A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. tauschii Wheats
Abstract
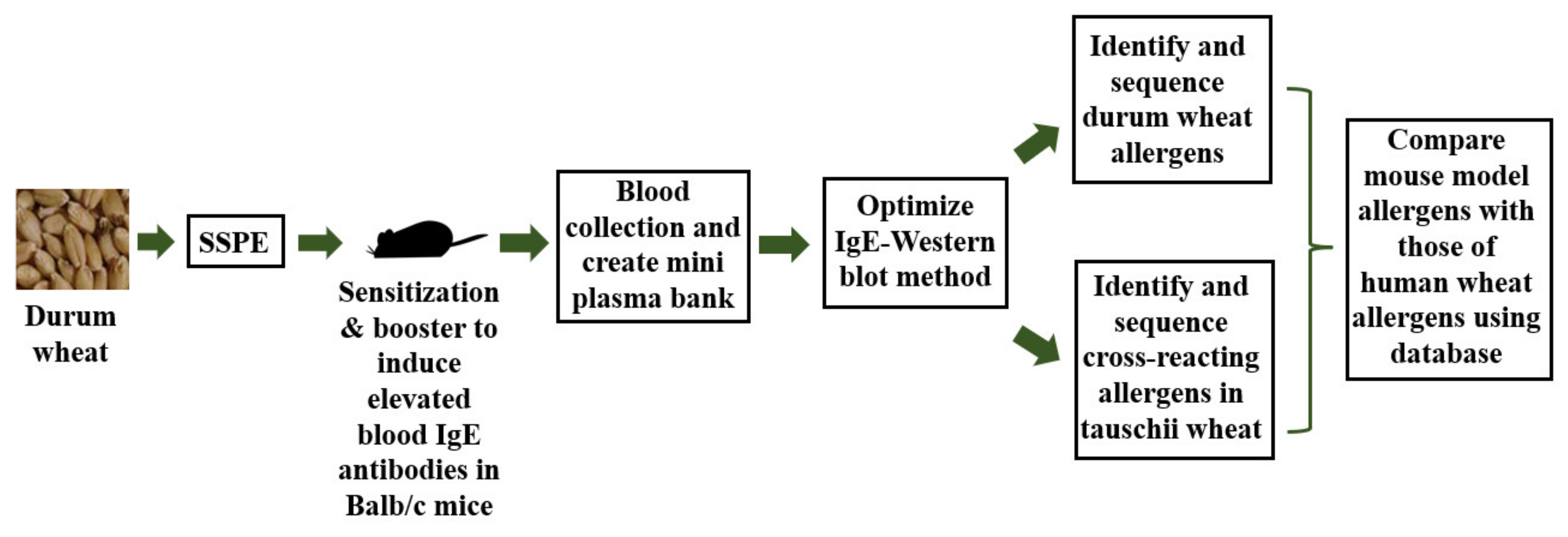
:1. Introduction
2. Results
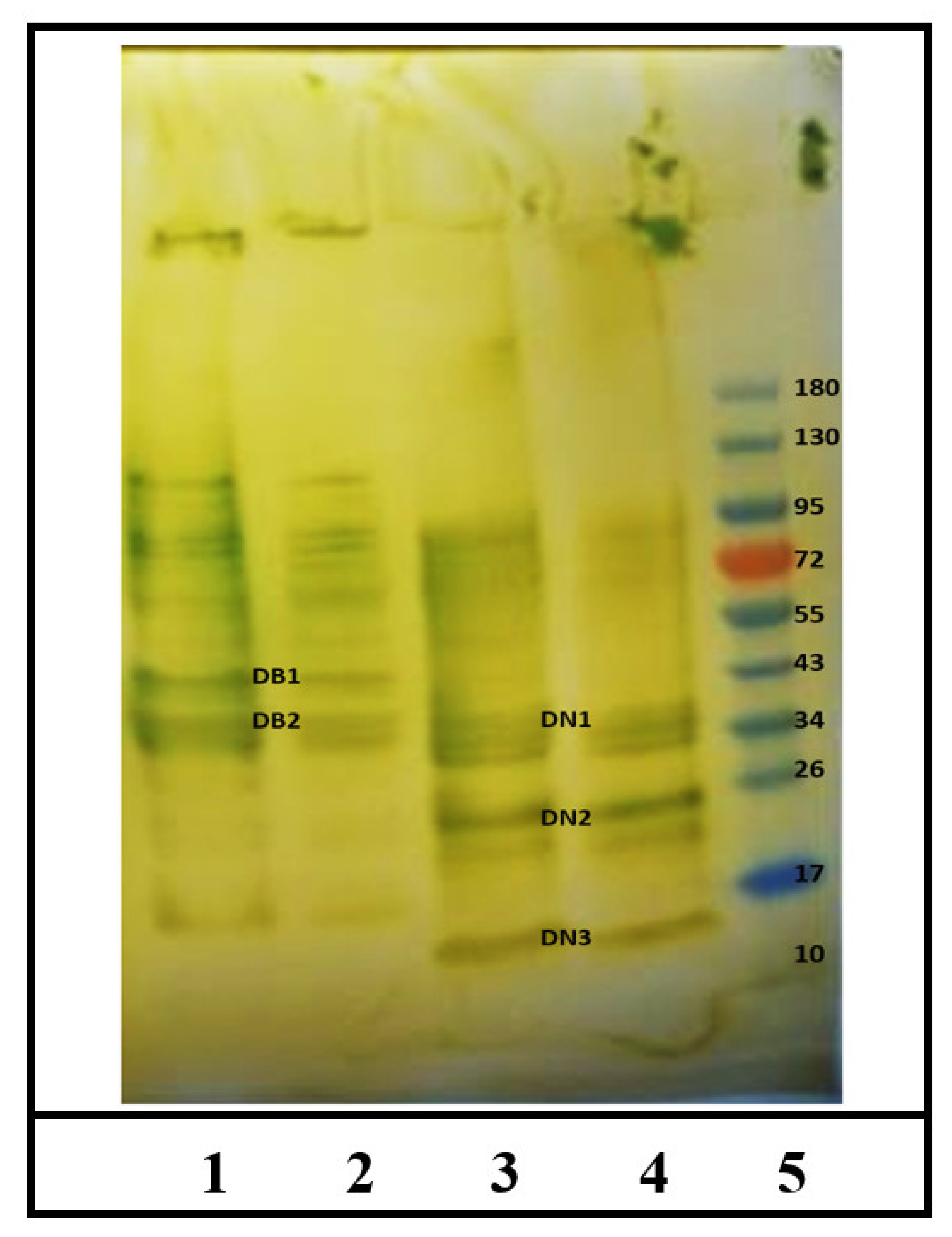
2.1. Identification of IgE Binding Protein Bands in Boiled/Reduced and Native SSPE from Durum Wheat
2.2. Sequencing and Identification of Allergens in Boiled/Reduced SSPE and Native SSPE from Durum Wheat
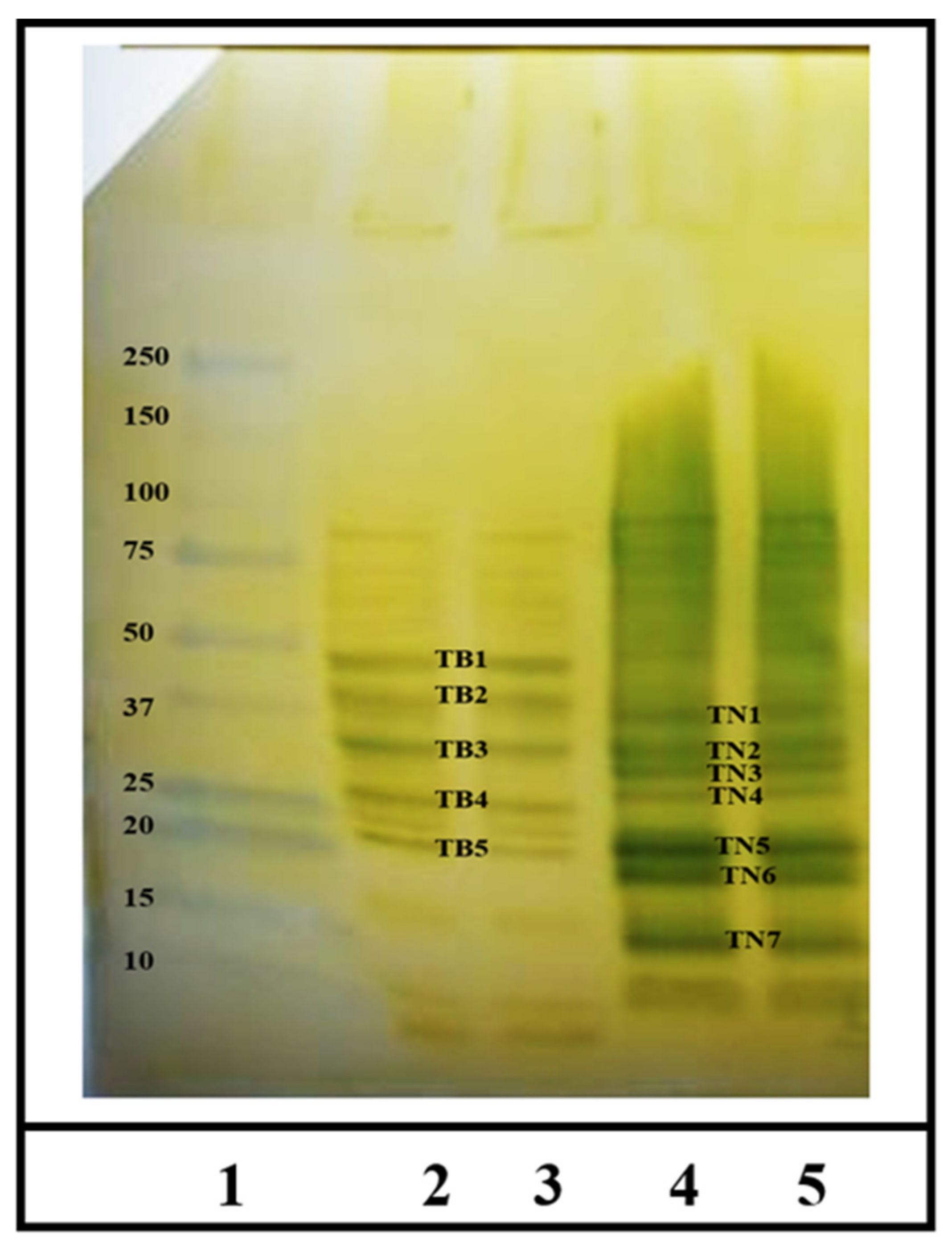
2.3. Identification of IgE Binding Protein Bands in Boiled/Reduced SSPE and Native SSPE from the A. tauschii Wheat
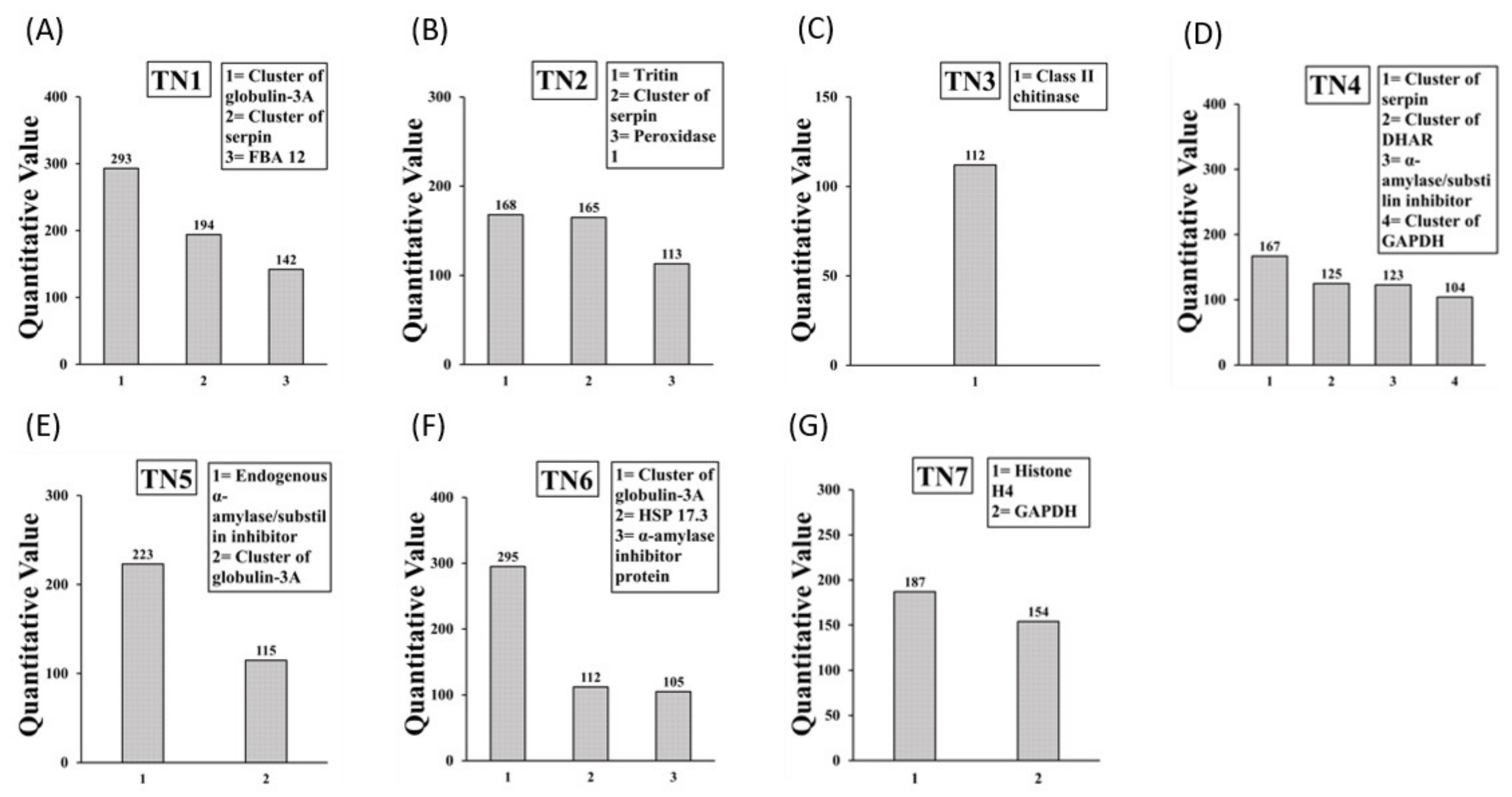
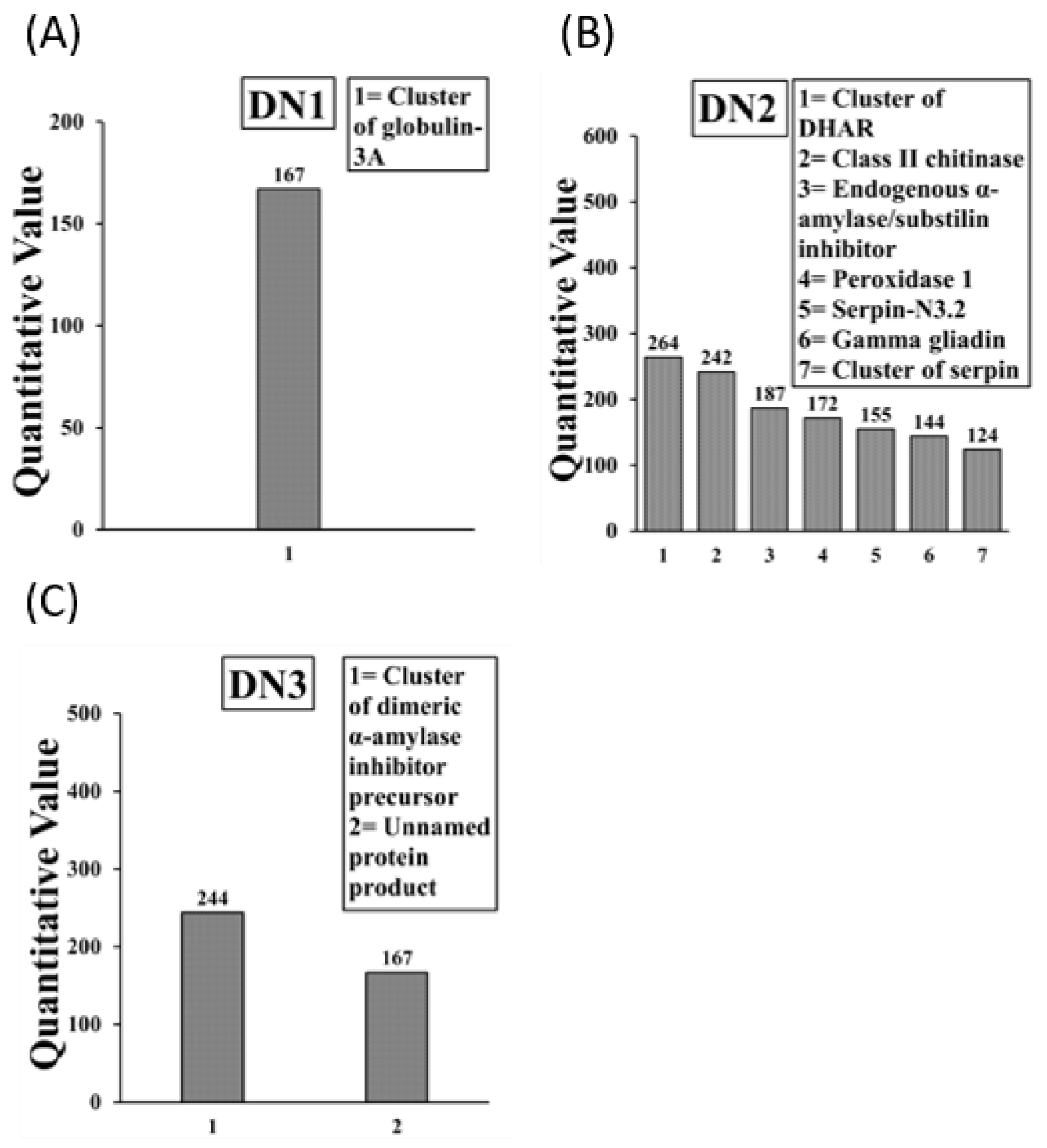
2.4. Sequencing and Identification of Allergens in Boiled/Reduced and Native A. tauschii Wheat SSPE
2.5. Comparison of the Wheat Allergens in the Mouse Model to Those Reported as Human Wheat Allergens in the Database
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice Breeding Generation of a Plant-Protein-Free Mice Colony
4.3. Preparation of Salt-Soluble Wheat Protein Extract from Durum Wheat
4.4. Growing Ancient A. tauschii Wheat and Preparation of Salt-Soluble Wheat Protein Extract
4.5. Preparing a Hyper-IgE Plasma Mini-Bank from Durum Wheat-Allergic Mice
4.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.7. Optimization of an IgE Western Blot Method
4.8. Identification of Wheat Allergens by LC-MS/MS Sequencing Method
4.9. Comparison of Mouse Wheat Allergens to Human Wheat Allergens Reported in the Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US FDA Recalls, Market Withdrawals, and Safety Alerts. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts (accessed on 20 February 2022).
- Leonard, M.M.; Vasagar, B. US perspective on gluten-related diseases. Clin. Exp. Gastroenterol. 2014, 7, 25–37. [Google Scholar] [PubMed] [Green Version]
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Roberts, G.; Higgins, B.; Dean, T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J. Allergy Clin. Immunol. 2006, 117, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: A population-based study. Pediatr. Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food Allergy from Infancy Through Adulthood. J. Allergy Clin. Immunol. 2020, 8, 1854–1864. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and burden of food allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. 2018, 2, e185630. [Google Scholar] [CrossRef]
- Biagini, R.E.; MacKenzie, B.A.; Sammons, D.L.; Smith, J.P.; Striley, C.A.F.; Robertson, S.K.; Snawder, J.E. Evaluation of the prevalence of antiwheat-, anti-flour dust, and anti-α-amylase specific IgE antibodies in US blood donors. Ann. Allergy Asthma Immunol. 2004, 92, 649–653. [Google Scholar] [CrossRef]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Niitsuma, T.; Ito, H. A study of factors contributing to bakers’ allergy symptoms. Arerugi 1994, 43, 625–633. [Google Scholar]
- Baur, X.; Degens, P.O.; Sander, I. Baker’s asthma: Still among the most frequent occupational respiratory disorders. J. Allergy Clin. Immunol. 1998, 102, 984–997. [Google Scholar] [CrossRef]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat allergens associated with Baker’s asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–92. [Google Scholar]
- Mimura, T.; Yamagami, S.; Noma, H.; Kamei, Y.; Goto, M.; Kondo, A.; Matsubara, M. Specific IgE for wheat in tear fluid of patients with allergic conjunctivitis. Cutan. Ocul. Toxicol. 2015, 34, 25–34. [Google Scholar] [CrossRef]
- Beaudouin, E.; Renaudin, J.M.; Morisset, M.; Codreanu, F.; Kanny, G.; Moneret-Vautrin, D.A. Food-dependent exercise-induced anaphylaxis–update and current data. Eur. Ann. Allergy Clin. Immunol. 2006, 38, 45–51. [Google Scholar]
- Morita, E.; Matsuo, H.; Chinuki, Y.; Takahashi, H.; Dahlstrom, J.; Tanaka, A. Food-dependent exercise-induced anaphylaxis—Importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol. Int. 2009, 58, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef]
- Furuta, G.T.; Katzka, D.A. Eosinophilic Esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648. [Google Scholar] [CrossRef] [Green Version]
- Kottyan, L.C.; Rothenberg, M.E. Genetics of eosinophilic esophagitis. Mucosal Immunol. 2017, 10, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in molecular mechanisms of wheat allergenicity in animal models: A comprehensive review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-mediated food allergy in european patients: α-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins-Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K.; Alenius, H.; Varjonen, E.; Koivuluhta, M.; Mikkola, J.; Keskinen, H.; Kalkkinen, N.; Reunala, T. A novel wheat gliadin as a cause of exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 1999, 103, 912–917. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Mihara, S.; Morimoto, K.; Savage, A.W.; Tatham, A.S. Fast omega-gliadin is a major allergen in wheat-dependent exercise-induced anaphylaxis. J. Dermatol. Sci. 2003, 33, 99–104. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E. The allergy epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Nagisetty, S.; Ng, P.K.W.; Gangur, V. Creating hypo-/nonallergenic wheat products using processing methods: Fact or fiction? Compr. Rev. Food Sci. Food Saf. 2021, 20, 6089–6115. [Google Scholar] [CrossRef]
- FAO. Global Cereal Supply and Demand Brief. 2020. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 20 February 2022).
- USDA Economic Research Services. Per Capita Availability of Corn Products More Than Doubled between 1988 and 2018. 2016. Available online: https://www.ers.usda.gov/dataproducts/chartgallery/gallery/chartdetail/?chartId=8509 (accessed on 20 February 2022).
- Leonard, M.M.; Sapone, A.; Catassi, C.; Fasano, A. Celiac disease and nonceliac gluten sensitivity: A review. JAMA 2017, 318, 647–656. [Google Scholar] [CrossRef]
- Pilolli, R.; Gadaleta, A.; Stasio, L.D.I.; Lamonaca, A.; De Angelis, E.; Nigro, D.; De Angelis, M.; Mamone, G.; Monaci, L. A comprehensive peptidomic approach to characterize the protein profile of selected durum wheat genotypes: Implication for coeliac disease and wheat allergy. Nutrients 2019, 11, 2321. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Powers, S.; Field, J.M.; Fido, R.J.; Jones, H.D.; Arnold, G.M.; West, J.; Lazzeri, P.A.; Barcelo, P.; Barro, F.; et al. Comparative field performance over 3 years and two sites of transgenic wheat lines expressing HMW subunit transgenes. Theor. Appl. Genet. 2006, 113, 128–136. [Google Scholar] [CrossRef]
- Lupi, R.; Denery-Papini, S.; Rogniaux, H.; Lafiandra, D.; Rizzi, C.; De Carli, M.; Moneret-Vautrin, D.A.; Masci, S.; Larré, C. How much does transgenesis affect wheat allergenicity: Assessment in two GM lines over-expressing endogenous genes. J. Proteom. 2013, 27, 281–291. [Google Scholar] [CrossRef]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a hypoallergenic wheat line lacking ω-5 gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Domingo, J.L. Safety assessment of GM plants: An updated review of the scientific literature. Food Chem. Toxicol. 2016, 95, 12–18. [Google Scholar] [CrossRef]
- Hollingworth, R.M.; Bjeldanes, L.F.; Bolger, M.; Kimber, I.; Meade, B.J.; Taylor, S.L.; Wallace, K.B. The safety of genetically modified foods produced through biotechnology. Toxicol. Sci. 2003, 71, 2–8. [Google Scholar]
- Selgrade, M.K.; Bowman, C.C.; Ladics, G.S.; Privalle, L.; Laessig, S.A. Safety assessment of biotechnology products for potential risk of food Allergy: Implications of new research. Toxicol. Sci. 2009, 110, 31–39. [Google Scholar] [CrossRef]
- Beale, M.H.; Ward, J.L.; Baker, J.M. Establishing substantial equivalence: Metabolomics. Methods Mol. Biol. 2009, 478, 289–303. [Google Scholar]
- Jin, Y.; Gao, H.; Jorgensen, R.; Salloum, J.; Jian, D.I.; Ng, P.K.W.; Gangur, V. Mechanisms of wheat allergenicity in mice: Comparison of adjuvant-free vs. Alum-adjuvant models. Int. J. Mol. Sci. 2020, 21, 3205. [Google Scholar] [CrossRef]
- Gonipeta, B.; Para, R.; He, Y.; Srkalovic, I.; Ortiz, T.; Kim, E.; Parvataneni, S.; Gangur, V. Cardiac mMCP-4+ mast cell expansion and elevation of IL-6, and CCR1/3 and CXCR2 signaling chemokines in an adjuvant-free mouse model of tree nut allergy. Immunobiology 2015, 220, 663–672. [Google Scholar] [CrossRef]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse Model of Anaphylaxis and Atopic Dermatitis to Salt-Soluble Wheat Protein Extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef]
- Birmingham, N.; Payankaulam, S.; Thanesvorakul, S.; Stefura, B.; HayGlass, K.; Gangur, V. An ELISA-based method for measurement of food-specific IgE antibody in mouse serum: An alternative to the passive cutaneous anaphylaxis assay. J. Immunol. Methods 2003, 275, 89–98. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and validation of a mouse-based primary screening method for testing relative allergenicity of proteins from different wheat genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, N.; Gangur, V.; Samineni, S.; Navuluri, L.; Kelly, C. Hazelnut Allergy: Evidence that hazelnut can directly elicit specific IgE antibody response via activating type 2 cytokines in mice. Int. Arch. Allergy Immunol. 2005, 137, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, N.P.; Parvataneni, S.; Hassan, H.M.; Harkema, J.; Samineni, S.; Navuluri, L.; Kelly, C.J.; Gangur, V. An adjuvant-free mouse model of tree nut allergy using hazelnut as a model tree nut. Int. Arch. Allergy Immunol. 2007, 144, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Parvataneni, S.; Gonipeta, B.; Tempelman, R.J.; Gangur, V. Development of an adjuvant-free cashew nut allergy mouse model. Int. Arch. Allergy Immunol. 2009, 149, 299–304. [Google Scholar] [CrossRef]
- Gonipeta, B.; Parvataneni, S.; Paruchuri, P.; Gangur, V. Long-term characteristics of hazelnut allergy in an adjuvant-free mouse model. Int. Arch. Allergy Immunol. 2010, 152, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse models of food allergy: How well do they simulate the human disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef]
- Denery-Papini, S.; Bodinier, M.; Pineau, F.; Triballeau, S.; Tranquet, O.; Adel-Patient, K.; Moneret-Vautrin, D.A.; Bakan, B.; Marion, D.; Mothes, T.; et al. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clin. Exp. Allergy 2011, 41, 1478–1492. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, S.; Li, H. Introgressing the Aegilops tauschii genome into wheat as a basis for cereal improvement. Nat. Plants 2021, 7, 774–786. [Google Scholar] [CrossRef]
- Bodinier, M.; Leroy, M.; Ah-Leung, S.; Blanc, F.; Tranquet, O.; Denery-Papini, S.; Wal, J.M.; Adel-Patient, K. Sensitization and elicitation of an allergic reaction to wheat gliadins in mice. J. Agric. Food Chem. 2009, 57, 1219–1225. [Google Scholar] [CrossRef]
- Bruce, T.J.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.J.; Napier, J.A.; et al. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015, 5, 11183. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Tanabe, S.; Watanabe, J.; Makino, T. Primary screening of relatively less allergenic wheat varieties. J. Nutr. Sci. Vitaminol. 2005, 51, 204–206. [Google Scholar] [CrossRef]
- Larré, C.; Lupi, R.; Gombaud, G.; Brossard, C.; Branlard, G.; Moneret-Vautrin, D.A.; Rogniaux, H.; Denery-Papini, S. Assessment of allergenicity of diploid and hexaploid wheat genotypes: Identification of allergens in the albumin/globulin fraction. J. Proteom. 2011, 74, 1279–1289. [Google Scholar] [CrossRef]
- Venter, C.; Maslin, K.; Arshad, S.H.; Patil, V.; Grundy, J.; Glasbey, G.; Twiselton, R.; Dean, T. Very low prevalence of IgE mediated wheat allergy and high levels of cross-sensitisation between grass and wheat in a UK birth cohort. Clin. Transl. Allergy 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Shavrukov, Y.; Bazanova, N.; Chirkova, L.; Borisjuk, N.; Kovalchuk, N.; Ismagul, A.; Parent, B.; Langridge, P.; Hrmova, M.; et al. Constitutive overexpression of the TaNF-YB4 gene in transgenic wheat significantly improves grain yield. J. Exp. Bot. 2015, 66, 6635–6650. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.B.; Song-Wilson, Y.; Foetzki, A.; Luginbühl, C.; Winzeler, M.; Kneubühler, Y.; Matasci, C.; Mascher-Frutschi, F.; Kalinina, O.; Boller, T.; et al. Does wheat genetically modified for disease resistance affect root-colonizing pseudomonads and arbuscular mycorrhizal fungi? PLoS ONE 2013, 8, e53825. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]

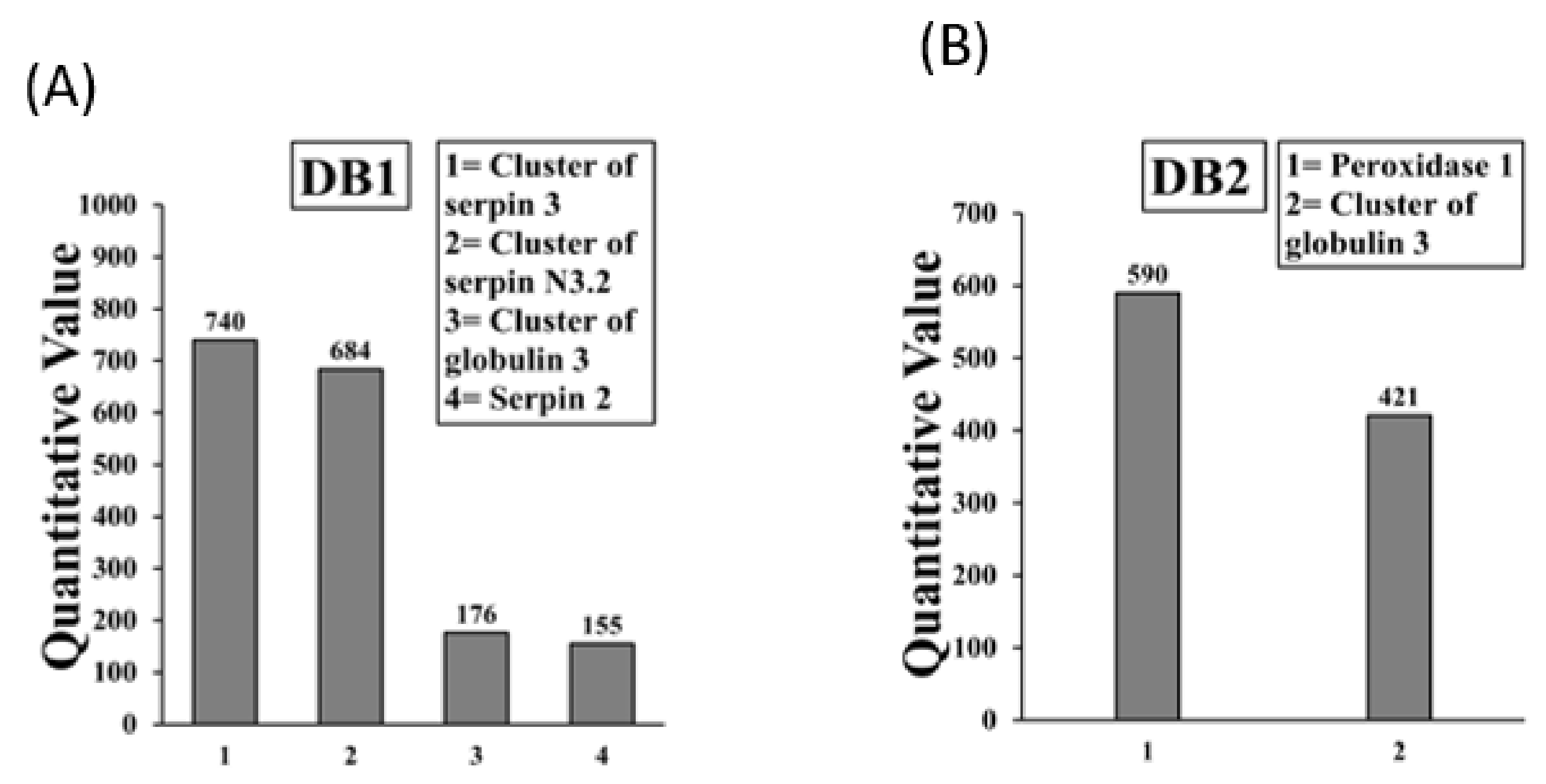
| Durum Mouse Allergens | Accession Number | Human Allergen |
|---|---|---|
| Boiled/reduced SSPE (7) | ||
| Tri Glo (Globulin 3 A, B) | AFM30909.1 [3], ACJ65515.1 [5] | Yes |
| Cluster of Globulin 1 *,** | ABG68034.1 [2] | Yes |
| Tri a 33 (Serpin) | CAB52710.1 [5], CAA90071.1 [5] | Yes |
| Tri a 34 (GAPDH) | ANW11922.1 (+1), ALE18232.1 [3] | Yes |
| Tri a aASI (Endogenous α-amylase/substilin inhibitor) | AAR10959.1 [4], IAAS_WHEAT (+1) | Yes |
| Tri a Chitinase | AAX83262.1 [13] | Yes |
| Cluster of fructose-1,6-bisphophate aldolase 12 ** | AVL25144.1 [5] | Yes |
| Native SSPE (12) | ||
| Tri Glo (Globulin 3 A) | AFM30909.1 [4] | Yes |
| Tri a 33 (Serpin) | CAB52710.1 [7], CAA90071.1 [6] | Yes |
| Tri a Tritin | BAA0248.1 | Yes |
| Tri a Peroxidase 1 | AAM88383.1 (+4) | Yes |
| Tri a Chitinase | AAX83262.1 | Yes |
| Cluster of dehydroascorbate reductase ** | AAL71851.1 [2] | Yes |
| Tri a aASI (Endogenous α-amylase/substilin inhibitor) | AAR10959.1 (+2) | Yes |
| Tri a aASI (Endogenous α-amylase/substilin inhibitor) | P16347.1 (+1) | Yes |
| Cluster of GAPDH ** | ALE18233.1 [3], ANW11922.1 (+1) | Yes |
| Histone H4 ** | AAA34292.1 (+21) | ? |
| Cluster of fructose-1,6-bisphophate aldolase 12 ** | AVL25144.1 [5] | Yes |
| Cluster of heat shock protein 17.3 ** | CAA41218.1 [5] | ? |
| A. tauschii Mouse Allergens | Accession Number | Human Allergen |
|---|---|---|
| Boiled/reduced SSPE (3) | ||
| Tri a 33 (Serpin 2 *, 3 *, N3.2) | ACN59484.1 (+1), ACN59485.1 [6], AFC89429.1 [5] | Yes |
| Tri Glo (Globulin 3) | ACJ65514.1 [4] | Yes |
| Tri a Peroxidase 1 | AAM88383.1 (+4) | Yes |
| Raw SSPE (8) | ||
| Tri Glo (Globulin 3A) | AFM30909.1 [4] | Yes |
| Cluster of dehydroascorbate reductase ** | ACV89491.1 [2] | Yes |
| Tri a Chitinase | AAX83262.1 | Yes |
| Tri a aASI (Endogenous α-amylase/substilin inhibitor) | P16347.1 (+1) | Yes |
| Tri a Peroxidase 1 | AAM8838.1 (+4) | Yes |
| Tri a 33 (Serpin N3.2) | AFC89429.1 | Yes |
| Tri a 20 (Gamma gliadin) | ABO37959.1 | Yes |
| Tri a 28 (dimeric α-amylase inhibitor precursor) | ABF93411.1 [8] | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorgensen, R.; Raghunath, R.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. tauschii Wheats. Int. J. Mol. Sci. 2022, 23, 6505. https://doi.org/10.3390/ijms23126505
Jorgensen R, Raghunath R, Gao H, Olson E, Ng PKW, Gangur V. A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. tauschii Wheats. International Journal of Molecular Sciences. 2022; 23(12):6505. https://doi.org/10.3390/ijms23126505
Chicago/Turabian StyleJorgensen, Rick, Rajsri Raghunath, Haoran Gao, Eric Olson, Perry K. W. Ng, and Venu Gangur. 2022. "A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. tauschii Wheats" International Journal of Molecular Sciences 23, no. 12: 6505. https://doi.org/10.3390/ijms23126505
APA StyleJorgensen, R., Raghunath, R., Gao, H., Olson, E., Ng, P. K. W., & Gangur, V. (2022). A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. tauschii Wheats. International Journal of Molecular Sciences, 23(12), 6505. https://doi.org/10.3390/ijms23126505





