Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps?
Abstract
:1. Background
2. Results
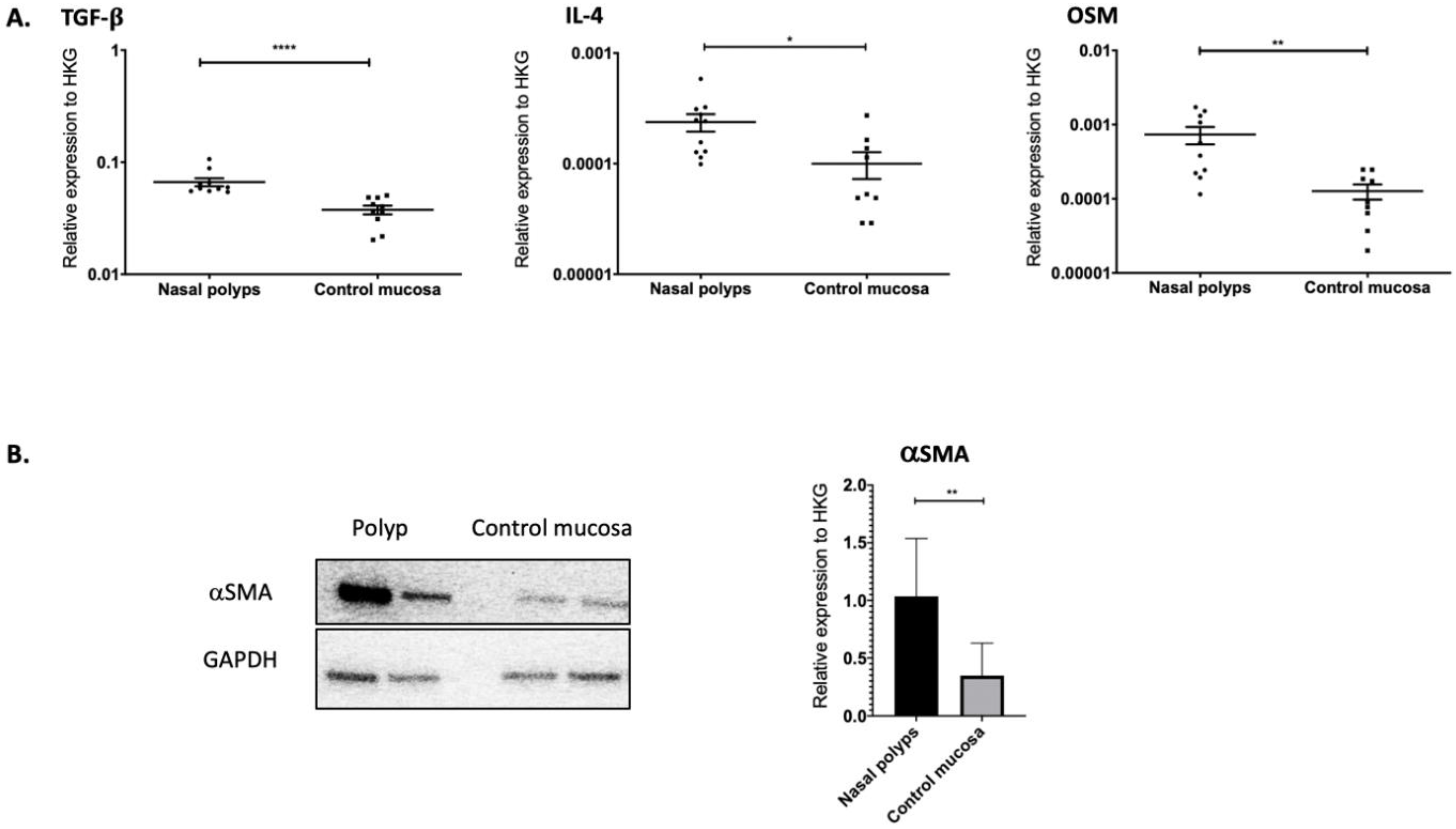
2.1. TGF-β1, IL-4 and OSM as Well as αSMA Were Overexpressed in Nasal Polyps
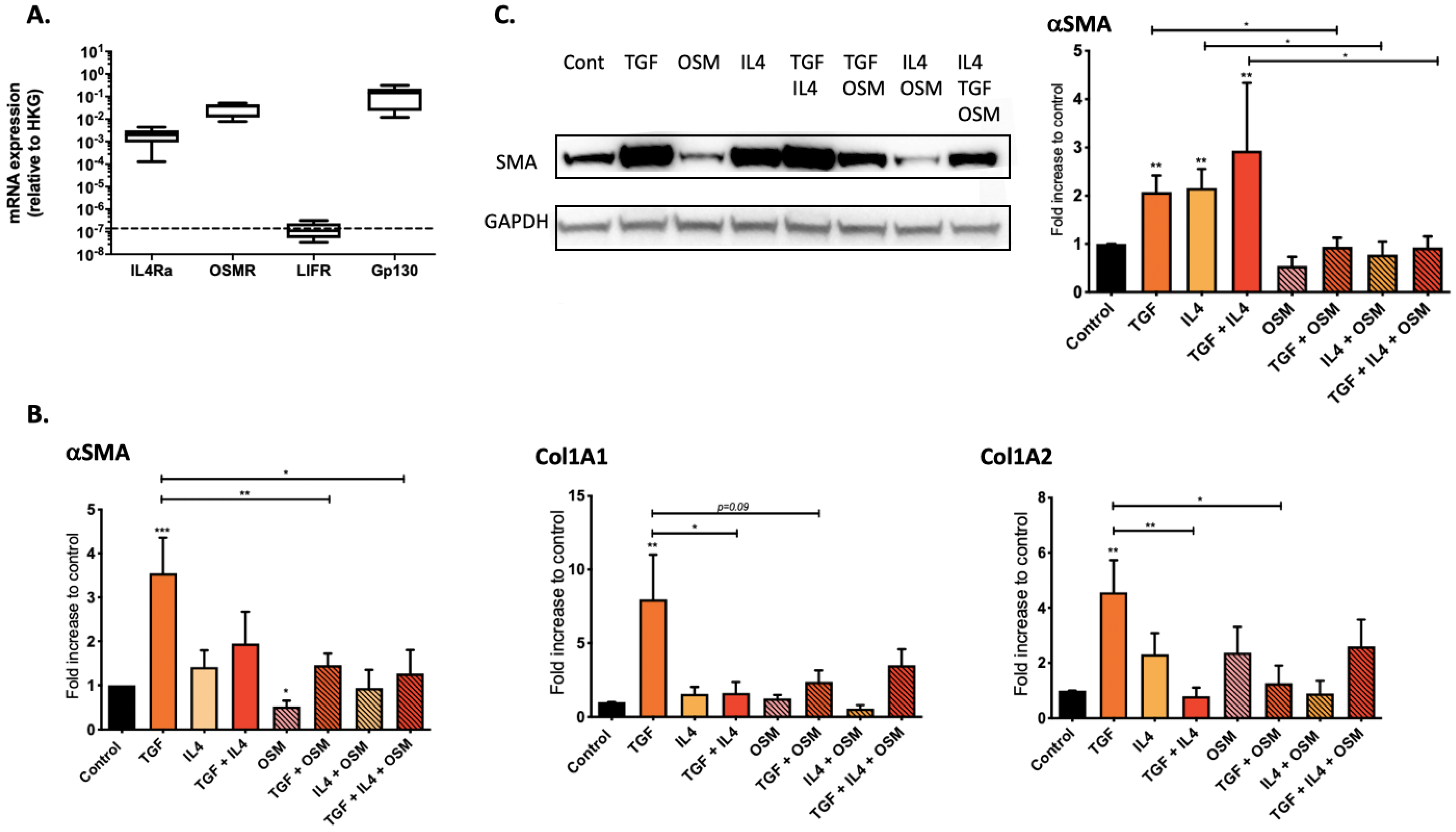
2.2. Regulation of Nasal-Polyp-Derived Fibroblasts ECM Components and αSMA by TGF-β1, IL-4 and OSM
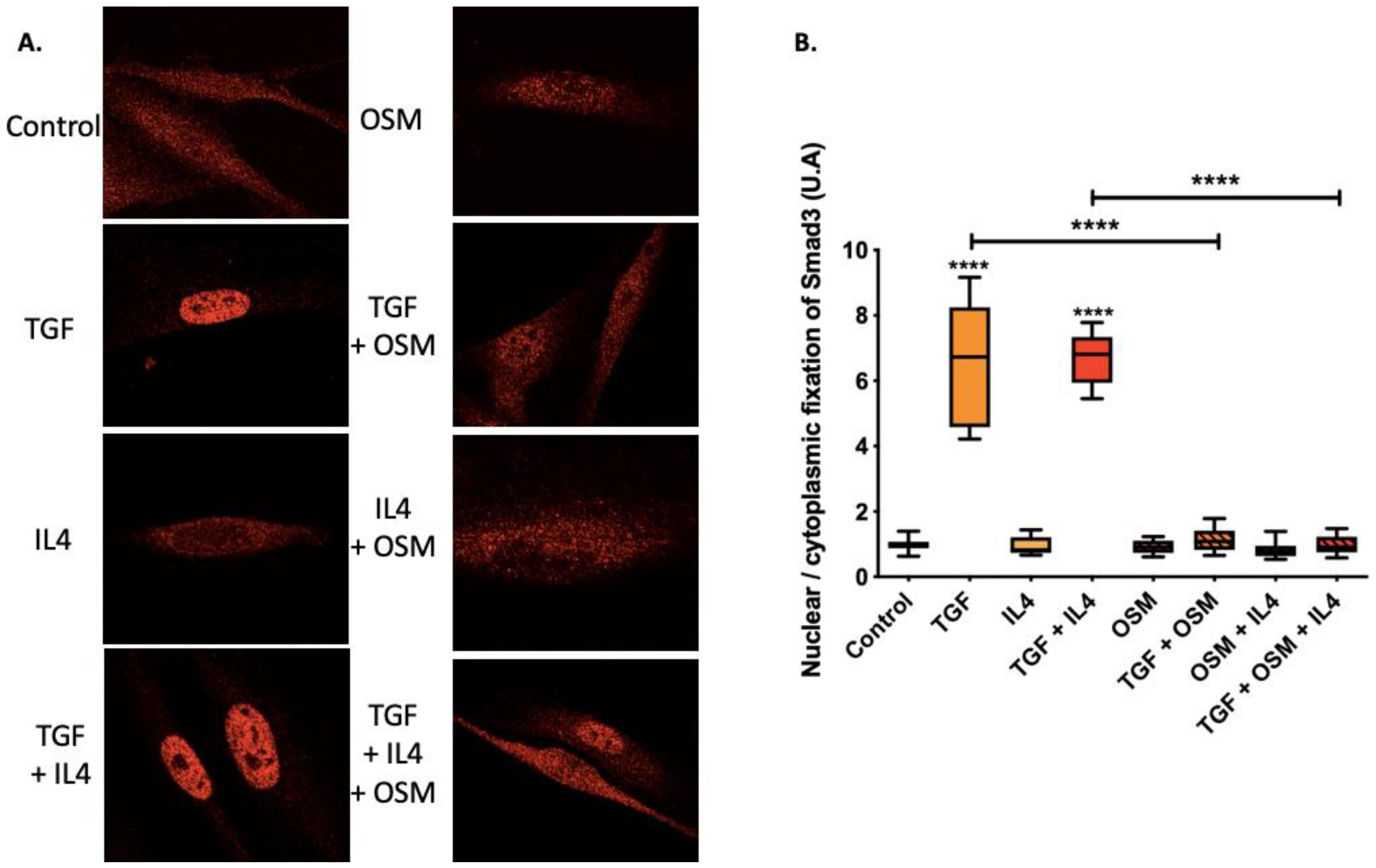
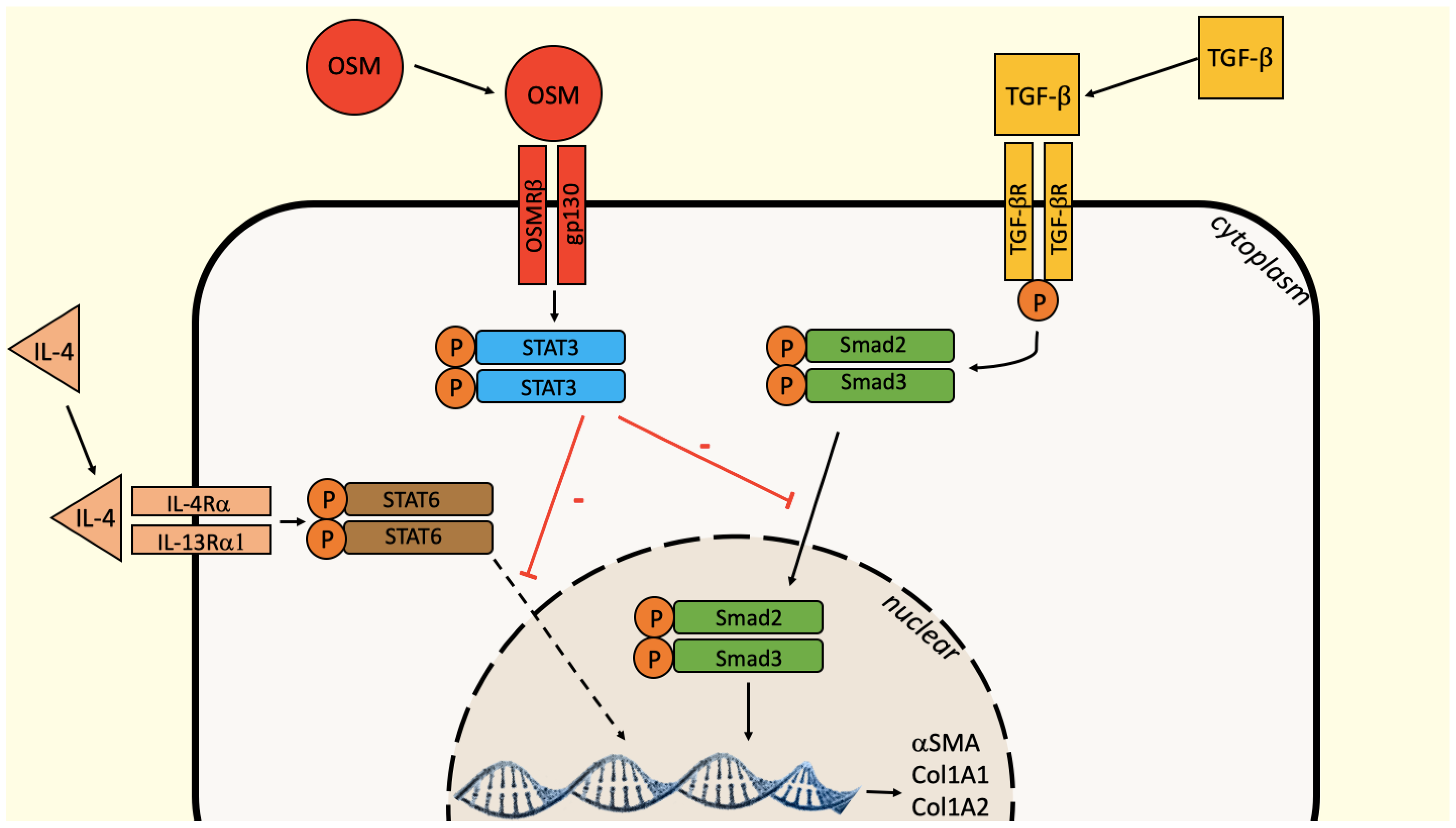
2.3. OSM Counteracted TGF-β1 Effects via the Inhibition of Smad3 Nuclear Translocation
3. Discussion
4. Materials and Methods
4.1. Prospective Clinical Study
4.2. Cell Extraction and Culture
4.3. Quantitative RT-PCR Analysis
4.4. Western Blotting
4.5. Immunofluorescence
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Nasal polyposis: An update: Editorial review. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Haruna, S.; Nakanishi, M.; Otori, N.; Moriyama, H. Histopathological features of nasal polyps with asthma association: An immunohistochemical study. Am. J. Rhinol. 2004, 18, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.P.; Escudier, E.; Roudot-Thoraval, F.; Abd-Al Samad, I.; Peynegre, R.; Coste, A. Myofibroblast accumulation induced by transforming growth factor-beta is involved in the pathogenesis of nasal polyps. Laryngoscope 1997, 107, 926–931. [Google Scholar] [CrossRef]
- Van Zele, T.; Gevaert, P.; Holtappels, G.; van Cauwenberge, P.; Bachert, C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007, 37, 1840–1847. [Google Scholar] [CrossRef]
- Steinke, J.W.; Crouse, C.D.; Bradley, D.; Hise, K.; Lynch, K.; Kountakis, S.E.; Borish, L. Characterization of Interleukin-4–Stimulated nasal polyp fibroblasts. Am. J. Respir Cell Mol. Biol. 2004, 30, 212–219. [Google Scholar] [CrossRef]
- Carsuzaa, F.; Béquignon, É.; Dufour, X.; de Bonnecaze, G.; Lecron, J.-C.; Favot, L. Cytokine signature and involvement in chronic rhinosinusitis with nasal polyps. Int. J. Mol. Sci. 2021, 23, 417. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Norton, J.E.; Hulse, K.E.; Suh, L.A.; Carter, R.G.; Rocci, E.; Harris, K.E.; Shintani-Smith, S.; Conley, D.B.; Chandra, R.K.; et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J. Allergy Clin. Immunol. 2015, 136, 737–746.e4. [Google Scholar] [CrossRef] [Green Version]
- Hanlon, M.M.; Rakovich, T.; Cunningham, C.C.; Ansboro, S.; Veale, D.J.; Fearon, U.; McGarry, T. STAT3 Mediates the Differential Effects of Oncostatin M and TNFα on RA Synovial Fibroblast and Endothelial Cell Function. Front Immunol. 2019, 10, 2056. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Sato, S.; Fujimoto, M.; Ihn, H.; Kikuchi, K.; Takehara, K. Serum levels of interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J. Rheumatol. 1998, 25, 308–313. [Google Scholar]
- Mozaffarian, A.; Brewer, A.W.; Trueblood, E.S.; Luzina, I.G.; Todd, N.W.; Atamas, S.P.; Arnett, H.A. Mechanisms of Oncostatin M-Induced Pulmonary Inflammation and Fibrosis. J. Immunol. 2008, 181, 7243–7253. [Google Scholar] [CrossRef]
- Huguier, V.; Giot, J.-P.; Simonneau, M.; Levillain, P.; Charreau, S.; Garcia, M.; Jégou, J.-F.; Bodet, C.; Morel, F.; Lecronet, J.-C.; et al. Oncostatin M exerts a protective effect against excessive scarring by counteracting the inductive effect of TGFβ1 on fibrosis markers. Sci. Rep. 2019, 9, 2113. [Google Scholar] [CrossRef] [PubMed]
- Botelho, F.; Dubey, A.; Ayaub, E.A.; Park, R.; Yip, A.; Humbles, A.; Kolbeck, R.; Richards, C.D. IL-33 Mediates Lung Inflammation by the IL-6-Type Cytokine Oncostatin, M. Mediat. Inflamm. 2020, 2020, 4087315. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Tsurusaki, S.; Miyata, N.; Saijou, E.; Okochi, H.; Miyajima, A.; Tanaka, M. Oncostatin M causes liver fibrosis by regulating cooperation between hepatic stellate cells and macrophages in mice. Hepatology 2018, 67, 296–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boniface, K.; Diveu, C.; Morel, F.; Pedretti, N.; Froger, J.; Ravon, E.; Garcia, M.; Venereau, E.; Preisser, L.; Guignouard, E.; et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J. Immunol. 2007, 178, 4615–4622. [Google Scholar] [CrossRef] [Green Version]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.X.; Lecron, J.C.; Morel, F. Skin inflammation induced by the synergistic action of IL-17A, IL-22, Oncostatin M, IL-1α, and TNF-α recapitulates some features of psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [Green Version]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.F.L.; Guillet, G.; Huguier, V.; Lecron, J.C.; et al. Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef] [Green Version]
- Pohin, M.; Guesdon, W.; Mekouo, A.A.T.; Rabeony, H.; Paris, I.; Atanassov, H.; Favot, L.; Mcheik, J.; Bernard, F.X.; Richards, C.D.; et al. Oncostatin M overexpression induces skin inflammation but is not required in the mouse model of imiquimod-induced psoriasis-like inflammation. Eur. J. Immunol. 2016, 46, 1737–1751. [Google Scholar] [CrossRef]
- Giot, J.-P.; Paris, I.; Levillain, P.; Huguier, V.; Charreau, S.; Delwail, A.; Garcia, M.; Garnier, J.; Bernard, F.X.; Dagregorio, G.; et al. Involvement of IL-1 and oncostatin M in acanthosis associated with hypertensive leg ulcer. Am. J. Pathol. 2013, 182, 806–818. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef]
- Scaffidi, A.K.; Mutsaers, S.E.; Moodley, Y.P.; McAnulty, R.J.; Laurent, G.J.; Thompson, P.J.; Knight, D.A. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br. J. Pharmacol. 2002, 136, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Sarközi, R.; Hauser, C.; Noppert, S.-J.; Kronbichler, A.; Pirklbauer, M.; Haller, V.M.; Grillari, J.; Grillari-Voglauer, R.; Mayer, G.; Schramek, H. Oncostatin M is a novel inhibitor of TGF-β1-induced matricellular protein expression. Am. J. Physiol. Renal. Physiol. 2011, 301, F1014–F1025. [Google Scholar] [CrossRef]
- Shin, J.-M.; Um, J.-Y.; Lee, S.-A.; Park, I.-H.; Lee, S.-H.; Lee, H.-M. Effect of MeCP2 on TGF- β1-induced Extracellular Matrix Production in Nasal Polyp-derived Fibroblasts. Am. J. Rhinol. Allergy 2018, 32, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Lefaucheur, J.P.; Wang, Q.P.; Lesprit, E.; Poron, F.; Peynegre, R.; Escudier, E. Expression of the transforming growth factor beta isoforms in inflammatory cells of nasal polyps. Arch. Otolaryngol.-Head Neck Surg. 1998, 124, 1361–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serpero, L.; Petecchia, L.; Sabatini, F.; Giuliani, M.; Silvestri, M.; Di Blasi, P.; Rossi, G.A. The effect of transforming growth factor (TGF)-beta1 and (TGF)-beta2 on nasal polyp fibroblast activities involved upper airway remodeling: Modulation by fluticasone propionate. Immunol. Lett. 2006, 105, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017, 25, 1118–1134.e7. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Saitoh, M.; Miyazawa, K. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim. Biophys. Sin. 2018, 50, 82–90. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, R.J.J.; Knight, D.A.; Richards, C.D.; Prêle, C.M.; Lau, H.L.; Jarnicki, A.G.; Jones, J.; Bozinovski, S.; Vlahos, R.; Thiem, S.; et al. Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Mol. Med. 2012, 4, 939–951. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsuzaa, F.; Béquignon, É.; Bainaud, M.; Jégou, J.-F.; Dufour, X.; Lecron, J.-C.; Favot, L. Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps? Int. J. Mol. Sci. 2022, 23, 6308. https://doi.org/10.3390/ijms23116308
Carsuzaa F, Béquignon É, Bainaud M, Jégou J-F, Dufour X, Lecron J-C, Favot L. Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps? International Journal of Molecular Sciences. 2022; 23(11):6308. https://doi.org/10.3390/ijms23116308
Chicago/Turabian StyleCarsuzaa, Florent, Émilie Béquignon, Matthieu Bainaud, Jean-François Jégou, Xavier Dufour, Jean-Claude Lecron, and Laure Favot. 2022. "Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps?" International Journal of Molecular Sciences 23, no. 11: 6308. https://doi.org/10.3390/ijms23116308
APA StyleCarsuzaa, F., Béquignon, É., Bainaud, M., Jégou, J.-F., Dufour, X., Lecron, J.-C., & Favot, L. (2022). Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps? International Journal of Molecular Sciences, 23(11), 6308. https://doi.org/10.3390/ijms23116308





