Transcriptome and Proteome Analyses Reveal Stage-Specific DNA Damage Response in Embryos of Sturgeon (Acipenser ruthenus)
Abstract
:1. Introduction
2. Results
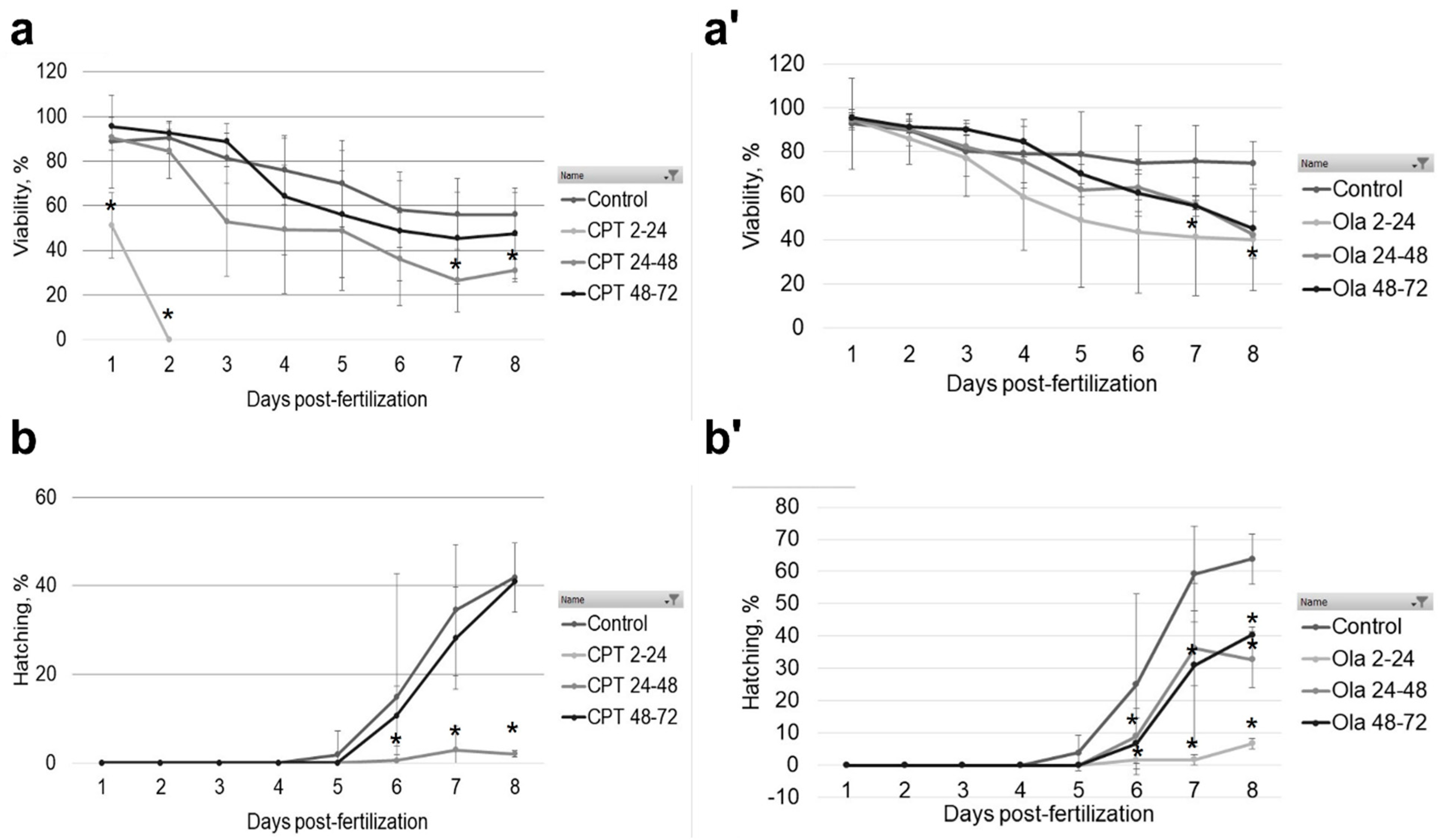
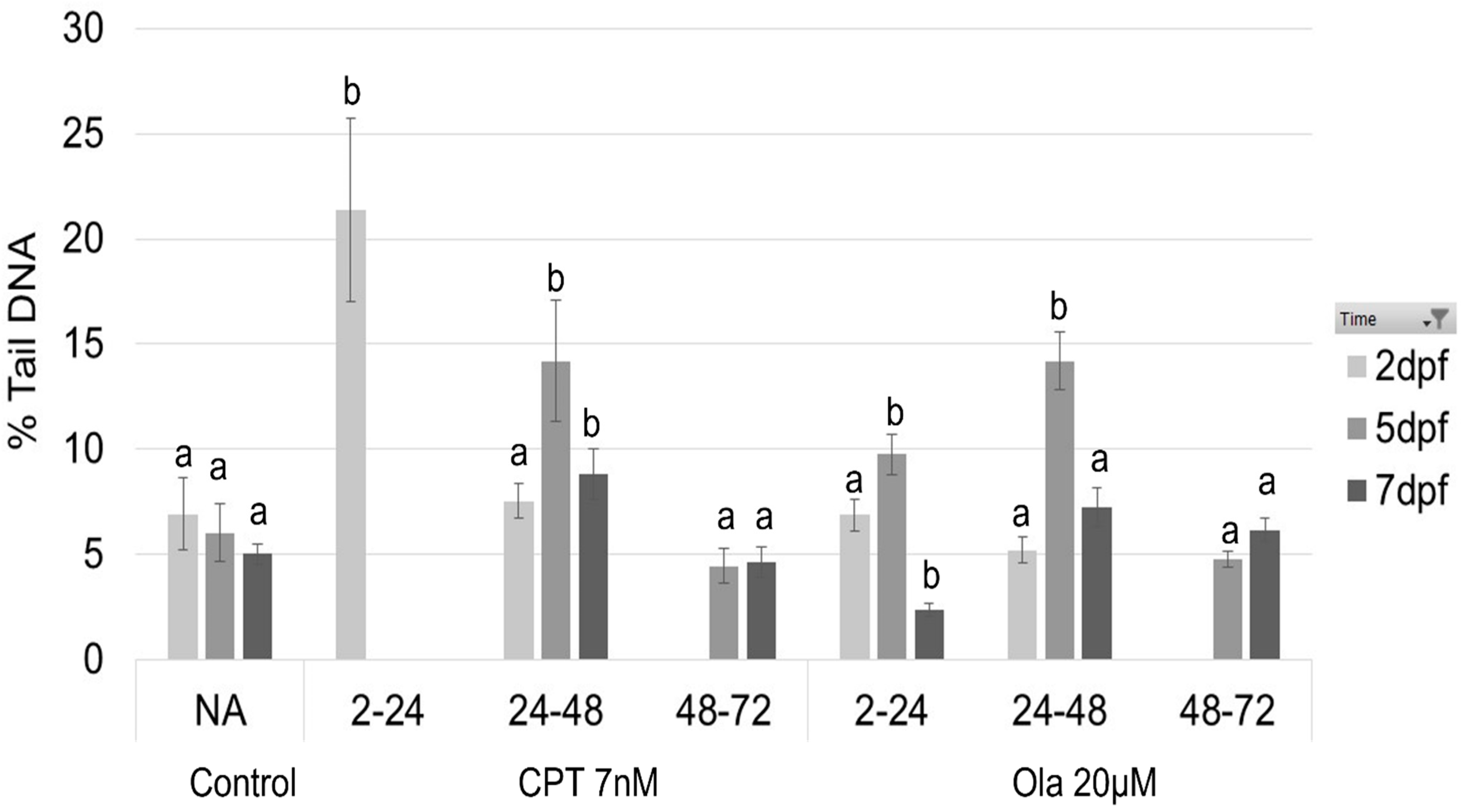
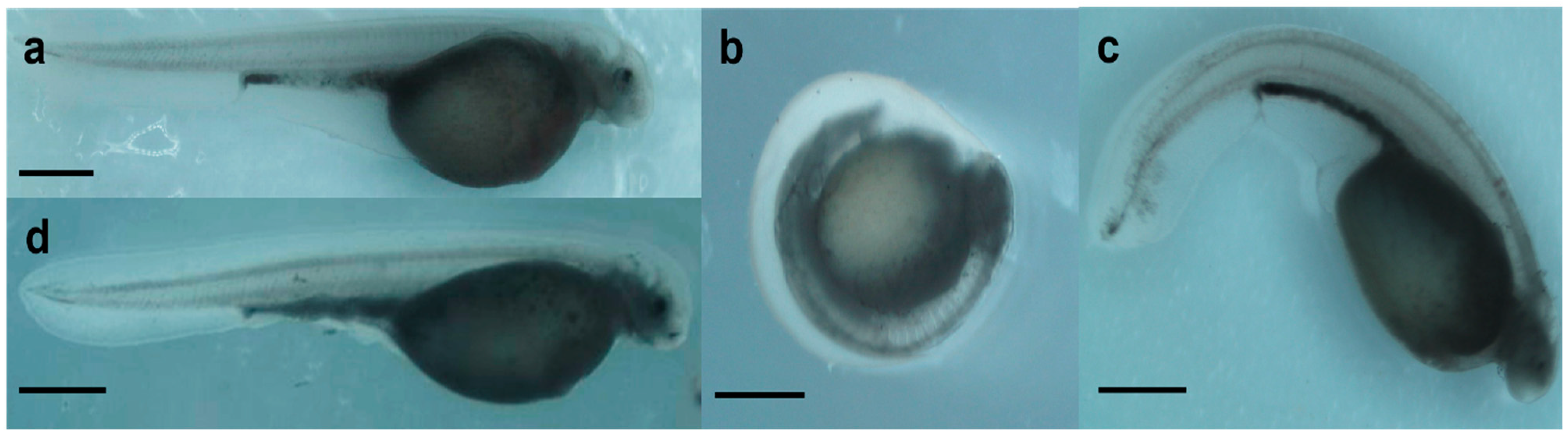
2.1. Effect of Genotoxicants on Embryo DNA Integrity and Phenotype Formation
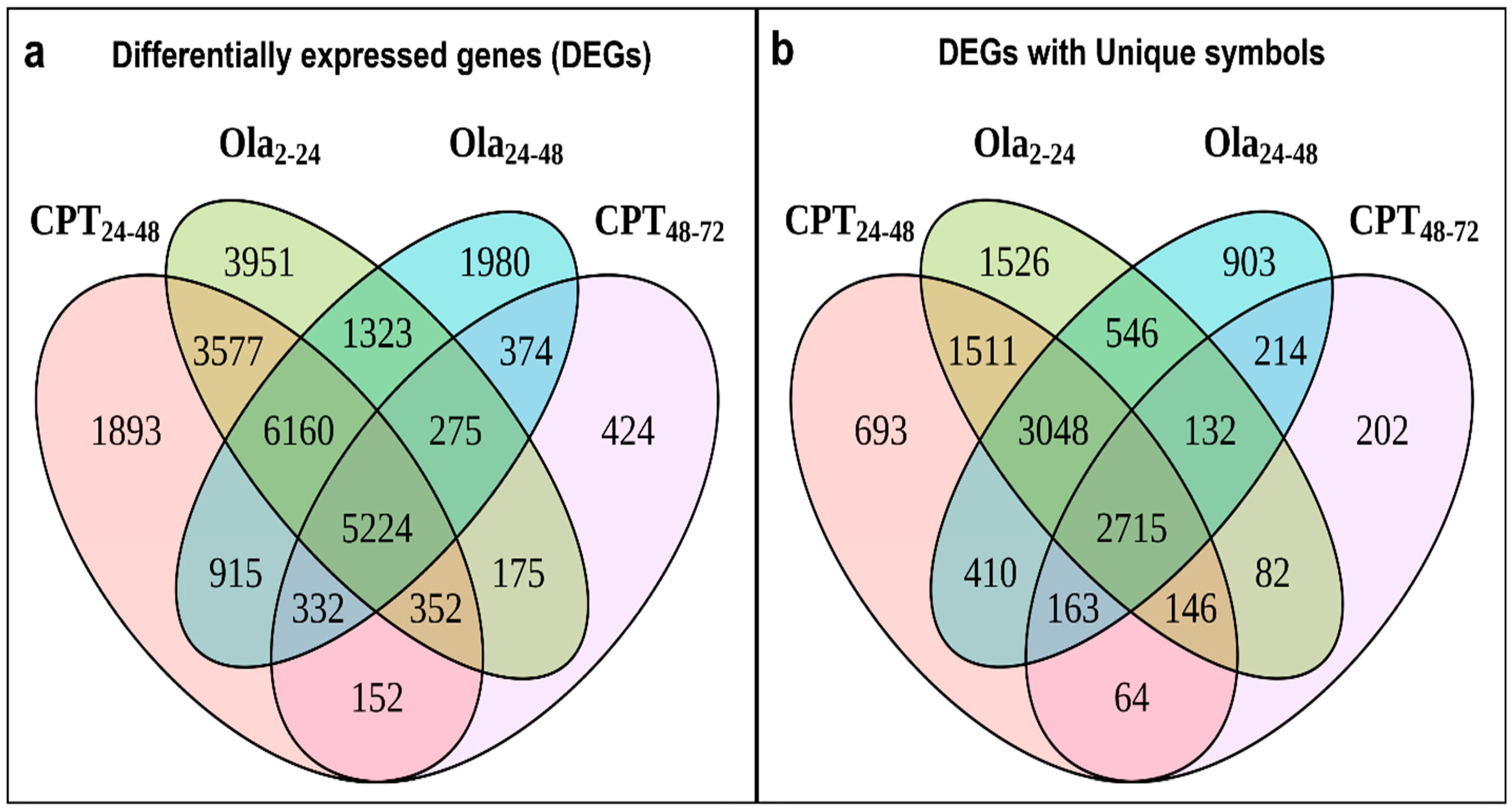
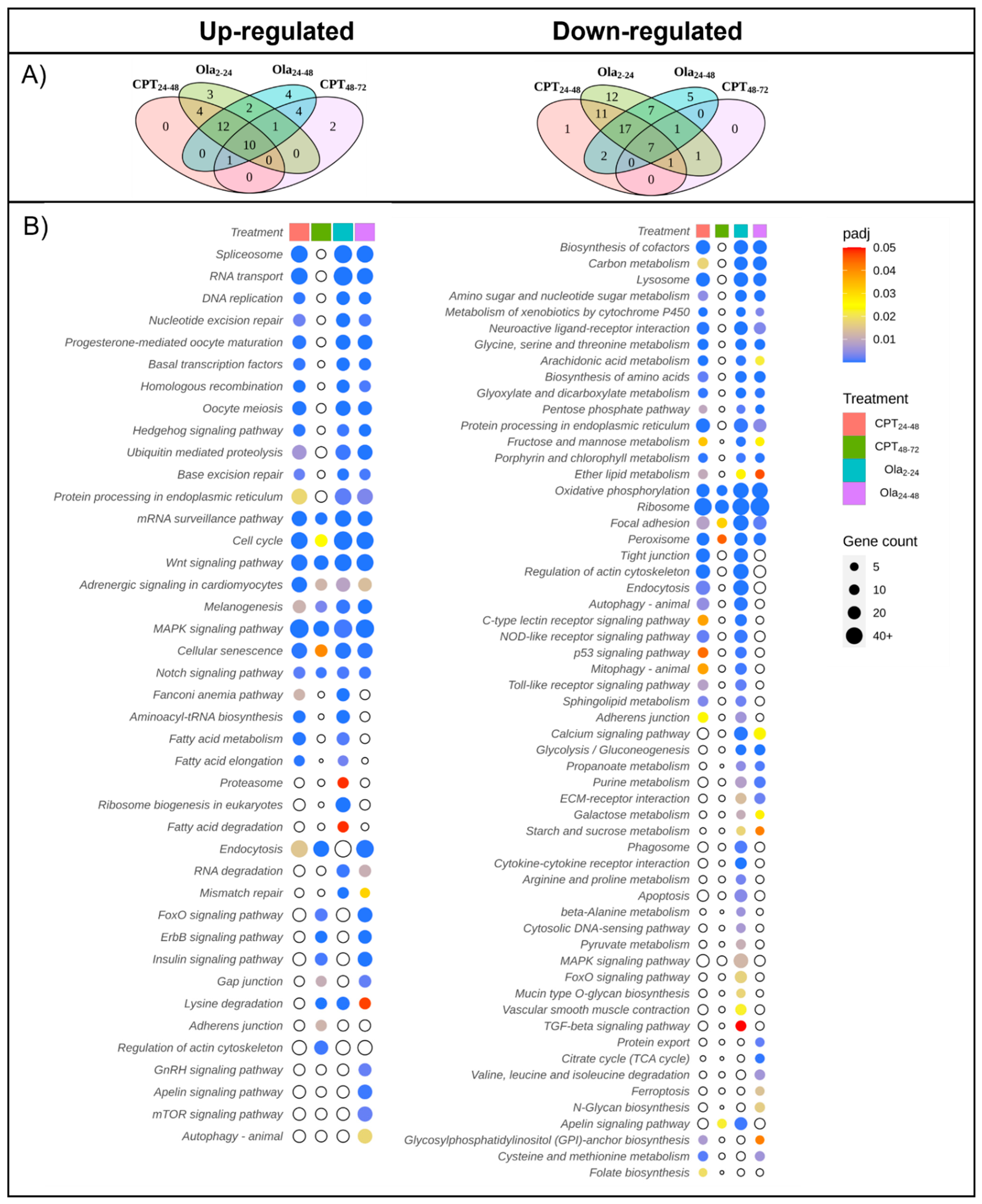
2.2. Main Changes in Transcriptomic Patterns in Sturgeon Embryos after DNA Damage
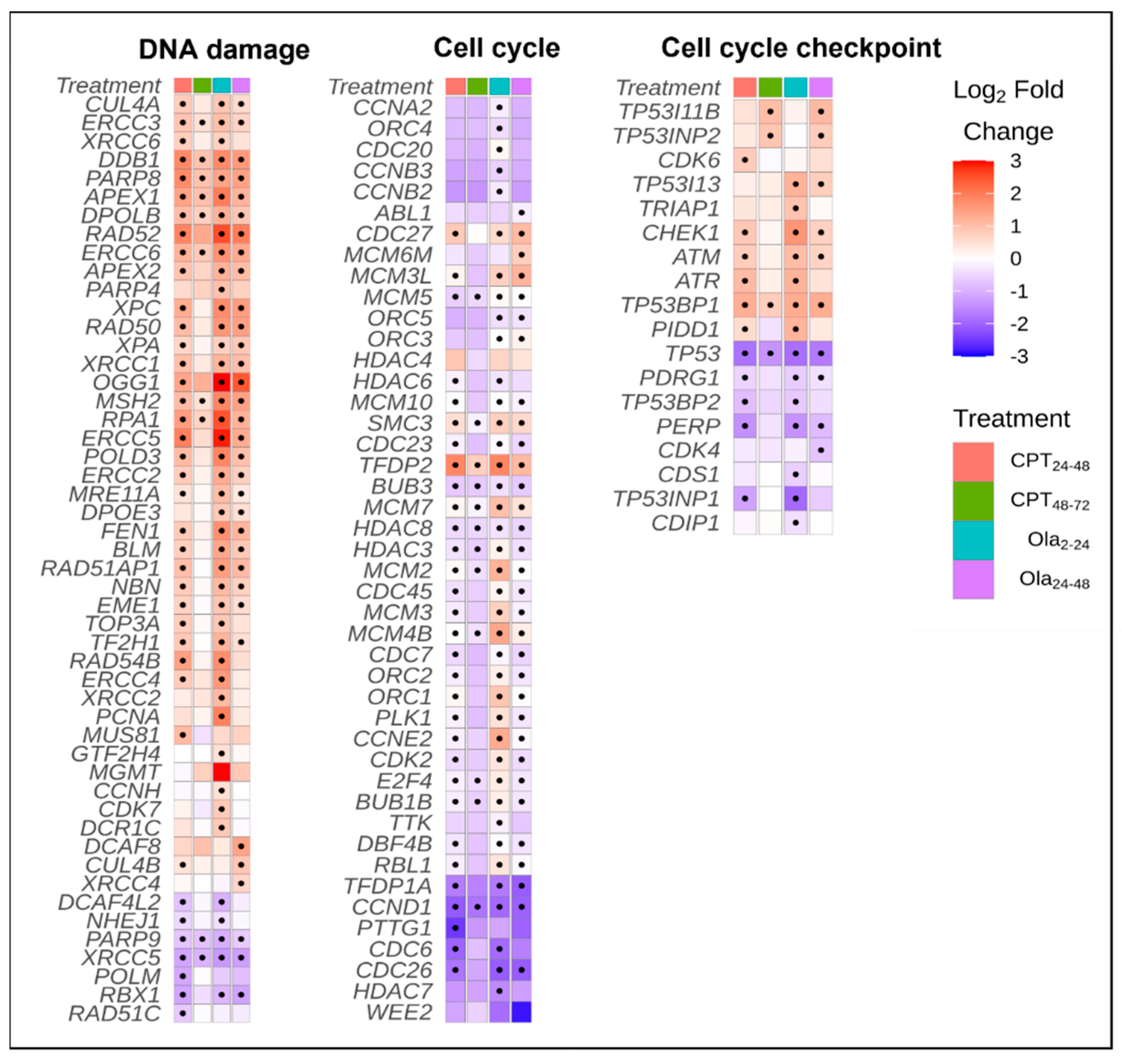
2.3. Differentially Expressed Genes Associated with Cell Cycle and DNA Repair Pathways
2.4. Protein Profiles in Sturgeon Embryos following DNA Damage
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animals
4.3. Experimental Design
- embryonic viability, % (number of live embryos/number of fertilized eggs × 100).
- hatching rate, % (number of hatched larvae/number of fertilized eggs × 100).
- occurrence of malformations.
4.4. Comet Assay
4.5. mRNA Extraction and Illumina Sequencing
4.6. RNAseq Data Analysis
4.7. Protein Extraction and Two-Dimensional Gel Electrophoresis
4.8. In-Gel Digestion and Protein Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canedo, A.; Rocha, T.L. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: Historical review, current status and trends. Sci. Total Environ. 2021, 762, 144084. [Google Scholar] [CrossRef] [PubMed]
- Akcha, F.; Barranger, A.; Bachere, E. Genotoxic and epigenetic effects of diuron in the Pacific oyster: In vitro evidence of interaction between DNA damage and DNA methylation. Environ. Sci. Pollut. Res. Int. 2021, 28, 8266–8280. [Google Scholar] [CrossRef] [PubMed]
- Devaux, A.; Fiat, L.; Gillet, C.; Bony, S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus). Aquat. Toxicol. 2011, 101, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Palos-Ladeiro, M.; Besnard, A.; Porcher, J.M.; Bony, S.; Sanchez, W.; Devaux, A. Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reprod. Toxicol. 2013, 36, 6–11. [Google Scholar] [CrossRef]
- Kermi, C.; Lo Furno, E.; Maiorano, D. Regulation of DNA Replication in Early Embryonic Cleavages. Genes 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Chenevert, J.; Roca, M.; Besnardeau, L.; Ruggiero, A.; Nabi, D.; McDougall, A.; Copley, R.R.; Christians, E.; Castagnetti, S. The Spindle Assembly Checkpoint Functions during Early Development in Non-Chordate Embryos. Cells 2020, 9, 1087. [Google Scholar] [CrossRef]
- Ikegami, R.; Hunter, P.; Yager, T.D. Developmental activation of the capability to undergo checkpoint-induced apoptosis in the early zebrafish embryo. Dev. Biol. 1999, 209, 409–433. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.M.; Lehmann, A.R. Conservation of eukaryotic DNA repair mechanisms. Int. J. Radiat. Biol. 1998, 74, 277–286. [Google Scholar]
- Jaroudi, S.; SenGupta, S. DNA repair in mammalian embryos. Mutat. Res. 2007, 635, 53–77. [Google Scholar] [CrossRef]
- Gazo, I.; Franek, R.; Sindelka, R.; Lebeda, I.; Shivaramu, S.; Psenicka, M.; Steinbach, C. Ancient Sturgeons Possess Effective DNA Repair Mechanisms: Influence of Model Genotoxicants on Embryo Development of Sterlet, Acipenser ruthenus. Int. J. Mol. Sci. 2020, 22, 6. [Google Scholar] [CrossRef]
- Bemis, W.E.; Findeis, E.K.; Grande, L. An overview of Acipenseriformes. Environ. Biol. Fishes 1997, 48, 25–71. [Google Scholar] [CrossRef]
- Chandra, G.; Fopp-Bayat, D. Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 2021, 13, 119–137. [Google Scholar] [CrossRef]
- Du, K.; Stöck, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.; Kichigin, I.; et al. The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasil’eva, E.D.; Vasil’ev, V.P.; Ponomareva, E.N.; Lapukhin, Y.A. Triple hybrids obtained by artificial hybridization of the Russian sturgeon Acipenser gueldenstaedtii with the hybrid of the starred sturgeon A. stellatus and the great sturgeon A. huso (Acipenseridae): The kind of inheritance of some morphological characters and fertility of the parental hybrid form. J. Ichthyol. 2010, 50, 605–617. [Google Scholar]
- Gille, D.A.; Famula, T.R.; May, B.P.; Schreier, A.D. Evidence for a maternal origin of spontaneous autopolyploidy in cultured white sturgeon (Acipenser transmontanus). Aquaculture 2015, 435, 467–474. [Google Scholar] [CrossRef]
- Van Eenennaam, J.P.; Fiske, A.J.; Leal, M.J.; Cooley-Rieders, C.; Todgham, A.E.; Conte, F.S.; Schreier, A.D. Mechanical shock during egg de-adhesion and post-ovulatory ageing contribute to spontaneous autopolyploidy in white sturgeon culture (Acipenser transmontanus). Aquaculture 2020, 515, 734530. [Google Scholar] [CrossRef]
- Lebeda, I.; Rab, P.; Majtanova, Z.; Flajshans, M. Artificial whole genome duplication in paleopolyploid sturgeons yields highest documented chromosome number in vertebrates. Sci. Rep. 2020, 10, 19705. [Google Scholar] [CrossRef]
- Voskarides, K.; Dweep, H.; Chrysostomou, C. Evidence that DNA repair genes, a family of tumor suppressor genes, are associated with evolution rate and size of genomes. Hum. Genom. 2019, 13, 26. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.P.; Gong, Z.; Tse, W.K.F. Zebrafish as the toxicant screening model: Transgenic and omics approaches. Aquat. Toxicol. 2021, 234, 105813. [Google Scholar] [CrossRef]
- Schuttler, A.; Reiche, K.; Altenburger, R.; Busch, W. The Transcriptome of the Zebrafish Embryo After Chemical Exposure: A Meta-Analysis. Toxicol. Sci. 2017, 157, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Marx-Stoelting, P.; Braeuning, A.; Buhrke, T.; Lampen, A.; Niemann, L.; Oelgeschlaeger, M.; Rieke, S.; Schmidt, F.; Heise, T.; Pfeil, R.; et al. Application of omics data in regulatory toxicology: Report of an international BfR expert workshop. Arch. Toxicol. 2015, 89, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.J.; Taher, L.; Drexler, H.; Suzuki, Y.; Makalowski, W.; Schwarzer, C.; Wang, B.; Fuellen, G.; Boiani, M. Differences in embryo quality are associated with differences in oocyte composition: A proteomic study in inbred mice. Proteomics 2015, 15, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Diez, C.; Gonzalez-Rojo, S.; Lombo, M.; Herraez, M.P. Tolerance to paternal genotoxic damage promotes survival during embryo development in zebrafish (Danio rerio). Biol. Open 2018, 7, bio030130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Nahrgang, J.; Tollefsen, K.E. Transcriptomic analysis reveals dose-dependent modes of action of benzo(a)pyrene in polar cod (Boreogadus saida). Sci. Total Environ. 2019, 653, 176–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.C. DNA topoisomerases. Annu. Rev. Biochem. 1996, 65, 635–692. [Google Scholar] [CrossRef]
- Karapetian, M.; Tsikarishvili, S.; Kulikova, N.; Kurdadze, A.; Zaalishvili, G. Genotoxic effects of topoisomerase poisoning and PARP inhibition on zebrafish embryos. DNA Repair (Amst) 2020, 87, 102772. [Google Scholar] [CrossRef]
- Hengel, S.R.; Spies, M.A.; Spies, M. Small-Molecule Inhibitors Targeting DNA Repair and DNA Repair Deficiency in Research and Cancer Therapy. Cell Chem. Biol. 2017, 24, 1101–1119. [Google Scholar] [CrossRef] [Green Version]
- Vierstraete, J.; Fieuws, C.; Willaert, A.; Vral, A.; Claes, K.B.M. Zebrafish as an in vivo screening tool to establish PARP inhibitor efficacy. DNA Repair (Amst) 2021, 97, 103023. [Google Scholar] [CrossRef]
- Tsikarishvili, S.; Karapetian, M.; Kulikova, N.; Zaalishvili, G. PARP inhibition suppresses topoisomerase 1 poisoning induced Caspase-3 dependent cell death in zebrafish embryos. Biochem. Biophys. Res. Commun. 2021, 550, 166–170. [Google Scholar] [CrossRef]
- Honjo, Y.; Ichinohe, T. Stage-Specific Effects of Ionizing Radiation during Early Development. Int. J. Mol. Sci. 2020, 21, 3975. [Google Scholar] [CrossRef]
- Gordon, W.E.; Espinoza, J.A.; Leerberg, D.M.; Yelon, D.; Hamdoun, A. Xenobiotic transporter activity in zebrafish embryo ionocytes. Aquat. Toxicol. 2019, 212, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Kluver, N.; Burkhardt-Medicke, K.; Pietsch, M.; Schmidt, A.M.; Wellner, P.; Schirmer, K.; Luckenbach, T. Abcb4 acts as multixenobiotic transporter and active barrier against chemical uptake in zebrafish (Danio rerio) embryos. BMC Biol. 2013, 11, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eachus, H.; Choi, M.K.; Ryu, S. The Effects of Early Life Stress on the Brain and Behaviour: Insights From Zebrafish Models. Front. Cell Dev. Biol. 2021, 9, 657591. [Google Scholar] [CrossRef] [PubMed]
- Vindas, M.A.; Fokos, S.; Pavlidis, M.; Hoglund, E.; Dionysopoulou, S.; Ebbesson, L.O.E.; Papandroulakis, N.; Dermon, C.R. Early life stress induces long-term changes in limbic areas of a teleost fish: The role of catecholamine systems in stress coping. Sci. Rep. 2018, 8, 5638. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, H.C.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012, 28, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Stacey, D.W. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr. Opin. Cell Biol. 2003, 15, 158–163. [Google Scholar] [CrossRef]
- Choi, E.H.; Yoon, S.; Park, K.S.; Kim, K.P. The Homologous Recombination Machinery Orchestrates Post-replication DNA Repair During Self-renewal of Mouse Embryonic Stem Cells. Sci. Rep. 2017, 7, 11610. [Google Scholar] [CrossRef]
- De Silva, I.U.; McHugh, P.J.; Clingen, P.H.; Hartley, J.A. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol. Cell Biol. 2000, 20, 7980–7990. [Google Scholar] [CrossRef]
- Kumar, N.; Moreno, N.C.; Feltes, B.C.; Menck, C.F.; Houten, B.V. Cooperation and interplay between base and nucleotide excision repair pathways: From DNA lesions to proteins. Genet. Mol. Biol. 2020, 43 (Suppl. S1), e20190104. [Google Scholar] [CrossRef]
- Marconi, D.; Ranfley, H.; Menck, C.F.M.; Ferreira, C.P.; Câmara, N.O.S. Interface of DNA Repair and Metabolism. Curr. Tissue Microenviron. Rep. 2020, 1, 209–220. [Google Scholar] [CrossRef]
- Moretton, A.; Loizou, J.I. Interplay between Cellular Metabolism and the DNA Damage Response in Cancer. Cancers 2020, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yin, N.; Peng, M.; Stamatiades, E.G.; Shyu, A.; Li, P.; Zhang, X.; Do, M.H.; Wang, Z.; Capistrano, K.J.; et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 2021, 371, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Patinote, A.; Com, E.; Pineau, C.; Bobe, J. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genom. 2021, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Gundel, U.; Benndorf, D.; von Bergen, M.; Altenburger, R.; Kuster, E. Vitellogenin cleavage products as indicators for toxic stress in zebra fish embryos: A proteomic approach. Proteomics 2007, 7, 4541–4554. [Google Scholar] [CrossRef]
- Sano, K.; Kawaguchi, M.; Katano, K.; Tomita, K.; Inokuchi, M.; Nagasawa, T.; Hiroi, J.; Kaneko, T.; Kitagawa, T.; Fujimoto, T.; et al. Comparison of Egg Envelope Thickness in Teleosts and its Relationship to the Sites of ZP Protein Synthesis. J. Exp. Zool. B Mol. Dev. Evol. 2017, 328, 240–258. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Z.; Xu, X.; Liu, Q.; Zhang, X.; Kong, B.; Wei, J.J.; Gong, Y.; Shao, C. Increased oxidative stress mediates the antitumor effect of PARP inhibition in ovarian cancer. Redox Biol. 2018, 17, 99–111. [Google Scholar] [CrossRef]
- Wang, X.; Tanaka, M.; Krstin, S.; Peixoto, H.S.; Moura, C.C.M.; Wink, M. Cytoskeletal interference—A new mode of action for the anticancer drugs camptothecin and topotecan. Eur. J. Pharmacol. 2016, 789, 265–274. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Dettlaff, T.A. The Russian Sturgeon Acipenser Güldenstädti. Part I. Gametes And Early Development Up To Time Of Hatching. In Animal Species for Developmental Studies: Vertebrates; Dettlaff, T.A., Vassetzky, S.G., Eds.; Springer: Boston, MA, USA, 1991; pp. 15–65. [Google Scholar]
- Park, C.; Lee, S.Y.; Kim, D.; Nam, Y. Embryonic Development of Siberian Sturgeon Acipenser baerii under Hatchery Conditions: An Image Guide with Embryological Descriptions. Fish. Aquat. Sci. 2013, 16, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Kopylova, E.; Noe, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Hübschmann, D. simplifyEnrichment: An R/Bioconductor package for Clustering and Visualizing Functional Enrichment Results. bioRxiv 2020. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [Green Version]
- Pei, D.-S.; Strauss, P.R. Zebrafish as a model system to study DNA damage and repair. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2013, 743–744, 151–159. [Google Scholar] [CrossRef]
| Parameter | Pathway | Treatment Groups | |||||
|---|---|---|---|---|---|---|---|
| CPT 2–24 | CPT 24–48 | CPT 48–72 | Ola 2–24 | Ola 24–48 | Ola 48–72 | ||
| Viability | 0% | 31% | 47.6% | 40.2% | 42% | 45.2% | |
| Hatching rate | 0% | 2% | 40.9% | 6.8% | 32.8% | 40.4% | |
| DNA fragmentation (max) | 21.3% | 14.2% | 4.6% | 9.8% | 14.1% | 5.2% | |
| RNASeq | √ | √ | √ | √ | |||
| Upregulated KEGG |
| √ | √ | √ | √ | ||
| √ | √ | √ | √ | |||
| √ | √ | √ | √ | |||
| √ | √ | √ | ||||
| √ | √ | √ | ||||
| √ | √ | √ | ||||
| √ | √ | |||||
| √ | ||||||
| √ | ||||||
| Downregulated KEGG |
| √ | √ | √ | √ | ||
| √ | √ | |||||
| Proteomics | √ | √ | √ | √ | |||
| Upregulated KEGG |
| √ | √ | √ | √ | ||
| √ | √ | |||||
| √ | √ | |||||
| Downregulated KEGG |
| √ | √ | ||||
| √ | √ | |||||
| Viability | Hatching | DNA Fragmentation | DEGs | DAPs | |
|---|---|---|---|---|---|
| Viability | 1.00 | 0.95 * | −0.73 | −0.91 * | −0.88 * |
| Hatching | 0.95 * | 1.00 | −0.62 | −0.93 * | −0.87 |
| DNA fragmentation | −0.73 | -0.62 | 1.00 | 0.73 | 0.38 |
| DEGs | −0.91 * | −0.93 * | 0.73 | 1.00 | 0.68 |
| DAPs | −0.88 * | −0.87 | 0.38 | 0.68 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazo, I.; Naraine, R.; Lebeda, I.; Tomčala, A.; Dietrich, M.; Franěk, R.; Pšenička, M.; Šindelka, R. Transcriptome and Proteome Analyses Reveal Stage-Specific DNA Damage Response in Embryos of Sturgeon (Acipenser ruthenus). Int. J. Mol. Sci. 2022, 23, 6392. https://doi.org/10.3390/ijms23126392
Gazo I, Naraine R, Lebeda I, Tomčala A, Dietrich M, Franěk R, Pšenička M, Šindelka R. Transcriptome and Proteome Analyses Reveal Stage-Specific DNA Damage Response in Embryos of Sturgeon (Acipenser ruthenus). International Journal of Molecular Sciences. 2022; 23(12):6392. https://doi.org/10.3390/ijms23126392
Chicago/Turabian StyleGazo, Ievgeniia, Ravindra Naraine, Ievgen Lebeda, Aleš Tomčala, Mariola Dietrich, Roman Franěk, Martin Pšenička, and Radek Šindelka. 2022. "Transcriptome and Proteome Analyses Reveal Stage-Specific DNA Damage Response in Embryos of Sturgeon (Acipenser ruthenus)" International Journal of Molecular Sciences 23, no. 12: 6392. https://doi.org/10.3390/ijms23126392
APA StyleGazo, I., Naraine, R., Lebeda, I., Tomčala, A., Dietrich, M., Franěk, R., Pšenička, M., & Šindelka, R. (2022). Transcriptome and Proteome Analyses Reveal Stage-Specific DNA Damage Response in Embryos of Sturgeon (Acipenser ruthenus). International Journal of Molecular Sciences, 23(12), 6392. https://doi.org/10.3390/ijms23126392