An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research
Abstract
:1. Introduction
2. Metallic Nanoparticles
2.1. Gold Nanoparticles (Au NPs)
2.1.1. Mechanism of Action of Gold Nanoparticles
2.1.2. Limitations of the Application of Gold Nanoparticles
2.2. Silver Nanoparticles (Ag NPs)
2.2.1. Mechanism of Action of Silver Nanoparticles
2.2.2. Limitations of the Application of Silver Nanoparticles
2.3. Platinum Nanoparticles (Pt NPs)
2.3.1. Mechanism of Action of Platinum Nanoparticles
2.3.2. Limitations of the Application of Platinum Nanoparticles
2.4. Palladium Nanoparticles (Pd NPs)
2.4.1. Mechanism of Action of Palladium Nanoparticles
2.4.2. Limitations of the Application of Palladium Nanoparticles
2.5. Copper Nanoparticles (Cu NPs)
2.5.1. Mechanism of Action of Copper Nanoparticles
2.5.2. Limitations of the Application of Copper Nanoparticles
2.6. Zinc Nanoparticles (Zn NPs)
2.6.1. Mechanism of Action of Zinc Nanoparticles
2.6.2. Limitations of the Application of Zinc Nanoparticles
2.7. Ruthenium Nanoparticles (Ru NPs)
2.7.1. Mechanism of Action of Ruthenium Nanoparticles
2.7.2. Limitations of the Application of Ruthenium Nanoparticles
2.8. Titanium Nanoparticles (Ti NPs)
2.8.1. Mechanism of Action of Titanium Nanoparticles
2.8.2. Limitations of the Application of Titanium Nanoparticles
2.9. Vanadium Nanoparticles (V NPs)
2.9.1. Mechanism of Action of Vanadium Nanoparticles
2.9.2. Limitations of the Application of Vanadium Nanoparticles
2.10. Iron Nanoparticles (I NPs)
2.10.1. Mechanism of Action of Iron Nanoparticles
2.10.2. Limitations of the Application of Iron Nanoparticles
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Li, H.; Qiao, X.; Jiang, T.; Fu, X.; He, Y.; Zhao, X. Agarose oligosaccharide- silver nanoparticle- antimicrobial peptide-composite for wound dressing. Carbohydr. Polym. 2021, 269, 118258. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Ahmad, A.; Khan, M.A.; Siddiqui, S. Zinc Oxide Nanoparticle Induces Apoptosis in Human Epidermoid Carcinoma Cells Through Reactive Oxygen Species and DNA Degradation. Biol. Trace Elem. Res. 2021, 199, 2172–2181. [Google Scholar] [CrossRef]
- Hsueh, C.-T.; Selim, J.H.; Tsai, J.Y. Nanovectors for anti-cancer drug delivery in the treatment of advanced pancreatic adenocarcinoma. World J. Gastroenterol. 2016, 22, 7080–7090. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Irani, A.S.; Ander, E.W.; Ludolph, C.M.; Venkataraman, A.K.; Zhong, J.X.; Peppas, N.A. Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications. Sci. Adv. 2019, 5, eaax7946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1210–S1220. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Ju, E.J.; Park, S.S.; Choi, J.; Lee, J.H.; Lee, K.J.; Shin, S.H.; Ko, E.J.; Park, I.; et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J. Control. Release 2015, 207, 77–85. [Google Scholar] [CrossRef]
- Peng, J.; Liang, X. Progress in research on gold nanoparticles in cancer management. Medicine 2019, 98, e15311. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold nanoparticles and angiogenesis: Molecular mechanisms and biomedical applications. Int. J. Nanomed. 2019, 14, 7643–7663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Lupusoru, R.V.; Pricop, D.A.; Uritu, C.M.; Arvinte, A.; Coroaba, A.; Esanu, I.; Zaltariov, M.F.; Silion, M.; Stefanescu, C.; Pinteala, M. Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells. Sci. Rep. 2020, 10, 6591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Omri, A.; Krishnan, L.; McCluskie, M.J. Potential applications of nanoparticles in cancer immunotherapy. Hum. Vaccines Immunother. 2017, 13, 63–74. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Davidi, E.S.; Dreifuss, T.; Motiei, M.; Shai, E.; Bragilovski, D.; Lubimov, L.; Kindler, M.J.J.; Popovtzer, A.; Don, J.; Popovtzer, R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck 2018, 40, 70–78. [Google Scholar] [CrossRef]
- Cui, L.; Her, S.; Dunne, M.; Borst, G.R.; De Souza, R.; Bristow, R.; Jaffray, D.; Allen, C. Significant Radiation Enhancement Effects by Gold Nanoparticles in Combination with Cisplatin in Triple Negative Breast Cancer Cells and Tumor Xenografts. Radiat. Res. 2017, 187, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Paknejadi, M.; Bayat, M.; Salimi, M.; Razavilar, V. Concentration- and Time-Dependent Cytotoxicity of Silver Nanoparticles on Normal Human Skin Fibroblast Cell Line. Iran. Red Crescent Med. J. 2018, 20, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Choi, D.Y.; Song, H.; Park, C.; Kim, J.-H.; Hong, K. Cytotoxicity and Transcriptomic Analysis of Silver Nanoparticles in Mouse Embryonic Fibroblast Cells. Int. J. Mol. Sci. 2018, 19, 3618. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Li, L.; Meng, X.; Liu, T.; Hu, Q.; Miao, L. Cytotoxicity of Silver Nanoparticles on Human Periodontal Ligament Fibroblasts. Nanosci. Nanotechnol. Lett. 2017, 9, 1015–1022. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Zhang, S.; Hwang, J.-Y.; Kong, I.-K. Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxidative Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef]
- Danışman-Kalındemirtaş, F.; Karíper, İ.A.; Hepokur, C.; Erdem-Kuruca, S. Selective cytotoxicity of paclitaxel bonded silver nanoparticle on different cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102265. [Google Scholar] [CrossRef]
- Hepokur, C.; Kariper, İ.A.; Mısır, S.; Ay, E.; Tunoğlu, S.; Ersez, M.S.; Zeybek, Ü.; Kuruca, S.E.; Yaylım, I. Silver nanoparticle/capecitabine for breast cancer cell treatment. Toxicol. Vitr. 2019, 61, 104600. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Li, S.; Shi, J.; Gao, H.; Leong, W.S.; Wu, Y.; Li, M.; Liu, C.; Li, P.; et al. Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent. Nat. Commun. 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berber, M.R.; Elkhenany, H.; Hafez, I.H.; El-Badawy, A.; Essawy, M.; El-Badri, N. Efficient tailoring of platinum nanoparticles supported on multiwalled carbon nanotubes for cancer therapy. Nanomedicine 2020, 15, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Xing, L.; Chang, X.; Zhou, T.-J.; Bi, Y.-Y.; Yu, Z.-Q.; Zhang, Z.-Q.; Jiang, H.-L. Synergistic Platinum(II) Prodrug Nanoparticles for Enhanced Breast Cancer Therapy. Mol. Pharm. 2020, 17, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef]
- Gu, T.; Wang, Y.; Lu, Y.; Cheng, L.; Feng, L.; Zhang, H.; Li, X.; Han, G.; Liu, Z. Platinum Nanoparticles to Enable Electrodynamic Therapy for Effective Cancer Treatment. Adv. Mater. 2019, 31, e1806803. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Graphene Oxide–Platinum Nanoparticle Nanocomposites: A Suitable Biocompatible Therapeutic Agent for Prostate Cancer. Polymers 2019, 11, 733. [Google Scholar] [CrossRef] [Green Version]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-Coated Platinum Nanoparticles with Selective Toxicity against Liver Cancer Cells. Angew. Chem. Int. Ed. 2019, 58, 4901–4905. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Rokade, S.S.; Joshi, K.A.; Mahajan, K.; Patil, S.; Tomar, G.; Dubal, D.S.; Parihar, V.S.; Kitture, R.; Bellare, J.R.; Ghosh, S. Gloriosa superba Mediated Synthesis of Platinum and Palladium Nanoparticles for Induction of Apoptosis in Breast Cancer. Bioinorg. Chem. Appl. 2018, 2018, 4924186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Fahdawi, M.Q.; Rasedee, A.; Al-Doghachi, F.A.; Rosli, R.; Taufiq-Yap, Y.; Al-Qubaisi, M.S. Anticancer palladium-doped magnesia nanoparticles: Synthesis, characterization, and in vitro study. Nanomedicine 2020, 15, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Laprise-Pelletier, M.; Lagueux, J.; Côté, M.-F.; Lagrange, T.; Fortin, M.-A. Low-Dose Prostate Cancer Brachytherapy with Radioactive Palladium-Gold Nanoparticles. Adv. Health Mater. 2017, 6, 1601120. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Phan, T.T.V.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Leso, V.; Iavicoli, I. Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment. Int. J. Mol. Sci. 2018, 19, 503. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Qasim, M.; Park, C.H.; Iqbal, M.A.; Yoo, H.; Hwang, J.H.; Uhm, S.J.; Song, H.; Park, C.; Choi, Y.; et al. Cytotoxicity and Transcriptomic Analyses of Biogenic Palladium Nanoparticles in Human Ovarian Cancer Cells (SKOV3). Nanomaterials 2019, 9, 787. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Soe, Z.C.; Yang, K.Y.; Phung, C.D.; Nguyen, L.T.-T.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-conjugated pH-sensitive platform for effective delivery of porous palladium nanoparticles and paclitaxel in cancer treatment. Colloids Surf. B Biointerfaces 2019, 176, 265–275. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Combination of palladium nanoparticles and tubastatin-A potentiates apoptosis in human breast cancer cells: A novel therapeutic approach for cancer. Int. J. Nanomed. 2017, 12, 6503–6520. [Google Scholar] [CrossRef] [Green Version]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Adeyemi, J.A.; Machado, A.R.T.; Ogunjimi, A.; Alberici, L.C.; Antunes, L.M.G.; Barbosa, F. Cytotoxicity, mutagenicity, oxidative stress and mitochondrial impairment in human hepatoma (HepG2) cells exposed to copper oxide, copper-iron oxide and carbon nanoparticles. Ecotoxicol. Environ. Saf. 2019, 189, 109982. [Google Scholar] [CrossRef]
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Aivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 12613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wan, F.; Ma, L.; Phan, J.B.; Lim, R.X.; Li, C.; Chen, J.; Deng, J.; Li, Y.; Chen, W.; et al. Investigation of copper-cysteamine nanoparticles as a new photosensitizer for anti-hepatocellular carcinoma. Cancer Biol. Ther. 2019, 20, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct. Target. Ther. 2020, 5, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, Z.; Wang, B.; Xu, G.; Wu, Q.; Zhang, Y.; Yuan, Z.; Yang, X.; Yu, C. Lysosomal deposition of copper oxide nanoparticles triggers HUVEC cells death. Biomaterials 2018, 161, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qin, J.; Zhou, C.; Wan, G.; Liu, Y.; Zhang, M.; Yang, X.; Zhang, N.; Wang, Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials 2019, 195, 86–99. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.; Wang, X.; Sun, R. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy. Mater. Sci. Eng. C 2018, 96, 129–137. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Ramani, M.; Mudge, M.C.; Morris, R.T.; Zhang, Y.; Warcholek, S.A.; Hurst, M.N.; Riviere, J.E.; DeLong, R.K. Zinc Oxide Nanoparticle–Poly I:C RNA Complexes: Implication as Therapeutics against Experimental Melanoma. Mol. Pharm. 2017, 14, 614–625. [Google Scholar] [CrossRef]
- He, G.; Pan, X.; Liu, X.; Zhu, Y.; Ma, Y.; Du, C.; Liu, X.; Mao, C. HIF-1α-Mediated Mitophagy Determines ZnO Nanoparticle-Induced Human Osteosarcoma Cell Death both In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 48296–48309. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140. [Google Scholar] [CrossRef]
- Park, Y.-H.; Bae, H.C.; Kim, J.; Jeong, S.H.; Yang, S.I.; Son, S.W. Zinc oxide nanoparticles induce HIF-1α protein stabilization through increased reactive oxygen species generation from electron transfer chain complex III of mitochondria. J. Dermatol. Sci. 2018, 91, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Wang, X.; Qiu, L.; Li, Y.; Marraiki, N.; Elgorban, A.M.; Xue, L. Green synthesized zinc oxide nanoparticles regulates the apoptotic expression in bone cancer cells MG-63 cells. J. Photochem. Photobiol. B Biol. 2019, 202, 111644. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2019, 94, 103423. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Qiu, F.; Zhu, F.; Qi, L. Therapeutic Potential of Zinc Oxide-Loaded Syringic Acid Against in vitro and in vivo Model of Lung Cancer. Int. J. Nanomed. 2020, 15, 8249–8260. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, K.; Choudhary, C.; Mishra, P.K.; Vaidya, B. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif. Cells Nanomed. Biotechnol. 2016, 44, 672–679. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, J.-S.; Zhang, P.; Chen, J.; Kong, J.-L.; Sun, L.-H.; Xiong, H.-M.; Möhwald, H. Self-Assembled ZnO Nanoparticle Capsules for Carrying and Delivering Isotretinoin to Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 18474–18481. [Google Scholar] [CrossRef]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Alves, S.R.; Colquhoun, A.; Wu, X.Y.; Silva, D.D.O. Synthesis of terpolymer-lipid encapsulated diruthenium(II,III)-anti-inflammatory metallodrug nanoparticles to enhance activity against glioblastoma cancer cells. J. Inorg. Biochem. 2019, 205, 110984. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Maly, M.; Ramirez, R.G.; de la Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, X.; Cao, C.; Sun, J.; Liu, J. Transferrin modified ruthenium nanoparticles with good biocompatibility for photothermal tumor therapy. J. Colloid Interface Sci. 2018, 511, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, M.; Zheng, W.; Xu, T.; Deng, H.; Liu, J. Se/Ru-Decorated Porous Metal–Organic Framework Nanoparticles for The Delivery of Pooled siRNAs to Reversing Multidrug Resistance in Taxol-Resistant Breast Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 6712–6724. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Huang, F.-Y.; Cao, R.; Zhang, L.; Tan, G.-H.; He, N.; Huang, J.; Wang, G.; Zhang, Z. Long Blood Residence and Large Tumor Uptake of Ruthenium Sulfide Nanoclusters for Highly Efficient Cancer Photothermal Therapy. Sci. Rep. 2017, 7, 41571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, B.A.; Reddy, A.S.; Sangubotla, R.; Hong, J.W.; Kim, S. Ruthenium(II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B Biointerfaces 2021, 204, 111773. [Google Scholar] [CrossRef]
- Liu, J.; Lai, H.; Xiong, Z.; Chen, B.; Chen, T. Functionalization and cancer-targeting design of ruthenium complexes for precise cancer therapy. Chem. Commun. 2019, 55, 9904–9914. [Google Scholar] [CrossRef]
- Xu, M.; Wen, Y.; Liu, Y.; Tan, X.; Chen, X.; Zhu, X.; Wei, C.; Chen, L.; Wang, Z.; Liu, J.; et al. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale 2019, 11, 9661–9678. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Zhu, X.; Chen, X.; Zhou, Y.; Yuan, G.; Gong, Y.; Liu, J. Iridium/ruthenium nanozyme reactors with cascade catalytic ability for synergistic oxidation therapy and starvation therapy in the treatment of breast cancer. Biomaterials 2020, 238, 119848. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2018, 83, 37–54. [Google Scholar] [CrossRef]
- Xu, F. Review of analytical studies on TiO2 nanoparticles and particle aggregation, coagulation, flocculation, sedimentation, stabilization. Chemosphere 2018, 212, 662–677. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, D.; Oh, S.; Nam, K.; Son, S.; Gye, M.C.; Shin, I. Titanium dioxide nanoparticles induce apoptosis by interfering with EGFR signaling in human breast cancer cells. Environ. Res. 2019, 175, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Haynes, V.N.; Ward, J.E.; Russell, B.J.; Agrios, A.G. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms—Current knowledge and suggestions for future research. Aquat. Toxicol. 2017, 185, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Ghany, S.; Raslan, S.; Tombuloglu, H.; Shamseddin, A.; Cevik, E.; Said, O.A.; Madyan, E.F.; Senel, M.; Bozkurt, A.; Rehman, S.; et al. Vorinostat-loaded titanium oxide nanoparticles (anatase) induce G2/M cell cycle arrest in breast cancer cells via PALB2 upregulation. 3 Biotech 2020, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zeng, L.; Shen, Z.; Xiang, L.; Gong, A.; Zhang, J.; Mao, C.; Li, A.; Paunesku, T.; Woloschak, G.E.; et al. Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv. 2013, 3, 20855–20861. [Google Scholar] [CrossRef]
- Shen, J.; Karges, J.; Xiong, K.; Chen, Y.; Ji, L.; Chao, H. Cancer cell membrane camouflaged iridium complexes functionalized black-titanium nanoparticles for hierarchical-targeted synergistic NIR-II photothermal and sonodynamic therapy. Biomaterials 2021, 275, 120979. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a com-prehensive overview of its pharmacotoxicological mechanisms and multi-apply with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Bueloni, B.; Sanna, D.; Garribba, E.; Castro, G.R.; León, I.E.; Islan, G.A. Design of nalidixic acid-vanadium complex loaded into chitosan hybrid nanoparticles as smart strategy to inhibit bacterial growth and quorum sensing. Int. J. Biol. Macromol. 2020, 161, 1568–1580. [Google Scholar] [CrossRef]
- Suma, P.R.; Padmanabhan, R.A.; Telukutla, S.R.; Ravindran, R.; Velikkakath, A.K.G.; Dekiwadia, C.D.; Paul, W.; Laloraya, M.; Srinivasula, S.M.; Bhosale, S.V.; et al. Vanadium pentoxide nanoparticle mediated perturbations in cellular redox balance and the paradigm of autophagy to apoptosis. Free Radic. Biol. Med. 2020, 161, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roy, A.; Barui, A.K.; Alabbasi, M.M.A.; Kuncha, M.; Sistla, R.; Sreedhar, B.; Patra, C.R. Anti-angiogenic vanadium pentoxide nanoparticles for the treatment of melanoma and their in vivo toxicity study. Nanoscale 2020, 12, 7604–7621. [Google Scholar] [CrossRef]
- Xi, W.-S.; Tang, H.; Liu, Y.-Y.; Liu, C.-Y.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H. Cytotoxicity of vanadium oxide nanoparticles and titanium dioxide-coated vanadium oxide nanoparticles to human lung cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Elstrott, B.; Khan, L.; Olson, S.; Raghunathan, V.; Deloughery, T.; Shatzel, J.J. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 2021, 104, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.-D.; Maghsoodi, F.; Panahandeh, F.; Yazdian-Robati, R.; Reisi-Vanani, A.; Tafaghodi, M. Doxorubicin delivery via magnetic nanomicelles comprising from reduction-responsive poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-SS-PCL) and loaded with superparamagnetic iron oxide (SPIO) nanoparticles: Preparation, characterization and simulation. Mater. Sci. Eng. C 2018, 92, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, K.; Zhang, X.; Ouyang, B.; Liu, H.; Pang, Z.; Yang, W. Platelet Membrane-Camouflaged Magnetic Nanoparticles for Ferroptosis-Enhanced Cancer Immunotherapy. Small 2020, 16, e2001704. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2020, 138, 302–325. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Mahdavi, M.; Amini, E.; Yeap, S.K.; Chartrand, M.S.; Rahman, H.S. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed. 2014, 9, 2479–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, M.; Mollania, N.; Momeni-Moghaddam, M.; Sadeghifar, F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: Characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J. Biotechnol. 2018, 270, 1–11. [Google Scholar] [CrossRef]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
- Antal, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Khmara, I.; Lucanska, D.; Jelenska, L.; Vidlickova, I.; Zatovicova, M.; Pastorekova, S.; et al. d,l-lysine functionalized Fe3O4 nanoparticles for detection of cancer cells. Colloids Surf. B Biointerfaces 2018, 163, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Hui Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
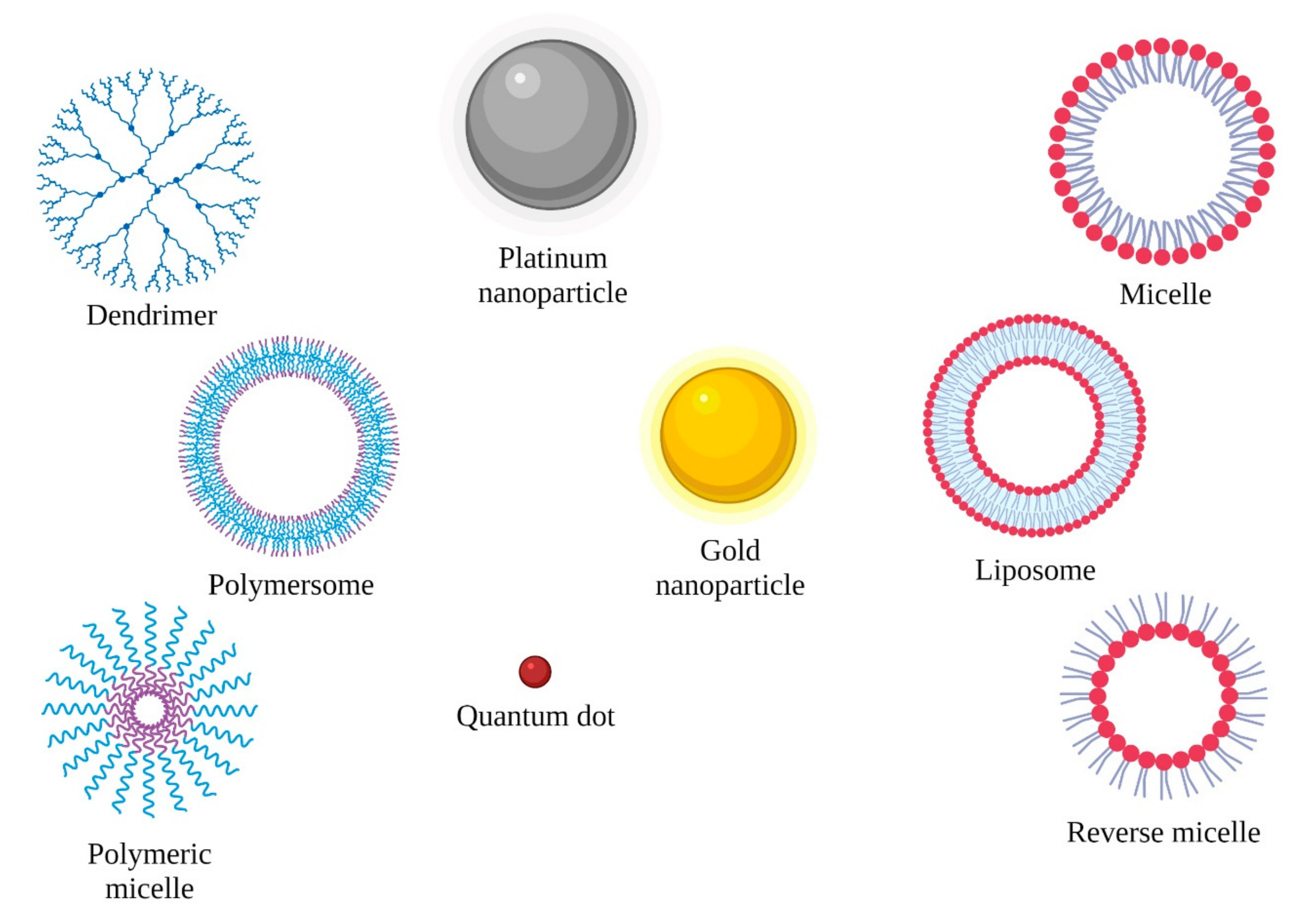
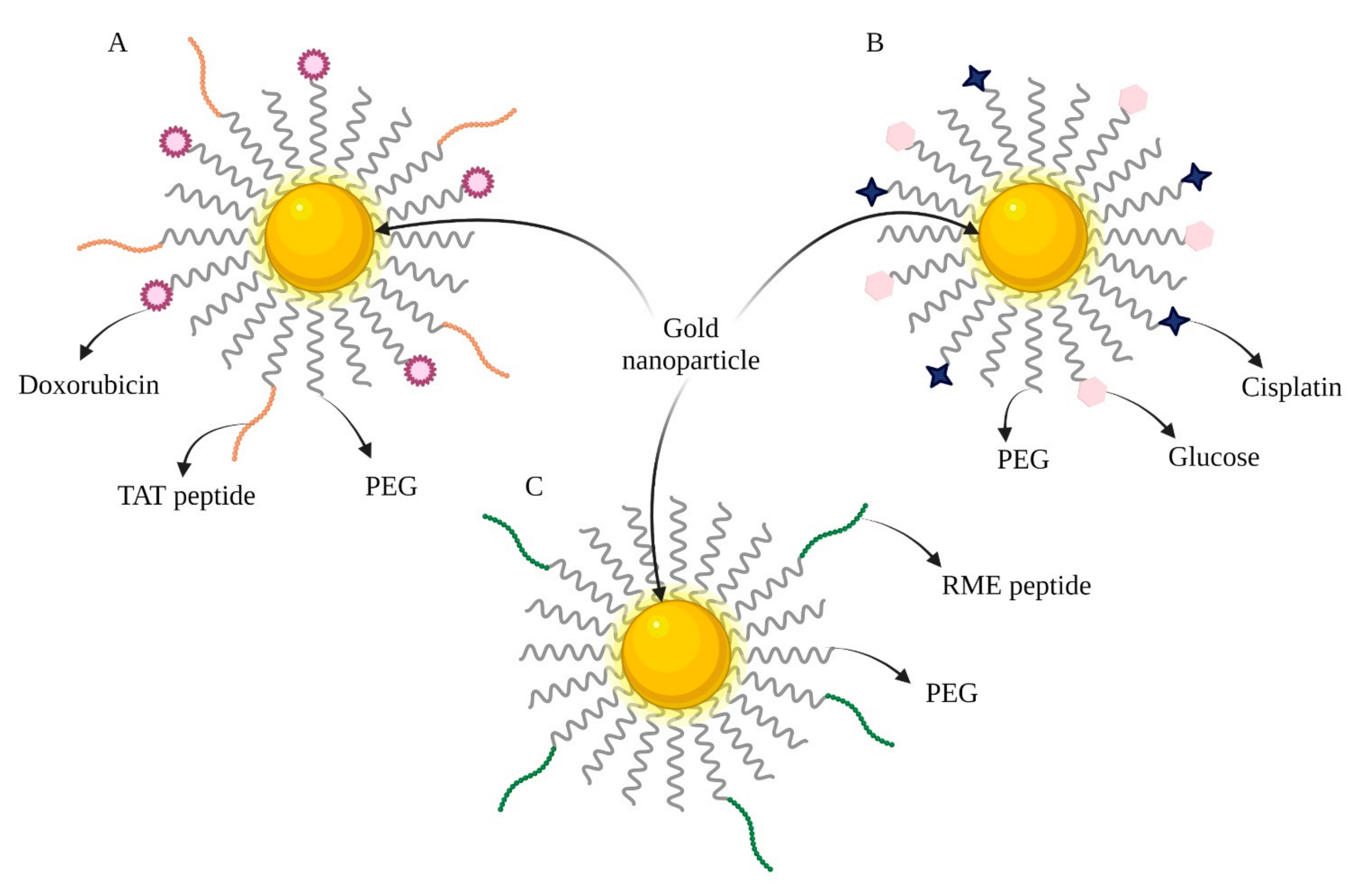

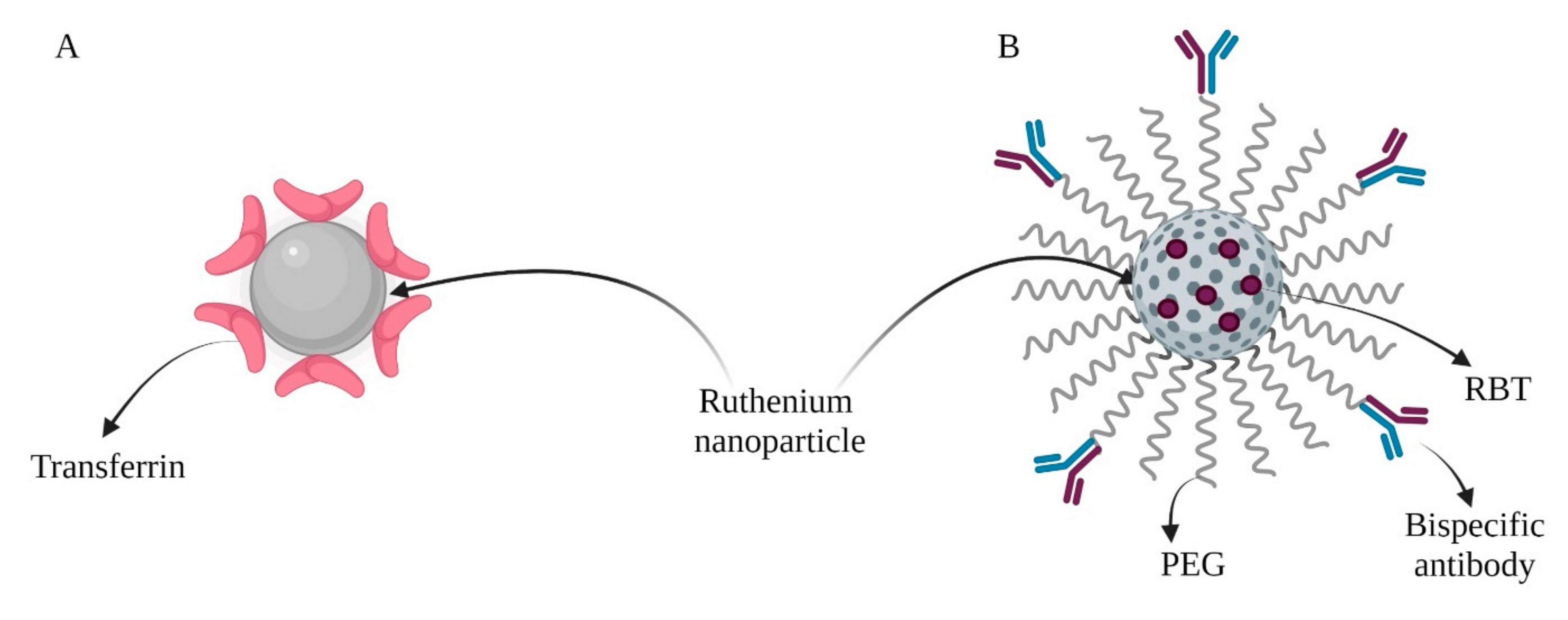

| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT/RT | Cisplatin, Glucose (Figure 2B) | A431 cells Mice bearing A431 tumor | Simultaneously acts as a radiosensitizer, drug carrier, and tumor imaging agent. | [20] |
| RT | An adenoviral receptor-mediated endocytosis (RME) peptide (Figure 2C) | MDA-MB-231 cells NOD/SCID mice bearing MDA-MB-231 tumor | Acts as a radiosensitizer. | [21] |
| CHT | Doxorubicin (DOX) TAT peptide | HOS and NHDF cell lines | Cytotoxic effect of pure doxorubicin with 3% of the substance incorporated into the nanoparticles. | [16] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT | Camptothecin (CPT) | HeLa cells | CPT and Ag NPs caused cell death by inducing a mitochondrial-membrane permeability change and activation of caspase 9, 6, and 3. | [28] |
| CHT | Paclitaxel (PTX) | MDA-MB-231, MCF-7, 4T1, Saos-2, and HUVEC cells | Ag NPs-PTX reduced the PTX dose significantly, which may prevent serious side effects. | [29] |
| CHT | Capecitabine | MCF-7 | Lower doses of capecitabine bonded with Ag NPs can reduce unwanted side effects. | [30] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| PDT/CHT | Camptothecin (CPT) 2-(1-hexyloxyethyl)-2-divinyl pyropheophorbide-a (HPPH) | CT26 cells CT26 tumor-bearing BALB/c mice | Pt NPs could decompose H2O2 into oxygen, leading to improvement in the ROS-generation ability of HPPH. The fluorogenic nature of HPPH enabled the visualization of the color cellular uptake in vitro and tissue distribution in vivo via fluorescence imaging and photoacoustic imaging. | [34] |
| CHT | H-Lys-[Pro-Gly- Lys]2-NH2 | HepG2, MCF-7, HeLa, PC3, A431, A549, A2780 and HT-29 cell lines | The combination of high cellular uptake and an oxidative environment was the reason that peptide-coated Pt NPs had the highest cytotoxicity, combined with selectivity, for hepatic cancer cells. | [37] |
| CHT/PTT | Doxorubicin hydrochloride (Dox) | MCF-7/ADR cells | Pt NPs had a high loading capacity for chemotherapeutic drugs and could deliver Dox into tumor cells. Moreover, they had great photothermal conversion capabilities and photostability. | [38] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT/PDT | Paclitaxel (PTX) Transferrin (Tf) (Figure 3B) | MDA-MB-231 and MCF-7 cells MCF-7 tumor-bearing BALB/c nude mice | Synergistic anticancer effect in combination with PTX. Transferrin on the surface improved their uptake into cancer cells. | [47] |
| PTT | RGD (arginine-glycine-aspartic acid) Chitosan oligosaccharide (COS) | MDA-MB-231, HEK-293, MG-63 cells BALB/c nude mice bearing MDA-MB-231 tumor | Chitosan oligosaccharide (COS) improved biocompatibility. Particles were functionalized with RGD peptide, which improved their accumulation. | [44] |
| CHT | Tubastatin-A | MDA-MB-231 | TUB-A and Pd NPs synergistically induced apoptosis by decreasing cell viability and inhibiting HDAC activity. This effect was more significant in cytotoxicity, loss of mitochondrial membrane potential and increases in caspase-3 activity, DNA fragmentation, and expression of proapoptotic genes. | [48] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| PDT | Cysteamine | HepG2 cells, nude mice bearing HepG2 tumor; B16F10 cell line, nude mice bearing B16F10 tumor | Generation of substantial ROS levels, induction of an antitumor immune response. The presence of oxygen led to the creation of the photodynamic reaction. | [52,53] |
| CHT | Triethylene- tetramine-bis(dithiocarbamate) (TETA-DTC), poly-L-histidine (PHis), Arg- Gly-Asp peptide (RGD)-conjugated poly(ethylene glycol) (RGD-PEG) | HUVECs, BEAS-2B, MCF-7, MDA-MB-231, and 4T1 cells Female BALB/c mice bearing 4T1 tumor | Suppression of angiogenesis through RPTDH-induced copper deficiency and stimulation of antitumor immunity in vivo | [55] |
| PTT | Quaternized chitosan (QCS) | 4T1 cell lines 4T1 tumor-bearing mice | Under NIR light, Cu ions from the CuS nanoparticles led redox reactions to generate ROS production, stimulating inflammation and initiating proapoptotic cellular signaling. | [56] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT | Quercetin (Q) PBA acid (3-carboxybenzeneboronic acid) | MCF-7 and MCF-10a Ehrlich’s ascites carcinoma, solid tumor-bearing male Swiss albino mice | Induction of cytotoxic effect via ROS enhancement effects of ZnO and free Q in cancer cells. Nanoparticles did not show systemic toxicity in tumor-bearing mice and were found to reduce tumor-associated toxicity in the liver, kidney, and spleen. | [60] |
| CHT | Syringic acid | A549 cell line Male adult swiss albino mouse model of induced lung cancer | Moderate ROS generation, disrupted mitochondrial membrane potential, morphological modification by dual staining and viability, and non-viability by cell adhesion assay. | [64] |
| CHT | Isotretinoin Nintedanib Crizotinib | DU145, HeLa, MCF-7, and A549 cell lines | The loading capacity of the capsules was higher than on NP surfaces. The pH sensitivity of ZnO−ISO was also higher. | [66] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| PTT | Transferrin | A549 and HEK-293 cell lines Male mice bearing A549 tumor | Acts as a photothermal agent. High absorption under NIR irradiation and efficient heat transformation for photothermal therapy. | [71] |
| PTT/PDT/IMT | Bispecific antibodies (SS-Fc) Fluorescent anti-tumor complex ([Ru(bpy)2(tip)]2+, RBT (Figure 4B) | HIEC-6 cells, Caco-2, SW480, HCT116, CT26.WT cell lines Female BALB/c mice bearing CT26-CEA cells | Nanoparticles delivered RBT to solid tumors for combined HMRu-based PTT, RBT-induced PDT, and SS-Fc-mediated immunotherapy. | [76] |
| Starvation therapy and Oxidation therapy | Glucose oxidase (GOx) | 4T1 and U87 cell lines BALB/c nude mice with 4T1 xenograft tumors | Compound converted H2O2 to toxic 1O2, thereby inducing tumor cell apoptosis and also catalyzing the conversion of H2O2 to O2. | [77] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT | Erlotinib (ERL) and Vorinostat (SAHA) | WISH, MDA-MB-231, and MCF-7 cell lines | Increase in total apoptosis in all treatments. ERL- and SAHA-loaded TiO2 NP treatments arrested cells at the G2/M phase. PLAB2 was upregulated in ERL- and SAHA-loaded TiO2 NPs compared with control cells. | [84] |
| CHT | Doxorubicin (DOX) | MCF-7 and MCF-7/ADM cells | DOX can be released from the surface of TiO2 nanoparticles in the acidic environment of endosomes or lysosomes. | [85] |
| PTT, SDT | [Ir(2-phenylbenzo[d]thiazole)2(4-(1-phenyl-1H- imidazo [4,5-f][1,10]phenanthrolin-2-yl)benzoic dopamine amide)]Cl | HeLa cells HeLa tumor-bearing mice | Localized and accumulated in cancerous over non-cancerous cells. Upon irradiation in the near-infrared- II region at 1064 nm or ultrasound radiation and their combination, acted as an imaging agent and as a therapeutic agent. | [86] |
| Therapy | Connected Particles | Experimental Model | Molecular Mechanism | Reference |
|---|---|---|---|---|
| CHT | Doxorubicin (DOX) | CHO and HFLF cells | Redox-responsive properties resulted from a disulfide bond; the rapid release was observed in intracellular reducing potential. | [95] |
| CHT | 5-Fluorouracil (5-FU) | Female athymic nude mice bearing HT-29 tumor | DNA damage and increased stress levels in cells, impact on second messenger and nuclear receptor signaling, caveolar-mediated endocytosis with DAMPs. | [101] |
| Immunotherapy | d,l-lysine M75 monoclonal antibody | B16 mouse melanoma cells, C33a human cervical cancer cells | Antibody-conjugated nanoparticles can target malignant cells and accumulate in the cytoplasm. | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, O.K.; Roszczenko, P.; Czarnomysy, R.; Bielawska, A.; Bielawski, K. An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research. Int. J. Mol. Sci. 2022, 23, 6688. https://doi.org/10.3390/ijms23126688
Szewczyk OK, Roszczenko P, Czarnomysy R, Bielawska A, Bielawski K. An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research. International Journal of Molecular Sciences. 2022; 23(12):6688. https://doi.org/10.3390/ijms23126688
Chicago/Turabian StyleSzewczyk, Olga Klaudia, Piotr Roszczenko, Robert Czarnomysy, Anna Bielawska, and Krzysztof Bielawski. 2022. "An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research" International Journal of Molecular Sciences 23, no. 12: 6688. https://doi.org/10.3390/ijms23126688
APA StyleSzewczyk, O. K., Roszczenko, P., Czarnomysy, R., Bielawska, A., & Bielawski, K. (2022). An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research. International Journal of Molecular Sciences, 23(12), 6688. https://doi.org/10.3390/ijms23126688







