Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review
Abstract
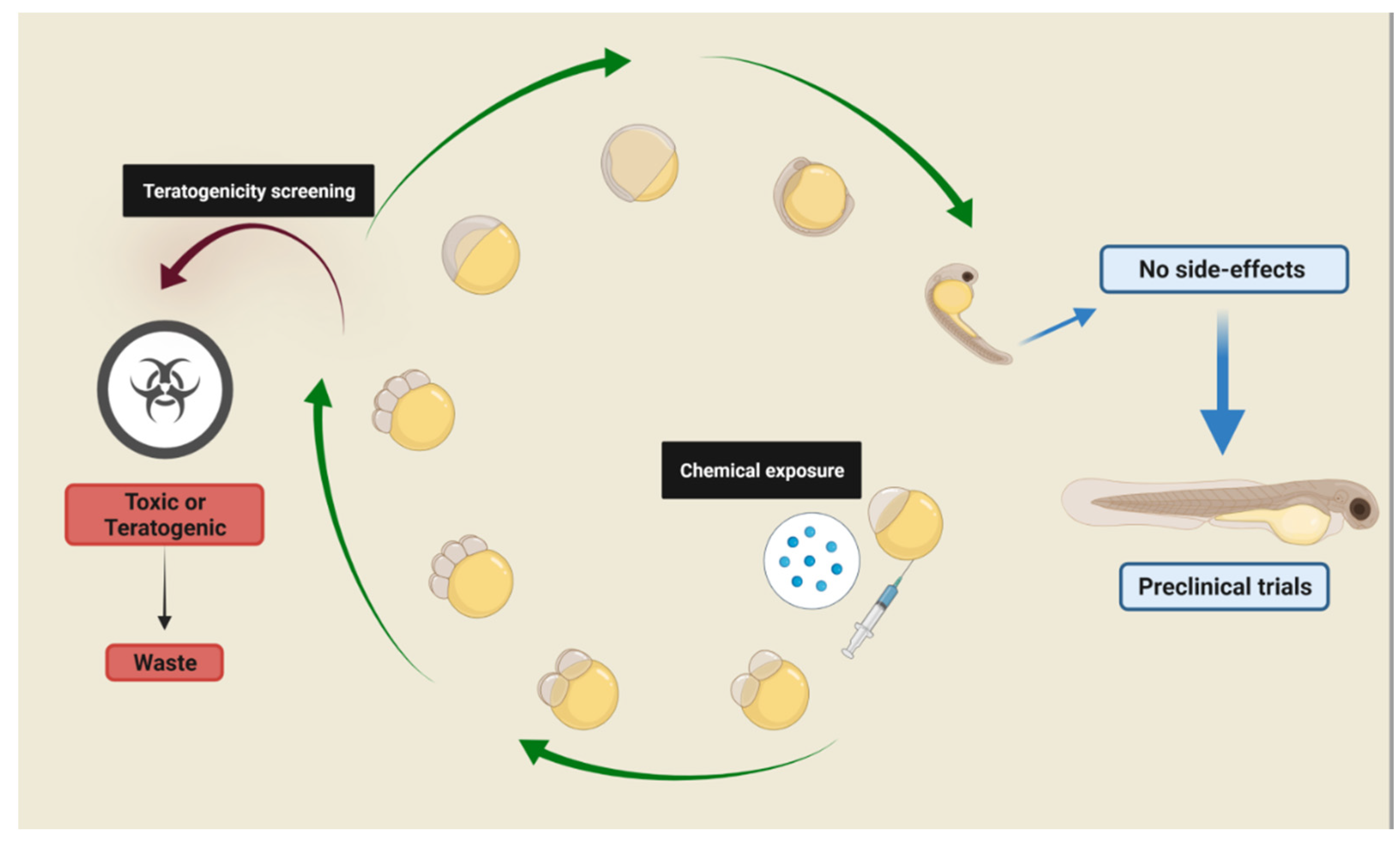
:1. Zebrafish as a Model for Phenotype-Based Screening
2. Zebrafish Neurotransmitter Systems
3. Neurological Functions and Behavior Models toward Pre-Clinical Assays
3.1. Anxiety-like Behavioral Models
3.2. Visual Behavior
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Swinney, D.C.; Anthony, J. How Were New Medicines Discovered? Nat. Rev. Drug Discov. 2011, 10, 507–519. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and Challenges in Phenotypic Drug Discovery: An Industry Perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Lowery, L.A.; De Rienzo, G.; Gutzman, J.H.; Sive, H. Characterization and Classification of Zebrafish Brain Morphology Mutants. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009, 292, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Geisler, R.; Köhler, A.; Dickmeis, T.; Strähle, U. Archiving of Zebrafish Lines Can Reduce Animal Experiments in Biomedical Research. EMBO Rep. 2017, 18, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Saint-Amant, L.; Drapeau, P. Time Course of the Development of Motor Behaviors in the Zebrafish Embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Orger, M.B.; de Polavieja, G.G. Zebrafish Behavior: Opportunities and Challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [Green Version]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Förster, D.; Arnold-Ammer, I.; Laurell, E.; Barker, A.J.; Fernandes, A.M.; Finger-Baier, K.; Filosa, A.; Helmbrecht, T.O.; Kölsch, Y.; Kühn, E.; et al. Genetic Targeting and Anatomical Registration of Neuronal Populations in the Zebrafish Brain with a New Set of BAC Transgenic Tools. Sci. Rep. 2017, 7, 5230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Alex, D.; Siu, S.O.; Chu, I.K.; Renn, J.; Winkler, C.; Lou, S.; Tsui, S.K.-W.; Zhao, H.Y.; Yan, W.R.; et al. Combined in Vivo Imaging and Omics Approaches Reveal Metabolism of Icaritin and Its Glycosides in Zebrafish Larvae. Mol. Biosyst. 2011, 7, 2128. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Kwon, H.-B.; Ahn, J.-C.; Kang, D.; Kwon, S.-H.; Park, J.A.; Kim, K.-W. Functional and Developmental Analysis of the Blood–Brain Barrier in Zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and Developmental Expression of the Full Complement of Cytochrome P450 Genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randlett, O.; Wee, C.L.; Naumann, E.A.; Nnaemeka, O.; Schoppik, D.; Fitzgerald, J.E.; Portugues, R.; Lacoste, A.M.B.; Riegler, C.; Engert, F.; et al. Whole-Brain Activity Mapping onto a Zebrafish Brain Atlas. Nat. Methods 2015, 12, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Blader, P. Zebrafish Developmental Genetics and Central Nervous System Development. Hum. Mol. Genet. 2000, 9, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kizil, C.; Kaslin, J.; Kroehne, V.; Brand, M. Adult Neurogenesis and Brain Regeneration in Zebrafish. Dev. Neurobiol. 2012, 72, 429–461. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Steenaerts, L.; Philippe, C.; Grégoir, A.F.; Brendonck, L.; Pinceel, T. Improving the Reliability and Ecological Validity of Pharmaceutical Risk Assessment: Turquoise Killifish (Nothobranchius furzeri) as a Model in Behavioral Ecotoxicology. Environ. Toxicol. Chem. 2019, 38, 262–270. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.J.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid Behavior-Based Identification of Neuroactive Small Molecules in the Zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolman, M.A.; Jain, R.A.; Liss, L.; Granato, M. Chemical Modulation of Memory Formation in Larval Zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 15468–15473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.O.; Gaviria, J.; Haigh, A.; Millington, M.E.; Brown, V.J.; Combe, F.J.; Brennan, C.H. Discrimination Reversal and Attentional Sets in Zebrafish (Danio rerio). Behav. Brain Res. 2012, 232, 264–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish Models in Neuropsychopharmacology and CNS Drug Discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish Models of Neurodevelopmental Disorders: Past, Present, and Future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef] [Green Version]
- Haug, M.F.; Gesemann, M.; Mueller, T.; Neuhauss, S.C.F. Phylogeny and Expression Divergence of Metabotropic Glutamate Receptor Genes in the Brain of Zebrafish (Danio rerio). J. Comp. Neurol. 2013, 521, 1533–1560. [Google Scholar] [CrossRef] [Green Version]
- Doldn, M.J.; Prego, B.; Holmqvist, B.I.; de Miguel, E. Distribution of GABA-Immunolabeling in the Early Zebrafish (Danio rerio) Brain. Eur. J. Morphol. 1999, 37, 126–129. [Google Scholar] [CrossRef]
- Assad, N.; Luz, W.L.; Santos-Silva, M.; Carvalho, T.; Moraes, S.; Picanço-Diniz, D.L.W.; Bahia, C.P.; de Oliveira Batista, E.J.; da Conceição Passos, A.; Oliveira, K.R.H.M.; et al. Acute Restraint Stress Evokes Anxiety-Like Behavior Mediated by Telencephalic Inactivation and GabAergic Dysfunction in Zebrafish Brains. Sci. Rep. 2020, 10, 5551. [Google Scholar] [CrossRef] [Green Version]
- Mueller, T.; Vernier, P.; Wullimann, M.F. A Phylotypic Stage in Vertebrate Brain Development: GABA Cell Patterns in Zebrafish Compared with Mouse. J. Comp. Neurol. 2006, 494, 620–634. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.C.; Bill, B.R.; Glanzman, D.L. Learning and Memory in Zebrafish Larvae. Front. Neural Circuits 2013, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- McLean, D.L.; Fetcho, J.R. Ontogeny and Innervation Patterns of Dopaminergic, Noradrenergic, and Serotonergic Neurons in Larval Zebrafish. J. Comp. Neurol. 2004, 480, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Kaslin, J.; Panula, P. Comparative Anatomy of the Histaminergic and Other Aminergic Systems in Zebrafish (Danio rerio). J. Comp. Neurol. 2001, 440, 342–377. [Google Scholar] [CrossRef] [PubMed]
- Ek, F.; Malo, M.; Åberg Andersson, M.; Wedding, C.; Kronborg, J.; Svensson, P.; Waters, S.; Petersson, P.; Olsson, R. Behavioral Analysis of Dopaminergic Activation in Zebrafish and Rats Reveals Similar Phenotypes. ACS Chem. Neurosci. 2016, 7, 633–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, J.; Driever, W. Development of the Dopamine Systems in Zebrafish. In Development and Engineering of Dopamine Neurons; Springer: Berlin/Heidelberg, Germany, 2009; Volume 651, pp. 1–14. [Google Scholar] [CrossRef]
- Tay, T.L.; Ronneberger, O.; Ryu, S.; Nitschke, R.; Driever, W. Comprehensive Catecholaminergic Projectome Analysis Reveals Single-Neuron Integration of Zebrafish Ascending and Descending Dopaminergic Systems. Nat. Commun. 2011, 2, 171. [Google Scholar] [CrossRef] [Green Version]
- Alsop, D.; Vijayan, M. The Zebrafish Stress Axis: Molecular Fallout from the Teleost-Specific Genome Duplication Event. Gen. Comp. Endocrinol. 2009, 161, 62–66. [Google Scholar] [CrossRef]
- Clemente, D.; Porteros, Á.; Weruaga, E.; Alonso, J.R.; Arenzana, F.J.; Aijón, J.; Arévalo, R. Cholinergic Elements in the Zebrafish Central Nervous System: Histochemical and Immunohistochemical Analysis. J. Comp. Neurol. 2004, 474, 75–107. [Google Scholar] [CrossRef]
- Mueller, T.; Vernier, P.; Wullimann, M.F. The Adult Central Nervous Cholinergic System of a Neurogenetic Model Animal, the Zebrafish Danio rerio. Brain Res. 2004, 1011, 156–169. [Google Scholar] [CrossRef]
- Papke, R.L.; Ono, F.; Stokes, C.; Urban, J.M.; Boyd, R.T. The Nicotinic Acetylcholine Receptors of Zebrafish and an Evaluation of Pharmacological Tools Used for Their Study. Biochem. Pharmacol. 2012, 84, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, P.; Thomas, A.K.; Cosacak, M.I.; Papadimitriou, C.; Mashkaryan, V.; Zhang, Y.; Kizil, C. Modeling Amyloid-Β42 Toxicity and Neurodegeneration in Adult Zebrafish Brain. J. Vis. Exp. 2017, 128, 56014. [Google Scholar] [CrossRef]
- Koehler, D.; Shah, Z.A.; Williams, F.E. The GSK3β Inhibitor, TDZD-8, Rescues Cognition in a Zebrafish Model of Okadaic Acid-Induced Alzheimer’s Disease. Neurochem. Int. 2019, 122, 31–37. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Woodard, A.; Barbery, B.; Wilkinson, R.; Strozyk, J.; Milner, M.; Doucette, P.; Doran, J.; Appleby, K.; Atwill, H.; Bell, W.E.; et al. The Role of Neuronal Nitric Oxide and Its Pathways in the Protection and Recovery from Neurotoxin-Induced de Novo Hypokinetic Motor Behaviors in the Embryonic Zebrafish (Danio rerio). AIMS Neurosci. 2019, 6, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Lundegaard, P.R.; Anastasaki, C.; Grant, N.J.; Sillito, R.R.; Zich, J.; Zeng, Z.; Paranthaman, K.; Larsen, A.P.; Armstrong, J.D.; Porteous, D.J.; et al. MEK Inhibitors Reverse CAMP-Mediated Anxiety in Zebrafish. Chem. Biol. 2015, 22, 1335–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.; Stewart, A.; Gilder, T.; Wu, N.; Frank, K.; Gaikwad, S.; Suciu, C.; DiLeo, J.; Utterback, E.; Chang, K.; et al. Modeling Seizure-Related Behavioral and Endocrine Phenotypes in Adult Zebrafish. Brain Res. 2010, 1348, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rahn, J.J.; Bestman, J.E.; Josey, B.J.; Inks, E.S.; Stackley, K.D.; Rogers, C.E.; Chou, C.J.; Chan, S.S.L. Novel Vitamin K Analogs Suppress Seizures in Zebrafish and Mouse Models of Epilepsy. Neuroscience 2014, 259, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lin, J.; Peng, X.; Zhang, Q.; Zhang, Y.; Guo, N.; Zhou, S.; Li, Q. Effects of Picrotoxin on Zebrafish Larvae Behaviors: A Comparison Study with PTZ. Epilepsy Behav. 2017, 70, 224–231. [Google Scholar] [CrossRef]
- Patten, S.A.; Parker, J.A.; Wen, X.-Y.; Drapeau, P. Simple Animal Models for Amyotrophic Lateral Sclerosis Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 797–804. [Google Scholar] [CrossRef]
- Ferguson, R.; Holloway, D.E.; Chandrasekhar, A.; Acharya, K.R.; Subramanian, V. The Catalytic Activity and Secretion of Zebrafish RNases Are Essential for Their in Vivo Function in Motor Neurons and Vasculature. Sci. Rep. 2019, 9, 1107. [Google Scholar] [CrossRef] [Green Version]
- Martineau, P.R.; Mourrain, P. Tracking Zebrafish Larvae in Group—Status and Perspectives. Methods 2013, 62, 292–303. [Google Scholar] [CrossRef]
- Mirat, O.; Sternberg, J.R.; Severi, K.E.; Wyart, C. ZebraZoom: An Automated Program for High-Throughput Behavioral Analysis and Categorization. Front. Neural Circuits 2013, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Kirsten, K.; Soares, S.M.; Koakoski, G.; Carlos Kreutz, L.; Barcellos, L.J.G. Characterization of Sickness Behavior in Zebrafish. Brain. Behav. Immun. 2018, 73, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The Habenula Is Crucial for Experience-Dependent Modification of Fear Responses in Zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Perathoner, S.; Cordero-Maldonado, M.L.; Crawford, A.D. Potential of Zebrafish as a Model for Exploring the Role of the Amygdala in Emotional Memory and Motivational Behavior. J. Neurosci. Res. 2016, 94, 445–462. [Google Scholar] [CrossRef]
- Kenney, J.W.; Scott, I.C.; Josselyn, S.A.; Frankland, P.W. Contextual Fear Conditioning in Zebrafish. Learn. Mem. 2017, 24, 516–523. [Google Scholar] [CrossRef]
- Braida, D.; Ponzoni, L.; Martucci, R.; Sala, M. A New Model to Study Visual Attention in Zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 80–86. [Google Scholar] [CrossRef]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel Object Recognition and Object Location Tasks in Zebrafish: Influence of Habituation and NMDA Receptor Antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef]
- Santacà, M.; Dadda, M.; Miletto Petrazzini, M.E.; Bisazza, A. Stimulus Characteristics, Learning Bias and Visual Discrimination in Zebrafish (Danio rerio). Behav. Processes 2021, 192, 104499. [Google Scholar] [CrossRef] [PubMed]
- Al-Imari, L.; Gerlai, R. Sight of Conspecifics as Reward in Associative Learning in Zebrafish (Danio rerio). Behav. Brain Res. 2008, 189, 216–219. [Google Scholar] [CrossRef]
- Maximino, C.; Meinerz, D.L.; Fontana, B.D.; Mezzomo, N.J.; Stefanello, F.V.; de Prestes, A.S.; Batista, C.B.; Rubin, M.A.; Barbosa, N.V.; Rocha, J.B.T.; et al. Extending the Analysis of Zebrafish Behavioral Endophenotypes for Modeling Psychiatric Disorders: Fear Conditioning to Conspecific Alarm Response. Behav. Processes 2018, 149, 35–42. [Google Scholar] [CrossRef]
- Menezes, F.P.; Amorim, R.R.; Silva, P.F.; Luchiari, A.C. Alcohol Exposure and Environmental Enrichment Effects on Contextual Fear Conditioning in Zebrafish. Behav. Processes 2022, 197, 104608. [Google Scholar] [CrossRef]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory Neurotransmitter Systems and Behavior: Towards Zebrafish Models of Neurodegenerative Diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Vijayan, M.M. Development of the Corticosteroid Stress Axis and Receptor Expression in Zebrafish. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-Body Cortisol Increases after Direct and Visual Contact with a Predator in Zebrafish, Danio rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-Dimensional Neurophenotyping of Adult Zebrafish Behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, Chlordiazepoxide and Diazepam Effects in a Zebrafish Model of Anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, R.E.; Chadwick, L.; McGinnis, G.C. Behavioral Measures of Anxiety in Zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef]
- Basnet, R.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Suryanto, M.E.; Audira, G.; Uapipatanakul, B.; Hussain, A.; Saputra, F.; Siregar, P.; Chen, K.H.-C.; Hsiao, C.-D. Antidepressant Screening Demonstrated Non-Monotonic Responses to Amitriptyline, Amoxapine and Sertraline in Locomotor Activity Assay in Larval Zebrafish. Cells 2021, 10, 738. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Locomotor Behaviors in Zebrafish (Danio rerio) Larvae. Behav. Processes 2011, 86, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Afrikanova, T.; Serruys, A.-S.K.; Buenafe, O.E.M.; Clinckers, R.; Smolders, I.; de Witte, P.A.M.; Crawford, A.D.; Esguerra, C.V. Validation of the Zebrafish Pentylenetetrazol Seizure Model: Locomotor versus Electrographic Responses to Antiepileptic Drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef] [Green Version]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.M.; Fero, K.; Arrenberg, A.B.; Bergeron, S.A.; Driever, W.; Burgess, H.A. Deep Brain Photoreceptors Control Light-Seeking Behavior in Zebrafish Larvae. Curr. Biol. 2012, 22, 2042–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emran, F.; Rihel, J.; Dowling, J.E. A Behavioral Assay to Measure Responsiveness of Zebrafish to Changes in Light Intensities. J. Vis. Exp. 2008, 20, e923. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, G.; Jelfs, B.; Carmer, R.; Venkatraman, P.; Ghadami, M.; Brown, S.A.; Pang, C.P.; Leung, Y.F.; Chan, R.H.M.; et al. Computational Classification of Different Wild-Type Zebrafish Strains Based on Their Variation in Light-Induced Locomotor Response. Comput. Biol. Med. 2016, 69, 1–9. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Bellot, M.; Gomez-Canela, C.; Raldúa, D. Pharmacological Modulation of Serotonin Levels in Zebrafish Larvae: Lessons for Identifying Environmental Neurotoxicants Targeting the Serotonergic System. Toxics 2021, 9, 118. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; De Paula Cognato, G.; Bonan, C.D. Embryological Exposure to Valproic Acid Induces Social Interaction Deficits in Zebrafish (Danio rerio): A Developmental Behavior Analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef]
- Thompson, W.A.; Arnold, V.I.; Vijayan, M.M. Venlafaxine in Embryos Stimulates Neurogenesis and Disrupts Larval Behavior in Zebrafish. Environ. Sci. Technol. 2017, 51, 12889–12897. [Google Scholar] [CrossRef]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring Thigmotaxis in Larval Zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Han, S.; Zhang, D.; Dong, Q.; Wang, X.; Wang, L. Overexpression of Neuroserpin in Larval and Adult Zebrafish Shows Different Behavioral Phenotypes. Neurosci. Lett. 2021, 762, 136175. [Google Scholar] [CrossRef]
- Maphanga, V.B.; Skalicka-Wozniak, K.; Budzynska, B.; Skiba, A.; Chen, W.; Agoni, C.; Enslin, G.M.; Viljoen, A.M. Mesembryanthemum tortuosum L. Alkaloids Modify Anxiety-like Behaviour in a Zebrafish Model. J. Ethnopharmacol. 2022, 290, 115068. [Google Scholar] [CrossRef]
- Copmans, D.; Kildgaard, S.; Rasmussen, S.A.; Ślęzak, M.; Dirkx, N.; Partoens, M.; Esguerra, C.V.; Crawford, A.D.; Larsen, T.O.; de Witte, P.A.M. Zebrafish-Based Discovery of Antiseizure Compounds from the North Sea: Isoquinoline Alkaloids TMC-120A and TMC-120B. Mar. Drugs 2019, 17, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganzen, L.; Ko, M.J.; Zhang, M.; Xie, R.; Chen, Y.; Zhang, L.; James, R.; Mumm, J.; van Rijn, R.M.; Zhong, W.; et al. Drug Screening with Zebrafish Visual Behavior Identifies Carvedilol as a Potential Treatment for an Autosomal Dominant Form of Retinitis Pigmentosa. Sci. Rep. 2021, 11, 11432. [Google Scholar] [CrossRef] [PubMed]
- Viewpoint LifeSciences. Available online: https://viewpoint.fr/en/home (accessed on 16 February 2022).
- Liu, Y.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.-P.; Zhang, M.; Ma, P.; Leung, Y.F. Statistical Analysis of Zebrafish Locomotor Response. PLoS ONE 2015, 10, e0139521. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.A.; Marsden, A.N.; Slusarski, D.C. Automated, High-Throughput, in vivo Analysis of Visual Function Using the Zebrafish. Dev. Dyn. 2016, 245, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorn, R.J.; Dombroski, A.; Eller, K.; Dominguez-Gonzalez, T.M.; Clift, D.E.; Baek, P.; Seto, R.J.; Kahn, E.S.; Tucker, S.K.; Colwill, R.M.; et al. Analysis of Vertebrate Vision in a 384-Well Imaging System. Sci. Rep. 2019, 9, 13989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noldus. Available online: http://www.noldus.com/daniovision (accessed on 16 February 2022).
- Ali, S.; Champagne, D.L.; Richardson, M.K. Behavioral Profiling of Zebrafish Embryos Exposed to a Panel of 60 Water-Soluble Compounds. Behav. Brain Res. 2012, 228, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, J.H. The Zebrafish Visual System: From Circuits to Behavior. Annu. Rev. Vis. Sci. 2019, 5, 269–293. [Google Scholar] [CrossRef]
- Ganzen, L.; Venkatraman, P.; Pang, C.; Leung, Y.; Zhang, M. Utilizing Zebrafish Visual Behaviors in Drug Screening for Retinal Degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; Herculano, A.M. A Review of Monoaminergic Neuropsychopharmacology in Zebrafish. Zebrafish 2010, 7, 359–378. [Google Scholar] [CrossRef]
- Richendrfer, H.; Pelkowski, S.D.; Colwill, R.M.; Creton, R. On the Edge: Pharmacological Evidence for Anxiety-Related Behavior in Zebrafish Larvae. Behav. Brain Res. 2012, 228, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Huang, I.J.; Sirotkin, H.I.; McElroy, A.E. Varying the Exposure Period and Duration of Neuroactive Pharmaceuticals and Their Metabolites Modulates Effects on the Visual Motor Response in Zebrafish (Danio rerio) Larvae. Neurotoxicol. Teratol. 2019, 72, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine Modulates the Transcription of Genes Involved in Serotonin, Dopamine and Adrenergic Signalling in Zebrafish Embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval Zebrafish Model for Studying the Effects of Valproic Acid on Neurodevelopment: An Approach towards Modeling Autism. J. Pharmacol. Toxicol. Methods 2018, 95, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Rosemberg, D.B. Measures of Anxiety in Zebrafish (Danio rerio): Dissociation of Black/White Preference and Novel Tank Test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [Green Version]
- Thoré, E.S.J.; Brendonck, L.; Pinceel, T. Conspecific Density and Environmental Complexity Impact Behaviour of Turquoise Killifish (Nothobranchius furzeri). J. Fish Biol. 2020, 97, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.B.; Deb, D.; Bahl, A.; Engert, F. Algorithms Underlying Flexible Phototaxis in Larval Zebrafish. J. Exp. Biol. 2021, 224, jeb.238386. [Google Scholar] [CrossRef] [PubMed]
- Copmans, D.; Meinl, T.; Dietz, C.; van Leeuwen, M.; Ortmann, J.; Berthold, M.R.; de Witte, P.A.M. A KNIME-Based Analysis of the Zebrafish Photomotor Response Clusters the Phenotypes of 14 Classes of Neuroactive Molecules. SLAS Discov. 2016, 21, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The Zebrafish Eye—A Paradigm for Investigating Human Ocular Genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef] [Green Version]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef] [Green Version]
- Lovett-Barron, M.; Andalman, A.S.; Allen, W.E.; Vesuna, S.; Kauvar, I.; Burns, V.M.; Deisseroth, K. Ancestral Circuits for the Coordinated Modulation of Brain State. Cell 2017, 171, 1411–1423. [Google Scholar] [CrossRef] [Green Version]
- Yokogawa, T.; Hannan, M.C.; Burgess, H.A. The Dorsal Raphe Modulates Sensory Responsiveness during Arousal in Zebrafish. J. Neurosci. 2012, 32, 15205–15215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, T.; Zwart, M.F.; Yang, C.-T.; Mensh, B.D.; Ahrens, M.B. The Serotonergic System Tracks the Outcomes of Actions to Mediate Short-Term Motor Learning. Cell 2016, 167, 933–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinig, S.; Driever, W.; Arrenberg, A.B. The Descending Diencephalic Dopamine System Is Tuned to Sensory Stimuli. Curr. Biol. 2017, 27, 318–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| BEHAVIORAL TEST | ENDPOINTS | REFERENCE |
|---|---|---|
| LIGHT-DARK TEST | Total distance traveled | [68] |
| VISUAL MOTOR RESPONSE | Velocity, total distance moved, and mobility time | [69] |
| LOCOMOTOR ACTIVITY | Velocity, total distance moved, and mobility time | [70] |
| LOCOMOTOR ACTIVITY | Total distance traveled | [71] |
| ACOUSTIC STARTLE RESPONSE | Head angle | [72] |
| VISUAL MOTOR RESPONSE | Total distance traveled | [73] |
| VISUAL MOTOR RESPONSE | Average distance traveled | [74] |
| VISUAL MOTOR RESPONSE | Burst swim | [75] |
| VISUAL MOTOR RESPONSE | Total distance traveled | [76] |
| VIBRATIONAL STARTLE RESPONSE | Total distance traveled | [76] |
| LOCOMOTOR ACTIVITY | Total distance traveled, mean speed, turn angle | [77] |
| THIGMOTAXIS | Entries in outer area | [77] |
| LIGHT-DARK TEST | Total distance traveled | [78] |
| THIGMOTAXIS | Distance traveled in outer area | [78] |
| THIGMOTAXIS | Percentage of distance moved in outer zone | [79] |
| VISUAL MOTOR RESPONSE | Total distance traveled | [80] |
| THIGMOTAXIS | Distance traveled/time spent in each zone | [80] |
| THIGMOTAXIS | Percentage of distance moved in outer zone | [81] |
| LOCOMOTOR ACTIVITY | Average distance traveled | [81] |
| PHOTOMOTOR RESPONSE | Movements/5 min | [82] |
| LOCOMOTOR ACTIVITY | Total distance traveled | [82] |
| VISUAL MOTOR RESPONSE | Total distance traveled | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. https://doi.org/10.3390/ijms23126647
Rosa JGS, Lima C, Lopes-Ferreira M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. International Journal of Molecular Sciences. 2022; 23(12):6647. https://doi.org/10.3390/ijms23126647
Chicago/Turabian StyleRosa, João Gabriel Santos, Carla Lima, and Monica Lopes-Ferreira. 2022. "Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review" International Journal of Molecular Sciences 23, no. 12: 6647. https://doi.org/10.3390/ijms23126647
APA StyleRosa, J. G. S., Lima, C., & Lopes-Ferreira, M. (2022). Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. International Journal of Molecular Sciences, 23(12), 6647. https://doi.org/10.3390/ijms23126647








