Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance
Abstract
1. Introduction
2. The Notch Signaling Pathway
3. Notch Signaling Dysregulation in BC
4. Notch Signaling Regulates BC Progression
4.1. The Breast TME and Disease Progression
4.2. Notch in the Breast TME
4.2.1. Notch Signaling and CAFs
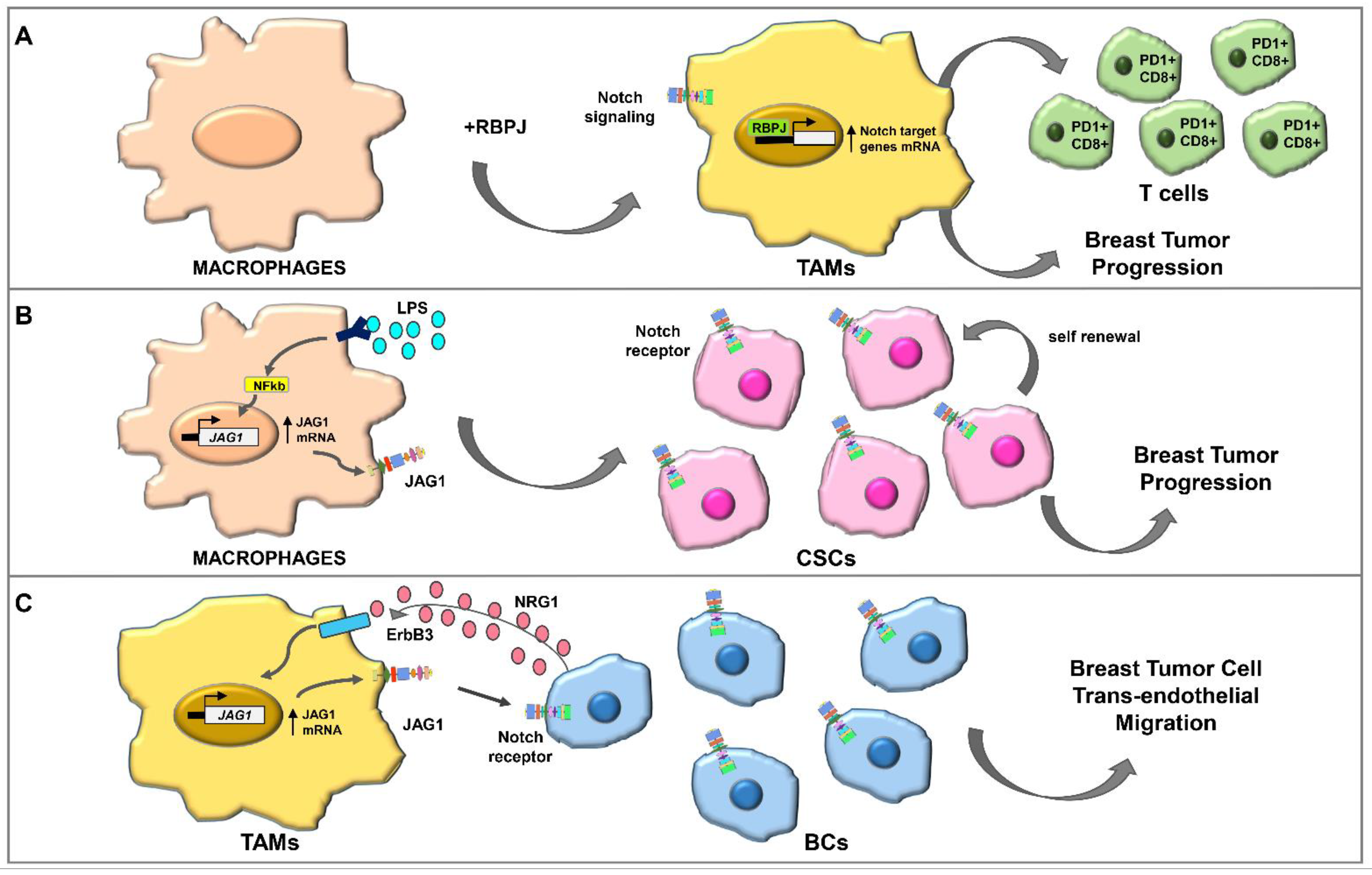
4.2.2. Notch Signaling and TAMs
4.2.3. Notch and the ECM
4.2.4. Notch Signaling and Angiogenesis
5. Notch Signaling in the Emergence of Drug Resistance Dependent on TME
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | aromatase inhibitors |
| ALDH+ | aldehyde dehydrogenase positive |
| ARG-1 | arginase-1 |
| BC | breast cancer |
| BCs | breast cancer cells |
| BCSCs | breast cancer stem cells |
| bHLH | Hes/Hey family of basic helix–loop–helix |
| CAFs | cancer-associated fibroblasts |
| CBF-1 | centromere binding factor 1 |
| CCL17 | C-C motif chemokine ligand 17 |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL22 | C-C motif chemokine ligand 22 |
| CCND1 | cyclin D1 |
| CCR2 | C-C chemokine receptor type 2 |
| CDKI | cyclin-dependent kinase inhibitor |
| ChemoR | chemokines receptors |
| CSCs | cancer stem-like cells |
| CSF-1 | colony stimulating factor-1 |
| DCIS | ductal carcinoma in situ |
| E2 | estradiol |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EMT | epithelial mesenchymal transition |
| ER | estrogen receptor |
| ER+ | estrogen receptor alpha positive |
| ER− | estrogen receptor alpha negative |
| ErbB1 | human epidermal growth factor receptor |
| ErbB2 | human epidermal growth factor receptor 2 |
| ErbB3 | human epidermal growth factor receptor 3 |
| ERα | estrogen receptor alpha |
| GFRs | growth factor receptors |
| GFs | growth factors |
| GPER | G protein-coupled estrogen receptor 1 |
| GSIs | γ-secretase inhibitors |
| GSK3-β | glycogen synthase kinase 3 beta |
| HER2 | human epidermal growth factor receptor 2 |
| HER2+ | human epidermal growth factor receptor 2 positive |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| HIF-2α | hypoxia-inducible factor 2 alpha |
| IL-10 | interleukin-10 |
| IL-1β | interleukin-1 beta |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| IRF8 | interferon regulatory factor 8 |
| JAG | membrane-bound ligands Jagged |
| LN | lymph nodes |
| LPS | lipopolysaccharide |
| MAML | mastermind-like |
| MAPK | mitogen-activated protein kinase |
| MMP-2 | matrix metalloproteinase-2 |
| MMP-9 | matrix metalloproteinase-9 |
| MMPs | matrix metalloproteinases |
| MMTV | mouse mammary tumor virus |
| MSCs | mesenchymal cells |
| NF-κB | nuclear factor kappa B |
| NECD | Notch extracellular domain |
| NICD | Notch intracellular domain |
| NRG1 | neuregulin 1 |
| Oct-4 | octamer binding transcription factor 4 |
| PA | plasminogen activator |
| PD1+ | programmed death 1 positive |
| PR | progesterone receptor |
| PR+ | progesterone receptor positive |
| PTEN | phosphatase and tensin homolog |
| RBPJ | recombination signal binding protein for immunoglobulin kappa J region |
| SDF-1 | stromal cell-derived factor-1 |
| STAT1 | signal transducer and activator of transcription 1 |
| TAMs | tumor-associated macrophages |
| TGF-β | transforming growth factor beta |
| TILs | tumor-infiltrating lymphocytes |
| TME | tumor microenvironment |
| TNBCs | triple negative breast cancers |
| TNF-α | tumor necrosis factor alpha |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial growth factor |
| VEGFR3 | VEGF receptor 3 |
| VEGFRs | vascular endothelial growth factor receptors |
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Guido, C.; Santoro, M.; Giordano, F.; Donà, A.; Rizza, P.; Pellegrino, M.; Perrotta, I.; Bonofiglio, D.; Sisci, D.; et al. Ligand activated progesterone receptor B drives autophagy-senescence transition through a Beclin-1/Bcl-2 dependent mechanism in human breast cancer cells. Oncotarget 2016, 7, 57955–57969. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Amicis, F.; Guido, C.; Santoro, M.; Lanzino, M.; Panza, S.; Avena, P.; Panno, M.L.; Perrotta, I.; Aquila, S.; Andò, S. A novel functional interplay between Progesterone Receptor-B and PTEN, via AKT, modulates autophagy in breast cancer cells. J. Cell. Mol. Med. 2014, 18, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.M.; Nagarajan, S.; Cosulich, S.; Carroll, J.S. Genome-Wide Estrogen Receptor Activity in Breast Cancer. Endocrinology 2021, 162, bqaa224. [Google Scholar] [CrossRef]
- Shin, I. HER2 Signaling in Breast Cancer. Adv. Exp. Med. Biol. 2021, 1187, 53–79. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef]
- Schmidt, M.; Böhm, D.; von Törne, C.; Steiner, E.; Puhl, A.; Pilch, H.; Lehr, H.A.; Hengstler, J.G.; Kölbl, H.; Gehrmann, M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008, 68, 5405–5413. [Google Scholar] [CrossRef]
- Schmidt, M.; Hengstler, J.G.; von Törne, C.; Koelbl, H.; Gehrmann, M.C. Coordinates in the universe of node-negative breast cancer revisited. Cancer Res. 2009, 69, 2695–2698. [Google Scholar] [CrossRef]
- Callari, M.; Cappelletti, V.; D’Aiuto, F.; Musella, V.; Lembo, A.; Petel, F.; Karn, T.; Iwamoto, T.; Provero, P.; Daidone, M.G.; et al. Subtype-Specific Metagene-Based Prediction of Outcome after Neoadjuvant and Adjuvant Treatment in Breast Cancer. Clin. Cancer Res. 2016, 22, 337–345. [Google Scholar] [CrossRef]
- Jubair, S.; Alkhateeb, A.; Tabl, A.A.; Rueda, L.; Ngom, A. A novel approach to identify subtype-specific network biomarkers of breast cancer survivability. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 43. [Google Scholar] [CrossRef]
- Kwon, M.J. Emerging immune gene signatures as prognostic or predictive biomarkers in breast cancer. Arch. Pharmacal Res. 2019, 42, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Chakrabarti, R. The many facets of Notch signaling in breast cancer: Toward overcoming therapeutic resistance. Genes Dev. 2020, 34, 1422–1438. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.V.; Giuliani, E.; Screpanti, I.; Bellavia, D.; Checquolo, S. Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype. J. Oncol. 2019, 2019, 8707053. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Crabtree, J.S.; Golde, T.E.; Minter, L.M.; Osborne, B.A.; Miele, L. Targeting Notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021, 20, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Orzechowska, M.; Anusewicz, D.; Bednarek, A.K. Functional Gene Expression Differentiation of the Notch Signaling Pathway in Female Reproductive Tract Tissues—A Comprehensive Review With Analysis. Front. Cell Dev. Biol. 2020, 8, 592616. [Google Scholar] [CrossRef]
- Montalto, F.I.; Giordano, F.; Chiodo, C.; Marsico, S.; Mauro, L.; Sisci, D.; Aquila, S.; Lanzino, M.; Panno, M.L.; Andò, S.; et al. Progesterone Receptor B signaling Reduces Breast Cancer Cell Aggressiveness: Role of Cyclin-D1/Cdk4 Mediating Paxillin Phosphorylation. Cancers 2019, 11, 1201. [Google Scholar] [CrossRef]
- Sterneck, E.; Poria, D.K.; Balamurugan, K. Slug and E-Cadherin: Stealth Accomplices? Front. Mol. Biosci. 2020, 7, 138. [Google Scholar] [CrossRef]
- Sprinzak, D.; Blacklow, S.C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021, 50, 157–189. [Google Scholar] [CrossRef] [PubMed]
- Kashikar, N.D.; Zhang, W.; Massion, P.P.; Gonzalez, A.L.; Datta, P.K. Role of STRAP in regulating GSK3β function and Notch3 stabilization. Cell Cycle 2011, 10, 1639–1654. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Rizzo, P.; Osipo, C.; Pannuti, A.; Wyatt, D.; Cheung, L.W.; Sonenshein, G.; Osborne, B.A.; Miele, L. Notch-1 activates estrogen receptor-α-dependent transcription via IKKα in breast cancer cells. Oncogene 2010, 29, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Kim, H.; Ki, H.; Yang, I.; Yang, N.; Lee, K.Y.; Kim, N.; Park, H.S.; Kim, K. Beta-catenin modulates the level and transcriptional activity of Notch1/NICD through its direct interaction. Biochim. Biophys. Acta 2009, 1793, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Blokzijl, A.; Dahlqvist, C.; Reissmann, E.; Falk, A.; Moliner, A.; Lendahl, U.; Ibáñez, C.F. Cross-talk between the Notch and TGF-β signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 2003, 163, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Tiemeijer, L.A.; Sanlidag, S.; Bouten, C.V.C.; Sahlgren, C.M. Engineering tissue morphogenesis: Taking it up a Notch. Trends Biotechnol. 2022; in press. [Google Scholar] [CrossRef]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 245–275. [Google Scholar] [CrossRef]
- Misiorek, J.O.; Przybyszewska-Podstawka, A.; Kałafut, J.; Paziewska, B.; Rolle, K.; Rivero-Müller, A.; Nees, M. Context Matters: NOTCH Signatures and Pathway in Cancer Progression and Metastasis. Cells 2021, 10, 94. [Google Scholar] [CrossRef]
- Ellisen, L.W.; Bird, J.; West, D.C.; Soreng, A.L.; Reynolds, T.C.; Smith, S.D.; Sklar, J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991, 66, 649–661. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, A.K.; Damodaran, C. A Review on Notch Signaling and Colorectal Cancer. Cells 2020, 9, 1549. [Google Scholar] [CrossRef]
- Bazzoni, R.; Bentivegna, A. Role of Notch Signaling Pathway in Glioblastoma Pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Mollen, E.W.J.; Ient, J.; Tjan-Heijnen, V.C.G.; Boersma, L.J.; Miele, L.; Smidt, M.L.; Vooijs, M. Moving Breast Cancer Therapy up a Notch. Front. Oncol. 2018, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Tamagnone, L.; Zacchigna, S.; Rehman, M. Taming the Notch Transcriptional Regulator for Cancer Therapy. Molecules 2018, 23, 431. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Precision medicine for human cancers with Notch signaling dysregulation (Review). Int. J. Mol. Med. 2020, 45, 279–297. [Google Scholar] [CrossRef]
- Reedijk, M.; Odorcic, S.; Chang, L.; Zhang, H.; Miller, N.; McCready, D.R.; Lockwood, G.; Egan, S.E. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005, 65, 8530–8537. [Google Scholar] [CrossRef]
- Gallahan, D.; Callahan, R. Mammary tumorigenesis in feral mice: Identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J. Virol. 1987, 61, 66–74. [Google Scholar] [CrossRef]
- Callahan, R.; Smith, G.H. Common integration sites for MMTV in viral induced mouse mammary tumors. J. Mammary Gland. Biol. Neoplasia 2008, 13, 309–321. [Google Scholar] [CrossRef]
- Hu, C.; Dievart, A.; Lupien, M.; Calvo, E.; Tremblay, G.; Jolicoeur, P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am. J. Pathol. 2006, 168, 973–990. [Google Scholar] [CrossRef]
- Raafat, A.; Bargo, S.; Anver, M.R.; Callahan, R. Mammary development and tumorigenesis in mice expressing a truncated human Notch4/Int3 intracellular domain (h-Int3sh). Oncogene 2004, 23, 9401–9407. [Google Scholar] [CrossRef][Green Version]
- Stylianou, S.; Clarke, R.B.; Brennan, K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006, 66, 1517–1525. [Google Scholar] [CrossRef]
- Nagamatsu, I.; Onishi, H.; Matsushita, S.; Kubo, M.; Kai, M.; Imaizumi, A.; Nakano, K.; Hattori, M.; Oda, Y.; Tanaka, M.; et al. NOTCH4 is a potential therapeutic target for triple-negative breast cancer. Anticancer. Res. 2014, 34, 69–80. [Google Scholar] [PubMed]
- Rizzo, P.; Miao, H.; D′Souza, G.; Osipo, C.; Yun, J.; Zhao, H.; Mascarenhas, J.; Wyatt, D.; Antico, G.; Hao, L.; et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008, 68, 5226–5235. [Google Scholar] [CrossRef] [PubMed]
- Farnie, G.; Willan, P.M.; Clarke, R.B.; Bundred, N.J. Combined inhibition of ErbB1/2 and Notch receptors effectively targets breast ductal carcinoma in situ (DCIS) stem/progenitor cell activity regardless of ErbB2 status. PLoS ONE 2013, 8, e56840. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, A.; Balaji, S.A.; Gowda, M.C.; Dighe, R.R.; Kumar, R.V.; Rangarajan, A. Coordinate hyperactivation of Notch1 and Ras/MAPK pathways correlates with poor patient survival: Novel therapeutic strategy for aggressive breast cancers. Mol. Cancer Ther. 2014, 13, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Niessen, K.; Kulic, I.; Raouf, A.; Eaves, C.; Pollet, I.; Karsan, A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J. Exp. Med. 2007, 204, 2935–2948. [Google Scholar] [CrossRef]
- Sales-Dias, J.; Silva, G.; Lamy, M.; Ferreira, A.; Barbas, A. The Notch ligand DLL1 exerts carcinogenic features in human breast cancer cells. PLoS ONE 2019, 14, e0217002. [Google Scholar] [CrossRef]
- Chen, C.F.; Dou, X.W.; Liang, Y.K.; Lin, H.Y.; Bai, J.W.; Zhang, X.X.; Wei, X.L.; Li, Y.C.; Zhang, G.J. Notch3 overexpression causes arrest of cell cycle progression by inducing Cdh1 expression in human breast cancer cells. Cell Cycle 2016, 15, 432–440. [Google Scholar] [CrossRef]
- Pece, S.; Serresi, M.; Santolini, E.; Capra, M.; Hulleman, E.; Galimberti, V.; Zurrida, S.; Maisonneuve, P.; Viale, G.; Di Fiore, P.P. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J. Cell Biol. 2004, 167, 215–221. [Google Scholar] [CrossRef]
- Parr, C.; Watkins, G.; Jiang, W.G. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int. J. Mol. Med. 2004, 14, 779–786. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Maggiolini, M.; Musti, A.M. Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 2018, 19, 2011. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Gonzalez-Perez, R.R. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim. Biophys. Acta 2011, 1815, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Margolis, B.; Skolnik, E.Y. Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol. 1994, 5, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Davis, N.M.; Abrams, S.L.; Montalto, G.; Cervello, M.; Libra, M.; Nicoletti, F.; D’Assoro, A.B.; Cocco, L.; Martelli, A.M.; et al. Targeting breast cancer initiating cells: Advances in breast cancer research and therapy. Adv. Biol. Regul. 2014, 56, 81–107. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.V.; Mancusi, A.; Giuliani, E.; Screpanti, I.; Checquolo, S. Notch signaling in female cancers: A multifaceted node to overcome drug resistance. Cancer Drug Resist. 2021, 4, 805–836. [Google Scholar] [CrossRef] [PubMed]
- BeLow, M.; Osipo, C. Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells 2020, 9, 2204. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352. [Google Scholar] [CrossRef]
- Kontomanolis, E.; Panteliadou, M.; Giatromanolaki, A.; Pouliliou, S.; Efremidou, E.; Limberis, V.; Galazios, G.; Sivridis, E.; Koukourakis, M.I. Delta-like ligand 4 (DLL4) in the plasma and neoplastic tissues from breast cancer patients: Correlation with metastasis. Med. Oncol. 2014, 31, 945. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Alkasalias, T.; Moyano-Galceran, L.; Arsenian-Henriksson, M.; Lehti, K. Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int. J. Mol. Sci. 2018, 19, 1532. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Beningo, K.A. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers 2019, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Tian, W.Y.; Wang, Y.M.; Zhang, Y.F.; Guo, F.; Zhao, J.; Gao, C.; Xue, F.X. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J. Hematol. Oncol. 2016, 9, 8. [Google Scholar] [CrossRef]
- Colombo, M.; Mirandola, L.; Chiriva-Internati, M.; Basile, A.; Locati, M.; Lesma, E.; Chiaramonte, R.; Platonova, N. Cancer Cells Exploit Notch Signaling to Redefine a Supportive Cytokine Milieu. Front. Immunol. 2018, 9, 1823. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.P.; Lee, J.H.; Gao, M.-Q.; Gil Kim, B.; Kang, S.; Kim, S.H.; Cho, N.H. Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. Int. J. Cancer 2014, 135, 2024–2033. [Google Scholar] [CrossRef]
- Ueno, T.; Utsumi, J.; Toi, M.; Shimizu, K. Characteristic Gene Expression Profiles of Human Fibroblasts and Breast Cancer Cells in a Newly Developed Bilateral Coculture System. BioMed Res. Int. 2015, 2015, 960840. [Google Scholar] [CrossRef]
- Singer, C.F.; Gschwantler-Kaulich, D.; Fink-Retter, A.; Haas, C.; Hudelist, G.; Czerwenka, K.; Kubista, E. Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Res. Treat. 2008, 110, 273–281. [Google Scholar] [CrossRef]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Du, H.; Che, G. Genetic alterations and epigenetic alterations of cancer-associated fibroblasts. Oncol. Lett. 2017, 13, 3–12. [Google Scholar] [CrossRef]
- Fiori, M.E.; Di Franco, S.; Villanova, L.; Bianca, P.; Stassi, G.; De Maria, R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zhang, M.; Zhen, L.; Pang, D.; Zhang, Q.; Li, Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS ONE 2013, 8, e76147. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Campbell, M.J.; Baehner, F.; O’Meara, T.; Ojukwu, E.; Han, B.; Mukhtar, R.; Tandon, V.; Endicott, M.; Zhu, Z.; Wong, J.; et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 2017, 161, 17–28. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Ye, X.; Meeker, A.; Argani, P.; Emens, L.A. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: A pilot study. Hum. Pathol. 2013, 44, 2055–2063. [Google Scholar] [CrossRef]
- Pupo, M.; Pisano, A.; Abonante, S.; Maggiolini, M.; Musti, A.M. GPER activates Notch signaling in breast cancer cells and cancer-associated fibroblasts (CAFs). Int. J. Biochem. Cell Biol. 2014, 46, 56–67. [Google Scholar] [CrossRef]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef]
- Tsuyada, A.; Chow, A.; Wu, J.; Somlo, G.; Chu, P.; Loera, S.; Luu, T.; Li, A.X.; Wu, X.; Ye, W.; et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012, 72, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, A.W.; Storci, G.; Werbeck, J.L.; Sansone, P.; Sasser, A.K.; Tavolari, S.; Huang, T.; Chan, M.W.; Marini, F.C.; Rosol, T.J.; et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6—Dependent manner. Cancer Res. 2008, 68, 9087–9095. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Morein, D.; Rubinstein-Achiasaf, L.; Sprinzak, D.; Wiemann, S.; Körner, C.; Ehrlich, M.; Ben-Baruch, A. Notch-Mediated Tumor-Stroma-Inflammation Networks Promote Invasive Properties and CXCL8 Expression in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 804. [Google Scholar] [CrossRef]
- Wang, Y.C.; He, F.; Feng, F.; Liu, X.W.; Dong, G.Y.; Qin, H.Y.; Hu, X.B.; Zheng, M.H.; Liang, L.; Feng, L.; et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010, 70, 4840–4849. [Google Scholar] [CrossRef]
- Palaga, T.; Wongchana, W.; Kueanjinda, P. Notch Signaling in Macrophages in the Context of Cancer Immunity. Front. Immunol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhang, M.; Xuan, Q.; Wang, Z.; Lian, X.; Zhang, Q. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res. Treat. 2017, 166, 95–107. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef]
- Wang, H.; Zang, C.; Taing, L.; Arnett, K.L.; Wong, Y.J.; Pear, W.S.; Blacklow, S.C.; Liu, X.S.; Aster, J.C. NOTCH1–RBPJ complexes drive target gene expression through dynamic interactions with superenhancers. Proc. Natl. Acad. Sci. USA 2014, 111, 705–710. [Google Scholar] [CrossRef]
- Yamamoto, M.; Taguchi, Y.; Ito-Kureha, T.; Semba, K.; Yamaguchi, N.; Inoue, J. NF-κB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat. Commun. 2013, 4, 2299. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Mao, S.P.H.; Surve, C.R.; Condeelis, J.S.; Segall, J.E. A novel neuregulin—Jagged1 paracrine loop in breast cancer transendothelial migration. Breast Cancer Res. 2018, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, J.; Smith, S.; Foldi, J.; Zhao, B.; Chung, A.Y.; Outtz, H.; Kitajewski, J.; Shi, C.; Weber, S.; et al. Notch–RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 2012, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Liubomirski, Y.; Ben-Baruch, A. Notch-Inflammation Networks in Regulation of Breast Cancer Progression. Cells 2020, 9, 1576. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Cohen, B.; Zheng, W.; Rahbar, R.; Martin, B.; Murakami, K.; Lamorte, S.; Thompson, P.; Berman, H.; Zúñiga-Pflücker, J.C.; et al. Notch Shapes the Innate Immunophenotype in Breast Cancer. Cancer Discov 2017, 7, 1320–1335. [Google Scholar] [CrossRef]
- Xu, J.; Chi, F.; Guo, T.; Punj, V.; Lee, W.N.; French, S.W.; Tsukamoto, H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J. Clin. Investig. 2015, 125, 1579–1590. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, P.; Chen, Y.; Zheng, S.; Zheng, L.; Weng, Z. Inhibition of Notch Signaling Attenuates Schistosomiasis Hepatic Fibrosis via Blocking Macrophage M2 Polarization. PLoS ONE 2016, 11, e0166808. [Google Scholar] [CrossRef]
- Foldi, J.; Chung, A.Y.; Xu, H.; Zhu, J.; Outtz, H.H.; Kitajewski, J.; Li, Y.; Hu, X.; Ivashkiv, L.B. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J—Dependent induction of Jagged1. J. Immunol. 2010, 185, 5023–5031. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Rahman, F.A.; Krause, M.P. PAI-1, the Plasminogen System, and Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 7066. [Google Scholar] [CrossRef]
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868. [Google Scholar] [CrossRef]
- Heissig, B.; Salama, Y.; Osada, T.; Okumura, K.; Hattori, K. The Multifaceted Role of Plasminogen in Cancer. Int. J. Mol. Sci. 2021, 22, 2304. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, F.; Lu, J.; Li, T.; Yang, H.; Wu, C.; Liu, Y. Notch-1 signaling promotes the malignant features of human breast cancer through NF-κB activation. PLoS ONE 2014, 9, e95912. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Cohen, B.; Goldvasser, P.; Berman, H.; Virtanen, C.; Reedijk, M. Plasminogen activator uPA is a direct transcriptional target of the JAG1-Notch receptor signaling pathway in breast cancer. Cancer Res. 2011, 71, 277–286. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, W.; Zhou, Q.; Zhang, Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int. J. Mol. Med. 2018, 42, 3395–3403. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Lepucki, A.; Orlińska, K.; Mielczarek-Palacz, A.; Kabut, J.; Olczyk, P.; Komosińska-Vassev, K. The Role of Extracellular Matrix Proteins in Breast Cancer. J. Clin. Med. 2022, 11, 1250. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef]
- Oskarsson, T.; Massagué, J. Extracellular matrix players in metastatic niches. EMBO J. 2012, 31, 254–256. [Google Scholar] [CrossRef]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Cione, E.; Vincenza, D.; Donà, A.; Panno, M.L.; Aquila, S. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol. Rep. 2016, 35, 568–576. [Google Scholar] [CrossRef]
- Iessi, E.; Vona, R.; Cittadini, C.; Matarrese, P. Targeting the Interplay between Cancer Metabolic Reprogramming and Cell Death Pathways as a Viable Therapeutic Path. Biomedicines 2021, 9, 1942. [Google Scholar] [CrossRef]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The Tumor Suppressor PTEN as Molecular Switch Node Regulating Cell Metabolism and Autophagy: Implications in Immune System and Tumor Microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, C.; Zhang, Z.; Wang, Q.; Wei, H.; Shi, W.; Li, J.; Wang, Z.; Ou, Y.; Wang, W.; et al. Jagged1-Notch1-deployed tumor perivascular niche promotes breast cancer stem cell phenotype through Zeb1. Nat. Commun. 2020, 11, 5129. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.J.; Iruela-Arispe, M.L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822. [Google Scholar] [CrossRef] [PubMed]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting signalling pathways in tumour angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Boareto, M.; Jolly, M.K.; Ben-Jacob, E.; Onuchic, J.N. Jagged mediates differences in normal and tumor angiogenesis by affecting tip-stalk fate decision. Proc. Natl. Acad. Sci. USA 2015, 112, E3836–E3844. [Google Scholar] [CrossRef]
- Benedito, R.; Roca, C.; Sörensen, I.; Adams, S.; Gossler, A.; Fruttiger, M.; Adams, R.H. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009, 137, 1124–1135. [Google Scholar] [CrossRef]
- Shawber, C.J.; Funahashi, Y.; Francisco, E.; Vorontchikhina, M.; Kitamura, Y.; Stowell, S.A.; Borisenko, V.; Feirt, N.; Podgrabinska, S.; Shiraishi, K.; et al. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J. Clin. Investig. 2007, 117, 3369–3382. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Paulus, P.; Stanley, E.R.; Schäfer, R.; Abraham, D.; Aharinejad, S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006, 66, 4349–4356. [Google Scholar] [CrossRef]
- Sansone, P.; Ceccarelli, C.; Berishaj, M.; Chang, Q.; Rajasekhar, V.K.; Perna, F.; Bowman, R.L.; Vidone, M.; Daly, L.; Nnoli, J.; et al. Self-renewal of CD133hi cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat. Commun. 2016, 7, 10442. [Google Scholar] [CrossRef]
- Gelsomino, L.; Panza, S.; Giordano, C.; Barone, I.; Gu, G.; Spina, E.; Catalano, S.; Fuqua, S.; Andò, S. Mutations in the estrogen receptor alpha hormone binding domain promote stem cell phenotype through notch activation in breast cancer cell lines. Cancer Lett. 2018, 428, 12–20. [Google Scholar] [CrossRef]
- Simões, B.M.; O’Brien, C.S.; Eyre, R.; Silva, A.; Yu, L.; Sarmiento-Castro, A.; Alférez, D.G.; Spence, K.; Santiago-Gómez, A.; Chemi, F.; et al. Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4-Dependent Cancer Stem Cell Activity. Cell Rep. 2015, 12, 1968–1977. [Google Scholar] [CrossRef]
- Shah, D.; Wyatt, D.; Baker, A.T.; Simms, P.; Peiffer, D.S.; Fernandez, M.; Rakha, E.; Green, A.; Filipovic, A.; Miele, L.; et al. Inhibition of HER2 Increases JAGGED1-dependent Breast Cancer Stem Cells: Role for Membrane JAGGED1. Clin. Cancer Res. 2018, 24, 4566–4578. [Google Scholar] [CrossRef]
- Baker, A.; Wyatt, D.; Bocchetta, M.; Li, J.; Filipovic, A.; Green, A.; Peiffer, D.S.; Fuqua, S.; Miele, L.; Albain, K.S.; et al. Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast cancer cell proliferation and stem cell survival. Oncogene 2018, 37, 4489–4504. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Han, L.; Zhao, L.; Chen, J.; Olopade, O.I.; He, M.; Wei, M. HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J. Exp. Clin. Cancer Res. 2018, 37, 256. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, E.; Weigert, A. Breast Cancer CAFs: Spectrum of Phenotypes and Promising Targeting Avenues. Int. J. Mol. Sci 2021, 22, 11636. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, M.; Yang, L.; Tu, G.; Zhu, Q.; Chen, M.; Cheng, H.; Luo, H.; Fu, W.; Li, Z.; et al. Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: A new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and β1-integrin signaling pathway in tumor cells. Breast Cancer Res. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Zhang, J.G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2020, 11, 599965. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A.; Mayne, C.G.; Katzenellenbogen, B.S.; Greene, G.L.; Chandarlapaty, S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nat. Rev. Cancer 2018, 18, 377–388. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastav, R.K.; Wilkes, D.W.; Ross, T.; Kim, S.; Kowalski, J.; Chatla, S.; Zhang, Q.; Nayak, A.; Guha, M.; et al. Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer. Oncogene 2019, 38, 2092–2107. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Mahalingaiah, P.K.S.; Singh, K.P. Epigenetic reprogramming and potential application of epigenetic-modifying drugs in acquired chemotherapeutic resistance. Adv. Clin. Chem. 2020, 94, 219–259. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Sun, Q.; Zhang, Y.J.; Wang, G.M.; He, B.; Qi, T.; Zhou, Y.; Li, X.W.; Li, S.; He, L. Targeted inhibition of Notch1 gene enhances the killing effects of paclitaxel on triple negative breast cancer cells. Asian Pac. J. Trop. Med. 2017, 10, 179–183. [Google Scholar] [CrossRef]
- Zheng, H.; Bae, Y.; Kasimir-Bauer, S.; Tang, R.; Chen, J.; Ren, G.; Yuan, M.; Esposito, M.; Li, W.; Wei, Y.; et al. Therapeutic Antibody Targeting Tumor- and Osteoblastic Niche-Derived Jagged1 Sensitizes Bone Metastasis to Chemotherapy. Cancer Cell 2017, 32, 731–747.e736. [Google Scholar] [CrossRef]
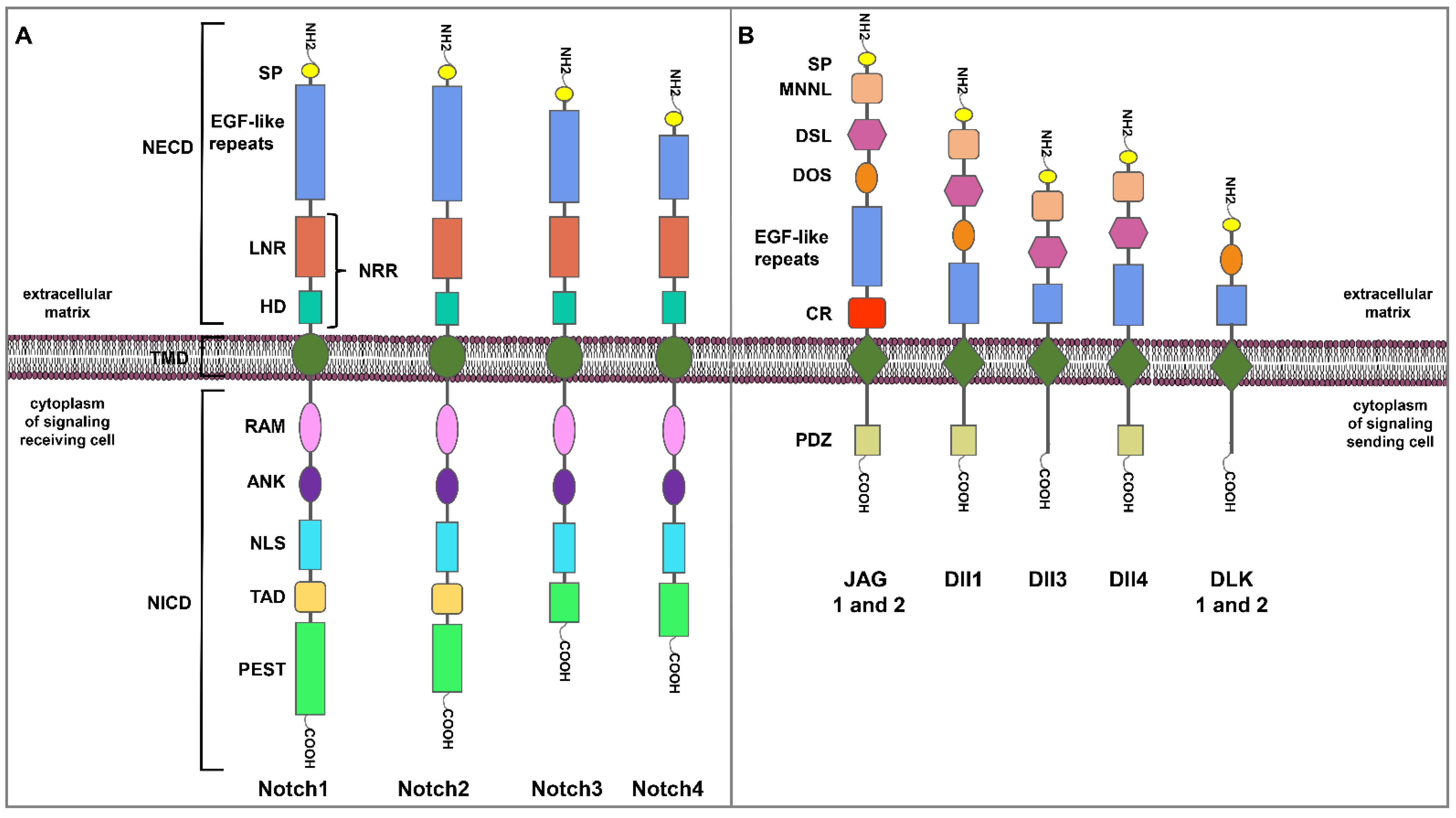

| Notch Receptors or Ligands | Experimental Models | Evidences | References |
|---|---|---|---|
| Notch1 ↑ Notch3 ↑ | MMTV/Notch1 transgenic mice MMTV/Notch3 transgenic mice | Mammary gland tumor formation | [39] |
| Notch1 ↑ | Normal and tumorigenic human mammary epithelial cell lines | Breast epithelial cells transformation Cell proliferation ↑ Apoptosis suppression | [41] |
| Notch4 ↑ | MDA-MB-231 cells BC xenograft models | Cell proliferation and invasiveness ↑ Apoptosis suppression Xenografts tumor growth ↑ | [42] |
| Notch1 Notch4 | ERα+ and ERα- BC cells ERα- BC xenograft models | E2/ ERα inhibition of Notch ERα- BC cells proliferation ↑ Xenografts tumor growth ↑ | [43] |
| Notch1 | ErbB2 normal and ErbB2 overexpressing human DCIS cell lines BC patient samples | Notch and ErbB1/2 crosstalk regulates DCIS acini size and mammosphere formation | [44] |
| Notch1 ↑ | ERα+ and ERα- BC cells BC xenograft models BC patient samples | Notch1 and Ras/MAPK crosstalk regulates BCs spheres formation and xenografts tumor growth | [45] |
| JAG1/Notch1 ↑ | MDA-MB-231 cells BC xenograft models | EMT Anoikis inhibition Xenografts tumor growth and metastasis ↑ | [46] |
| Dll1 ↑ | MCF-7, BT474 cells | Proliferation, migration, and invasion ↑ Apoptosis suppression | [47] |
| Drugs | Targeted Notch Receptors or Ligands | Experimental Models | Evidences | References |
|---|---|---|---|---|
| Anastrozole, Letrozole, Exemestane | JAG1 | AI resistant BC cells AI resistant BC patient samples | M2 TAM proportion ↑ | [88] |
| Tamoxifen, Fulvestrant | IL6/Notch3 signaling activation | Hormonal therapy resistant cells In vivo xenograft BC models | CD133 high/ ER low/IL6 high CSCs self-renewal ↑ | [127] |
| Tamoxifen | Notch 4 ↑ JAG1 ↑ Dll1-3 ↑ | MCF7 Y537S-ERα cells | Mammosphere-forming efficiency ↑ Endocrine resistance | [128] |
| Tamoxifen, Fulvestrant, | JAG1/Notch4 activation | ALDH+/ER− BCSCs patient-derived cells In vivo patient-derived xenograft BC models | BCSCs self-renewal ↑ | [129] |
| Lapatinib | JAG1 ↑ Notch1/3/4 ↑ | HER2 overexpressing BC cells | CSCs enrichment and tumor initiation | [130] |
| Trastuzumab | Notch1 ↑ | Trastuzumab resistant HER2+ BC cells | PTEN ↓ ERK1/2 ↑ BCSCs survival and self-renewal ↑ | [131] |
| Paclitaxel | Notch signaling activation | ER+ and TNBC cells Xenograft BC models | HIF2α ↑ Stem phenotype | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimento, A.; D’Amico, M.; Pezzi, V.; De Amicis, F. Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 6296. https://doi.org/10.3390/ijms23116296
Chimento A, D’Amico M, Pezzi V, De Amicis F. Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance. International Journal of Molecular Sciences. 2022; 23(11):6296. https://doi.org/10.3390/ijms23116296
Chicago/Turabian StyleChimento, Adele, Maria D’Amico, Vincenzo Pezzi, and Francesca De Amicis. 2022. "Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance" International Journal of Molecular Sciences 23, no. 11: 6296. https://doi.org/10.3390/ijms23116296
APA StyleChimento, A., D’Amico, M., Pezzi, V., & De Amicis, F. (2022). Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance. International Journal of Molecular Sciences, 23(11), 6296. https://doi.org/10.3390/ijms23116296







