesives via emulsion polymerisation. Green Chem. 2020, 22, 4561–4569. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Świderska, J. UV-crosslinkable acrylic pressure-sensitive adhesives for industrial application. Polym. Bull. 2012, 69, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Kajtna, J.; Likozar, B.; Golob, J.; Krajnc, M. The influence of the polymerization on properties of an ethylacrylate/2-ethyl hexylacrylate pressure-sensitive adhesive suspension. Int. J. Adhes. Adhes. 2008, 28, 382–390. [Google Scholar] [CrossRef]
- Degrandi-Contraires, E.; Lopez, A.; Reyes, Y.; Asua, J.M.; Creton, C. High-Shear-Strength Waterborne Polyurethane/Acrylic Soft Adhesives. Macromol. Mater. Eng. 2013, 298, 612–623. [Google Scholar] [CrossRef]
- Cilurzo, F.; Gennari, C.G.M.; Minghetti, P. Adhesive properties: A critical issue in transdermal patch development. Expert Opin. Drug Deliv. 2011, 9, 33–45. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Klebeko, J.; Świątek, E.; Bilska, K.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Struk, Ł.; Wenelska, K.; Klimowicz, A.; et al. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. Int. J. Mol. Sci. 2022, 23, 4158. [Google Scholar] [CrossRef]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and functional comparisons of four anatomical regions of porcine skin with human abdominal skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Kuntsche, J.; Bunjes, H.; Fahr, A.; Pappinen, S.; Rönkkö, S.; Suhonen, M.; Urtti, A. Interaction of lipid nanoparticles with human epidermis and an organotypic cell culture model. Int. J. Pharm. 2008, 354, 180–195. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Song, Y.H.; Gwak, H.S.; Chun, I.K. The effects of terpenes on the permeation of lidocaine and ofloxacin from moisture-activated patches. Drug Deliv. 2009, 16, 75–81. [Google Scholar] [CrossRef]


| Sample Code | Coat Weight (g/m2) | SWC (%) | Shear Strength | Adhesion (N/25 mm) | Tack (N) |
|---|---|---|---|---|---|
| DT54 | 32 | 98 | >72 h | 13.60 | 14.00 |
| TP-IBU | 40 | 97 | 10 min/c.f. | 11.90/c.f. | 13.50 |
| TP-IBUNa | 28 | 97 | 1 min 26 s/c.f. | 0.08/c.f. | 0.15/c.f. |
| TP-GlyIBU | 28 | 93 | 3 min 11 s/c.f. | 10.14/c.f. | 8.60 |
| TP-AlaIBU | 27 | 92 | 1 min 42 s/c.f. | 11.48/c.f. | 11.18 |
| TP-ValIBU | 17 | 96 | 2 min 16 s/c.f. | 6.77/c.f. | 2.61 |
| TP-SerIBU | 23 | 92 | 1 min/c.f. | 3.68/c.f. | 8.08/c.f. |
| TP-ThrIBU | 39 | 91 | 1 min 18 s/c.f. | 9.16/c.f. | 4.81/c.f. |
| TP-AspIBU | 38 | 90 | 48 s/c.f. | 7.78/c.f. | 9.68/c.f. |
| TP-LysIBU | 25 | 94 | 17 h 18 min | 3.17 | 2.03 |
| TP-LysIBU2 | 35 | 93 | 6 min 8 s/c.f. | 13.32/c.f. | 8.72 |
| TP-PheIBU | 22 | 90 | 11 min/c.f. | 11.26/c.f. | 8.04 |
| TP-ProIBU | 35 | 94 | 1 min 10 s/c.f. | 7.57/c.f. | 11.55 |
| Sample Code | First Day of Observation | Observation after 7 Days (Patches Not Protected with Siliconized Foil) | Observation after 3 Months (Patches Protected with Siliconized Foil) |
|---|---|---|---|
| TP-IBU | 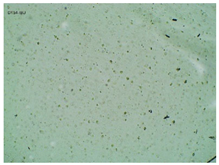 | 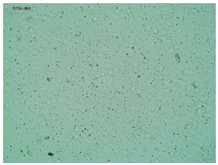 |  |
| TP-IBUNa | 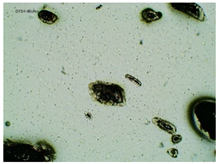 | 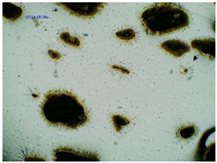 | 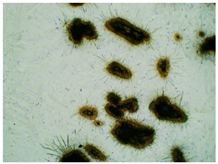 |
| TP-GlyIBU |  | 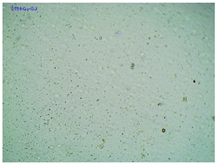 | 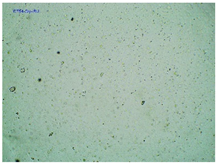 |
| TP-AlaIBU | 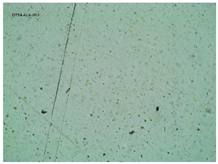 | 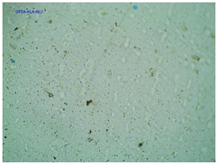 |  |
| TP-ValIBU |  | 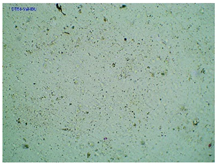 | 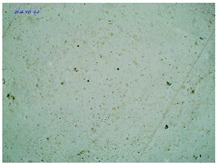 |
| TP-SerIBU | 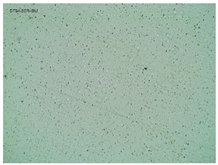 |  |  |
| TP-ThrIBU | 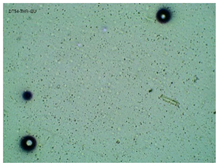 |  | 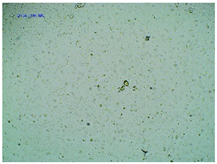 |
| TP-AspIBU | 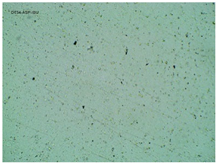 |  | 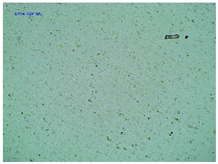 |
| TP-LysIBU | 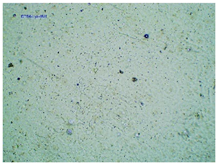 | 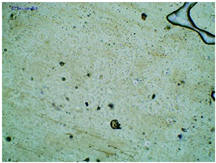 | 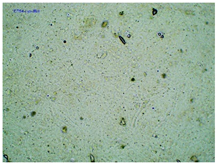 |
| TP-LysIBU2 | 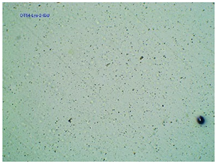 |  | 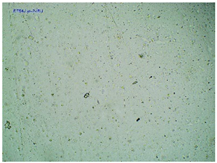 |
| TP-PheIBU |  | 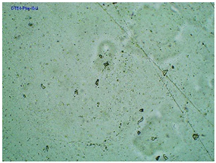 |  |
| TP-Pro-IBU | 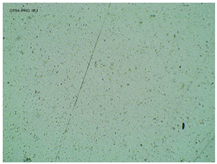 | 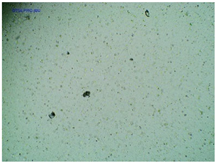 |  |
| Sample Code | First Day of Observation | Patches Not Protected with Siliconized Foil—Observation after 7 Days | Patches Protected with Siliconized Foil—Observation after 3 Months |
|---|---|---|---|
| TP-IBU | 26 ± 6 µm | 51 ± 4 µm | 19 ± 9 µm |
| TP-IBUNa | 168 ± 64 µm | 263 ± 112 µm | 231 ± 13 µm |
| TP-GlyIBU | 28 ± 5 µm | 38 ± 12 µm | 27 ± 4 µm |
| TP-AlaIBU | 32 ± 5 µm | 52 ± 9 µm | 24 ± 9 µm |
| TP-ValIBU | 24 ± 9 µm | 28 ± 7 µm | 24 ± 3 µm |
| TP-SerIBU | 13 ± 6 µm | 57 ± 14 µm | 13 ± 5 µm |
| TP-ThrIBU | 32 ± 9 µm | 36 ± 4 µm | 29 ± 6 µm |
| TP-AspIBU | 26 ± 9 µm | 36 ± 6 µm | 23 ± 6 µm |
| TP-LysIBU | 23 ± 3 µm | 45 ± 14 µm | 36 ± 4 µm |
| TP-LysIBU2 | 26 ± 2 µm | 67 ± 9 µm | 39 ± 4 µm |
| TP-PheIBU | 20 ± 7 µm | 41 ± 9 µm | 23 ± 5 µm |
| TP-ProIBU | 17 ± 13 µm | 38 ± 6 µm | 15 ± 2 µm |
| Sample Code | Tg (°C) | TIDT (°C) | Td50% (°C) | TMDT (°C) |
|---|---|---|---|---|
| DT54 | −45.98 | 301.1 | 357.6 | 347.1 |
| TP-IBU | −51.88 | 165.8 | 333.0 | 348.6 |
| TP-IBUNa | nd | 256.2 | 348.1 | 307.6 |
| TP-GlyIBU | −46.14 | 166.6 | 356.2 | 378.4 |
| TP-AlaIBU | −51.06 | 182.0 | 351.4 | 361.3 |
| TP-ValIBU | −48.35 | 168.3 | 350.1 | 361.6 |
| TP-SerIBU | −47.01 | 185.4 | 346.2 | 371.2 |
| TP-ThrIBU | −50.47 | 181.6 | 361.7 | 382.0 |
| TP-AspIBU | −51.23 | 180.9 | 338.8 | 392.0 |
| TP-LysIBU | −9.57 | 164.2 | 351.8 | 390.3 |
| TP-LysIBU2 | −26.89 | 184.3 | 333.8 | 392.5 |
| TP-PheIBU | −43.64 | 159.5 | 338.1 | 360.7 |
| TP-ProIBU | −51.34 | 187.1 | 354.8 | 381.9 |
| Sample Code | Cumulative Permeation Mass, µg IBU/cm2 | JSS, µg IBU/cm2·h | KP·103, cm/h | LT, min | D, cm2/h | Km·103 | Q%24 h |
|---|---|---|---|---|---|---|---|
| TP-IBU | 23.979 ± 0.547 a | 4.702 | 3.291 | 1.231 | 0.406 | 0.405 | 1.679 |
| TP-IBUNa | 17.378 ± 1.408 a | 0.771 | 0.539 | 28.770 | 0.017 | 1.552 | 0.406 |
| TP-GlyIBU | 53.019 ± 1.519 b | 6.005 | 4.204 | 3.492 | 0.143 | 1.468 | 3.711 |
| TP-AlaIBU | 65.601 ± 6.542 b | 4.852 | 3.396 | 11.003 | 0.045 | 3.736 | 4.592 |
| TP-ValIBU | 67.741 ± 7.244 b | 4.620 | 3.234 | 24.413 | 0.020 | 7.894 | 4.742 |
| TP-SerIBU | 93.343 ± 2.673 c | 11.431 | 8.002 | 2.271 | 0.222 | 1.817 | 6.534 |
| TP-ThrIBU | 102.211± 9.860 cd | 10.534 | 7.374 | 3.932 | 0.128 | 2.899 | 7.155 |
| TP-AspIBU | 59.143 ± 4.307 b | 5.716 | 4.001 | 0.126 | 3.969 | 0.050 | 4.140 |
| TP-LysIBU | 114.653 ± 6.375 d | 11.157 | 7.810 | 13.042 | 0.038 | 10.186 | 8.026 |
| TP-LysIBU2 | 147.356 ± 14.215 e | 15.112 | 10.578 | 0.655 | 0.127 | 4.159 | 10.315 |
| TP-PheIBU | 90.132 ± 6.563 c | 8.821 | 6.174 | 4.193 | 0.119 | 2.589 | 6.309 |
| TP-ProIBU | 56.765 ± 6.071 b | 5.328 | 3.729 | 0.126 | 3.964 | 0.047 | 3.974 |
| Commercial product | 28.817 ± 2.158 a | 5.226 | 3.658 | 3.487 | 0.143 | 1.276 | 2.017 |
| Symbol | Name | Mmol (g/mol) | Chemical Structure |
|---|---|---|---|
| IBU | (RS)-ibuprofen | 206.28 |  |
| IBUNa | ibuprofen sodium salts | 228.26 | 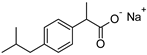 |
| Gly-IBU | [GlyOiPr][IBU] | 323.43 |  |
| Ala-IBU | [AlaOiPr][IBU] | 337.45 | 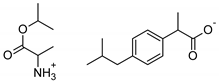 |
| Val-IBU | [ValOiPr][IBU] | 365.51 | 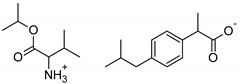 |
| Ser-IBU | [SerOiPr][IBU] | 353.45 | 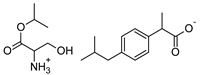 |
| Thr-IBU | [ThrOiPr][IBU] | 367.48 | 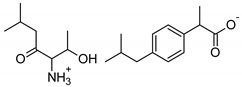 |
| Asp-IBU | [(AspOiPr)2][IBU] | 423.54 | 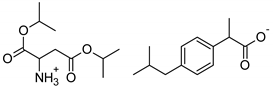 |
| Lys-IBU | [LysOiPr][IBU] | 394.55 | 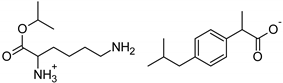 |
| Lys-IBU2 | [LysOiPr][IBU]2 | 600.83 |  |
| Phe-IBU | [PheOiPr][IBU] | 413.55 | 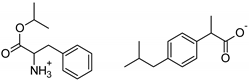 |
| Pro-IBU | [ProOiPr][IBU] | 363.49 | 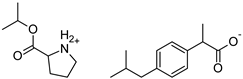 |
| Sample Code of the Adhesive Composition Containing the Active Substance | PSA Characteristics | Active Substance Characteristics | The Weight Ratio of the Adhesive to the Active Substance | |||||
|---|---|---|---|---|---|---|---|---|
| Symbol | SWC (%) | l (µm) | Coat Weight (g/m2) | Symbol | Mmol (g/mol) | PSA (g) | Active Substance (g) * | |
| TP-IBU | DT54 | 49.7 | 250 | 32 | IBU | 206.28 | 0.901 | 0.200 |
| TP-IBUNa | IBUNa | 228.26 | 0.221 | |||||
| TP-GlyIBU | Gly-IBU | 323.43 | 0.314 | |||||
| TP-AlaIBU | Ala-IBU | 337.45 | 0.327 | |||||
| TP-ValIBU | Val-IBU | 365.51 | 0.354 | |||||
| TP-Ser-IBU | Ser-IBU | 353.45 | 0.343 | |||||
| TP-ThrIBU | Thr-IBU | 367.48 | 0.356 | |||||
| TP-AspIBU | Asp-IBU | 423.54 | 0.411 | |||||
| TP-LysIBU | Lys-IBU | 394.55 | 0.383 | |||||
| TP-LysIBU2 | Lys-IBU2 | 600.83 | 0.583 | |||||
| TP-PheIBU | Phe-IBU | 413.55 | 0.401 | |||||
| TP-ProIBU | Pro-IBU | 363.49 | 0.352 | |||||
| Sample Code of the Adhesive Composition Containing the Active Substance | PSA | Active Substance | Solvent | |||
|---|---|---|---|---|---|---|
| Symbol | Weight (g) | Symbol | Weight (g) | Symbol | Weight (g) | |
| TP-IBU | DT54 | 4.51 | IBU | 1.00 | OE | 1.00 |
| TP-IBUNa | IBUNa | 1.11 | 2.00 | |||
| TP-GlyIBU | Gly-IBU | 1.57 | 4.00 | |||
| TP-AlaIBU | Ala-IBU | 1.64 | 2.00 | |||
| TP-ValIBU | Gly-IBU | 1.77 | 8.00 | |||
| TP-Ser-IBU | Ser-IBU | 1.71 | 6.00 | |||
| TP-ThrIBU | Thr-IBU | 1.78 | 2.00 | |||
| TP-AspIBU | Asp-IBU | 2.05 | 1.00 | |||
| TP-LysIBU | Lys-IBU | 1.91 | 6.00 | |||
| TP-LysIBU2 | Lys-IBU2 | 2.91 | 3.00 | |||
| TP-PheIBU | Phe-IBU | 2.01 | 7.00 | |||
| TP-ProIBU | Pro-IBU | 1.76 | 2.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowicz-Rupniewska, P.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klebeko, J.; Świątek, E.; Bilska, K.; Rokicka, J.; et al. Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch. Int. J. Mol. Sci. 2022, 23, 7752. https://doi.org/10.3390/ijms23147752
Ossowicz-Rupniewska P, Bednarczyk P, Nowak M, Nowak A, Duchnik W, Kucharski Ł, Klebeko J, Świątek E, Bilska K, Rokicka J, et al. Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch. International Journal of Molecular Sciences. 2022; 23(14):7752. https://doi.org/10.3390/ijms23147752
Chicago/Turabian StyleOssowicz-Rupniewska, Paula, Paulina Bednarczyk, Małgorzata Nowak, Anna Nowak, Wiktoria Duchnik, Łukasz Kucharski, Joanna Klebeko, Ewelina Świątek, Karolina Bilska, Joanna Rokicka, and et al. 2022. "Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch" International Journal of Molecular Sciences 23, no. 14: 7752. https://doi.org/10.3390/ijms23147752
APA StyleOssowicz-Rupniewska, P., Bednarczyk, P., Nowak, M., Nowak, A., Duchnik, W., Kucharski, Ł., Klebeko, J., Świątek, E., Bilska, K., Rokicka, J., Janus, E., Klimowicz, A., & Czech, Z. (2022). Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch. International Journal of Molecular Sciences, 23(14), 7752. https://doi.org/10.3390/ijms23147752









