Bos taurus and Cervus elaphus as Non-Seasonal/Seasonal Models for the Role of Melatonin Receptors in the Spermatozoon
Abstract
:1. Introduction
2. Results
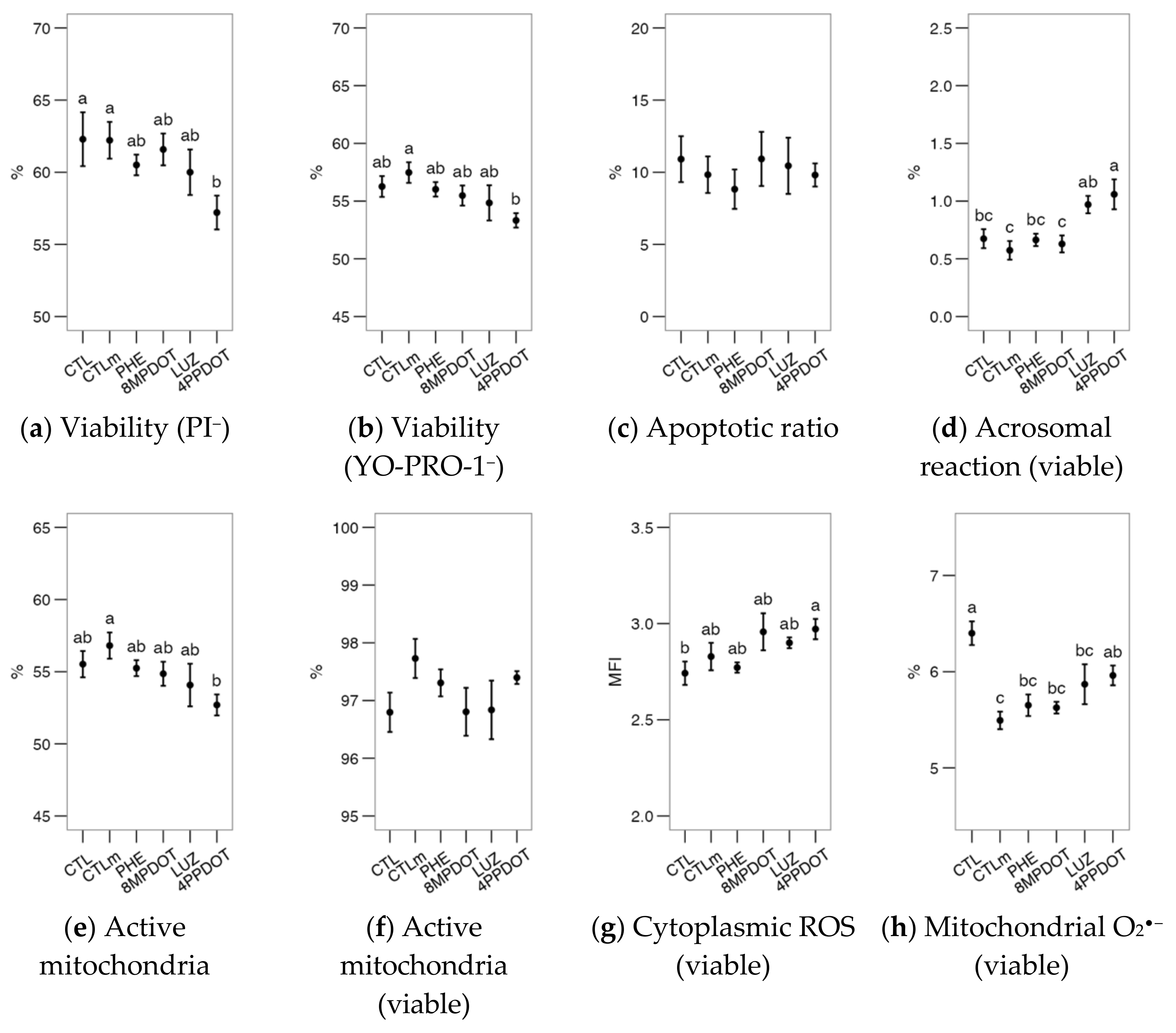
2.1. Melatonin Receptor MT2 Contributes to Maintaining Bull Sperm Functionality
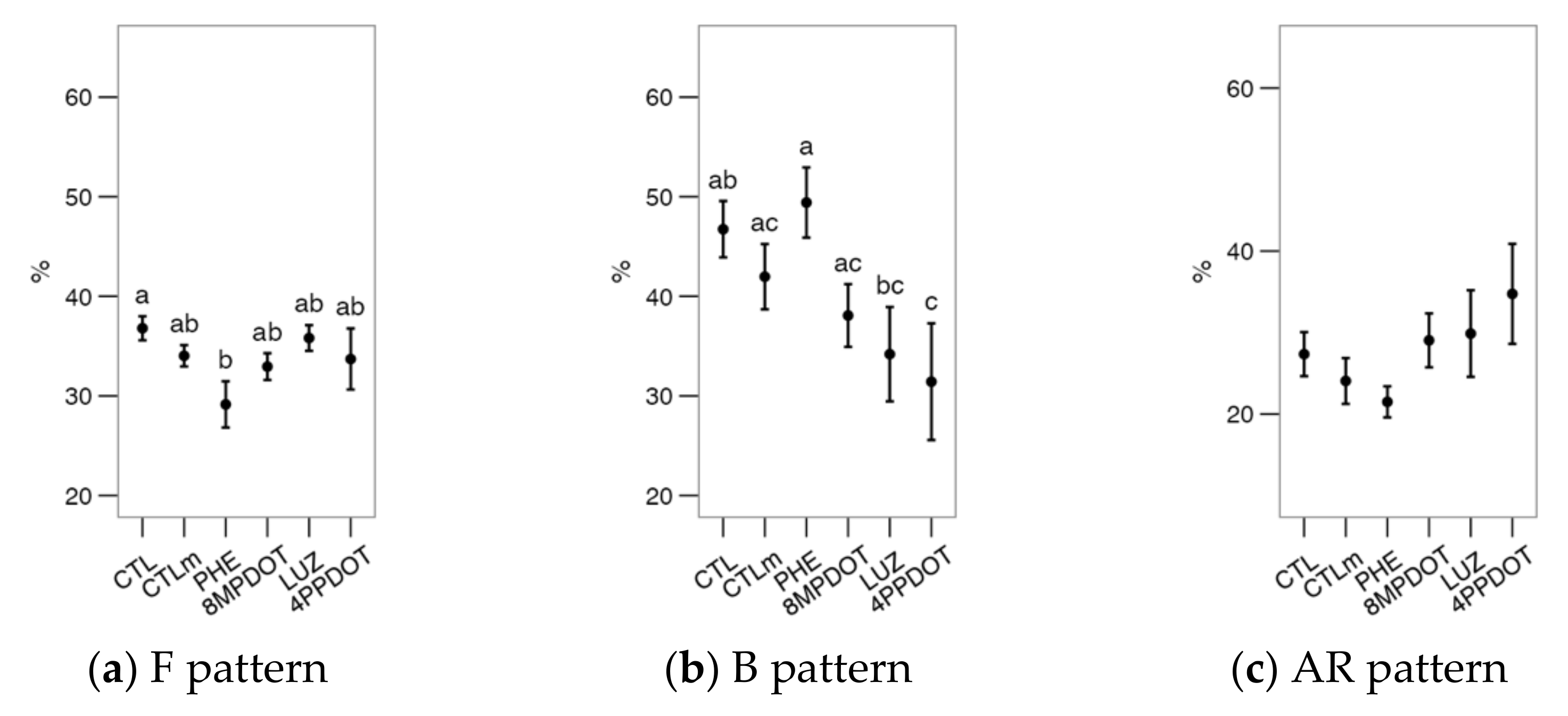
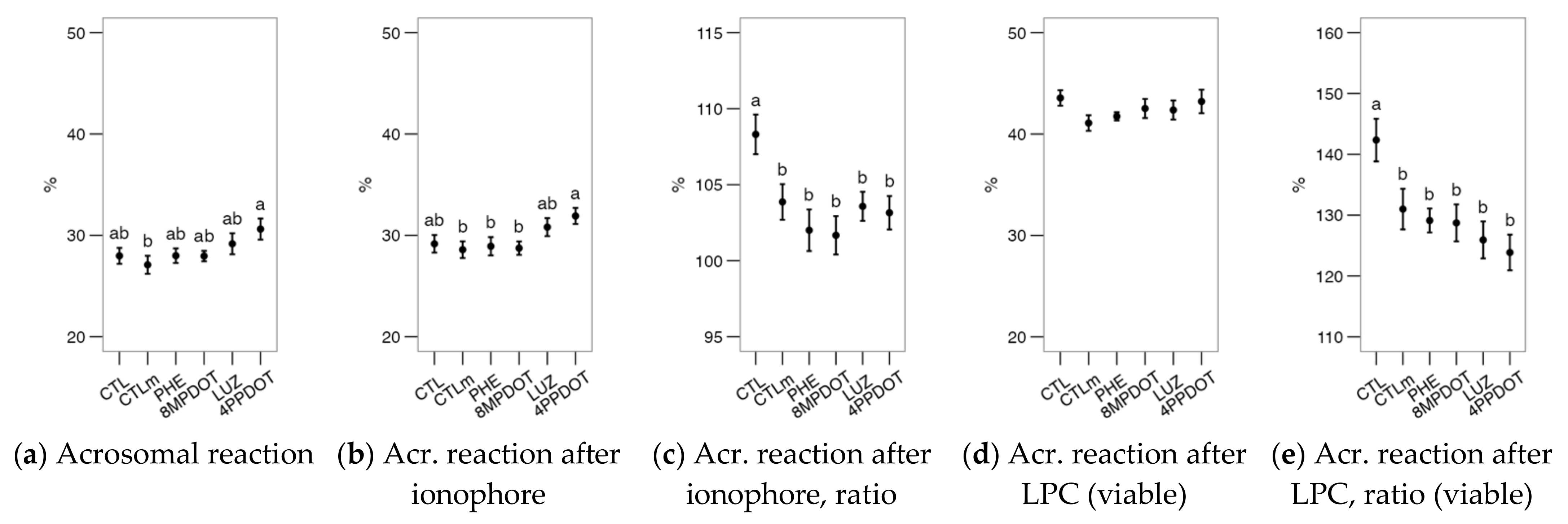
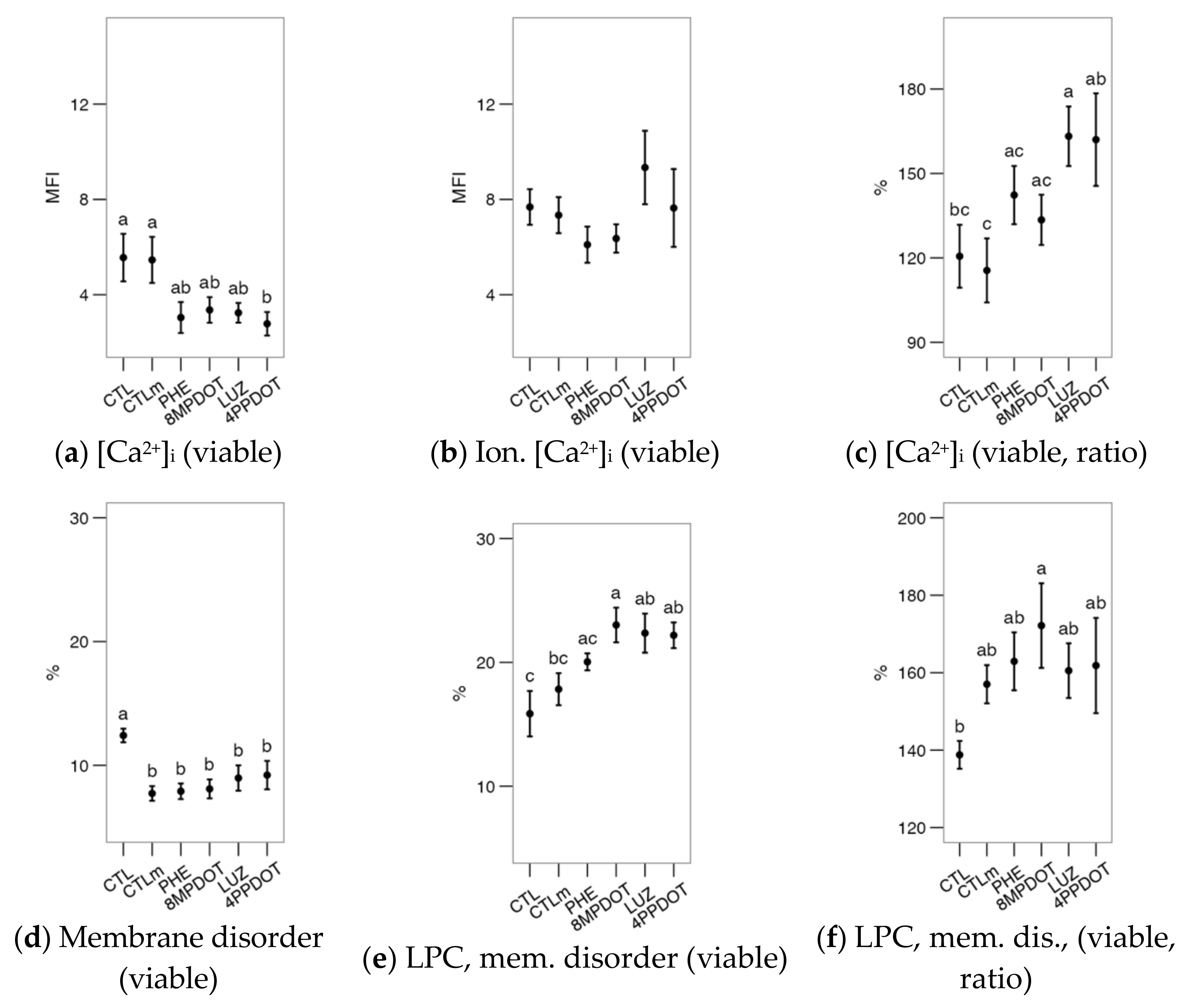
2.2. Melatonin Receptors Modulate the Response of Bull Spermatozoa to Triggering the Acrosomal Reaction and Intracellular Calcium Distribution

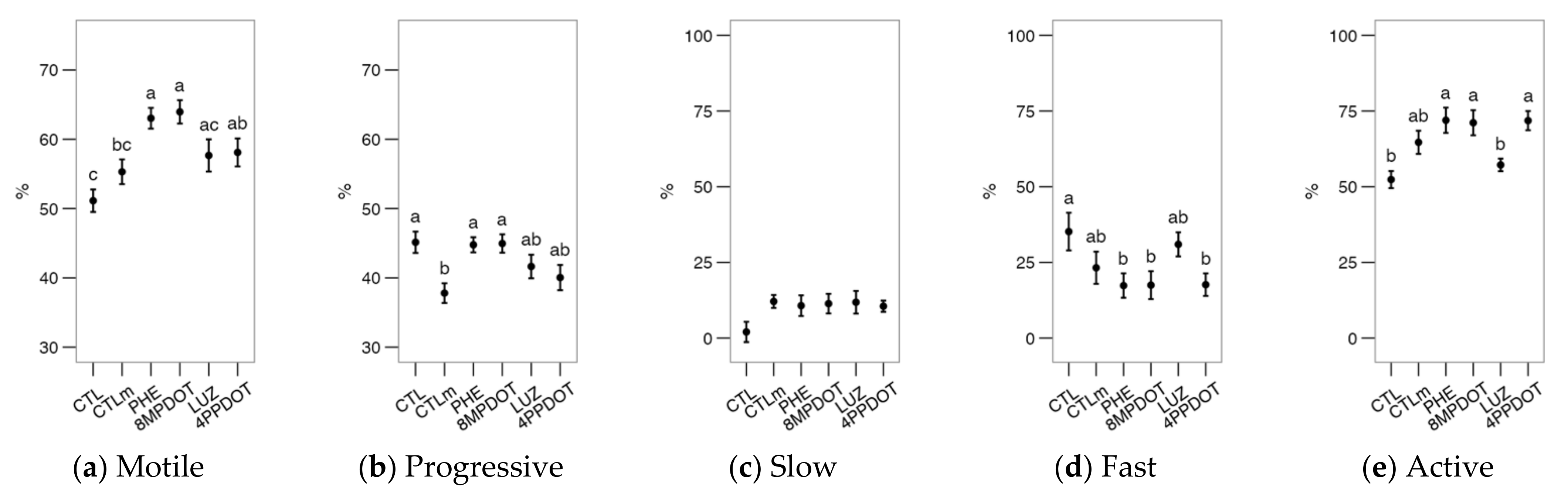
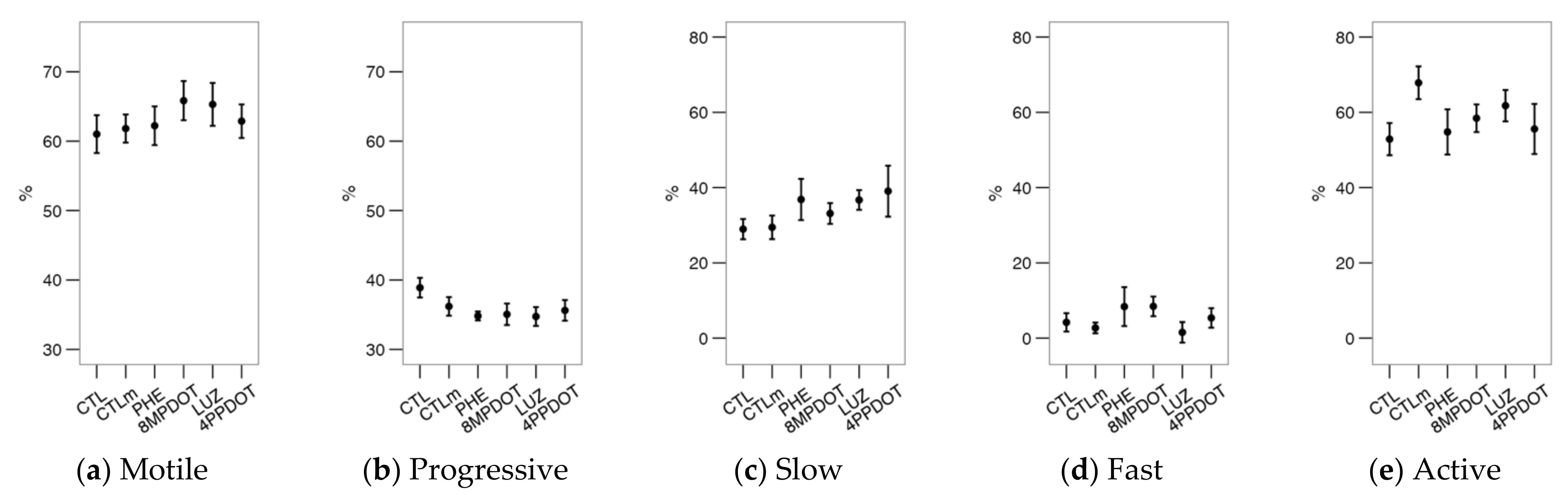
2.3. The Activation of Melatonin Receptors Improves Sperm Motility
2.4. Melatonin and the Receptors Agonists/Antagonists Showed Few Effects on Deer Spermatozoa
3. Discussion
4. Materials and Methods
4.1. Reagents, Animals, and Sample Collection and Preparation
4.2. Experimental Design
4.3. Flow Cytometry Analysis of Sperm Physiology
4.4. Ionophore and Lysophosphatidylcholine Challenges
4.5. Chlortetracycline (CTC) Stain for Assessing [Ca2+]i Patterns
4.6. Sperm Motility Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4P-PDOT | cis-4-phenyl-2-propionamidotetralin |
| 8M-PDOT | 8-methoxy-2-propionamidotetralin |
| ALH | Amplitude of the Lateral Displacement of the Sperm Head (µm) |
| BCF | Frequency of the Flagellar Beat (Hz) |
| CM-H2DCFDA | Chloromethyl dihydroxy 2′,7′-di-chlorofluorescein acetate |
| CTC | Chlortetracycline |
| DABCO | 1,4-diazabicyclo[2.2.2]octane, triethylenediamine |
| DNC | Sperm dance (VCL × ALH; µm2/s) |
| DNCm | Sperm mean dance (VCL × ALH/VSL; µm) |
| DMSO | Dimethylsulphoxide |
| LIN | Linearity (VCL/VSL) |
| LPC | Lysophosphatidylcholine |
| LUZ | Luzindole |
| MOT | Total Motility |
| PHE | Phenylmelatonin |
| PROG | Progressive Motility |
| PNA | Peanut agglutinin |
| PVP | Polyvinylpyrrolidone |
| VSL | Straight Path Velocity (µm/s) |
| STR | Straightness (VAP/VSL) |
| TALP | Tyrode-Albumin-Lactate-Pyruvate medium |
| VAP | Average Path Velocity (µm/s) |
| VCL | Curvilinear Velocity (µm/s) |
| WOB | Wobble (VAP/VCL) |
References
- González-Arto, M.; Aguilar, D.; Gaspar-Torrubia, E.; Gallego, M.; Carvajal-Serna, M.; Herrera-Marcos, L.V.; Serrano-Blesa, E.; Hamilton, T.R.D.S.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin MT₁ and MT₂ Receptors in the Ram Reproductive Tract. Int. J. Mol. Sci. 2017, 18, 662. [Google Scholar] [CrossRef] [PubMed]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin Receptors MT1 and MT2 Are Expressed in Spermatozoa from Several Seasonal and Nonseasonal Breeder Species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. Identification and Immunolocalisation of Melatonin MT(1) and MT(2) Receptors in Rasa Aragonesa Ram Spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, K.; Broda, D.; Romerowicz-Misielak, M.; Nowak, S.; Koziorowski, M. Melatonin Concentration in Peripheral Blood and Melatonin Receptors (MT1 and MT2) in the Testis and Epididymis of Male Roe Deer during Active Spermatogenesis. Theriogenology 2020, 149, 25–37. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-Related Changes in Melatonin Synthesis in Rat Extrapineal Tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Hamilton, T.R.d.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pé-rez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; et al. Evidence of Melatonin Synthesis in the Ram Reproductive Tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Gurer-Orhan, H.; Suzen, S. Melatonin, Its Metabolites and Its Synthetic Analogs as Multi-Faceted Compounds: Antioxidant, Prooxidant and Inhibitor of Bioactivation Reactions. Curr. Med. Chem. 2015, 22, 490–499. [Google Scholar] [CrossRef]
- Minguini, I.P.; Luquetti, C.M.; Baracat, M.C.P.; Maganhin, C.C.; Nunes, C.d.O.; Simões, R.S.; Veiga, E.C.d.A.; Cipolla Neto, J.; Baracat, E.C.; Soares, J.M., Jr. Melatonin Effects on Ovarian Follicular Cells: A Systematic Review. Rev. Assoc. Med. Bras. 2019, 65, 1122–1127. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and the Ovary: Physiological and Pathophysiological Implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef]
- Casao, A.; Vega, S.; Palacín, I.; Pérez-Pe, R.; Laviña, A.; Quintín, F.J.; Sevilla, E.; Abecia, J.A.; Cebrián-Pérez, J.A.; Forcada, F.; et al. Effects of Melatonin Implants during Non-Breeding Season on Sperm Motility and Reproductive Parameters in Rasa Aragonesa Rams. Reprod. Domest. Anim. 2010, 45, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M. Melatonin-Enhanced Hyperactivation of Hamster Sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigo, M.; Maňásková-Postlerová, P.; Zuidema, D.; Kerns, K.; Jonáková, V.; Tůmová, L.; Bubeníčková, F.; Sutovsky, P. Porcine Model for the Study of Sperm Capacitation, Fertilization and Male Fertility. Cell Tissue Res. 2020, 380, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, J.; Sheng, S.; Sun, Y.; Li, H.; Li, W.-P.; Zhang, C.; Chen, Z.-J. Melatonin Levels in Follicular Fluid as Markers for IVF Outcomes and Predicting Ovarian Reserve. Reproduction 2017, 153, 443–451. [Google Scholar] [CrossRef]
- Espino, J.; Ortiz, Á.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Rodríguez, A.B.; Pariente, J.A. Melatonin Protects Human Spermatozoa from Apoptosis via Melatonin Receptor- and Extracellular Signal-Regulated Kinase-Mediated Pathways. Fertil. Steril. 2011, 95, 2290–2296. [Google Scholar] [CrossRef]
- Li, C.-Y.; Hao, H.-S.; Zhao, Y.-H.; Zhang, P.-P.; Wang, H.-Y.; Pang, Y.-W.; Du, W.-H.; Zhao, S.-J.; Liu, Y.; Huang, J.-M.; et al. Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1. Int. J. Mol. Sci. 2019, 20, 3921. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Alegre, E.; Álvarez-Fernández, I.; Domínguez, J.C.; Casao, A.; Martínez-Pastor, F. Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions. Int. J. Mol. Sci. 2020, 21, 2701. [Google Scholar] [CrossRef]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.-A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin Prevents Capacitation and Apoptotic-like Changes of Ram Spermatozoa and Increases Fertility Rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; Casao, A.; Yeste, M.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. Melatonin Reduces CAMP-Stimulated Capacitation of Ram Spermatozoa. Reprod. Fertil. Dev. 2019, 31, 420–431. [Google Scholar] [CrossRef]
- Miguel-Jiménez, S.; Carvajal-Serna, M.; Calvo, S.; Casao, A.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pe, R. Does Melatonin Exert Its Effect on Ram Sperm Capacitation Through Nitric Oxide Synthase Regulation? Int. J. Mol. Sci. 2020, 21, 2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Arto, M.; Luna, C.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Casao, A. New Evidence of Melatonin Receptor Contribution to Ram Sperm Functionality. Reprod. Fertil. Dev. 2016, 28, 924–935. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Redox Regulation of Mammalian Sperm Capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s Meaning of Capacitation: A Molecular Perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebrián-Pérez, J.; Casao, A.; González-Arto, M.; Dos Santos Hamilton, T.; Pérez-Pé, R.; Muiño-Blanco, T. Melatonin in Sperm Biology: Breaking Paradigms. Reprod. Domest. Anim. 2014, 49 (Suppl. 4), 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef] [Green Version]
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin Receptor Signaling: Impact of Receptor Oligomerization on Receptor Function. Int. Rev. Cell Mol. Biol. 2018, 338, 59–77. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling Mechanisms of a Pleiotropic Agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin Signaling and Cell Protection Function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of Melatonin in the Field of Reproductive Medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Pastor, F.; Guerra, C.; Kaabi, M.; Garcia-Macias, V.; de Paz, P.; Alvarez, M.; Herraez, P.; Anel, L. Season Effect on Genitalia and Epididymal Sperm from Iberian Red Deer, Roe Deer and Cantabrian Chamois. Theriogenology 2005, 63, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, X. Melatonin and Male Reproduction. Clin. Chim. Acta 2015, 446, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian Disruption and Human Health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef]
- Beltran-Frutos, E.; Casarini, L.; Santi, D.; Brigante, G. Seasonal Reproduction and Gonadal Function: A Focus on Humans Starting from Animal Studies. Biol. Reprod. 2022, 106, 47–57. [Google Scholar] [CrossRef]
- Fernández-Alegre, E.; Lacalle, E.; Soriano-Úbeda, C.; Domínguez, J.C.; Casao, A.; Martínez-Pastor, F. Melatonin Affects Red Deer Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions. Reprod. Domest. Anim. 2022, in press. [Google Scholar] [CrossRef]
- Gadella, B.M.; Luna, C. Cell Biology and Functional Dynamics of the Mammalian Sperm Surface. Theriogenology 2014, 81, 74–84. [Google Scholar] [CrossRef]
- Casao, A.; Cebrián, I.; Asumpção, M.E.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Seasonal Variations of Melatonin in Ram Seminal Plasma Are Correlated to Those of Testosterone and Antioxidant Enzymes. Reprod. Biol. Endocrinol. 2010, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Pool, K.R.; Rickard, J.P.; de Graaf, S.P. Melatonin Improves the Motility and DNA Integrity of Frozen-Thawed Ram Spermatozoa Likely via Suppression of Mitochondrial Superoxide Production. Domest. Anim. Endocrinol. 2021, 74, 106516. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R. Targeting Host Defense System and Rescuing Compromised Mitochondria to Increase Tolerance against Pathogens by Melatonin May Impact Outcome of Deadly Virus Infection Pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the Effect of Incubation and Oxidative Stress on Thawed Spermatozoa from Red Deer by Using Different Antioxidant Treatments. Reprod. Fertil. Dev. 2010, 22, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, I.; Kohram, H.; Ardabili, F.F. Antioxidative Effects of Melatonin on Kinetics, Microscopic and Oxidative Parameters of Cryopreserved Bull Spermatozoa. Anim. Reprod. Sci. 2013, 139, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin Transport into Mitochondria. Cell Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Carrillo, A.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. The CatSper Channel Modulates Boar Sperm Motility during Capacitation. Reprod. Biol. 2017, 17, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.-W.; Sun, Y.-Q.; Jiang, X.-L.; Huang, Z.-Q.; Zhao, S.-J.; Du, W.-H.; Hao, H.-S.; Zhao, X.-M.; Zhu, H.-B. Protective Effects of Melatonin on Bovine Sperm Characteristics and Subsequent in Vitro Embryo Development. Mol. Reprod. Dev. 2016, 83, 993–1002. [Google Scholar] [CrossRef]
- Álvarez, M.; Tamayo-Canul, J.; Martínez-Rodríguez, C.; López-Urueña, E.; Gomes-Alves, S.; Anel, L.; Martínez-Pastor, F.; de Paz, P. Specificity of the Extender Used for Freezing Ram Sperm Depends of the Spermatozoa Source (Ejaculate, Electroejaculate or Epididymis). Anim. Reprod. Sci. 2012, 132, 145–154. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Martínez, F.; García-Macías, V.; Esteso, M.C.; Anel, E.; Fernández-Santos, M.R.; Soler, A.J.; de Paz, P.; Garde, J.; Anel, L. A Pilot Study on Post-Thawing Quality of Iberian Red Deer Spermatozoa (Epididymal and Electroejaculated) Depending on Glycerol Concentration and Extender Osmolality. Theriogenology 2006, 66, 1165–1172. [Google Scholar] [CrossRef]
- Tamayo-Canul, J.; Alvarez, M.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; de Paz, P.; Anel, L.; Martínez-Pastor, F. Effect of Storage Method and Extender Osmolality in the Quality of Cryopreserved Epididymal Ram Spermatozoa. Anim. Reprod. Sci. 2011, 129, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial Dynamics in the Epididymis: Role in the Maturation, Protection, and Storage of Spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Anel, L.; Guerra, C.; Alvarez, M.; Soler, A.J.; Garde, J.J.; Chamorro, C.; de Paz, P. Seminal Plasma Improves Cryopreservation of Iberian Red Deer Epididymal Sperm. Theriogenology 2006, 66, 1847–1856. [Google Scholar] [CrossRef]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin Receptors, Heterodimerization, Signal Transduction and Binding Sites: What’s New? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.A.; Levoye, A.; Delagrange, P.; Jockers, R. Preferential Formation of MT1/MT2 Melatonin Receptor Heterodimers with Distinct Ligand Interaction Properties Compared with MT2 Homodimers. Mol. Pharmacol. 2004, 66, 312–321. [Google Scholar] [CrossRef]
- Garcia-Macias, V.; de Paz, P.; Martinez-Pastor, F.; Alvarez, M.; Gomes-Alves, S.; Bernardo, J.; Anel, E.; Anel, L. DNA Fragmentation Assessment by Flow Cytometry and Sperm-Bos-Halomax (Bright-Field Microscopy and Fluorescence Microscopy) in Bull Sperm. Int. J. Androl. 2007, 30, 88–98. [Google Scholar] [CrossRef]
- Martínez, A.F.; Martínez-Pastor, F.; Alvarez, M.; Fernández-Santos, M.R.; Esteso, M.C.; de Paz, P.; Garde, J.J.; Anel, L. Sperm Parameters on Iberian Red Deer: Electroejaculation and Post-Mortem Collection. Theriogenology 2008, 70, 216–226. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Diaz-Corujo, A.R.; Anel, E.; Herraez, P.; Anel, L.; de Paz, P. Post Mortem Time and Season Alter Subpopulation Characteristics of Iberian Red Deer Epididymal Sperm. Theriogenology 2005, 64, 958–974. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Garcia-Macias, V.; Alvarez, M.; Chamorro, C.; Herraez, P.; de Paz, P.; Anel, L. Comparison of Two Methods for Obtaining Spermatozoa from the Cauda Epididymis of Iberian Red Deer. Theriogenology 2006, 65, 471–485. [Google Scholar] [CrossRef]
- Fernández-Gago, R.; Domínguez, J.C.; Martínez-Pastor, F. Seminal Plasma Applied Post-Thawing Affects Boar Sperm Physiology: A Flow Cytometry Study. Theriogenology 2013, 80, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, A.; Fernández-Alegre, E.; Cano, A.; Hozbor, F.; Martínez-Pastor, F.; Cesari, A. Seminal Plasma Proteins Interacting with Sperm Surface Revert Capacitation Indicators in Frozen-Thawed Ram Sperm. Anim. Reprod. Sci. 2016, 173, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-Félez, I.; Castañeda-Sampedro, A.; Sánchez, D.I.; Fernández-Alegre, E.; Álvarez-Rodríguez, M.; Domínguez, J.C.; Morrell, J.M.; Martínez-Pastor, F. Effect of Single Layer Centrifugation Porcicoll (70%, 80% and 90%) or Supplementation with Reduced Glutathione, Seminal Plasma and Bovine Serum Albumin on Frozen-Thawed Boar Sperm. Anim. Reprod. Sci. 2017, 187, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Grasa, P.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Signal Transduction Mechanisms Involved in in Vitro Ram Sperm Capacitation. Reproduction 2006, 132, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer Assisted Semen Analyzers in Andrology Research and Veterinary Practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fernández-Gago, R.; Álvarez-Rodríguez, M.; Alonso, M.E.; González, J.R.; Alegre, B.; Domínguez, J.C.; Martínez-Pastor, F. Thawing Boar Semen in the Presence of Seminal Plasma Improves Motility, Modifies Subpopulation Patterns and Reduces Chromatin Alterations. Reprod. Fertil. Dev. 2017, 29, 1576–1584. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]



| Subpopulation | VCL | VSL | VAP | LIN | STR | WOB | ALH | BCF | DNC | DNCm | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slow | 59.6 ± 29.3 | 16.7 ± 13.5 | 33.8 ± 17.8 | 29.5 ± 18.1 | 54.1 ± 26.8 | 57.4 ± 14.0 | 1.6 ± 0.6 | 7.9 ± 5.1 | 95.3 ± 74.9 | 5.5 ± 3.9 | 11.6 |
| Fast | 103.4 ± 31.1 | 68.1 ± 24.6 | 78.9 ± 22.8 | 68.0 ± 16.0 | 89.3 ± 8.6 | 77.4 ± 11.3 | 1.7 ± 0.5 | 21.6 ± 8.0 | 175.1 ± 93.1 | 2.5 ± 1.1 | 22.6 |
| Active | 212.7 ± 52.1 | 111.5 ± 42.0 | 136.5 ± 28 | 54.9 ± 17.4 | 88.0 ± 11.0 | 64.7 ± 10.9 | 3.8 ± 1.5 | 26.0 ± 5.9 | 809.0 ± 495.3 | 7.3 ± 4.5 | 65.8 |
| Subpopulation | VCL | VSL | VAP | LIN | STR | WOB | ALH | BCF | DNC | DNCm | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slow | 74.5 ± 37.0 | 13.5 ± 10.0 | 34.1 ± 17.2 | 20.5 ± 16.4 | 46.6 ± 29.6 | 48.9 ± 15.7 | 1.9 ± 0.8 | 9.0 ± 5.9 | 144.1 ± 120.2 | 9.4 ± 8.8 | 27.0 |
| Fast | 126.8 ± 52.8 | 6.9 ± 6.7 | 48.0 ± 24.9 | 5.5 ± 5.2 | 14.1 ± 12.9 | 40.0 ± 14.8 | 3.4 ± 1.0 | 9.6 ± 5.6 | 439.1 ± 305.7 | 55.8 ± 40.1 | 3.0 |
| Active | 176.5 ± 60.8 | 82.0 ± 50.6 | 126.8 ± 40.9 | 48.8 ± 27.9 | 75.0 ± 26.1 | 68.5 ± 16.2 | 2.9 ± 1.1 | 25.6 ± 9.5 | 499.2 ± 342.8 | 6.2 ± 5 | 70.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Alegre, E.; Lacalle, E.; Soriano-Úbeda, C.; González-Montaña, J.R.; Domínguez, J.C.; Casao, A.; Martínez-Pastor, F. Bos taurus and Cervus elaphus as Non-Seasonal/Seasonal Models for the Role of Melatonin Receptors in the Spermatozoon. Int. J. Mol. Sci. 2022, 23, 6284. https://doi.org/10.3390/ijms23116284
Fernández-Alegre E, Lacalle E, Soriano-Úbeda C, González-Montaña JR, Domínguez JC, Casao A, Martínez-Pastor F. Bos taurus and Cervus elaphus as Non-Seasonal/Seasonal Models for the Role of Melatonin Receptors in the Spermatozoon. International Journal of Molecular Sciences. 2022; 23(11):6284. https://doi.org/10.3390/ijms23116284
Chicago/Turabian StyleFernández-Alegre, Estela, Estíbaliz Lacalle, Cristina Soriano-Úbeda, José Ramiro González-Montaña, Juan Carlos Domínguez, Adriana Casao, and Felipe Martínez-Pastor. 2022. "Bos taurus and Cervus elaphus as Non-Seasonal/Seasonal Models for the Role of Melatonin Receptors in the Spermatozoon" International Journal of Molecular Sciences 23, no. 11: 6284. https://doi.org/10.3390/ijms23116284
APA StyleFernández-Alegre, E., Lacalle, E., Soriano-Úbeda, C., González-Montaña, J. R., Domínguez, J. C., Casao, A., & Martínez-Pastor, F. (2022). Bos taurus and Cervus elaphus as Non-Seasonal/Seasonal Models for the Role of Melatonin Receptors in the Spermatozoon. International Journal of Molecular Sciences, 23(11), 6284. https://doi.org/10.3390/ijms23116284








