Identification of Novel Genes for Cell Fusion during Osteoclast Formation
Abstract
1. Introduction
2. Results
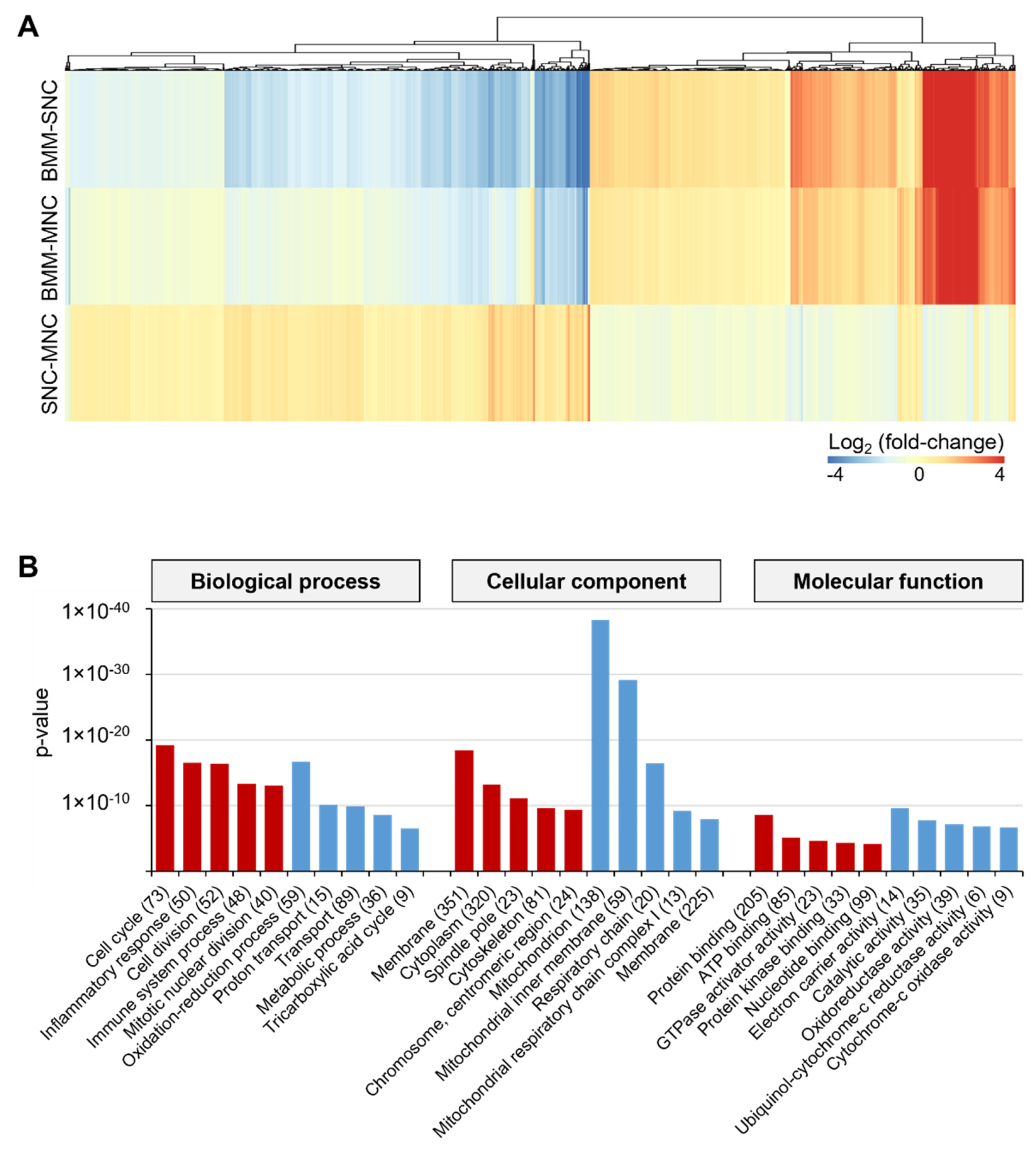
2.1. SNCs and MNCs Have Distinct Transcriptomic Profiles during Osteoclastogenesis
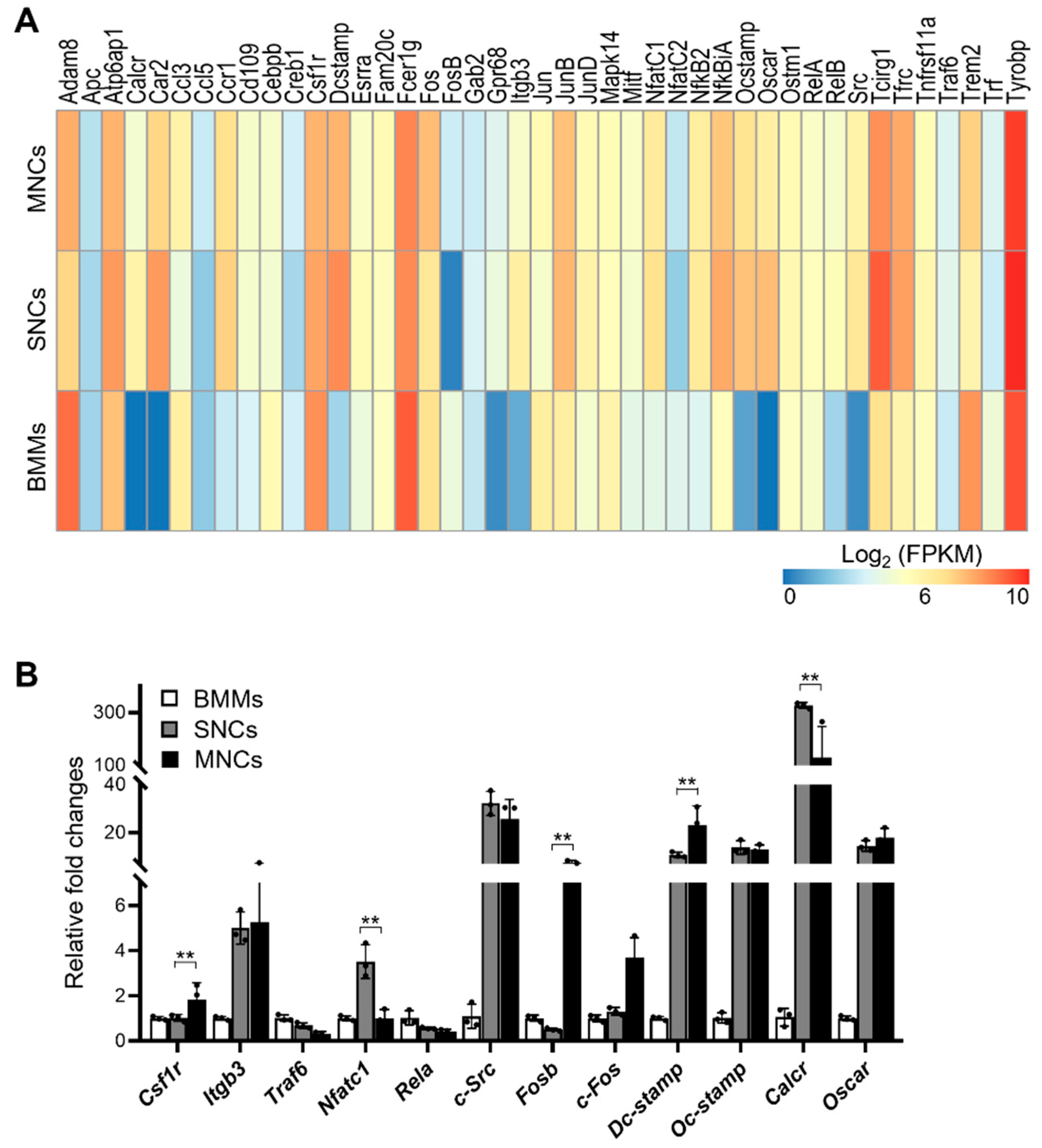
2.2. SNCs Include Preosteoclast Cells Committed to Osteoclasts
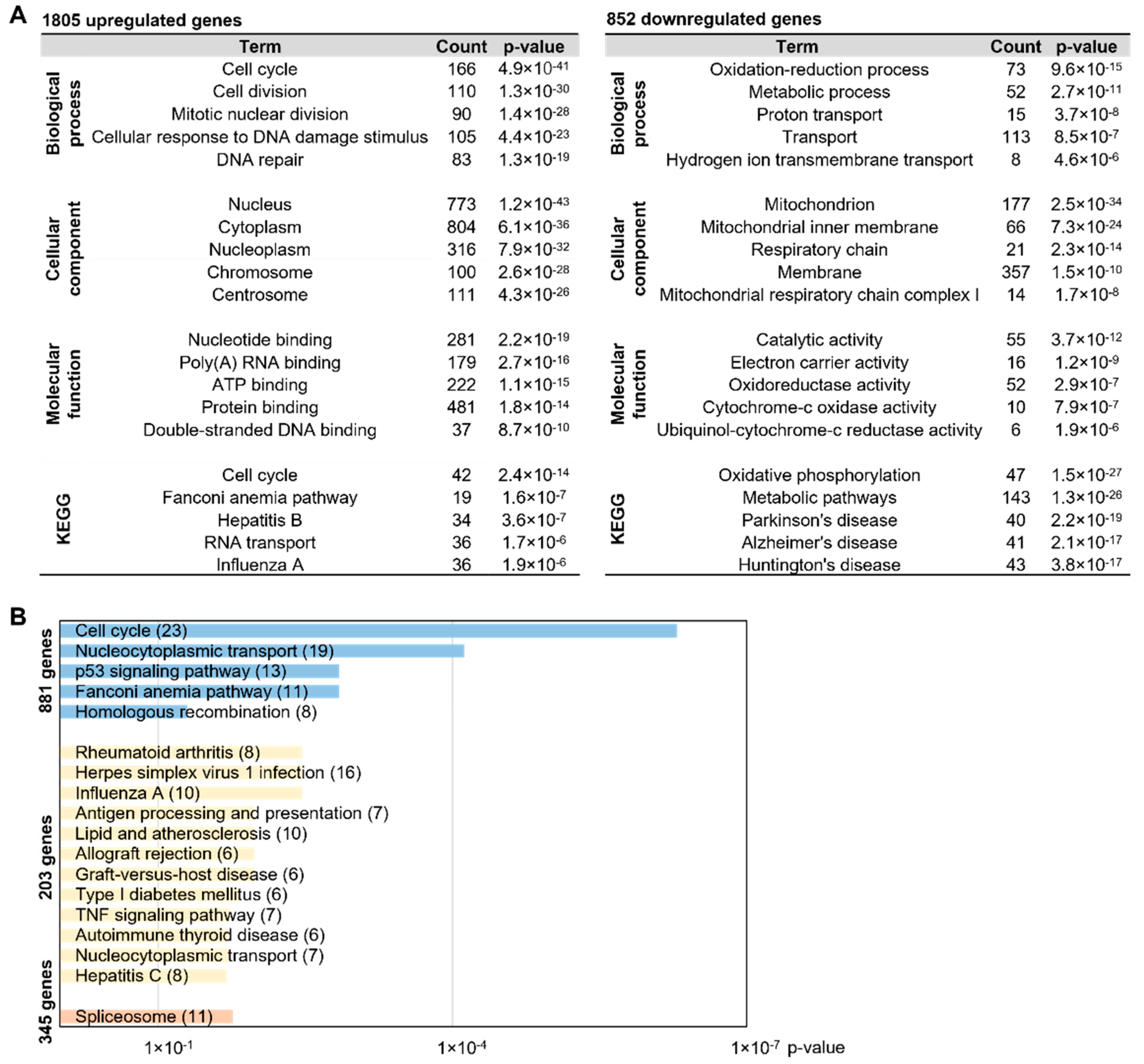
2.3. MNC-Specific DEGs Are Involved in Disease-Related Pathways
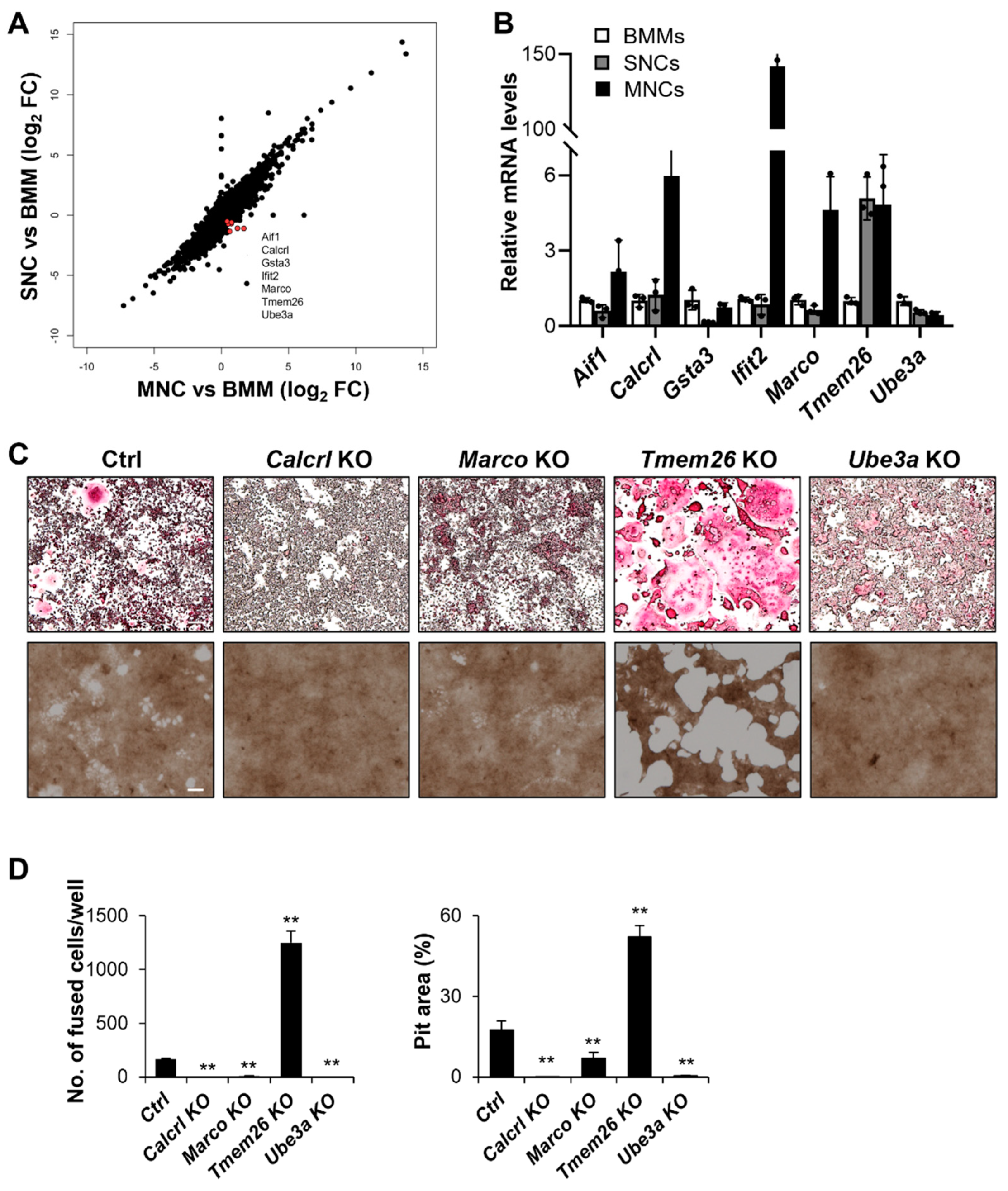
2.4. Calcrl, Marco, Tmem26, and Ube3a Control SNCs and MNCs
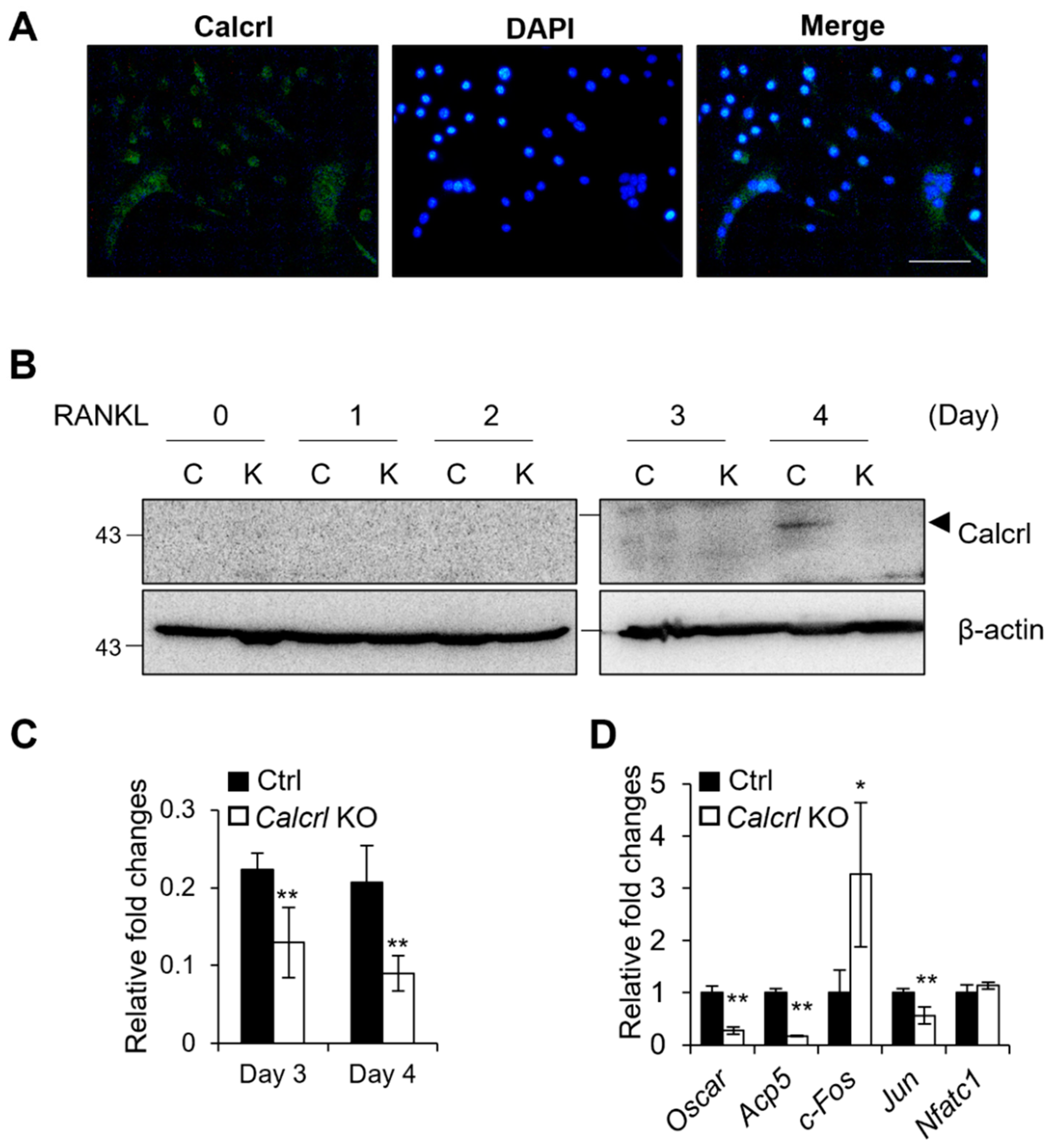
2.5. Calcrl Is Critical for MNC Development
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Osteoclast Differentiation
4.2. Bone Resorption Assay
4.3. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.4. Immunohistochemistry and Western Blotting
4.5. RNA Sequencing and Data Analyses
4.6. CRISPR-Cas9 Genome-Editing
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Novack, D.V.; Teitelbaum, S.L. The osteoclast: Friend or foe? Annu. Rev. Pathol. 2008, 3, 457–484. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347.e13. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Zhang, Z.; Bang, I.H.; Kwon, O.K.; Yoon, S.J.; Kim, J.R.; Lee, S.; Bae, E.J.; Park, B.H. Sirtuin 6 in preosteoclasts suppresses age- and estrogen deficiency-related bone loss by stabilizing estrogen receptor alpha. Cell Death Differ. 2019, 26, 2358–2370. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, Z.; Gu, Y.; Han, J.; Ye, K.; Zhang, Y. 7,8-Dihydroxyflavone modulates bone formation and resorption and ameliorates ovariectomy-induced osteoporosis. Elife 2021, 10, e64872. [Google Scholar] [CrossRef]
- Fierro, F.A.; Nolta, J.A.; Adamopoulos, I.E. Concise Review: Stem Cells in Osteoimmunology. Stem Cells 2017, 35, 1461–1467. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, T.; Yan, X.; Liu, Z.; Yan, Y.; Yang, K.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; et al. A Novel Rhein Derivative Modulates Bone Formation and Resorption and Ameliorates Estrogen-Dependent Bone Loss. J. Bone Miner. Res. 2019, 34, 361–374. [Google Scholar] [CrossRef]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-beta Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef]
- Cho, E.; Chen, Z.; Ding, M.; Seong, J.; Lee, S.; Min, S.H.; Choi, D.K.; Lee, T.H. PMSA prevents osteoclastogenesis and estrogen-dependent bone loss in mice. Bone 2021, 142, 115707. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.S.; Baylies, M.K.; Fleissner, A.; Helming, L.; Inoue, N.; Podbilewicz, B.; Wang, H.; Wong, M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013, 29, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef] [PubMed]
- Petrany, M.J.; Millay, D.P. Cell Fusion: Merging Membranes and Making Muscle. Trends Cell Biol. 2019, 29, 964–973. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibanez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.J.; et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. eLife 2020, 9, e54493. [Google Scholar] [CrossRef]
- Huang, R.; Wang, X.; Zhou, Y.; Xiao, Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Res. 2017, 5, 17019. [Google Scholar] [CrossRef]
- Soe, K. Osteoclast Fusion: Physiological Regulation of Multinucleation through Heterogeneity-Potential Implications for Drug Sensitivity. Int. J. Mol. Sci. 2020, 21, 7717. [Google Scholar] [CrossRef]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef]
- Sankar, U.; Patel, K.; Rosol, T.J.; Ostrowski, M.C. RANKL coordinates cell cycle withdrawal and differentiation in osteoclasts through the cyclin-dependent kinase inhibitors p27KIP1 and p21CIP1. J. Bone Miner. Res. 2004, 19, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, H.; Morozov, P.; Straus, A.; Sahasrabudhe, N.; Max, K.E.A.; Garzia, A.; Kustagi, M.; Tuschl, T.; Williams, Z. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018, 4, eaau4788. [Google Scholar] [CrossRef] [PubMed]
- Cappellen, D.; Luong-Nguyen, N.H.; Bongiovanni, S.; Grenet, O.; Wanke, C.; Susa, M. Transcriptional program of mouse osteoclast differentiation governed by the macrophage colony-stimulating factor and the ligand for the receptor activator of NFkappa B. J. Biol. Chem. 2002, 277, 21971–21982. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Angenendt, L.; Bormann, E.; Pabst, C.; Alla, V.; Gorlich, D.; Braun, L.; Dohlich, K.; Schwoppe, C.; Bohlander, S.K.; Arteaga, M.F.; et al. The neuropeptide receptor calcitonin receptor-like (CALCRL) is a potential therapeutic target in acute myeloid leukemia. Leukemia 2019, 33, 2830–2841. [Google Scholar] [CrossRef]
- Suekane, A.; Saito, Y.; Nakahata, S.; Ichikawa, T.; Ogoh, H.; Tsujikawa, K.; Morishita, K. CGRP-CRLR/RAMP1 signal is important for stress-induced hematopoiesis. Sci. Rep. 2019, 9, 429. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, S.Y.; Liang, X.; Zhang, H.; Xu, Z.; Liu, B.; Xu, M.J.; Jiang, C.; Shang, J.; Wang, X. Adrenomedullin 2 Enhances Beiging in White Adipose Tissue Directly in an Adipocyte-autonomous Manner and Indirectly through Activation of M2 Macrophages. J. Biol. Chem. 2016, 291, 23390–23402. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Fu, Y.; Shan, H.; Cui, S.; Wu, J. GSTA3 regulates TGF-beta1-induced renal interstitial fibrosis in NRK-52E cells as a component of the PI3K-Keap1/Nrf2 pathway. J. Int. Med. Res. 2019, 47, 5787–5801. [Google Scholar] [CrossRef]
- Bollag, A.E.; Guo, T.; Ding, K.H.; Choudhary, V.; Chen, X.; Zhong, Q.; Xu, J.; Yu, K.; Awad, M.E.; Elsalanty, M.; et al. Monomethylfumarate protects against ovariectomy-related changes in body composition. J. Endocrinol. 2019, 243, 15–26. [Google Scholar] [CrossRef]
- Gao, F.; Xu, F.; Wu, D.; Cheng, J.; Xia, P. Identification of novel genes associated with fracture healing in osteoporosis induced by Krm2 overexpression or Lrp5 deficiency. Mol. Med. Rep. 2017, 15, 3969–3976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komine, H.; Kuhn, L.; Matsushita, N.; Mule, J.J.; Pilon-Thomas, S. Examination of MARCO activity on dendritic cell phenotype and function using a gene knockout mouse. PLoS ONE 2013, 8, e67795. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Flaczyk, A.; Neal, L.M.; Fa, Z.; Eastman, A.J.; Malachowski, A.N.; Cheng, D.; Moore, B.B.; Curtis, J.L.; Osterholzer, J.J.; et al. Scavenger Receptor MARCO Orchestrates Early Defenses and Contributes to Fungal Containment during Cryptococcal Infection. J. Immunol. 2017, 198, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Joshi, V.; Amanullah, A.; Mishra, R.; Arora, N.; Prasad, A.; Mishra, A. E3 Ubiquitin Ligases Neurobiological Mechanisms: Development to Degeneration. Front. Mol. Neurosci. 2017, 10, 151. [Google Scholar] [CrossRef]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131, jcs216267. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, M.; Shah, A.M.; Tan, W.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution. Cell Rep. 2020, 33, 108472. [Google Scholar] [CrossRef]
- Gluexam, T.; Grandits, A.M.; Schlerka, A.; Nguyen, C.H.; Etzler, J.; Finkes, T.; Fuchs, M.; Scheid, C.; Heller, G.; Hackl, H.; et al. CGRP Signaling via CALCRL Increases Chemotherapy Resistance and Stem Cell Properties in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2019, 20, 5826. [Google Scholar] [CrossRef]
- Kukita, T.; Hiura, H.; Gu, J.Y.; Zhang, J.Q.; Kyumoto-Nakamura, Y.; Uehara, N.; Murata, S.; Sonoda, S.; Yamaza, T.; Takahashi, I.; et al. Modulation of osteoclastogenesis through adrenomedullin receptors on osteoclast precursors: Initiation of differentiation by asymmetric cell division. Lab. Investig. 2021, 101, 1449–1457. [Google Scholar] [CrossRef]
- Zampeta, F.I.; Sonzogni, M.; Niggl, E.; Lendemeijer, B.; Smeenk, H.; de Vrij, F.M.S.; Kushner, S.A.; Distel, B.; Elgersma, Y. Conserved UBE3A subcellular distribution between human and mice is facilitated by non-homologous isoforms. Hum. Mol. Genet. 2020, 29, 3032–3043. [Google Scholar] [CrossRef]
- Motiur Rahman, M.; Takeshita, S.; Matsuoka, K.; Kaneko, K.; Naoe, Y.; Sakaue-Sawano, A.; Miyawaki, A.; Ikeda, K. Proliferation-coupled osteoclast differentiation by RANKL: Cell density as a determinant of osteoclast formation. Bone 2015, 81, 392–399. [Google Scholar] [CrossRef]
- Buttitta, L.A.; Edgar, B.A. Mechanisms controlling cell cycle exit upon terminal differentiation. Curr. Opin. Cell Biol. 2007, 19, 697–704. [Google Scholar] [CrossRef] [PubMed]
- An, E.; Narayanan, M.; Manes, N.P.; Nita-Lazar, A. Characterization of functional reprogramming during osteoclast development using quantitative proteomics and mRNA profiling. Mol. Cell. Proteom. 2014, 13, 2687–2704. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; Alman, B.A. Macrophages promote osteoblastic differentiation in-vivo: Implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015, 30, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Chang, M.K.; Maylin, E.R.; Kohler, T.; Muller, R.; Wu, A.C.; Van Rooijen, N.; Sweet, M.J.; Hume, D.A.; Raggatt, L.J.; et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 2011, 26, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013, 34, 216–223. [Google Scholar] [CrossRef]
- Munoz, J.; Akhavan, N.S.; Mullins, A.P.; Arjmandi, B.H. Macrophage Polarization and Osteoporosis: A Review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef]
- Jeganathan, S.; Fiorino, C.; Naik, U.; Sun, H.S.; Harrison, R.E. Modulation of osteoclastogenesis with macrophage M1- and M2-inducing stimuli. PLoS ONE 2014, 9, e104498. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Le Nihouannen, D.; Fong, J.E.; Hussein, O.; Barralet, J.E.; Komarova, S.V. Regulation of Osteoclast Growth and Fusion by mTOR/raptor and mTOR/rictor/Akt. Front. Cell Dev. Biol. 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.M.; Delaisse, J.M.; Soe, K. Osteoclast Fusion: Time-Lapse Reveals Involvement of CD47 and Syncytin-1 at Different Stages of Nuclearity. J. Cell. Physiol. 2017, 232, 1396–1403. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ren, F.; Ye, Y.; Wang, F.; Zheng, C.; Qian, Y.; Zhang, M. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front. Immunol. 2021, 12, 664871. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Suzuki, T.; Miyauchi, Y.; Iwasaki, R.; Kobayashi, T.; Sato, Y.; Miyamoto, K.; Hoshi, H.; Hashimoto, K.; Yoshida, S.; et al. Osteoclast stimulatory transmembrane protein and dendritic cell-specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 2012, 27, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Hobolt-Pedersen, A.S.; Delaisse, J.M.; Soe, K. Osteoclast fusion is based on heterogeneity between fusion partners. Calcif. Tissue Int. 2014, 95, 73–82. [Google Scholar] [CrossRef]
- Ahn, S.H.; Chen, Z.; Lee, J.; Lee, S.W.; Min, S.H.; Kim, N.D.; Lee, T.H. Inhibitory Effects of 2N1HIA (2-(3-(2-Fluoro-4-Methoxyphenyl)-6-Oxo-1(6H)-Pyridazinyl)-N-1H-Indol-5-Ylacetamid e) on Osteoclast Differentiation via Suppressing Cathepsin K Expression. Molecules 2018, 23, 3139. [Google Scholar] [CrossRef]
- Cho, E.; Chen, Z.; Lee, J.; Lee, S.; Lee, T.H. PSTP-3,5-Me Inhibits Osteoclast Differentiation and Bone Resorption. Molecules 2019, 24, 3346. [Google Scholar] [CrossRef]
- Dou, C.; Cao, Z.; Yang, B.; Ding, N.; Hou, T.; Luo, F.; Kang, F.; Li, J.; Yang, X.; Jiang, H.; et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci. Rep. 2016, 6, 21499. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef]


| Sample | Number of Reads (Sum of Pairs) | Number of Reads after Trimming | Alignment Rate (%) |
|---|---|---|---|
| BMM 1 | 79,081,594 | 77,290,554 | 98.62% |
| BMM 2 | 73,952,744 | 72,383,444 | 98.17% |
| BMM 3 | 84,004,640 | 81,523,436 | 98.15% |
| MNC 1 | 75,068,812 | 73,463,268 | 98.82% |
| MNC 2 | 79,729,144 | 77,767,782 | 98.55% |
| MNC 3 | 81,017,248 | 79,417,460 | 98.74% |
| SNC 1 | 84,672,352 | 82,673,110 | 98.45% |
| SNC 2 | 87,103,520 | 85,087,954 | 98.45% |
| Gene | BMM–SNC | BMM–MNC | Description | ||
|---|---|---|---|---|---|
| Log2 FC | q-value | Log2 FC | q-value | ||
| Aif1 | −0.75 | 0.0096 | 0.77 | 0.0022 | Allograft inflammatory factor 1 |
| Calcrl | −1.41 | 0.0003 | 0.64 | 0.0020 | Calcitonin receptor-like |
| Gsta3 | −1.33 | 0.0003 | 1.30 | 0.0003 | Glutathione S-transferase, alpha 3 |
| Ifit2 | −0.90 | 0.0009 | 0.59 | 0.0335 | Interferon-induced protein with tetratricopeptide repeats 2 |
| Marco | −1.82 | 0.0003 | 1.98 | 0.0003 | Macrophage receptor with collagenous structure |
| Tmem26 | −0.80 | 0.0072 | 0.91 | 0.0003 | Transmembrane protein 26 |
| Ube3a | −0.55 | 0.0138 | 0.44 | 0.0297 | Ubiquitin protein ligase E3A |
| Gene | gRNA Sequence | Knockout Sequence |
|---|---|---|
| Aif1 | GTCCAAACTTGAAGCCTTCA TGCTGTATTTGGGATCATCG | Not detected |
| Calcrl | CCCAGGTCCTATTGCAGTAA | GCTGACCC---//---CTGGAATGAC (56-base pair deletion) |
| Gsta3 | GGAGCCTATCCGGTGGCTCT TCCTTCATTACTTTGATGGC | Not detected |
| Ifit2 | ATCAGAAGTCTGGTCACCTG | Not detected |
| Marco | CAGCACCCAATCTGAGAGAA | GGGGACCT--/TCTC/TGCATGGCA (2-base pair deletion or addition) |
| Tmem26 | TTCCAATTACACGAATTAAA | GCAGCACC---//---CACAGAACT (16-base pair deletion) |
| Ube3a | GTCCAAACTTGAAGCCTTCA ATATACAAGTGCATTCAGGA | AACTGCCTT-CTGAATGCACTTGT (1-base pair deletion) |
| Gene | Sequence |
|---|---|
| Csf1r | CTTCACTCCGGTGGTGGTGG and GCGCACCTGGTACTTCGGCT |
| Itgb3 | ACAGAGCGTGTCCCGTAATC and GTCTTCCATCCAGGGCAATA |
| Traf6 | AAAGCGAGAGATTCTTTCCCTG and ACTGGGGACAATTCACTAGAGC |
| Nfatc1 | CCCGTCACATTCTGGTCCAT and CAAGTAACCGTGTAGCTCCACAA |
| Rela | GCCCAGACCGCAGTATCC and GTCCCGCACTGTCACCTG |
| c-Src | CCAGGCTGAGGAGTGGTACT and GAGCTTGCGGATCTTGTAGT |
| Atf3 | GCTGCTGCCAAGTGTCGAAA and TACATGCTCAACCTGCACCG |
| Fosb | GATCGCCGAGCTGCAAAAAG and CCTTAGCGGATGTTGACCCTGG |
| DC-stamp | GGGAGTCCTGCACCATATGG and AGGCCAGTGCTGACTAGGATGA |
| OC-stamp | CAGAGTGACCACCTGAACAAACA and TGCCTGAGGTCCCTGTGACT |
| Calcr | CCTTCCAGAGGAGAAGAAACC and GGAGATTCCGCCTTTTCAC |
| c-Fos | CCAAGCGGAGACAGATCAACTT and TCCAGTTTTTCCTTCTCTTTCAGCAGA |
| Oscar | TGGCGGTTTGCACTCTTCA and GGAAGAACTCAGCCAGCTCAA |
| Aif1 | TGATGAGGATCTGCCGTCCAAACT and TCTCCAGCATTCGCTTCAAGGACA |
| Calcrl | CAAGATCATGACGGCTCAATA and CGTCATTCCAGCATAGCCAT |
| Gsta3 | AACCGTTACTTTCCTGCCTTTG and GCCCTGCTCAGCCTATTGC |
| Ifit2 | AAATGTCATGGGTACTGGAGTT and ATGGCAATTATCAAGTTTGTGG |
| Marco | AGGAAGACTTCTTGGGCAGC and GAGCAGGATCAGGTGGATGG |
| Tmem26 | GCACCATCACTAGAGACCAAC and ACAAGAATGCCAGAGACCAG |
| Ube3a | CAGCCTAGTTCAAGGACAGCAG and TCCACATACAACTGCTTCTTCAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Cheon, S.; Ding, M.; Lim, K.; Park, S.-W.; Park, C.; Lee, T.-H. Identification of Novel Genes for Cell Fusion during Osteoclast Formation. Int. J. Mol. Sci. 2022, 23, 6421. https://doi.org/10.3390/ijms23126421
Cho E, Cheon S, Ding M, Lim K, Park S-W, Park C, Lee T-H. Identification of Novel Genes for Cell Fusion during Osteoclast Formation. International Journal of Molecular Sciences. 2022; 23(12):6421. https://doi.org/10.3390/ijms23126421
Chicago/Turabian StyleCho, Eunjin, Seongmin Cheon, Mina Ding, Kayeong Lim, Sang-Wook Park, Chungoo Park, and Tae-Hoon Lee. 2022. "Identification of Novel Genes for Cell Fusion during Osteoclast Formation" International Journal of Molecular Sciences 23, no. 12: 6421. https://doi.org/10.3390/ijms23126421
APA StyleCho, E., Cheon, S., Ding, M., Lim, K., Park, S.-W., Park, C., & Lee, T.-H. (2022). Identification of Novel Genes for Cell Fusion during Osteoclast Formation. International Journal of Molecular Sciences, 23(12), 6421. https://doi.org/10.3390/ijms23126421






