Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice
Abstract
1. Introduction
2. Results
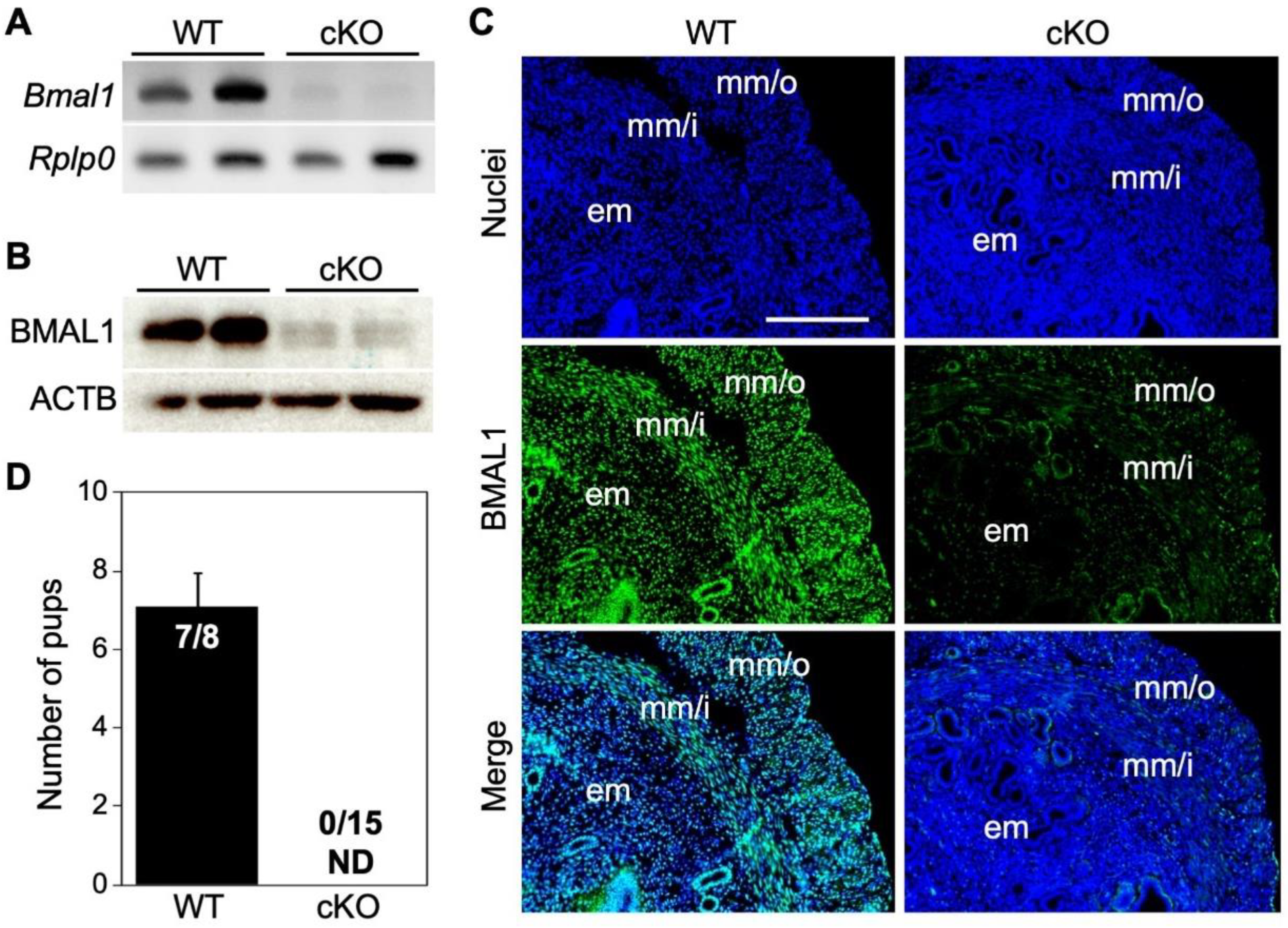
2.1. Uterine Bmal1 cKO Mice Gave No Live Birth
2.2. cKO Female Mice Increased Incomplete Miscarriage after Day Six of Pregnancy
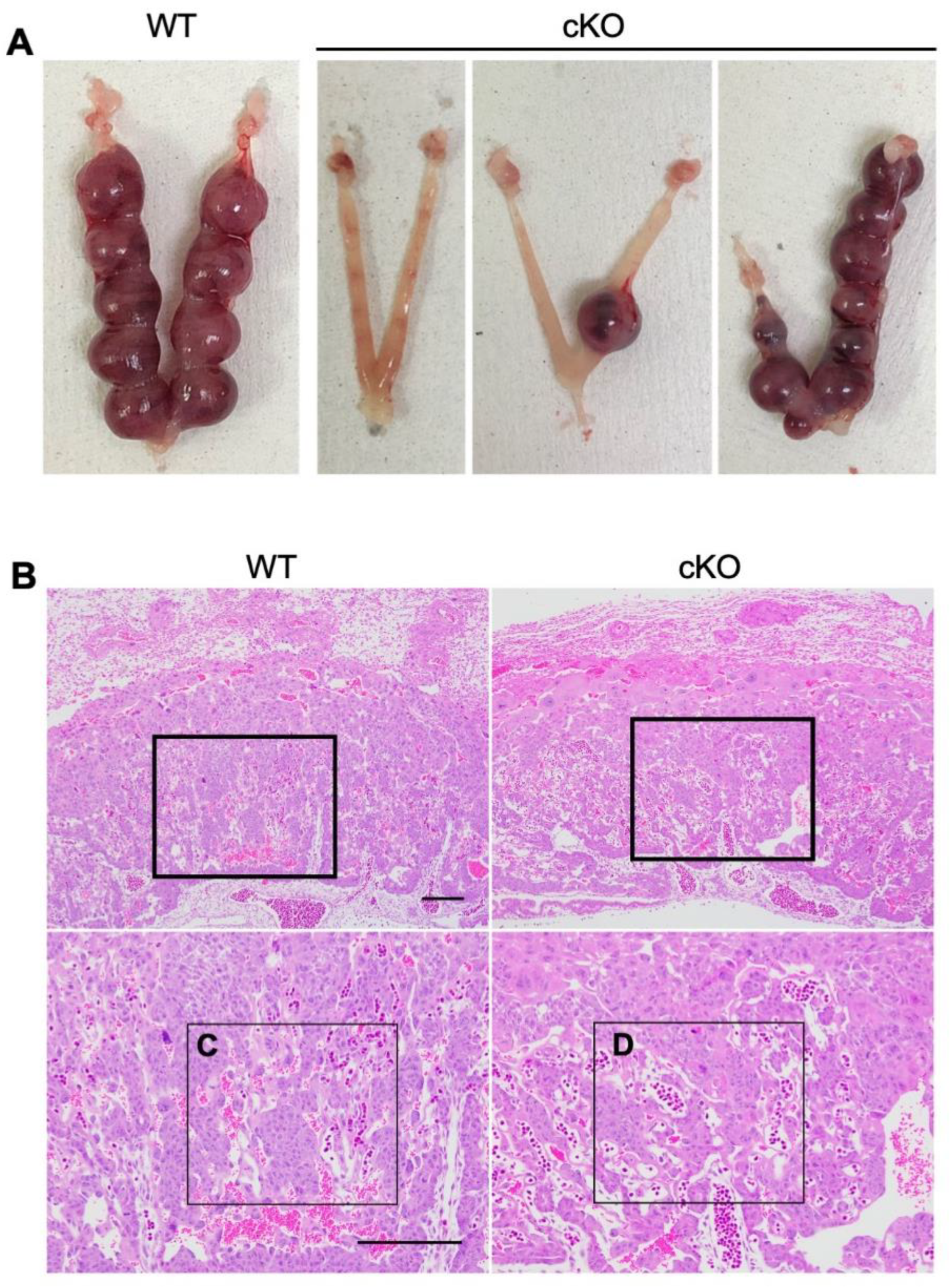
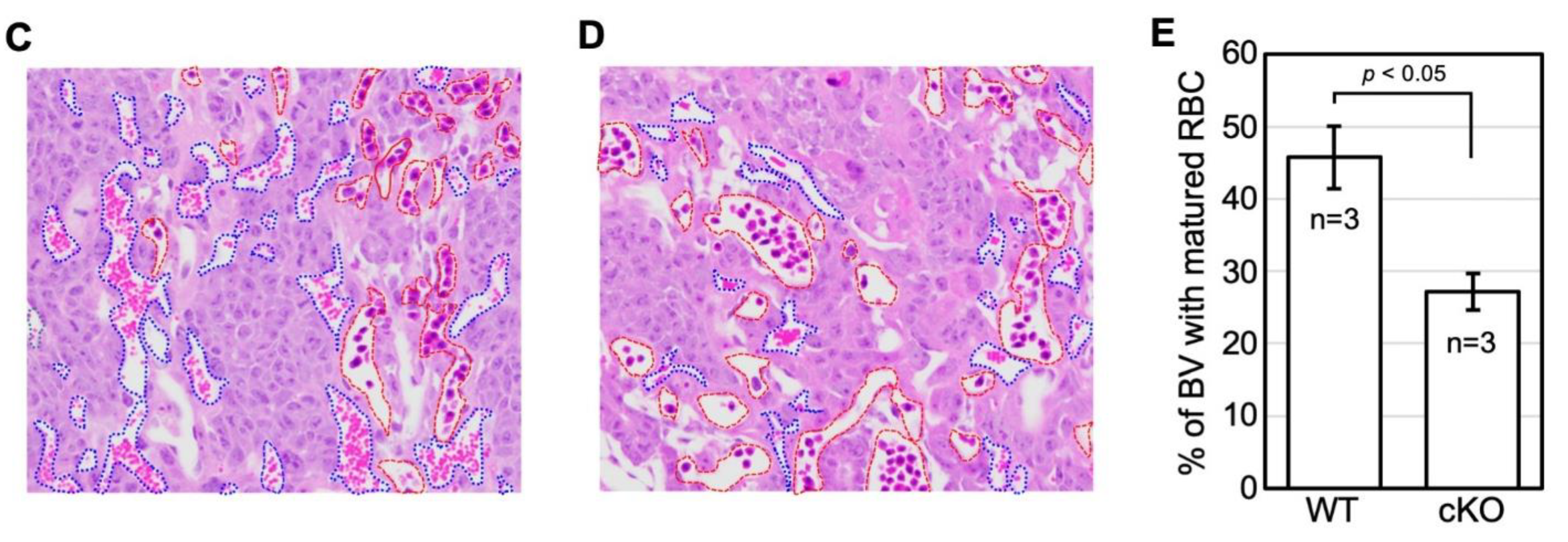
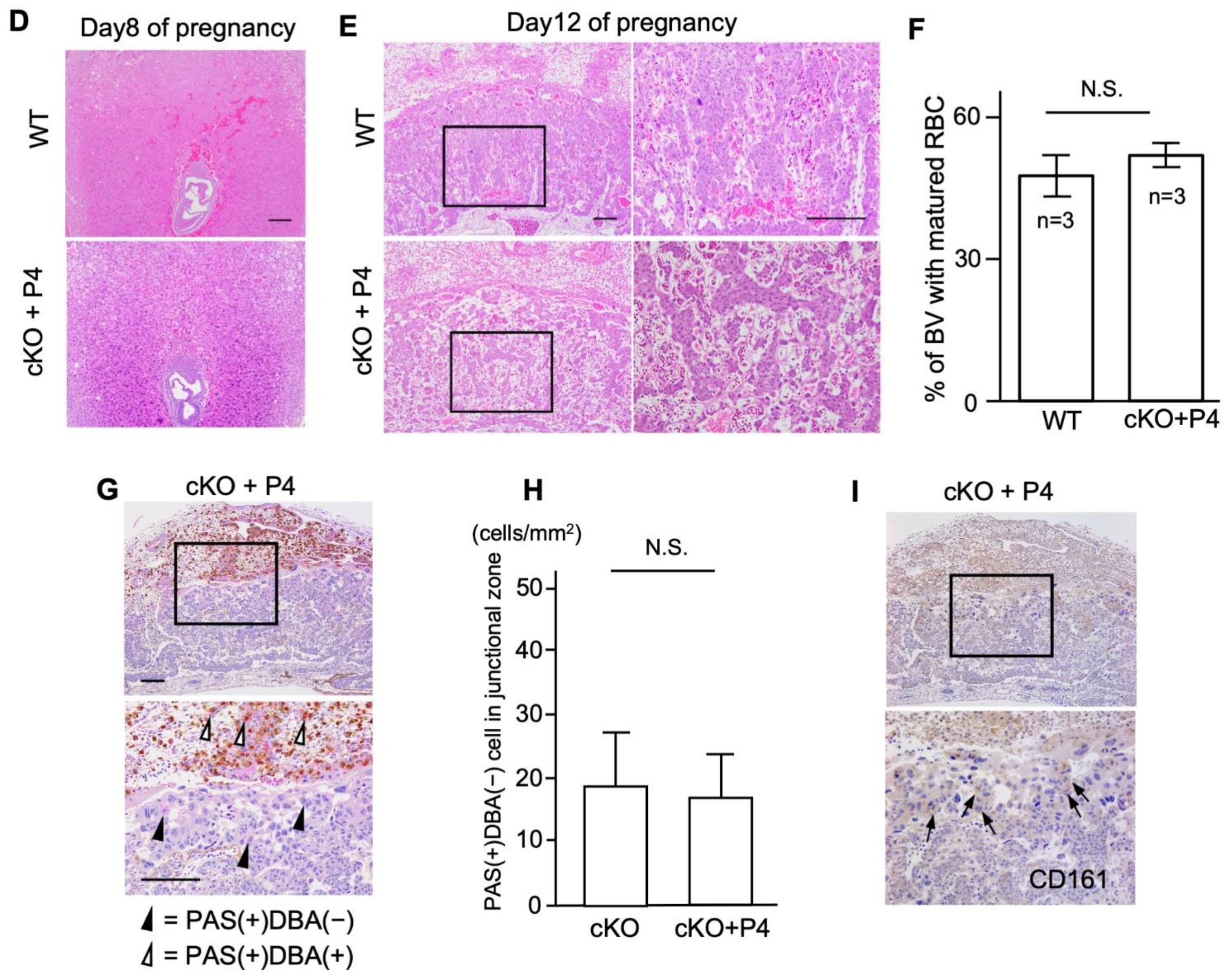
2.3. cKO Mice Showed Abnormal Placental Formation on Day Twelve of Pregnancy
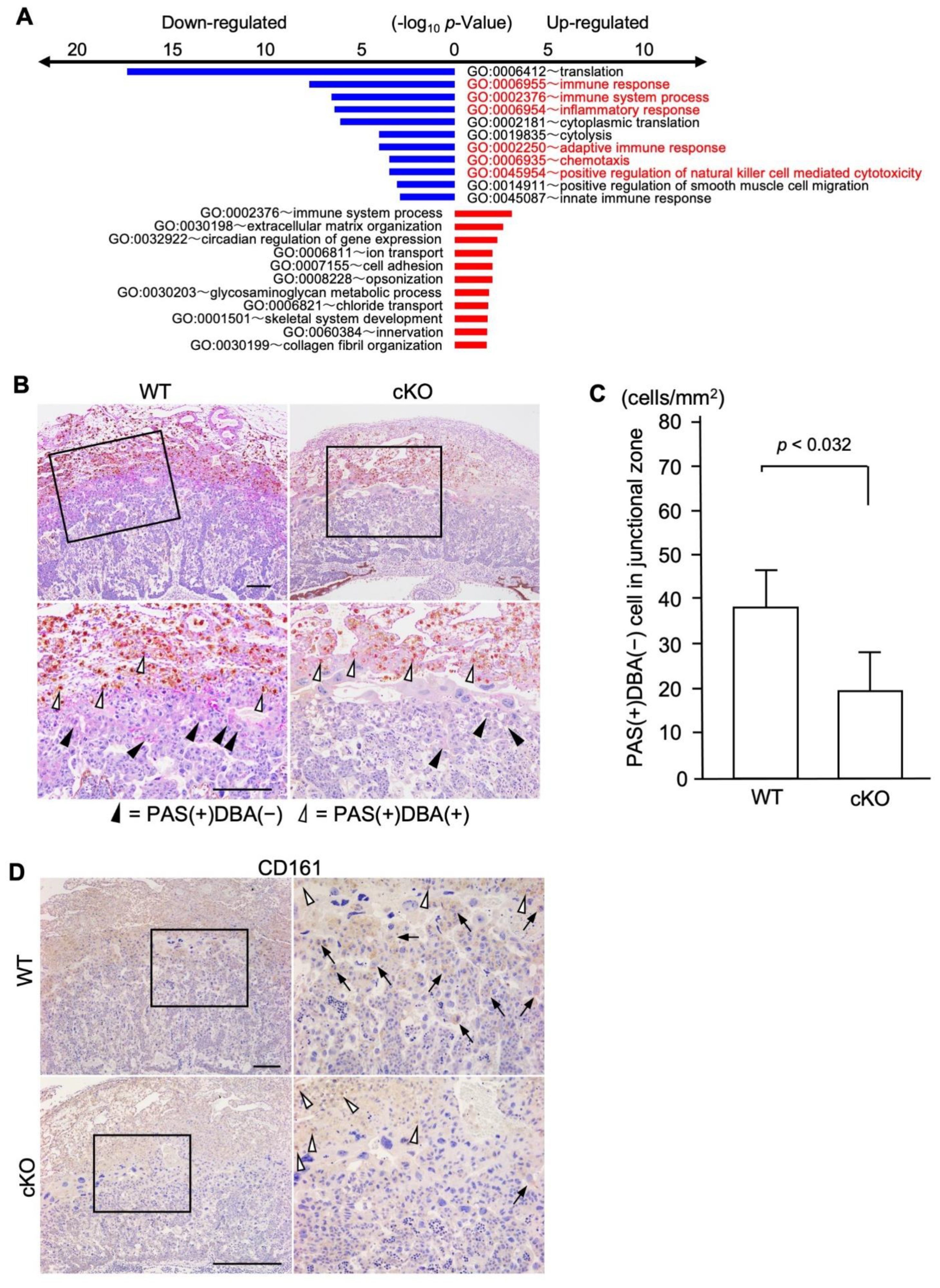
2.4. cKO Mice Showed Functional Changes in the Uterine Immune Environment
2.5. Progesterone Supplementation Rescues Perinatal Outcomes in cKO Female Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Analysis of Pregnancy Events
4.3. RT-PCR
4.4. Western Blot Analysis
4.5. Immunofluorescence
4.6. Histology and Measurement of Blood Vessel Area
4.7. Immunohistochemistry
4.8. DBA Lectin and PAS Dual Staining
4.9. Microarray Analysis
4.10. P4 Assay
5. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHOGD | adolescent dietary habit-induced obstetric and gynecologic disease |
| BMAL1 | brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 |
| CLOCK | circadian locomotor output cycles kaput |
| CRY | cryptochrome |
| HRP | horse-radish peroxidase |
| OCT | optimal cutting temperature |
| PER | Period |
| uNK | uterine natural killer |
| KO | knockout |
| cKO | conditional knockout |
| WT | wild type |
References
- Fujiwara, T. Skipping breakfast is associated with dysmenorrhea in young women in Japan. Int. J. Food Sci. Nutr. 2003, 54, 505–509. [Google Scholar] [CrossRef]
- Angelin, P.; Dileep, D.; Manju, T.; Veena, M.; Pradeep, D.; Amreen, K.; Soumitra, S. Effect of Skipping Breakfast on Young Girls’ Menstruation. Ind. J. Youth Adolesc. Health 2017, 4, 17–20. [Google Scholar]
- Abu Helwa, H.A.; Mitaeb, A.A.; Al-Hamshri, S.; Sweileh, W.M. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Womens Health 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tang, L.; Chen, L.; Kaminga, A.C.; Xu, H. Prevalence and Risk Factors Associated with Primary Dysmenorrhea among Chinese Female University Students: A Cross-sectional Study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Ono, M.; Iizuka, T.; Sekizuka-Kagami, N.; Maida, Y.; Adachi, Y.; Fujiwara, H.; Yoshikawa, H. Breakfast Skipping in Female College Students Is a Potential and Preventable Predictor of Gynecologic Disorders at Health Service Centers. Diagnostics 2020, 10, 476. [Google Scholar] [CrossRef]
- Nakayama, M.; Ono, M.; Iizuka, T.; Kagami, K.; Fujiwara, T.; Sekizuka-Kagami, N.; Maida, Y.; Obata, T.; Yamazaki, R.; Daikoku, T.; et al. Hypertensive disorders of pregnancy are associated with dysmenorrhea in early adulthood: A cohort study. J. Obstet. Gynaecol. Res. 2020, 46, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Ono, M.; Mieda, M.; Yoshikawa, H.; Nakata, R.; Daikoku, T.; Sekizuka-Kagami, N.; Maida, Y.; Ando, H.; Fujiwara, H. Adolescent Dietary Habit-induced Obstetric and Gynecologic Disease (ADHOGD) as a New Hypothesis-Possible Involvement of Clock System. Nutrients 2020, 12, 1294. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakata, R. Skipping breakfast is associated with reproductive dysfunction in post-adolescent female college students. Appetite 2010, 55, 714–717. [Google Scholar] [CrossRef]
- Hosono, T.; Ono, M.; Daikoku, T.; Mieda, M.; Nomura, S.; Kagami, K.; Iizuka, T.; Nakata, R.; Fujiwara, T.; Fujiwara, H.; et al. Time-Restricted Feeding Regulates Circadian Rhythm of Murine Uterine Clock. Curr. Dev. Nutr. 2021, 5, nzab064. [Google Scholar] [CrossRef]
- Boden, M.J.; Varcoe, T.J.; Voultsios, A.; Kennaway, D.J. Reproductive biology of female Bmal1 null mice. Reproduction 2010, 139, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Lim, A.; Fernando, D.; Day, M.L. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod. Biomed. Online 2002, 4, 140–145. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Moriya, T.; Inoue, S.; Shimazoe, T.; Watanabe, S.; Ebihara, S.; Shinohara, K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J. Neurosci. Res. 2005, 82, 622–630. [Google Scholar] [CrossRef]
- Lv, S.; Wang, N.; Ma, J.; Li, W.P.; Chen, Z.J.; Zhang, C. Impaired decidualization caused by downregulation of circadian clock gene BMAL1 contributes to human recurrent miscarriagedagger. Biol. Reprod. 2019, 101, 138–147. [Google Scholar] [CrossRef]
- Storch, K.F.; Paz, C.; Signorovitch, J.; Raviola, E.; Pawlyk, B.; Li, T.; Weitz, C.J. Intrinsic circadian clock of the mammalian retina: Importance for retinal processing of visual information. Cell 2007, 130, 730–741. [Google Scholar] [CrossRef]
- Daikoku, T.; Dey, S.K. Two faces of PTEN. Nat. Med. 2008, 14, 1192–1193. [Google Scholar] [CrossRef]
- Daikoku, T.; Hirota, Y.; Tranguch, S.; Joshi, A.R.; DeMayo, F.J.; Lydon, J.P.; Ellenson, L.H.; Dey, S.K. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008, 68, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.C.; Hemberger, M.; Lu, Y.; Nozaki, T.; Whiteley, K.; Masutani, M.; Adamson, S.L. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol. Cell Endocrinol. 2002, 187, 207–212. [Google Scholar] [CrossRef]
- Iizuka, T.; Wakae, K.; Ono, M.; Suzuki, T.; Mizumoto, Y.; Kitamura, K.; Horike, S.I.; Muramatsu, M.; Fujiwara, H. Activation-induced cytidine deaminase is a possible regulator of cross-talk between oocytes and granulosa cells through GDF-9 and SCF feedback system. Sci. Rep. 2021, 11, 3833. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Kiso, Y. Granulated metrial gland cells: A natural killer cell subset of the pregnant murine uterus. Microsc. Res. Tech. 1993, 25, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef] [PubMed]
- Paffaro, V.A., Jr.; Bizinotto, M.C.; Joazeiro, P.P.; Yamada, A.T. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003, 24, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Monsivais, D.; You, R.; Zhong, H.; Pangas, S.A.; Matzuk, M.M. Uterine activin receptor-like kinase 5 is crucial for blastocyst implantation and placental development. Proc. Natl. Acad. Sci. USA 2015, 112, E5098–E5107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Hatta, K.; Lima, P.D.; Yadi, H.; Colucci, F.; Yamada, A.T.; Croy, B.A. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol. Reprod. 2012, 87, 81. [Google Scholar] [CrossRef]
- Lima, P.D.; Tu, M.M.; Rahim, M.M.; Peng, A.R.; Croy, B.A.; Makrigiannis, A.P. Ly49 receptors activate angiogenic mouse DBA(+) uterine natural killer cells. Cell Mol. Immunol. 2014, 11, 467–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bogdan, A.; Berta, G.; Szekeres-Bartho, J. PIBF positive uterine NK cells in the mouse decidua. J. Reprod. Immunol. 2017, 119, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.A.; Di Santo, J.P.; Croy, B.A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 2000, 192, 259–270. [Google Scholar] [CrossRef]
- Zhang, J.H.; He, H.; Borzychowski, A.M.; Takeda, K.; Akira, S.; Croy, B.A. Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol. Reprod. 2003, 69, 404–411. [Google Scholar] [CrossRef][Green Version]
- Kurioka, A.; Cosgrove, C.; Simoni, Y.; van Wilgenburg, B.; Geremia, A.; Bjorkander, S.; Sverremark-Ekstrom, E.; Thurnheer, C.; Gunthard, H.F.; Khanna, N.; et al. CD161 Defines a Functionally Distinct Subset of Pro-Inflammatory Natural Killer Cells. Front. Immunol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Di, W.; Fan, W.; Wu, F.; Shi, Z.; Wang, Z.; Yu, M.; Zhai, Y.; Chang, Y.; Pan, C.; Li, G.; et al. Clinical characterization and immunosuppressive regulation of CD161 (KLRB1) in glioma through 916 samples. Cancer Sci. 2021, 113, 756. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Clark, D.D.; Dey, S.; Cao, N.V.; Semaan, S.J.; Chao, L.W.; Kauffman, A.S.; Stowers, L.; Mellon, P.L. Bmal1 Is Required for Normal Reproductive Behaviors in Male Mice. Endocrinology 2016, 157, 4914–4929. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.D.; Hansen, A.; Ord, T.; Bebas, P.; Chappell, P.E.; Giebultowicz, J.M.; Williams, C.; Moss, S.; Sehgal, A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythm. 2008, 23, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, S.; Xu, W.; Ying, J.; Qu, Y.; Jiang, X.; Zhang, A.; Yue, Y.; Zhou, R.; Ruan, T.; et al. Critical Roles of the Circadian Transcription Factor BMAL1 in Reproductive Endocrinology and Fertility. Front. Endocrinol. 2022, 13, 818272. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, C.K.; Boehle, K.L.; Muglia, L.J. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology 2009, 150, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Johnson, B.P.; Shen, A.L.; Wallisser, J.A.; Krentz, K.J.; Moran, S.M.; Sullivan, R.; Glover, E.; Parlow, A.F.; Drinkwater, N.R.; et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci. USA 2014, 111, 14295–14300. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knofler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Zhao, G.; Fan, H.; Hu, Y.; Hou, Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am. J. Reprod. Immunol. 2012, 67, 463–473. [Google Scholar] [CrossRef]
- Haas, D.M.; Hathaway, T.J.; Ramsey, P.S. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst. Rev. 2019, 2019, CD003511. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Lin, X.; He, J.; Wang, S.; Zhou, P. Pregnancy-related complications and perinatal outcomes following progesterone supplementation before 20 weeks of pregnancy in spontaneously achieved singleton pregnancies: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 165. [Google Scholar] [CrossRef]
- Barinov, S.V.; Tirskaya, Y.I.; Shamina, I.V.; Ledovskikh, I.O.; Atamanenko, O.J. Placental blood flow and pregnancy outcomes in women with abnormal placental localization and absence of placental “migration”. J. Matern.-Fetal Neonatal Med. 2021, 34, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef]
- Tranguch, S.; Wang, H.; Daikoku, T.; Xie, H.; Smith, D.F.; Dey, S.K. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J. Clin. Investig. 2007, 117, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Tranguch, S.; Cheung-Flynn, J.; Daikoku, T.; Prapapanich, V.; Cox, M.B.; Xie, H.; Wang, H.; Das, S.K.; Smith, D.F.; Dey, S.K. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 2005, 102, 14326–14331. [Google Scholar] [CrossRef]
- Daikoku, T.; Tranguch, S.; Trofimova, I.N.; Dinulescu, D.M.; Jacks, T.; Nikitin, A.Y.; Connolly, D.C.; Dey, S.K. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006, 66, 2527–2531. [Google Scholar] [CrossRef]
- Iizuka, T.; Wakae, K.; Nakamura, M.; Kitamura, K.; Ono, M.; Fujiwara, H.; Muramatsu, M. APOBEC3G is increasingly expressed on the human uterine cervical intraepithelial neoplasia along with disease progression. Am. J. Reprod. Immunol. 2017, 78, e12703. [Google Scholar] [CrossRef]
- Kadota, K.; Nakai, Y.; Shimizu, K. A weighted average difference method for detecting differentially expressed genes from microarray data. Algorithms Mol. Biol. 2008, 3, 8. [Google Scholar] [CrossRef]


| Day of Pregnancy | Genotype | No. of Mice | No. of Mice with Normal IS (%) | Mean No. of IS Sites (No. ± SE) * |
|---|---|---|---|---|
| Day 5 | WT | 8 | 100.0 | 8.3 ± 0.65 |
| cKO | 11 | 100.0 | 8.3 ± 0.38 | |
| Day 6 | WT | 14 | 85.7 | 9.4 ± 0.40 |
| cKO | 21 | 57.1 | 7.1 ± 0.67 | |
| Day 8 | WT | 16 | 81.3 | 8.8 ± 0.53 |
| cKO | 30 ** | 40.0 | 7.3 ± 0.56 | |
| Day 12 | WT | 20 | 85.0 | 9.5 ± 0.46 |
| cKO | 39 | 20.5 | 7.6 ± 1.00 |
| Day of Pregnancy | Genotype | No. of Mice | No. of Mice with IS (%) | Mean No. of IS Sites (No. ± SE) |
|---|---|---|---|---|
| Day 8 | WT + P4 | 4 | 100.0 | 8.5 ± 0.50 |
| cKO + P4 | 10 | 80.0 | 8.6 ± 0.46 | |
| Day 12 | WT + P4 | 8 | 100.0 | 6.5 ± 1.10 |
| cKO + P4 | 19 | 57.9 | 5.8 ± 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, M.; Toyoda, N.; Kagami, K.; Hosono, T.; Matsumoto, T.; Horike, S.-i.; Yamazaki, R.; Nakamura, M.; Mizumoto, Y.; Fujiwara, T.; et al. Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice. Int. J. Mol. Sci. 2022, 23, 7637. https://doi.org/10.3390/ijms23147637
Ono M, Toyoda N, Kagami K, Hosono T, Matsumoto T, Horike S-i, Yamazaki R, Nakamura M, Mizumoto Y, Fujiwara T, et al. Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice. International Journal of Molecular Sciences. 2022; 23(14):7637. https://doi.org/10.3390/ijms23147637
Chicago/Turabian StyleOno, Masanori, Natsumi Toyoda, Kyosuke Kagami, Takashi Hosono, Takeo Matsumoto, Shin-ichi Horike, Rena Yamazaki, Mitsuhiro Nakamura, Yasunari Mizumoto, Tomoko Fujiwara, and et al. 2022. "Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice" International Journal of Molecular Sciences 23, no. 14: 7637. https://doi.org/10.3390/ijms23147637
APA StyleOno, M., Toyoda, N., Kagami, K., Hosono, T., Matsumoto, T., Horike, S.-i., Yamazaki, R., Nakamura, M., Mizumoto, Y., Fujiwara, T., Ando, H., Fujiwara, H., & Daikoku, T. (2022). Uterine Deletion of Bmal1 Impairs Placental Vascularization and Induces Intrauterine Fetal Death in Mice. International Journal of Molecular Sciences, 23(14), 7637. https://doi.org/10.3390/ijms23147637







