Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF)
Abstract
:1. Introduction
2. Results
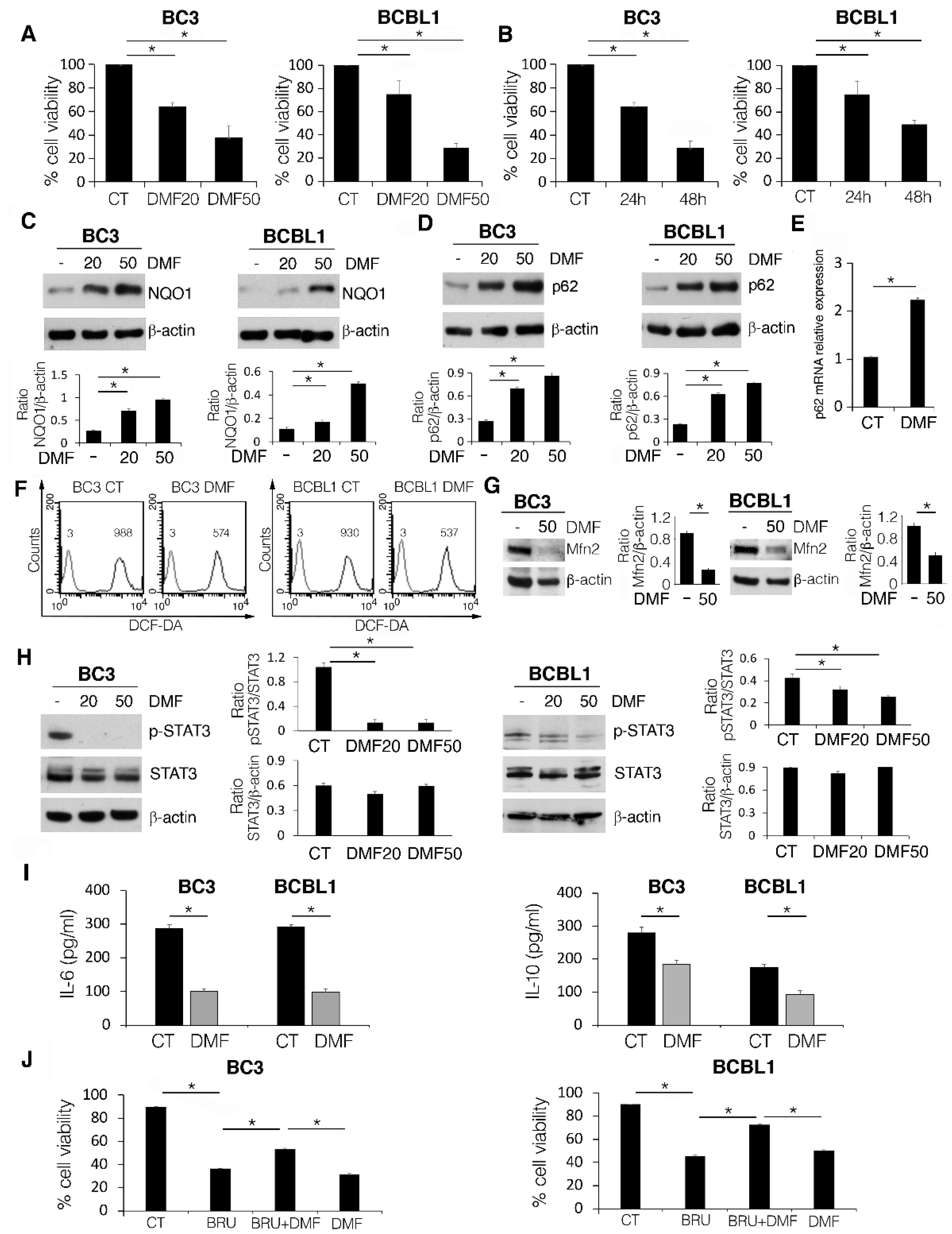
2.1. DMF Impairs PEL Cell Survival by Activating NRF2, Reducing Intracellular ROS and STAT3 Activation, and Counteracting the Release of IL-6 and IL-10 Cytokines
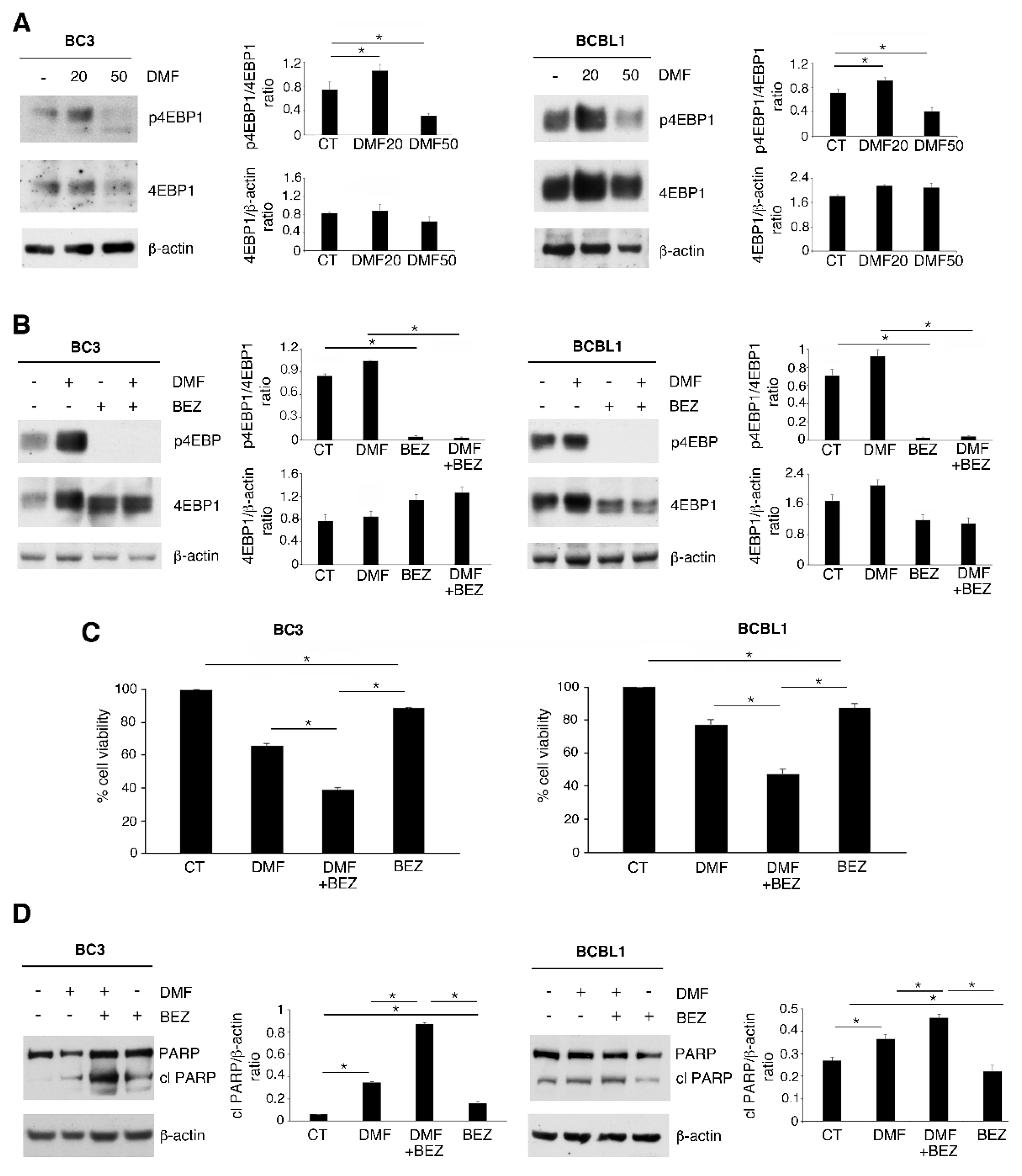
2.2. p-4EBP1 Activation Counteracts the Cytotoxic Effect of DMF at a Low Dose
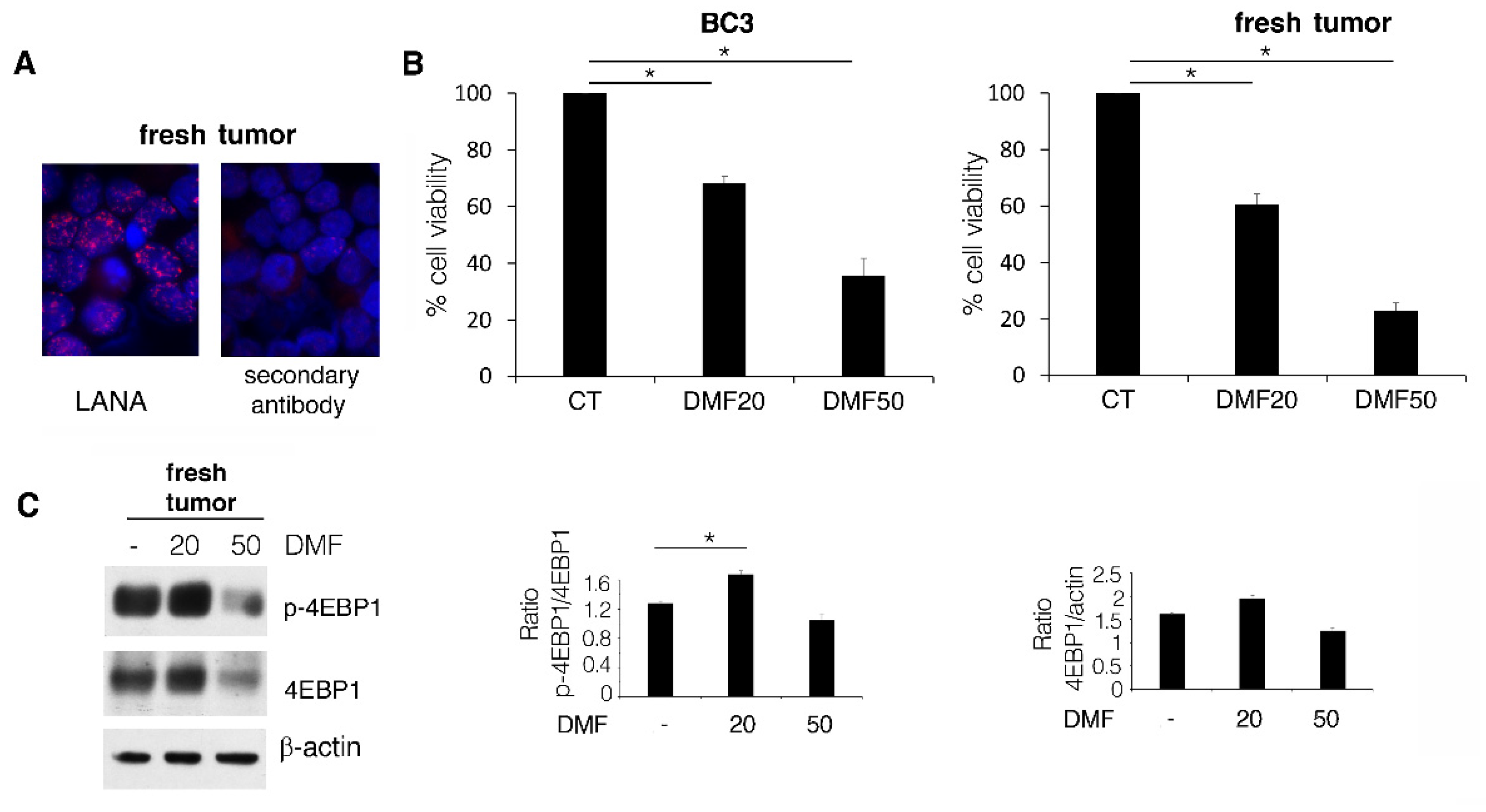
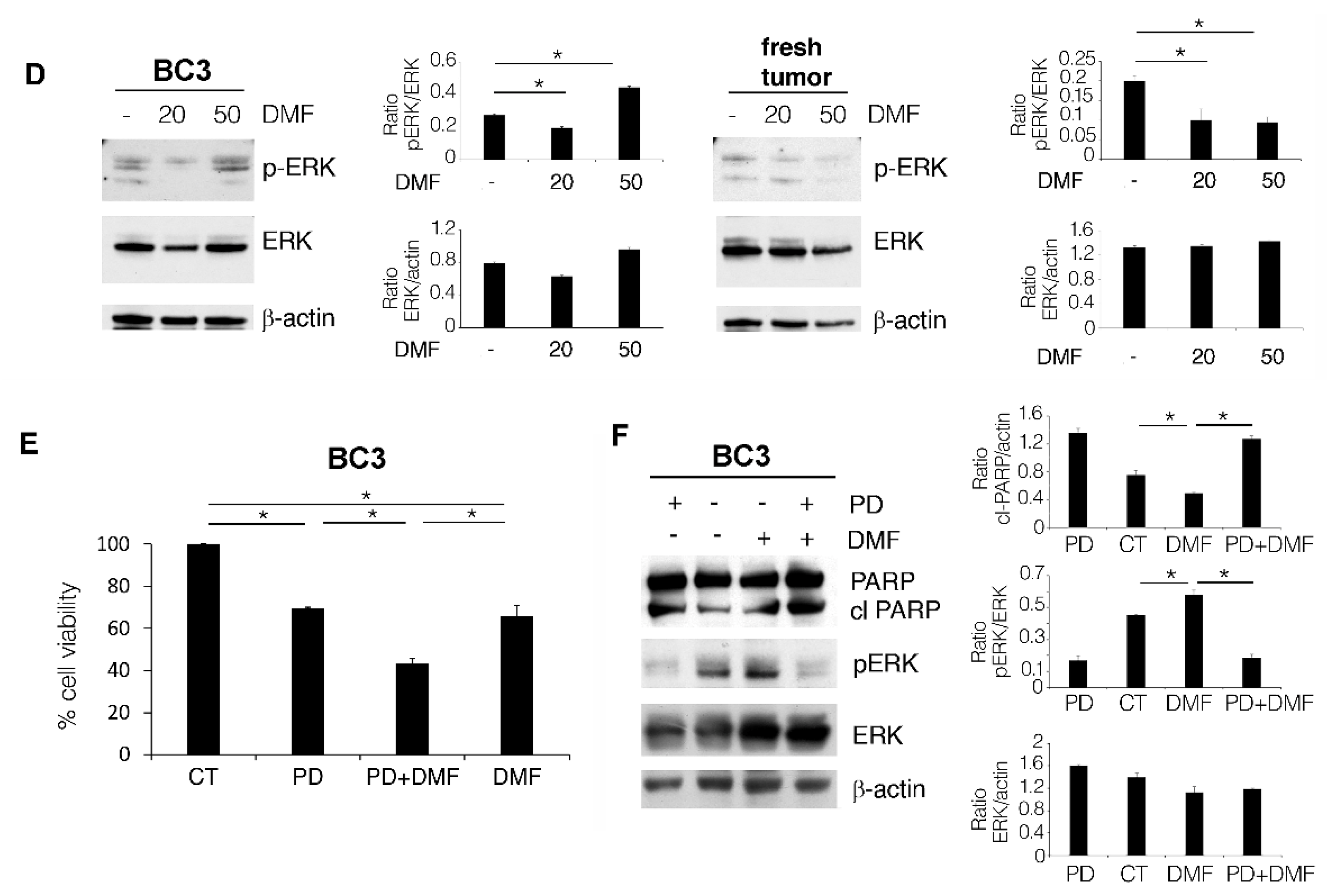
2.3. DMF Induces a Lower Cytotoxic Effect against BC3 Cell Line Compared to Fresh PEL Cells Due to ERK1/2 Activation
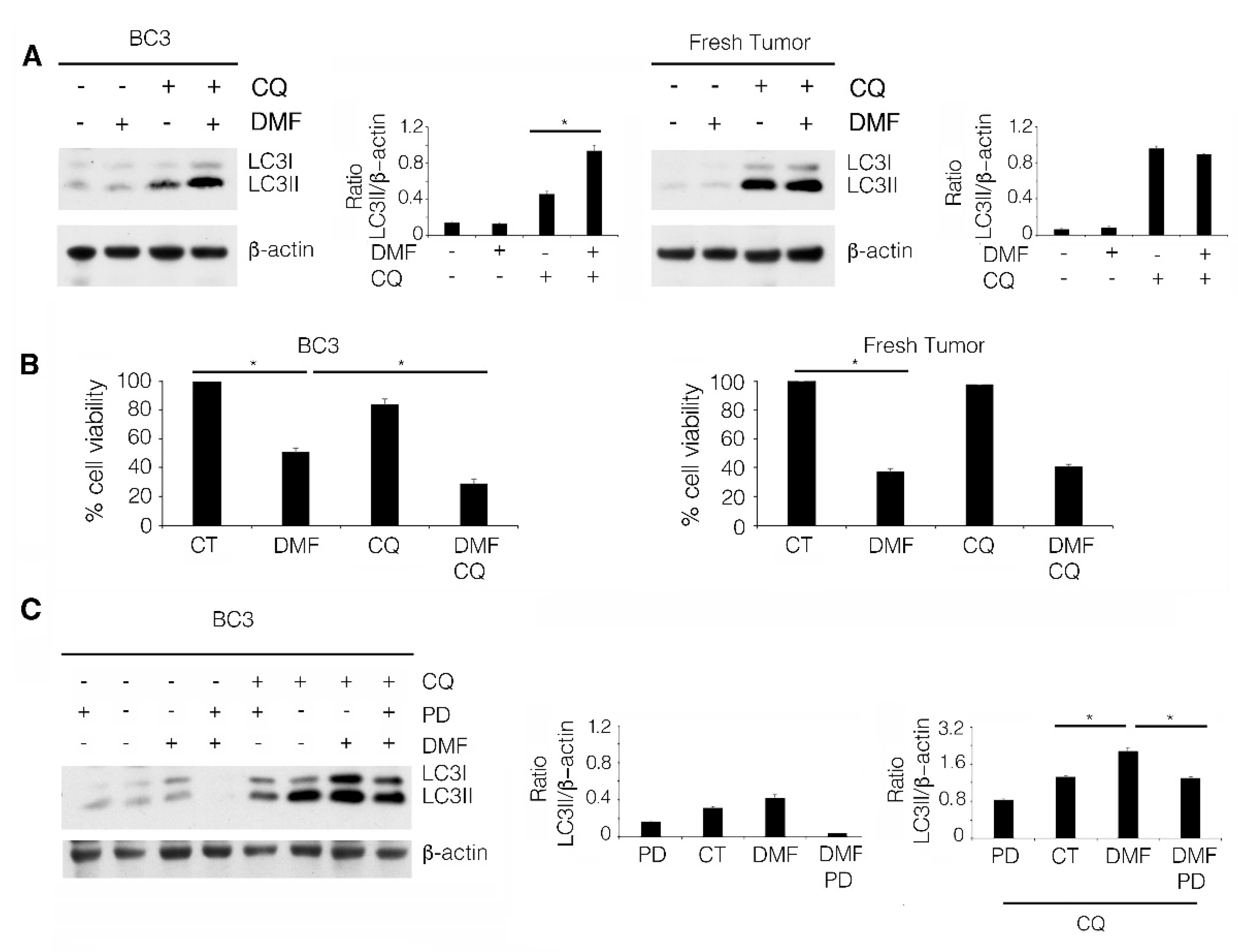
2.4. The Induction of a Pro-Survival Autophagy by ERK1/2 Activation Counteracts the Cytotoxic Effect of DMF in BC3 Cell Line
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Trypan Blue Exclusion Assay
4.3. Western Blot Analysis
4.4. Antibodies
- mouse monoclonal anti-NQO1 (1:500) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-32793),
- rabbit polyclonal anti-phospho-4E-BP1 (Thr37/46) (1:200) (Cell Signaling, Danvers, MA, USA, cat. n. 2855),
- rabbit polyclonal anti-4E-BP1 (1:200) (Cell Signaling, Danvers, MA, USA, cat. n. 9452),
- rabbit polyclonal anti-PARP1(1:1000) (Proteintech, Manchester, UK, cat. n. 13371-1),
- rabbit polyclonal anti-phospho STAT3 Tyr705 (1:500) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-8059),
- mouse monoclonal anti-STAT3 (1:100) (BD Transduction Lab, Franklin Lakes, NJ, USA, cat. n. 610189),
- rabbit polyclonal anti-LC3I/II (1:1000) (Novus, Littleton, CO, USA, cat. n. NB100-2220),
- mouse monoclonal anti-p62/SQSTM1 (1:300) (BD Transduction Lab, Franklin Lakes, NJ, USA, cat. n. 610832),
- rabbit polyclonal anti-ERK1 (1:200) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-93),
- rabbit polyclonal anti-ERK2 (1:200) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-154),
- mouse monoclonal anti-p-ERK (1:500) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-7383),
- mouse monoclonal anti-p-ERK (1:500) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-7383),
- mouse monoclonal anti-MFN2 (1:200) (Santa Cruz Biotechnologies, Dallas, TX, USA, cat. n. sc-515647).
- mouse monoclonal anti-β-actin (1:10,000) (Sigma Aldrich, St. Louis, MO, USA #A5441) was used as loading control.
4.5. Densitometric Analysis
4.6. Quantitative Real-Time PCR
4.7. Measurement of Intracellular Reactive Oxygen Species (ROS) Production
4.8. Chemiluminescence Immunometric Assay
4.9. Immunofluorescence Assay (IFA)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGuire, V.A.; Ruiz-Zorrilla Diez, T.; Emmerich, C.H.; Strickson, S.; Ritorto, M.S.; Sutavani, R.V.; Weibeta, A.; Houslay, K.F.; Knebel, A.; Meakin, P.J.; et al. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci. Rep. 2016, 6, 31159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewe, R.; Valero, T.; Kremling, S.; Pratscher, B.; Kunstfeld, R.; Pehamberger, H.; Petzelbauer, P. Dimethylfumarate impairs melanoma growth and metastasis. Cancer Res. 2006, 66, 11888–11896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastrati, I.; Siklos, M.I.; Calderon-Gierszal, E.L.; El-Shennawy, L.; Georgieva, G.; Thayer, E.N.; Thatcher, G.R.; Frasor, J. Dimethyl Fumarate Inhibits the Nuclear Factor kappaB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein. J. Biol. Chem. 2016, 291, 3639–3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidu, N.E.B.; Kavian, N.; Leroy, K.; Jacob, C.; Nicco, C.; Batteux, F.; Alexandre, J. Dimethyl fumarate, a two-edged drug: Current status and future directions. Med. Res. Rev. 2019, 39, 1923–1952. [Google Scholar] [CrossRef] [PubMed]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. DMF, but not other fumarates, inhibits NF-kappaB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Vanani, A.R.; Kalantari, H.; Mahdavinia, M.; Rashno, M.; Khorsandi, L.; Khodayar, M.J. Dimethyl fumarate reduces oxidative stress, inflammation and fat deposition by modulation of Nrf2, SREBP-1c and NF-kappaB signaling in HFD fed mice. Life Sci. 2021, 283, 119852. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Mohan, G.M.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [Green Version]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef] [Green Version]
- Granato, M.; Chiozzi, B.; Filardi, M.R.; Lotti, L.V.; Di Renzo, L.; Faggioni, A.; Cirone, M. Tyrosine kinase inhibitor tyrphostin AG490 triggers both apoptosis and autophagy by reducing HSF1 and Mcl-1 in PEL cells. Cancer Lett. 2015, 366, 191–197. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Filardi, M.; Faggioni, A.; Cirone, M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget 2015, 6, 29543–29554. [Google Scholar] [CrossRef] [Green Version]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Rahemtullah, A.; Hochberg, E. Primary effusion lymphoma. Oncologist 2007, 12, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Goto, H.; Yotsumoto, M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis. Res. 2014, 3, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Aoki, Y.; Feldman, G.M.; Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003, 101, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M.; Di Renzo, L.; Lotti, L.V.; Conte, V.; Trivedi, P.; Santarelli, R.; Gonnella, R.; Frati, L.; Faggioni, A. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS ONE 2012, 7, e31732. [Google Scholar] [CrossRef]
- Sin, S.H.; Roy, D.; Wang, L.; Staudt, M.R.; Fakhari, F.D.; Patel, D.D.; Henry, D.; Harrington, W.J., Jr.; Damania, B.A.; Dittmer, D.P. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 2007, 109, 2165–2173. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.P.; Bhende, P.M.; Sin, S.H.; Roy, D.; Dittmer, D.P.; Damania, B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood 2010, 115, 4455–4463. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Granato, M.; Santarelli, R.; Gonnella, R.; Farina, A.; Trivedi, P.; Faggioni, A.; Cirone, M. Targeting of prosurvival pathways as therapeutic approaches against primary effusion lymphomas: Past, present, and Future. Biomed. Res. Int. 2015, 2015, 104912. [Google Scholar] [CrossRef]
- Santarelli, R.; Pompili, C.; Gilardini Montani, M.S.; Romeo, M.A.; Gonnella, R.; D’Orazi, G.; Cirone, M. Lovastatin reduces PEL cell survival by phosphorylating ERK1/2 that blocks the autophagic flux and engages a cross-talk with p53 to activate p21. IUBMB Life 2021, 73, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, X.D.; Yang, B.; Tong, R.L.; Lu, Y.J.; Chen, D.Y.; Zhou, L.; Xie, H.Y.; Zheng, S.S.; Wu, J. Dimethyl fumarate suppresses hepatocellular carcinoma progression via activating SOCS3/JAK1/STAT3 signaling pathway. Am. J. Transl. Res. 2019, 11, 4713–4725. [Google Scholar] [PubMed]
- Li, H.; Wang, Y.; Su, R.; Jia, Y.; Lai, X.; Su, H.; Fan, Y.; Wang, Y.; Xing, W.; Qin, J. Dimethyl Fumarate Combined with Vemurafenib Enhances Anti-Melanoma Efficacy via Inhibiting the Hippo/YAP, NRF2-ARE, and AKT/mTOR/ERK Pathways in A375 Melanoma Cells. Front. Oncol. 2022, 12, 794216. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Aoki, Y.; Chang, Y.; Moore, P.S.; Yarchoan, R.; Tosato, G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 1999, 94, 2871–2879. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Zompetta, C.; Santarelli, R.; Gonnella, R.; Romeo, M.A.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization. Biomolecules 2019, 9, 482. [Google Scholar] [CrossRef] [Green Version]
- Cirone, M.; Gilardini Montani, M.S.; Granato, M.; Garufi, A.; Faggioni, A.; D’Orazi, G. Autophagy manipulation as a strategy for efficient anticancer therapies: Possible consequences. J. Exp. Clin. Cancer Res. 2019, 38, 262. [Google Scholar] [CrossRef]
- Akino, N.; Wada-Hiraike, O.; Isono, W.; Terao, H.; Honjo, H.; Miyamoto, Y.; Tanikawa, M.; Sone, K.; Hirano, M.; Harada, M.; et al. Activation of Nrf2/Keap1 pathway by oral Dimethylfumarate administration alleviates oxidative stress and age-associated infertility might be delayed in the mouse ovary. Reprod. Biol. Endocrinol. 2019, 17, 23. [Google Scholar] [CrossRef]
- Shen, J.; Rasmussen, M.; Dong, Q.R.; Tepel, M.; Scholze, A. Expression of the NRF2 Target Gene NQO1 Is Enhanced in Mononuclear Cells in Human Chronic Kidney Disease. Oxid Med. Cell Longev. 2017, 2017, 9091879. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Lamark, T.; Sjottem, E.; Larsen, K.B.; Awuh, J.A.; Overvatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [Green Version]
- Gwack, Y.; Hwang, S.; Lim, C.; Won, Y.S.; Lee, C.H.; Choe, J. Kaposi’s Sarcoma-associated herpesvirus open reading frame 50 stimulates the transcriptional activity of STAT3. J. Biol. Chem. 2002, 277, 6438–6442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarelli, R.; Gonnella, R.; Di Giovenale, G.; Cuomo, L.; Capobianchi, A.; Granato, M.; Gentile, G.; Faggioni, A.; Cirone, M. STAT3 activation by KSHV correlates with IL-10, IL-6 and IL-23 release and an autophagic block in dendritic cells. Sci. Rep. 2014, 4, 4241. [Google Scholar] [CrossRef] [Green Version]
- Morrison, P.J.; Suhrkamp, I.; Gerdes, S.; Mrowietz, U. Oral dimethyl fumarate induces changes within the peripheral neutrophil compartment of patients with psoriasis that are linked with skin improvement. Br. J Dermatol. 2021, 185, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Montes Diaz, G.; Hupperts, R.; Fraussen, J.; Somers, V. Dimethyl fumarate treatment in multiple sclerosis: Recent advances in clinical and immunological studies. Autoimmun. Rev. 2018, 17, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Bennett Saidu, N.E.; Bretagne, M.; Mansuet, A.L.; Just, P.A.; Leroy, K.; Cerles, O.; Chouzenoux, S.; Nicco, C.; Damotte, D.; Alifano, M.; et al. Dimethyl fumarate is highly cytotoxic in KRAS mutated cancer cells but spares non-tumorigenic cells. Oncotarget 2018, 9, 9088–9099. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wu, S.; Ye, S.; Huang, H.; Zhou, Y.; Zhou, H.; Wu, S.; Mao, Y.; Shangguan, F.; Lan, L.; et al. Dimethyl Fumarate Induces Metabolic Crisie to Suppress Pancreatic Carcinoma. Front. Pharmacol. 2021, 12, 617714. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Muller-Decker, K.; Schroeder, A.; Brechmann, M.; Mobs, M.; Geraud, C.; Assaf, C.; Goerdt, S.; Krammer, P.H.; Gulow, K. Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-kappaB. Blood 2016, 128, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Maeta, T.; Sato, T.; Asano, K.; Ito, S. Dimethyl Fumarate Induces Apoptosis via Inhibiting NF-kappaB and STAT3 Signaling in Adult T-cell Leukemia/Lymphoma Cells. Anticancer Res. 2022, 42, 2301–2309. [Google Scholar] [CrossRef]
- Bishnu, A.; Phadte, P.; Dhadve, A.; Sakpal, A.; Rekhi, B.; Ray, P. Molecular imaging of the kinetics of hyperactivated ERK1/2-mediated autophagy during acquirement of chemoresistance. Cell Death Dis. 2021, 12, 161. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Angiolillo, C.; D’Orazi, G.; Faggioni, A.; Cirone, M. Cytotoxic Drugs Activate KSHV Lytic Cycle in Latently Infected PEL Cells by Inducing a Moderate ROS Increase Controlled by HSF1, NRF2 and p62/SQSTM1. Viruses 2018, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Gonnella, R.; Yadav, S.; Gilardini Montani, M.S.; Granato, M.; Santarelli, R.; Garufi, A.; D’Orazi, G.; Faggioni, A.; Cirone, M. Oxidant species are involved in T/B-mediated ERK1/2 phosphorylation that activates p53-p21 axis to promote KSHV lytic cycle in PEL cells. Free Radic Biol. Med. 2017, 112, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, R.; Granato, M.; Farina, A.; Santarelli, R.; Faggioni, A.; Cirone, M. PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim. Biophys. Acta 2015, 1853, 1586–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonnella, R.; Zarrella, R.; Santarelli, R.; Germano, C.A.; Gilardini Montani, M.S.; Cirone, M. Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF). Int. J. Mol. Sci. 2022, 23, 6773. https://doi.org/10.3390/ijms23126773
Gonnella R, Zarrella R, Santarelli R, Germano CA, Gilardini Montani MS, Cirone M. Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF). International Journal of Molecular Sciences. 2022; 23(12):6773. https://doi.org/10.3390/ijms23126773
Chicago/Turabian StyleGonnella, Roberta, Roberta Zarrella, Roberta Santarelli, Concetta Anna Germano, Maria Saveria Gilardini Montani, and Mara Cirone. 2022. "Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF)" International Journal of Molecular Sciences 23, no. 12: 6773. https://doi.org/10.3390/ijms23126773
APA StyleGonnella, R., Zarrella, R., Santarelli, R., Germano, C. A., Gilardini Montani, M. S., & Cirone, M. (2022). Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF). International Journal of Molecular Sciences, 23(12), 6773. https://doi.org/10.3390/ijms23126773






