Fabrication of CL-20/HMX Cocrystal@Melamine–Formaldehyde Resin Core–Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization
Abstract
:1. Introduction
2. Results
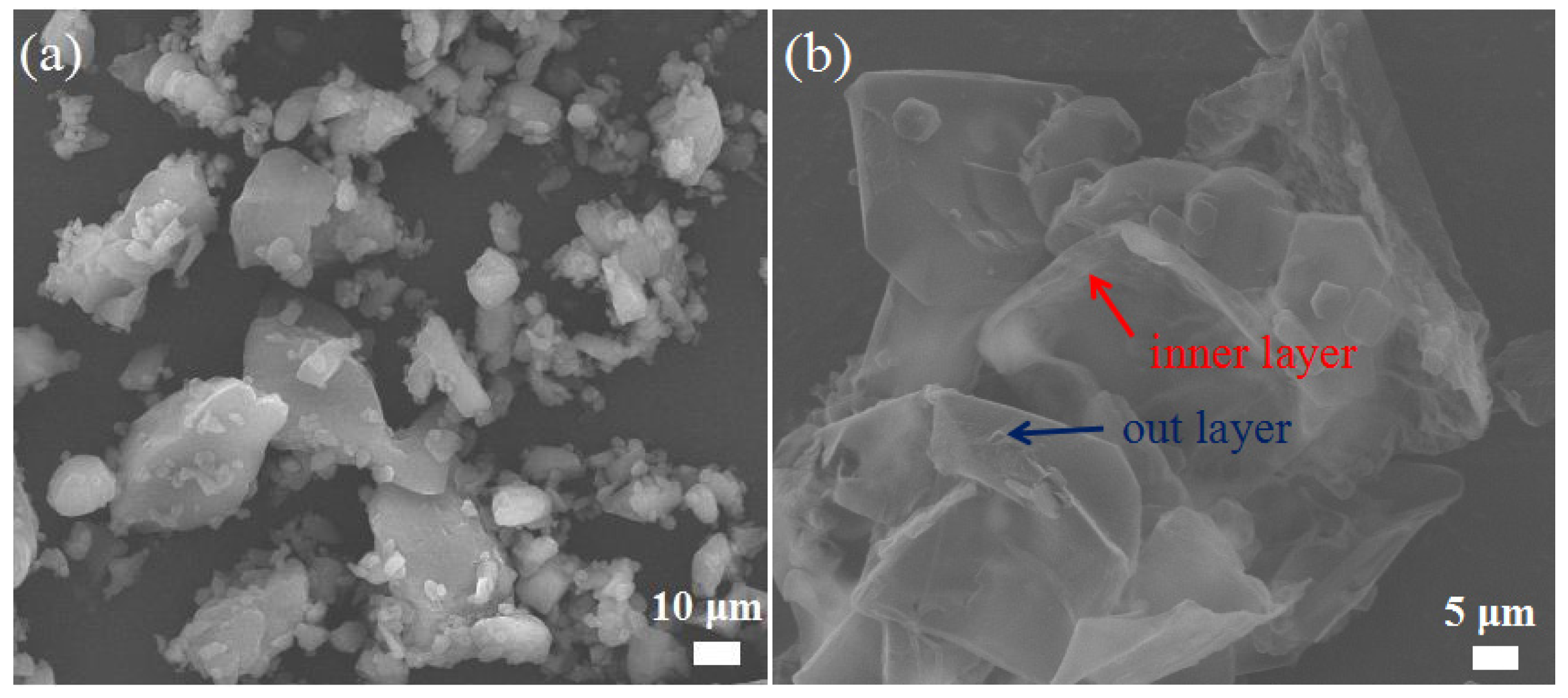
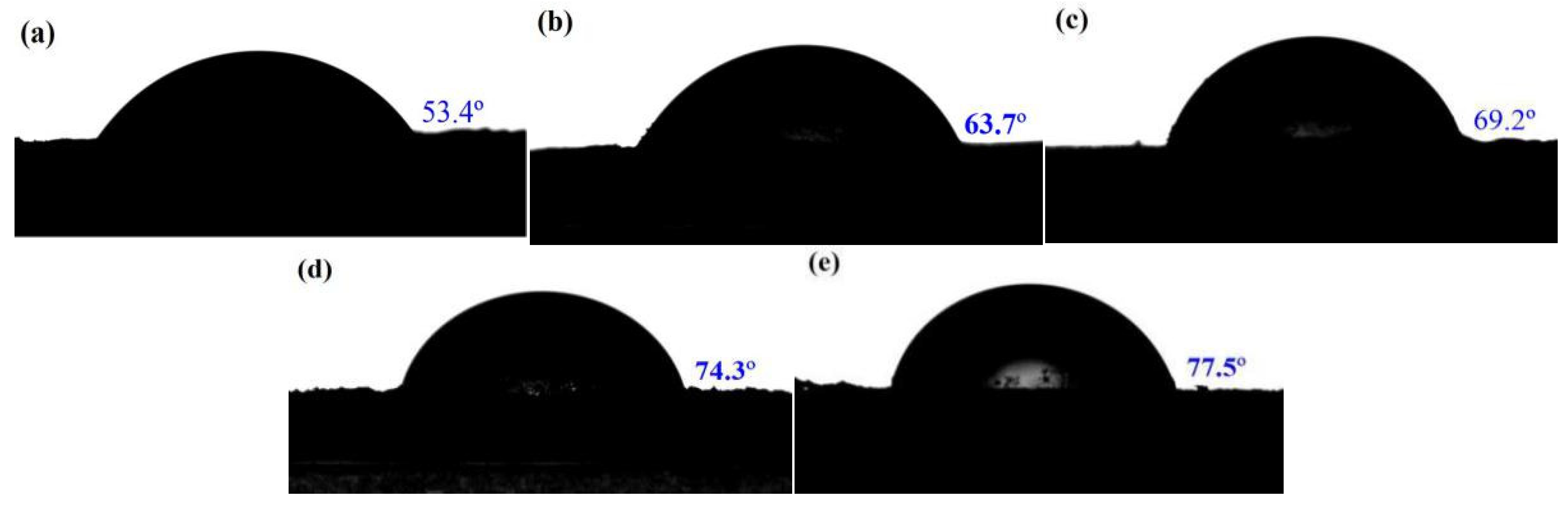
2.1. Morphologies
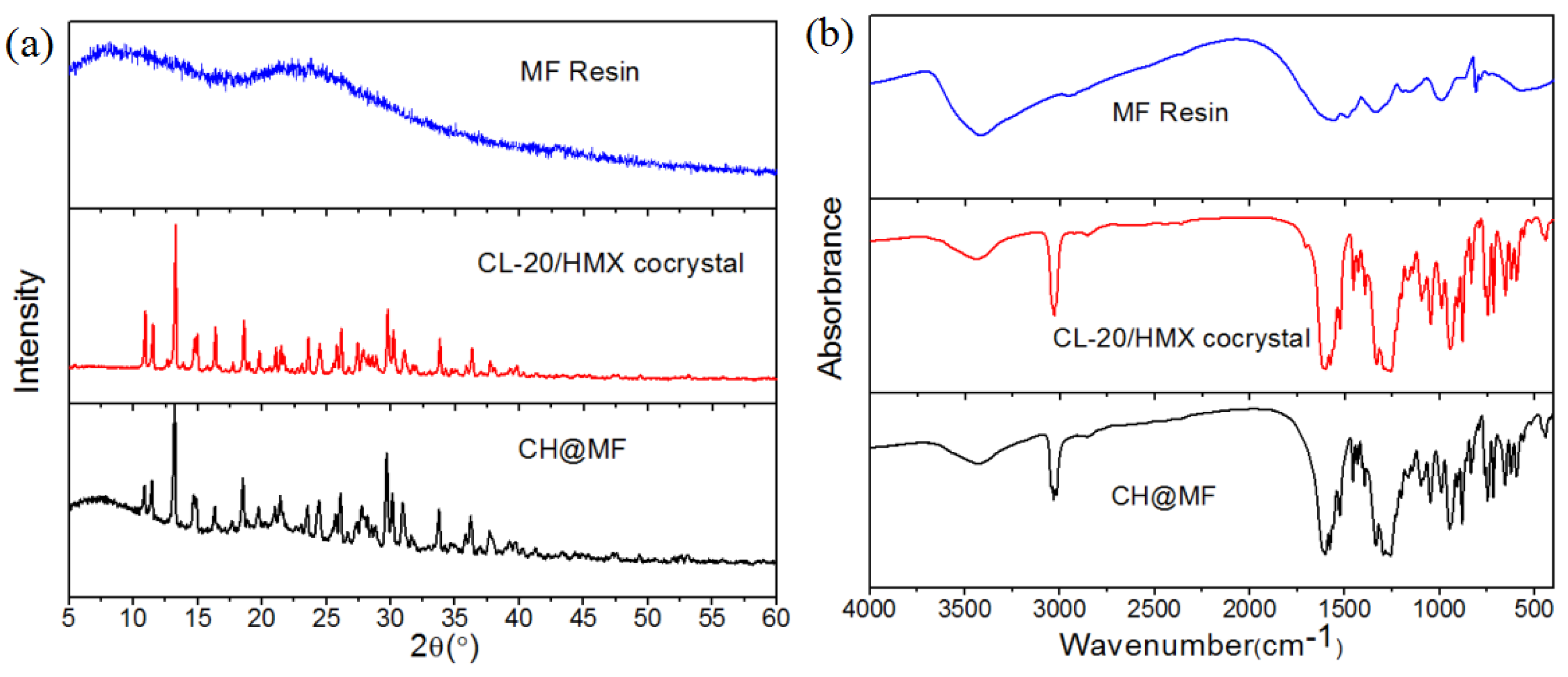
2.2. Structural Characterizations
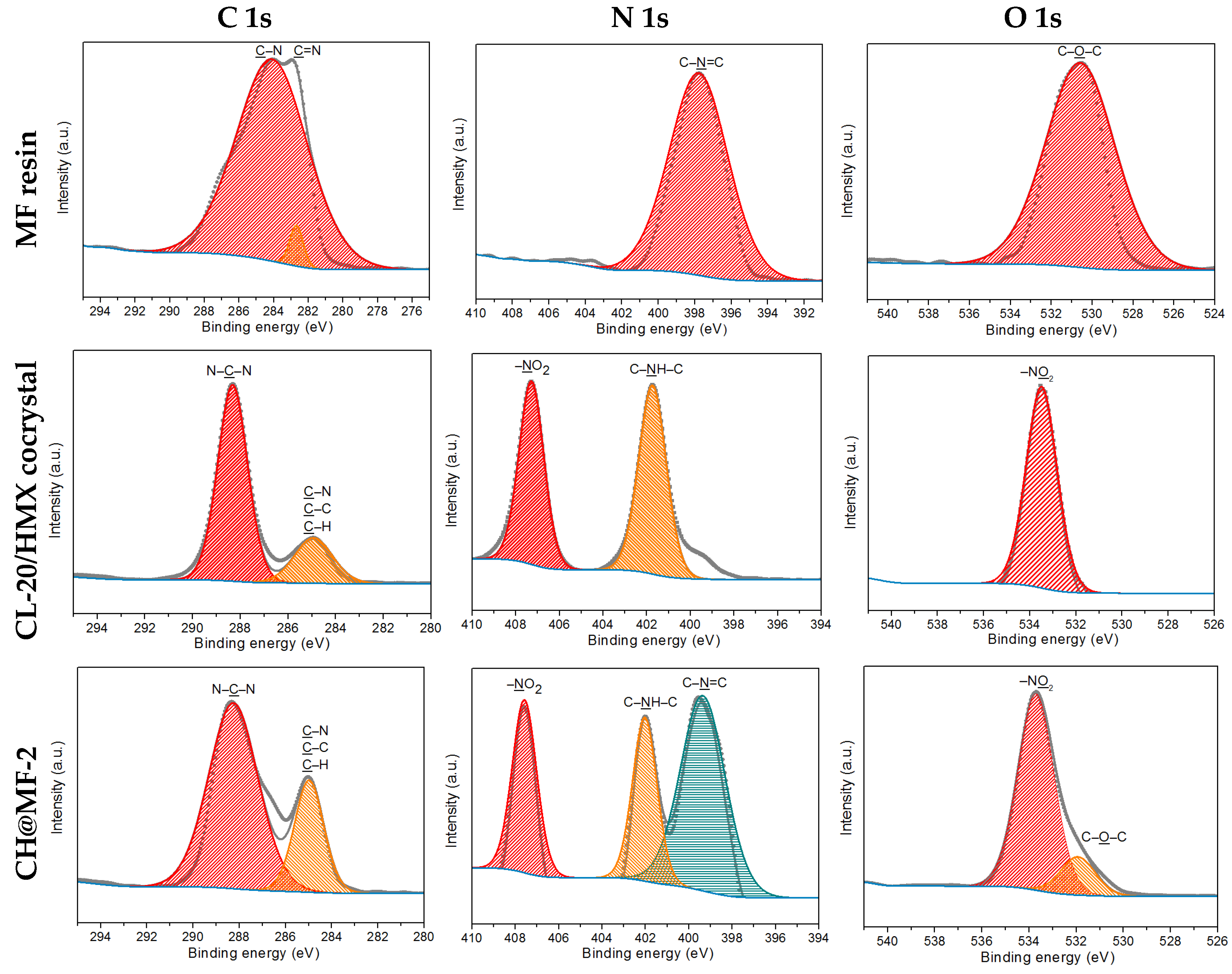
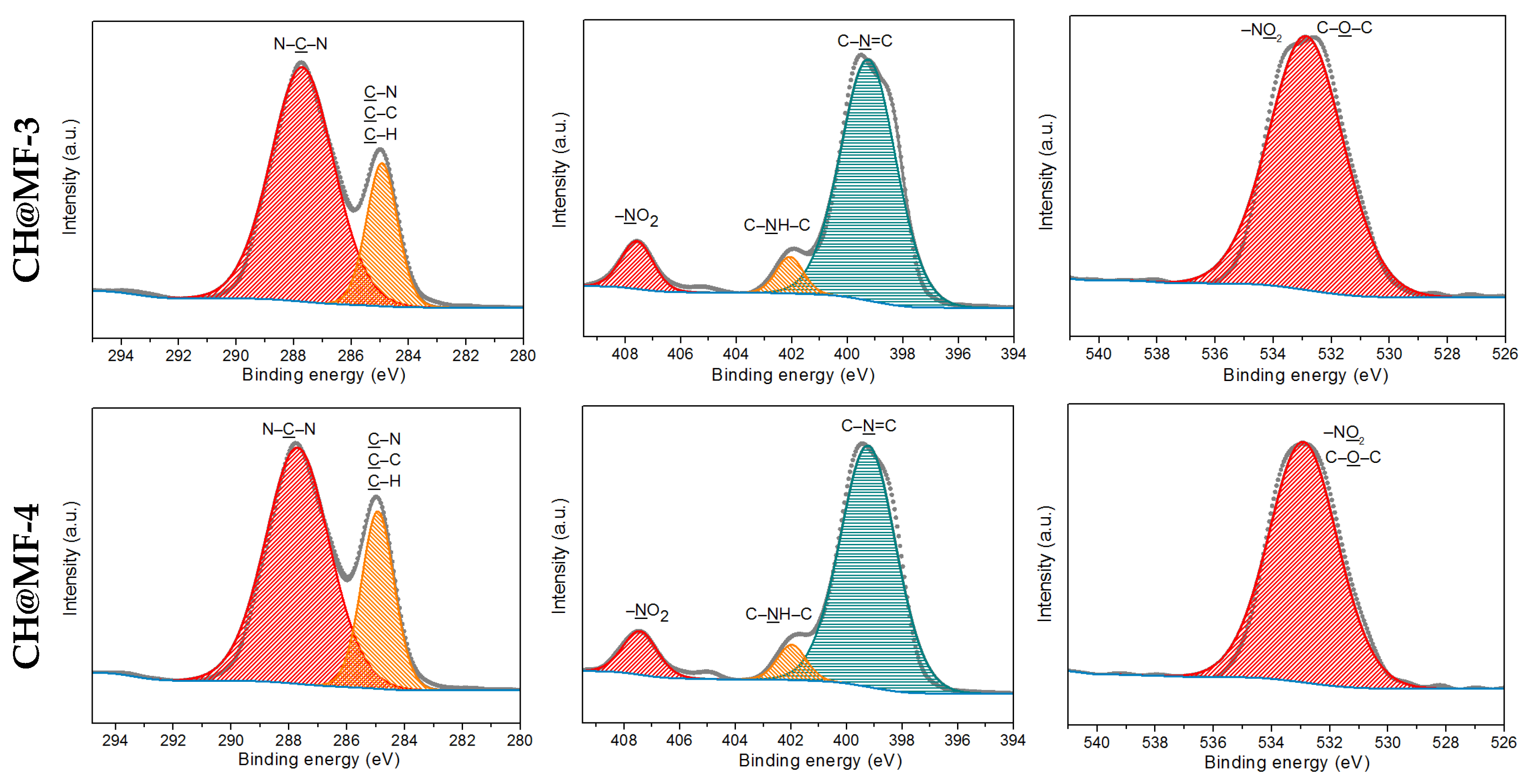
2.3. Surface Analysis
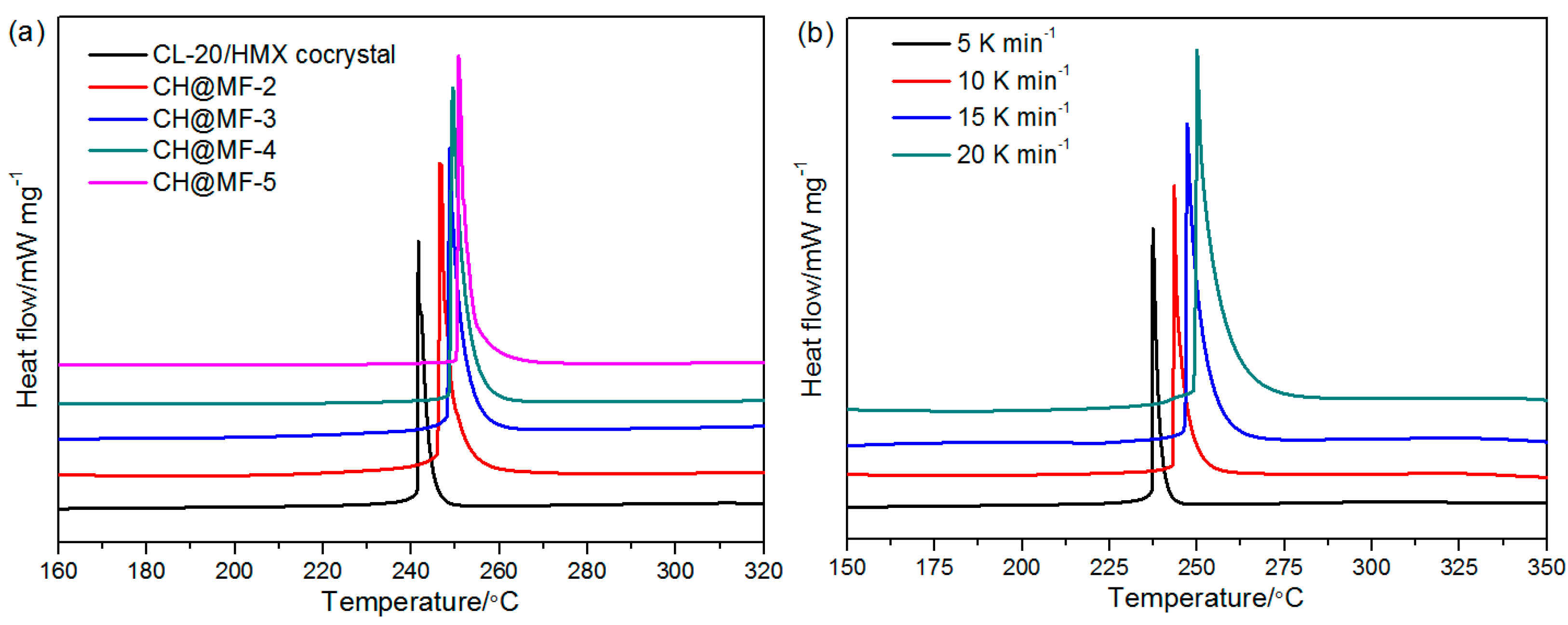
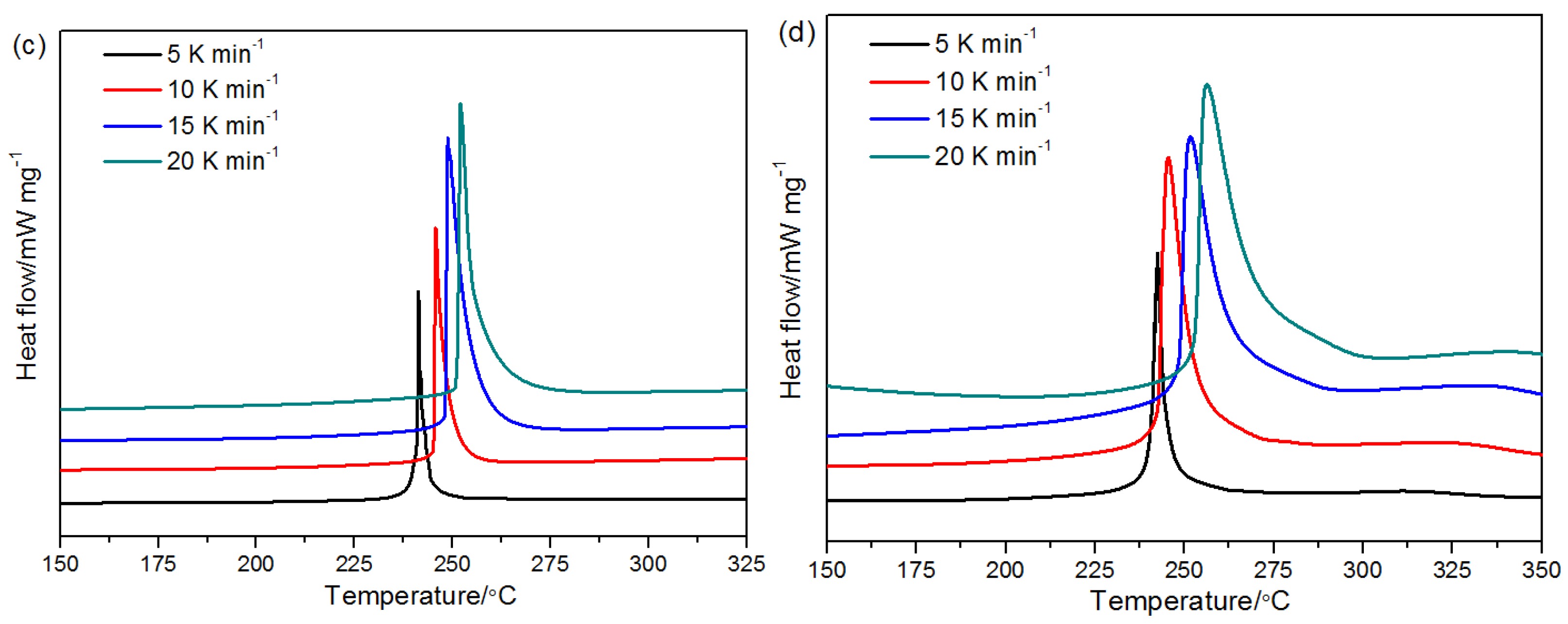
2.4. Thermal Properties
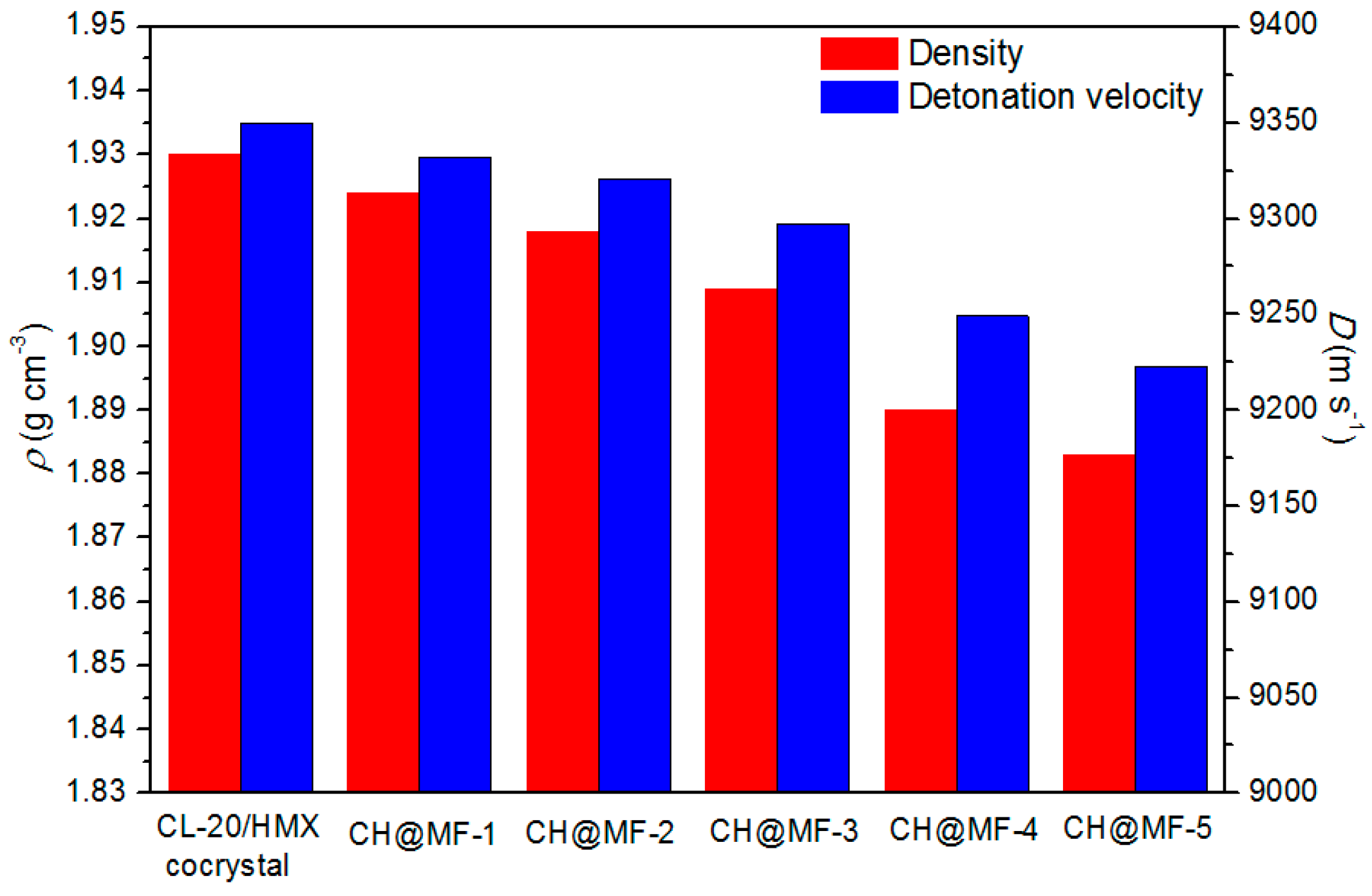
2.5. Sensitivity and Detonation Performance
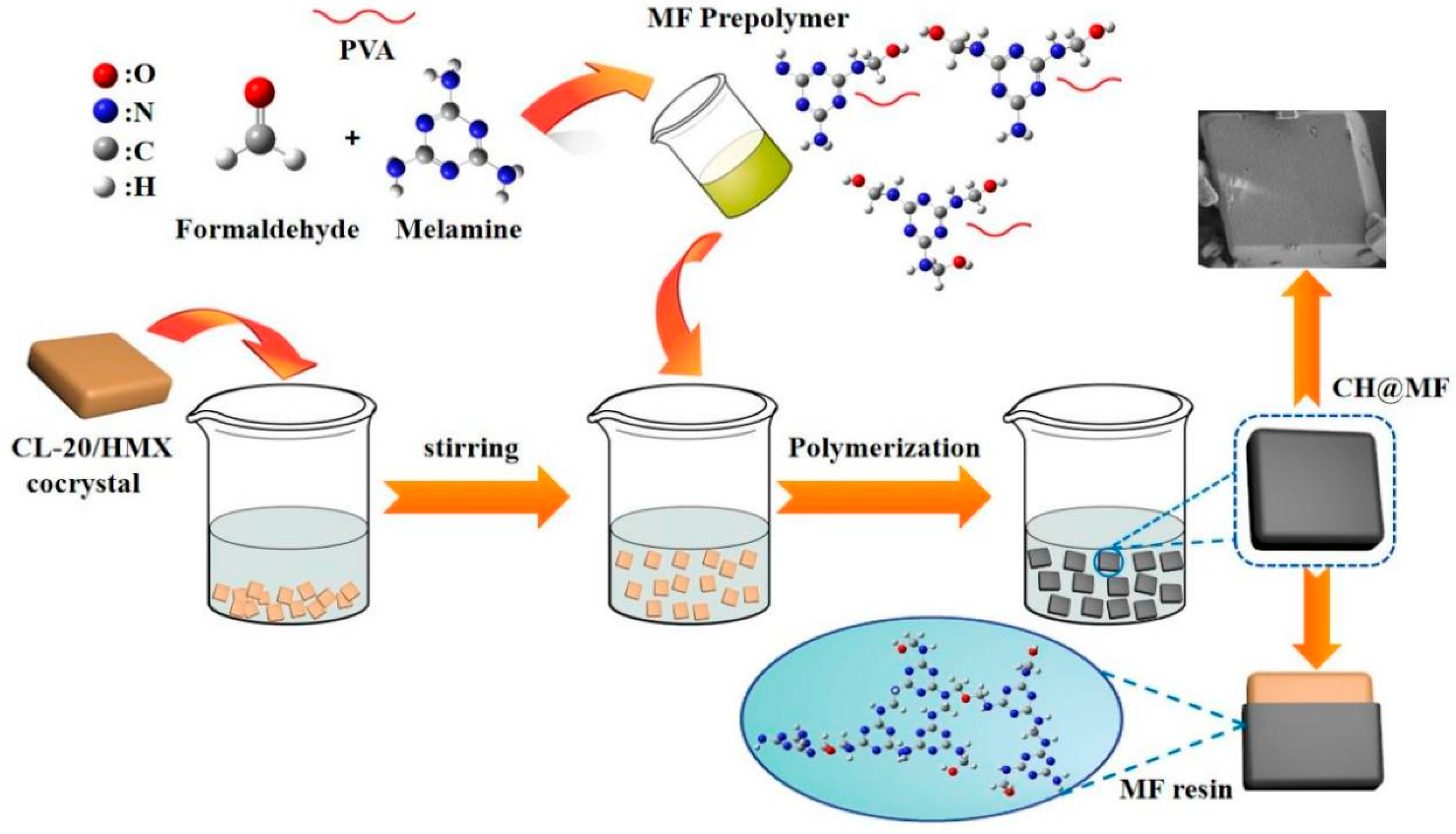
2.6. Proposed Formation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Symbols and Abbreviations
| CL-20 | 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane |
| HMX | 1,3,5,7-Tetranitro-1,3,5,7-tetrazacyclooctane |
| MF | Melamine–formaldehyde |
| EMs | Energetic materials |
| TNT | 2,4,6-Trinitrotoluene |
| BTF | Benzotrifuroxan |
| DNT | 2,5-Dinitrotoluene |
| MTNP | 1-Methyl-3,4,5-trinitropyrazole |
| MDNT | 1-Methyl-3,5-dinitro-1,2,4-triazole |
| CSEs | Core–shell EMs |
| CH | CL-20/HMX cocrystal |
| CH@MF | CL-20/HMX cocrystal@MF resin |
| F2314 | Fluoropolymer |
| PBX | Polymer-bonded explosive |
| PVA | Polyvinyl alcohol |
| CMDB | Composite–modified double base |
| γ | Surface energy |
| Wa | Interfacial adhesion work |
| Ea | Activation energy |
| α | Extent of conversion |
| r | Correlation coefficient |
| Ra | Mean value of roughness |
| Rq | Root mean square roughness |
| Rmax | Maximum height value from peak to valley |
| H50 | Characteristic height |
| P | Explosion probability |
| SEM | Scanning electron microscope |
| AFM | Atomic force microscope |
| FT-IR | Fourier transform infrared spectra |
| PXRD | Powder X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| DSC | Differential scanning calorimeter |
References
- Geetha, M.; Nair, U.R.; Sarwade, D.B.; Gore, G.M.; Asthana, S.N.; Singh, H. Studies on CL-20: The most powerful high energy material. J. Therm. Anal. Calorim. 2003, 73, 913–922. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shi, N.C. Crystal structure of ε-hexanitrohexaazaisowurtzitane. Chin. Sci. Bull. 1995, 40, 2158–2160. [Google Scholar]
- Liu, N.; Duan, B.H.; Lu, X.M.; Mo, H.C.; Xu, M.H.; Zhang, Q.; Wang, B.Z. Preparation of CL-20/DNDAP cocrystal by a rapid and continuous spray drying method: An alternative to cocrystal formation. CrystEngComm 2018, 20, 2060–2067. [Google Scholar] [CrossRef]
- Snyder, C.J.; Chavez, D.E.; Imler, G.H.; Byrd, E.F.; Leonard, P.W.; Parrish, D.A. Simple and efficient synthesis of explosive cocrystals containing 3,5-dimethylpyrazol-1-yl-substituted-1,2,4,5-tetrazines. Chem. Eur. J. 2017, 23, 16466–16471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Shreeve, J.M. Time for pairing: Cocrystals as advanced energetic materials. CrystEngComm 2016, 18, 6124–6133. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. 2011, 50, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2:1 cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhou, X.; Zhang, C.; Huang, H.; Li, J.; Nie, F. Characterization and properties of a novel energetic-energetic cocrystal explosive composed of HNIW and BTF. Cryst. Growth Des. 2012, 12, 5155–5158. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, G.; Luan, J.; Chen, Z.; Su, P.; Shu, Y. Crystal structure, spectrum character and explosive property of a new cocrystal CL-20/DNT. J. Mol. Struct. 2016, 1110, 91–96. [Google Scholar] [CrossRef]
- Anderson, S.R.; Dubé, P.; Krawiec, M.; Salan, J.S.; Ende, D.J.A.; Samuels, P. Promising CL-20-based energetic material by cocrystallization. Propellants Explos. Pyrotech. 2016, 41, 783–788. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, T.; Chi, Y.; Chen, Y.; Wang, J.; Huang, J.; Nie, F. A novel multi-nitrogen 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane-based energetic co-crystal with 1-methyl-3,4,5-trinitropyrazole as a donor: Experimental and theoretical investigations of intermolecular interactions. New J. Chem. 2017, 41, 4165–4172. [Google Scholar] [CrossRef]
- Anderson, S.R.; Am, D.J.; Ende, J.S.; Salan, P.S. Preparation of an energetic-energetic cocrystal using resonant acoustic mixing. Propellants Explos. Pyrotech. 2015, 39, 637–640. [Google Scholar] [CrossRef]
- Zhang, M.H.; Tan, Y.X.; Zhao, X.; Zhang, J.H.; Huang, S.L.; Zhai, Z.H.; Liu, Y.; Yang, Z. Seeking a novel energetic co-crystal strategy through the interfacial self-assembly of CL-20 and HMX nanocrystals. CrystEngComm 2020, 22, 61. [Google Scholar] [CrossRef]
- An, C.; Li, H.; Ye, B.; Wang, J. Nano-CL-20/HMX cocrystal explosive for significantly reduced mechanical sensitivity. J. Nanomater. 2017, 2017, 3791320. [Google Scholar] [CrossRef]
- Lian, P.; Kang, C.; Zhang, Y.X.; Chen, S.; Lai, W.P. Theoretical study on new high energetic density compounds with high power and specific impulse. FirePhysChem 2021, 1, 103–108. [Google Scholar] [CrossRef]
- Huang, B.; Xue, Z.; Fu, X.; Yan, Q.L. Advanced crystalline energetic materials modified by coating/intercalation techniques. Chem. Eng. J. 2021, 417, 128044. [Google Scholar] [CrossRef]
- Zeng, C.; Yang, Z.; Zhang, J.; Li, Y.; Gong, F. Enhanced interfacial and mechanical properties of PBX composites via surface modification on energetic crystals. Polymers 2019, 11, 1308. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.J.; Yan, Q.L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. Chem. Eng. J. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, J.; He, G.; Huang, C.; Yang, Z.; Liu, S.; Gong, F. Enhanced water resistance and energy performance of core–shell aluminum nanoparticles via in situ grafting of energetic glycidyl azide polymer. J. Mater. Sci. 2018, 53, 12091–12102. [Google Scholar] [CrossRef]
- Niu, C.; Jin, B.; Peng, R.; Shang, Y.; Liu, Q. Preparation and characterization of insensitive HMX/rGO/G composites via in situ reduction of graphene oxide. RSC Adv. 2017, 7, 32275–32281. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Gong, F.; Yang, Z.; Pan, L.; Liu, S.; Li, J.; Guo, S. Bio-inspired fabrication of core@shell structured TATB/polydopamine microparticles via in situ polymerization with tunable mechanical properties. Polym. Test. 2018, 68, 126–134. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, L.; Yang, Z.; Liu, X. Mussel-inspired coating of energetic crystals: A compact core–shell structure with highly enhanced thermal stability. Chem. Eng. J. 2017, 309, 140–150. [Google Scholar] [CrossRef]
- He, G.; Liu, J.; Gong, F.; Lin, C.; Yang, Z. Bioinspired mechanical and thermal conductivity reinforcement of highly explosive-filled polymer composites. Compos. Part A 2017, 107, 1–9. [Google Scholar] [CrossRef]
- Lin, C.; Huang, B.; Gong, F.; Yang, Z.; Guo, S. Core@double-shell structured energetic composites with reduced sensitivity and enhanced mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 30341–30351. [Google Scholar] [CrossRef]
- Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-shell structured HMX@polydopamine energetic microspheres: Synergistically enhanced mechanical, thermal, and safety performances. Polymers 2019, 11, 568. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Yang, Z.; Pan, L.; Zhang, J.; Liu, S.; Yan, Q.L. Bioinspired Interfacial Reinforcement of Polymer-based Energetic Composites with High Loading of Solid Explosive Crystals. J. Mater. Chem. A 2017, 5, 13499–13510. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Hussain, I.; Shen, R.; Yang, G.; Zhang, K. Core-shell structured nanoenergetic materials: Preparation and fundamental properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef]
- Duan, B.H.; Li, J.K.; Mo, H.C.; Lu, X.M.; Xu, M.H.; Wang, B.Z.; Liu, N. The art of framework construction: Core–shell structured micro-energetic materials. Molecules 2021, 26, 5650. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; He, W. Bio-inspired fabrication of energetic crystals@cellulose nanofibers core-shell composites with improved stability and reduced sensitivity. Compos. Commun. 2021, 27, 100868. [Google Scholar] [CrossRef]
- Lin, C.; Zeng, C.; Wen, Y.; Gong, F.; He, G.; Li, Y.; Yang, Z.; Ding, L.; Li, J.; Guo, S. Litchi-like core-Shell HMX@HPW@PDA microparticles for polymer-bonded energetic composites with low sensitivity and high mechanical properties. ACS Appl. Mater. Interfaces 2020, 12, 4002–4013. [Google Scholar] [CrossRef]
- Jia, X.; Cao, Q.; Guo, W.; Li, C.; Shen, J.; Geng, X.; Wang, J.; Hou, C. Synthesis, thermolysis, and solid spherical of RDX/PMMA energetic composite materials. J. Mater. Sci. 2019, 30, 20166–20173. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly reactive metastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Zhang, J.; Pan, L.; Ding, L.; Tian, X.; Zheng, X.; Gong, F. Fabrication and characterization of HMX@TPEE energetic microspheres with reduced sensitivity and superior toughness properties. Compos. Sci. Technol. 2017, 142, 253–263. [Google Scholar] [CrossRef]
- Huang, B.; Hao, X.; Zhang, H.; Yang, Z.; Ma, Z.; Li, H.; Nie, F.; Huang, H. Ultrasonic approach to the synthesis of HMX@TATB core-shell microparticles with improved mechanical sensitivity. Ultrason. Sonochem. 2014, 21, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhen, B.G.; Guo, X.D.; Liu, K.W.; Li, F.S. In-situ crystallization coating HMX by BAMO-THF copolyether. Chin. J. Explos. Propellants 2014, 37, 35–38. [Google Scholar]
- Salaün, F.; Devaux, E.; Bourbigot, S.; Rumeau, P. Influence of process parameters on microcapsules loaded with n-hexadecane prepared by in situ polymerization. Chem. Eng. J. 2009, 155, 457–465. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, L.; Wu, P.; Liu, Y.; Nie, F.; Huang, F. Fabrication of RDX, HMX and CL-20 based microcapsules via in situ polymerization of melamine–formaldehyde resins with reduced sensitivity. Chem. Eng. J. 2015, 268, 60–66. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- He, G.; Li, X.; Bai, L.; Meng, L.; Dai, Y. Multilevel core-shell strategies for improving mechanical properties of energetic polymeric composites by the “grafting-from” route. Compos. Part B 2020, 191, 107967. [Google Scholar] [CrossRef]
- Tan, L.; Lu, X.; Liu, N.; Yan, Q. Further enhancing thermal stability of thermostable energetic derivatives of dibenzotetraazapentene by polydopamine/graphene oxide coating. Appl. Surf. Sci. 2021, 543, 148825. [Google Scholar] [CrossRef]
- Xue, Z.H.; Zhang, X.X.; Huang, B.B.; Xin, B.; Yan, Q.L. The structural diversity of hybrid qy-HMX crystals with constraint of 2D dopants and the resulted changes in thermal reactivity. Chem. Eng. J. 2020, 390, 124565. [Google Scholar] [CrossRef]
- Wu, Z.K.; Liu, N.; Zheng, W.; Chen, J.B.; Song, X.D.; Wang, J.N. Application and properties of CL-20/HMX Cocrystal in composite modified double base propellants. Propellants Explos. Pyrotech. 2020, 45, 92–100. [Google Scholar] [CrossRef]
- Wu, Z.K.; Pei, J.F.; Song, X.D.; Liu, N.; Li, J.G.; Zhang, M. The catalytic effects of nano-Fe2O3 and rGO–Fe2O3 on the thermal decomposition properties of CL-20/HMX cocrystals. New J. Chem. 2020, 44, 1858. [Google Scholar] [CrossRef]


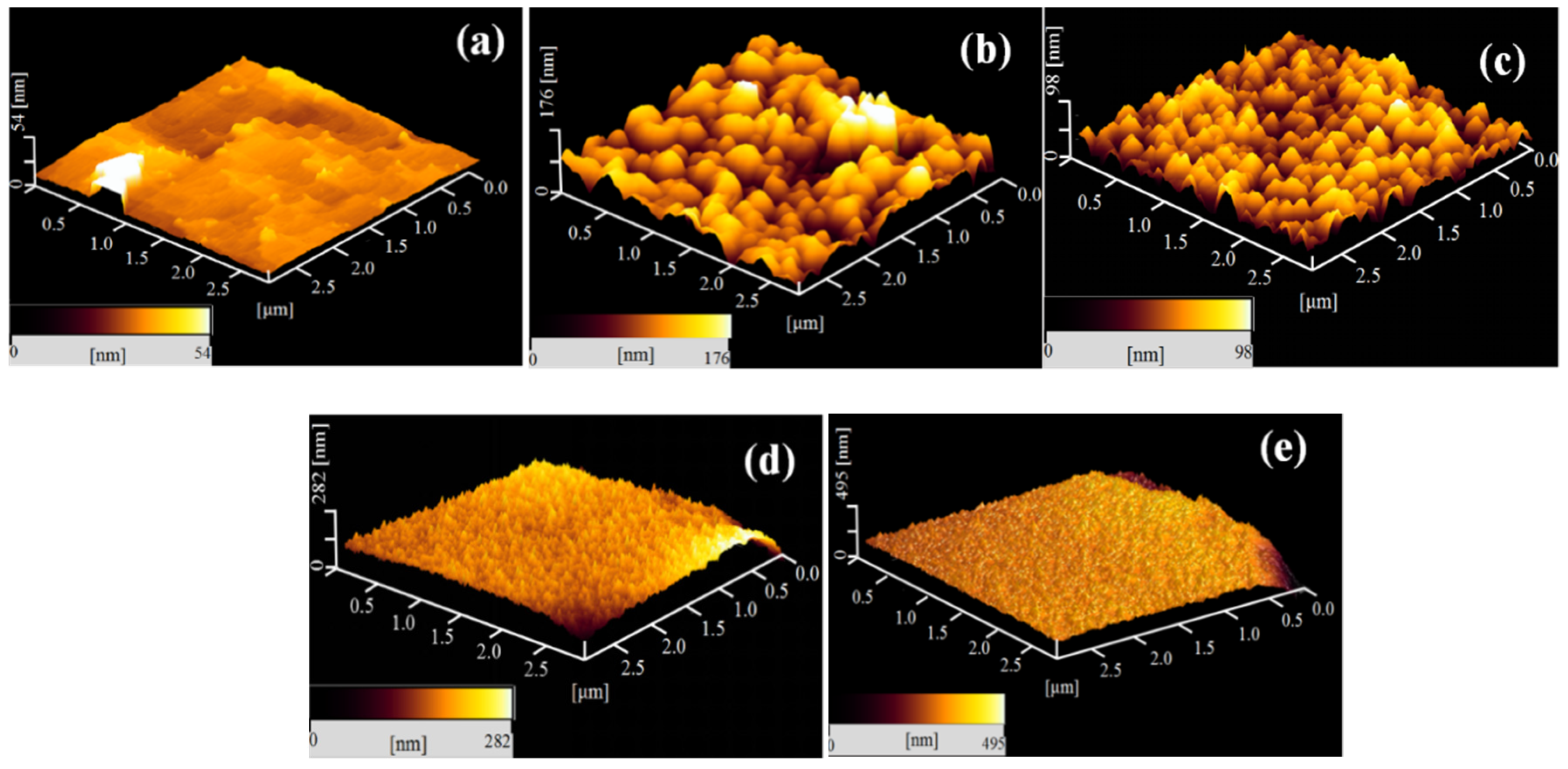
| Samples | Ra 1/nm | Rq 1/nm | Rmax 1/nm |
|---|---|---|---|
| CL-20/HMX cocrystal | 4.6 ± 0.30 | 3.3 ± 0.12 | 73 ± 0.58 |
| CH@MF–2 | 29.8 ± 0.62 | 22.7 ± 0.47 | 331 ± 0.79 |
| CH@MF–3 | 17.4 ± 0.69 | 10.1 ± 0.48 | 213 ± 0.88 |
| CH@MF–4 | 37.2 ± 0.78 | 23.9 ± 0.51 | 474 ± 1.24 |
| CH@MF–5 | 46.2 ± 0.60 | 26.4 ± 0.53 | 591 ± 1.08 |
| Samples | C 1s/% | N 1s/% | O 1s/% | C/N |
|---|---|---|---|---|
| MF resin | 72.72 | 19.24 | 8.04 | 3.78 |
| CL-20/HMX cocrystal | 28.63 | 38.28 | 33.09 | 0.75 |
| CH@MF–2 | 51.20 | 31.90 | 16.90 | 1.61 |
| CH@MF–3 | 58.76 | 27.95 | 13.29 | 2.10 |
| CH@MF–4 | 64.89 | 24.60 | 10.51 | 2.64 |
| Sample | Surface Energy (mN/m2) | Adhesion Work Wa (mN/m2) | ||
|---|---|---|---|---|
| γd | γp | γ | ||
| CL-20/HMX cocrystal | 31.86 | 6.18 | 38.04 | 64.07 |
| CH@MF–2 | 37.33 | 2.93 | 40.26 | 67.97 |
| CH@MF–3 | 38.02 | 2.67 | 40.69 | 68.40 |
| CH@MF–4 | 38.56 | 2.48 | 41.04 | 68.72 |
| CH@MF–5 | 39.58 | 2.10 | 41.68 | 69.28 |
| F2314 | 28.52 | 1.15 | 29.67 | __ |
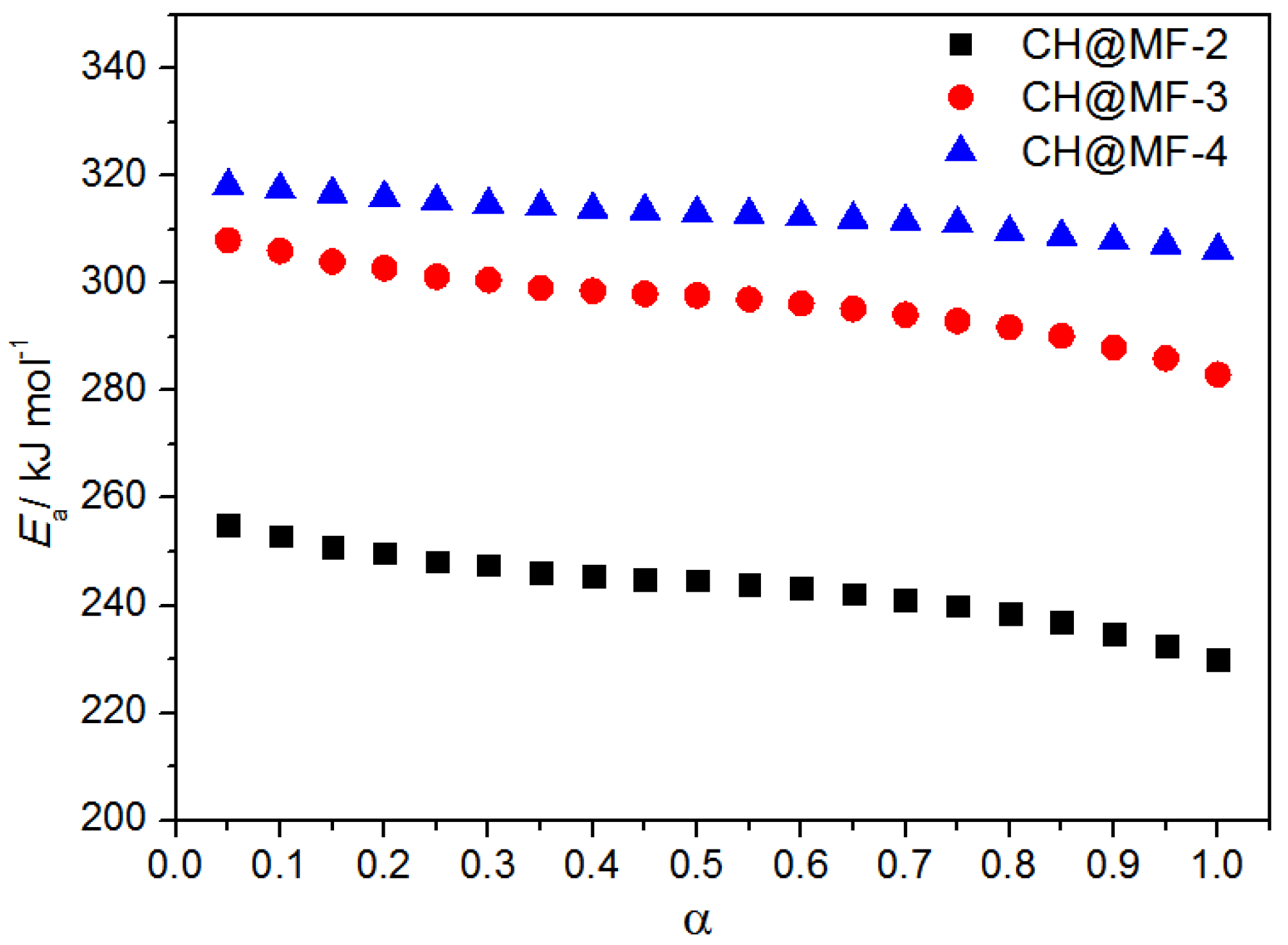
| Samples | Kissinger Method | Friedman Method | Combined Kinetic Method | |||||
|---|---|---|---|---|---|---|---|---|
| Ea(1) | logA | r | Ea(2) | r | m | n | Ea(3) | |
| CH@MF–2 | 233.42 | 17.86 | 0.9994 | 245.76 | 0.9912 | 0.786 | 0.968 | 258.14 |
| CH@MF–3 | 259.49 | 20.35 | 0.9965 | 297.62 | 0.9964 | 0.725 | 1.100 | 305.13 |
| CH@MF–4 | 278.11 | 22.24 | 0.9936 | 313.11 | 0.9951 | 0.762 | 1.230 | 315.60 |
| Samples | MF Content/% | Existent Form of MF | H50/cm | P/% |
|---|---|---|---|---|
| CL-20/HMX cocrystal | 0 | — | 27.1 | 72 |
| CH + MF | 1.0 | Physical mixture | 29.0 | 69 |
| CH + MF | 1.6 | Physical mixture | 31.2 | 65 |
| CH@MF–2 | 1.0 | Core–shell | 40.6 | 50 |
| CH@MF–3 | 1.6 | Core–shell | 56.4 | 38 |
| CH@MF–4 | 2.8 | Core–shell | 66.2 | 26 |
| CH@MF–5 | 3.5 | Core–shell | 75.0 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, B.; Lu, X.; Mo, H.; Tan, B.; Wang, B.; Liu, N. Fabrication of CL-20/HMX Cocrystal@Melamine–Formaldehyde Resin Core–Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization. Int. J. Mol. Sci. 2022, 23, 6710. https://doi.org/10.3390/ijms23126710
Duan B, Lu X, Mo H, Tan B, Wang B, Liu N. Fabrication of CL-20/HMX Cocrystal@Melamine–Formaldehyde Resin Core–Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization. International Journal of Molecular Sciences. 2022; 23(12):6710. https://doi.org/10.3390/ijms23126710
Chicago/Turabian StyleDuan, Binghui, Xianming Lu, Hongchang Mo, Bojun Tan, Bozhou Wang, and Ning Liu. 2022. "Fabrication of CL-20/HMX Cocrystal@Melamine–Formaldehyde Resin Core–Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization" International Journal of Molecular Sciences 23, no. 12: 6710. https://doi.org/10.3390/ijms23126710
APA StyleDuan, B., Lu, X., Mo, H., Tan, B., Wang, B., & Liu, N. (2022). Fabrication of CL-20/HMX Cocrystal@Melamine–Formaldehyde Resin Core–Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization. International Journal of Molecular Sciences, 23(12), 6710. https://doi.org/10.3390/ijms23126710







