Reproductive Immunology and Pregnancy
Funding
Conflicts of Interest
References
- Khosroshahi, L.M.; Parhizkar, F.; Kachalaki, S.; Aghebati-Maleki, A. Immune checkpoints and reproductive immunology: Pioneers in the future therapy of infertility related Disorders? Int. Immunopharmacol. 2021, 99, 107935. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, A.K.C. Neonatal lupus erythematosus. Autoimmune Dis. 2012, 2012, 301274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, D.M.; Rupel, A.; Buyon, J.P. Epidemiology, etiology, detection, and treatment of autoantibody-associated congenital heart block in neonatal lupus. Curr. Rheumatol. Rep. 2007, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gryka-Marton, M.; Szukiewicz, D.; Teliga-Czajkowska, J.; Olesinska, M. An Overview of Neonatal Lupus with Anti-Ro Characteristics. Int. J. Mol. Sci. 2021, 22, 9281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.S.; Chen, H.-L.; Miller, L. Tumor necrosis factors: Pivotal components of pregnancy? Biol. Reprod. 1996, 54, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Pazos, M.; Sperling, R.S.; Moran, T.M.; Kraus, T.A. The influence of pregnancy on systemic immunity. Immunol. Res. 2012, 54, 254–261. [Google Scholar] [CrossRef]
- Silva, L.C.; Ortigosa, L.C.; Benard, G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: Mechanisms of action and pitfalls. Immunotherapy 2010, 2, 817–833. [Google Scholar] [CrossRef]
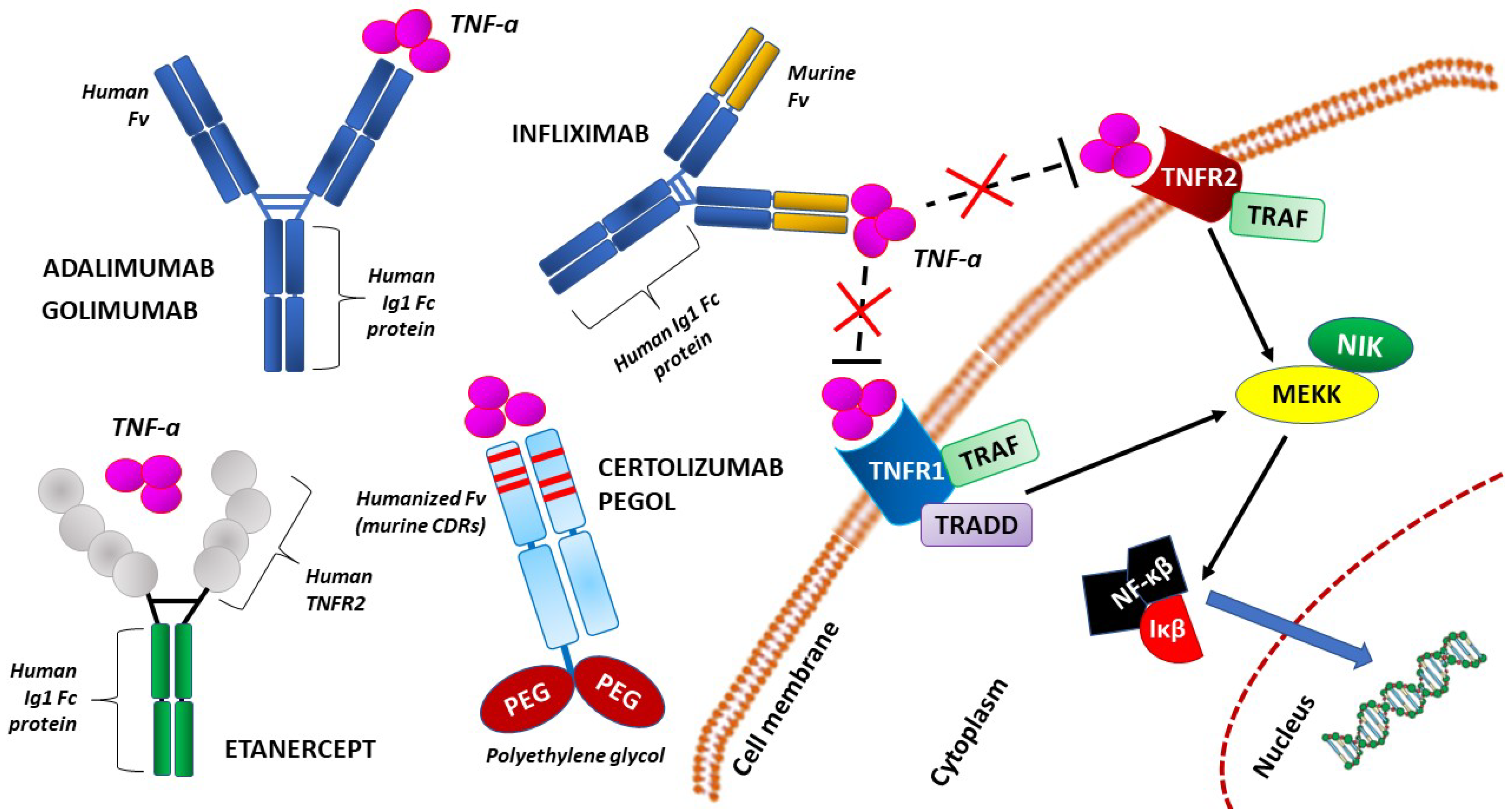
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef]
- Mårdh, P.A. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr. Opin. Infect. Dis. 2004, 17, 49–52. [Google Scholar] [CrossRef]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases and infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolarczyk, K.; Mlynarczyk-Bonikowska, B.; Rudnicka, E.; Szukiewicz, D.; Meczekalski, B.; Smolarczyk, R.; Pieta, W. The Impact of Selected Bacterial Sexually Transmitted Diseases on Pregnancy and Female Fertility. Int. J. Mol. Sci. 2021, 22, 2170. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Kim, S.H.; Cha, D.H.; Park, H.J. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 11202. [Google Scholar] [CrossRef] [PubMed]
- Hayder, H.; Fu, G.; Nadeem, L.; O’Brien, J.; Lye, S.; Peng, C. Overexpression of miR-210-3p Impairs Extravillous Trophoblast Functions Associated with Uterine Spiral Artery Remodeling. Int. J. Mol. Sci. 2021, 22, 3961. [Google Scholar] [CrossRef]
- Magatti, M.; Masserdotti, A.; Cargnoni, A.; Papait, A.; Stefani, F.; Silini, A.; Parolini, O. The Role of B Cells in PE Pathophysiology: A Potential Target for Perinatal Cell-Based Therapy? Int. J. Mol. Sci. 2021, 22, 3405. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Malinowska, M.; Kloska, A.; Jakóbkiewicz-Banecka, J.; Gujski, M.; Bojar, I.; Raczkiewicz, D.; Jakiel, G. Global Changes of 5-mC/5h-mC Ratio and Methylation of Adiponectin and Leptin Gene in Placenta Depending on Mode of Delivery. Int. J. Mol. Sci. 2021, 22, 3195. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Massot-Cladera, M.; Selma-Royo, M.; Parra-Llorca, A.; González, S.; García-Mantrana, I.; Castell, M.; Rodríguez-Lagunas, M.; Collado, M.; et al. Association of Maternal Microbiota and Diet in Cord Blood Cytokine and Immunoglobulin Profiles. Int. J. Mol. Sci. 2021, 22, 1778. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles for NHERF1 and NHERF2 on the regulation of C3a receptor signaling in human mast cells. PLoS ONE 2012, 7, e51355. [Google Scholar] [CrossRef] [Green Version]
- Kammala, A.K.; Sheller-Miller, S.; Radnaa, E.; Kechichian, T.; Subramanian, H.; Menon, R. Sodium Hydrogen Exchanger Regulatory Factor-1 (NHERF1) Regulates Fetal Membrane Inflammation. Int. J. Mol. Sci. 2020, 21, 7747. [Google Scholar] [CrossRef]
- Tikkanen, M. Placental abruption: Epidemiology, risk factors and consequences. Acta Obstet. Gynecol. Scand. 2011, 90, 140–149. [Google Scholar] [CrossRef]
- Bączkowska, M.; Zgliczyńska, M.; Faryna, J.; Przytuła, E.; Nowakowski, B.; Ciebiera, M. Molecular Changes on Maternal-Fetal Interface in Placental Abruption-A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6612. [Google Scholar] [CrossRef] [PubMed]
- Narang, K.; Cheek, E.; Enninga, E.; Theiler, R. Placental Immune Responses to Viruses: Molecular and Histo-Pathologic Perspectives. Int. J. Mol. Sci. 2021, 22, 2921. [Google Scholar] [CrossRef] [PubMed]
- Bukowska-Ośko, I.; Popiel, M.; Kowalczyk, P. The Immunological Role of the Placenta in SARS-CoV-2 Infection-Viral Transmission, Immune Regulation, and Lactoferrin Activity. Int. J. Mol. Sci. 2021, 22, 5799. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szukiewicz, D. Reproductive Immunology and Pregnancy. Int. J. Mol. Sci. 2022, 23, 6485. https://doi.org/10.3390/ijms23126485
Szukiewicz D. Reproductive Immunology and Pregnancy. International Journal of Molecular Sciences. 2022; 23(12):6485. https://doi.org/10.3390/ijms23126485
Chicago/Turabian StyleSzukiewicz, Dariusz. 2022. "Reproductive Immunology and Pregnancy" International Journal of Molecular Sciences 23, no. 12: 6485. https://doi.org/10.3390/ijms23126485
APA StyleSzukiewicz, D. (2022). Reproductive Immunology and Pregnancy. International Journal of Molecular Sciences, 23(12), 6485. https://doi.org/10.3390/ijms23126485




