Critical PDT Theory III: Events at the Molecular and Cellular Level
Abstract
:1. Introduction
2. Photodynamic Therapy
3. Photokilling Mechanisms
4. Recent Research Efforts
5. Conclusions
Funding
Conflicts of Interest
References
- Raab, O. Uber die Wirkung, fluorescirender Stoffe auf infusorien. Z. Biol. 1900, 39, 524–546. [Google Scholar]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The Use of a Derivative of Hematoporphyrin in Tumor Detection23. JNCI J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Dougherty, T.J. The Circuitous Route by a Group of Novices to a New FDA Approved Cancer Therapy; Outskirts Press: Denver, CO, USA, 2015. [Google Scholar]
- Pandey, R.K.; Siegel, M.M.; Tsao, R.; McReynolds, J.H.; Dougherty, T.J. Fast atom bombardment mass spectral analyses of Photofrin II® and its synthetic analogs. Biol. Mass Spectrom. 1990, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wieman, T.J.; Mang, T.S.; Fingar, V.H.; Hill, T.G.; Reed, M.W.; Corey, T.S.; Nguyen, V.Q.; Render, E.R., Jr. Effect of photody-namic therapy on blood flow in normal and tumor vessels. Surgery 1998, 104, 512–517. [Google Scholar]
- Schmidt-Erfurth, U.; Miller, J.; Sickenberg, M.; Bunse, A.; Laqua, H.; Gragoudas, E.; Zografos, L.; Birngruber, R.; Bergh, H.V.D.; Strong, A.; et al. Photodynamic therapy of subfoveal choroidal neovascularization: Clinical and angiographic examples. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 365–374. [Google Scholar] [CrossRef]
- Manyak, M.J.; Russo, A.; Smith, P.D.; Glatstein, E. Photodynamic therapy. J. Clin. Oncol. 1988, 6, 380–391. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, M.L.; Clay, M.E.; Harvey, E.J.; Evans, H.H.; Antunez, A.R.; Oleinick, N.L. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991, 51, 5993–5996. [Google Scholar]
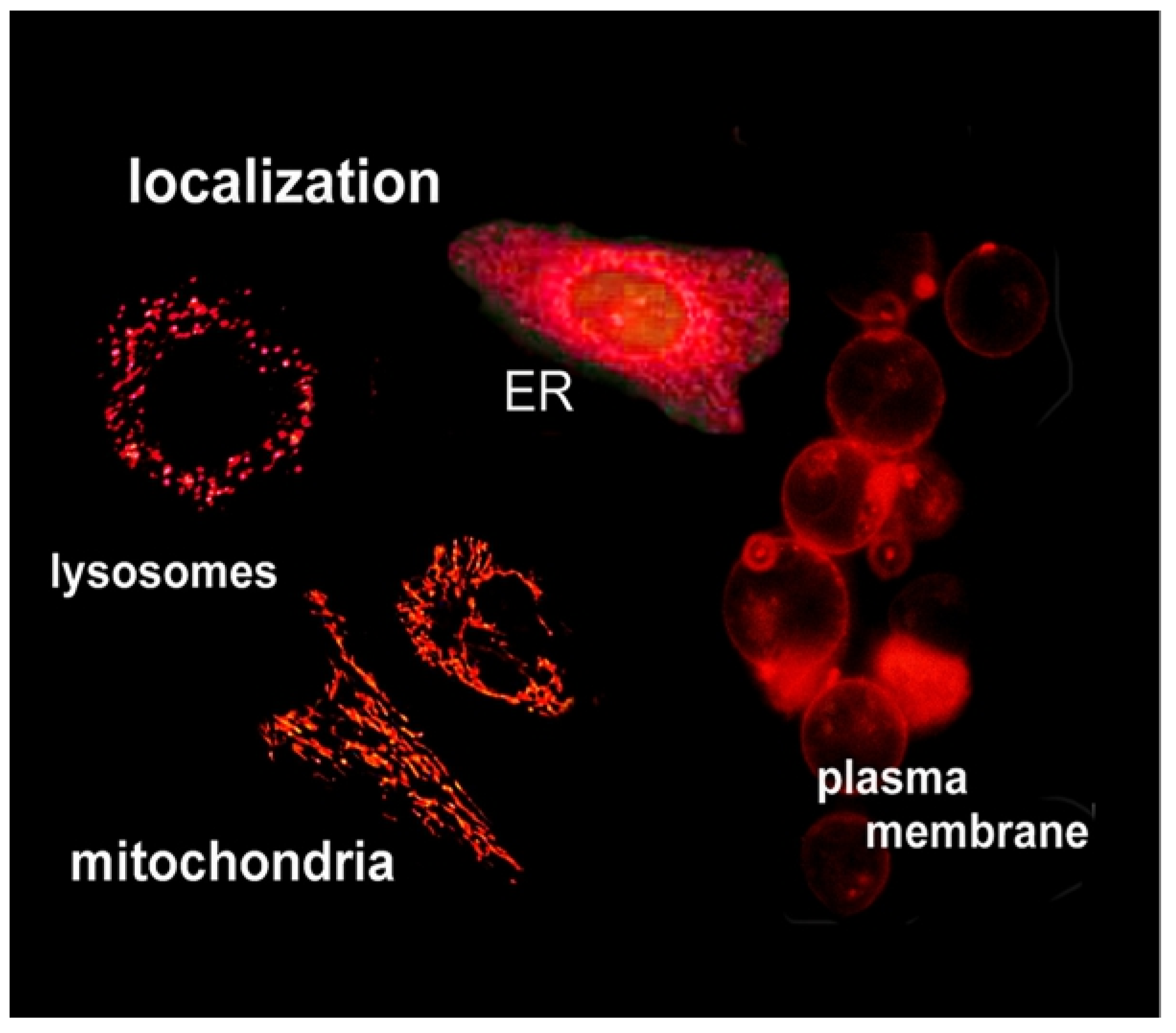
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C. The Role of Subcellular Localization in Initiation of Apoptosis by Photodynamic Therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Polo, L.; Valduga, G.; Jori, G.; Reddi, E. Low-density lipoprotein receptors in the uptake of tumour photosensitizers by human and rat transformed fibroblasts. Int. J. Biochem. Cell Biol. 2001, 34, 10–23. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-Inspired Nanoparticles for Cancer Theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Gomer, C.J.; Dougherty, T.J. Determination of [3H]- and [14C] hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979, 39, 146–151. [Google Scholar] [PubMed]
- Lilge, L.; Molpus, K.; Hasan, T.; Wilson, B.C. Light dosimetry for intraperitoneal photodynamic therapy in a murine xenograft model of human epithelial ovarian carcinoma. Photochem. Photobiol. 1998, 68, 281–288. [Google Scholar] [CrossRef]
- Grossweiner, L.I. PDT light dosimetry revisited. J. Photochem. Photobiol. B Biol. 1997, 38, 258–268. [Google Scholar] [CrossRef]
- Richter, A.M.; Waterfield, E.; Jain, A.K.; Allison, B.A.; Sternberg, E.D.; Dolphin, D.; Levy, J.G. Photosensitising potency of structural analogues of benzoporphyrin derivative (BPD) in a mouse tumour model. Br. J. Cancer 1991, 63, 87–93. [Google Scholar] [CrossRef]
- Kleemann, B.; Loos, B.; Scriba, T.J.; Lang, D.; Davids, L.M. St John’s Wort (Hypericum perforatum)-photodynamic therapy induces metastatic melanoma cell death. PLoS ONE 2014, 9, e103762. [Google Scholar] [CrossRef] [Green Version]
- Ris, H.-B.; Altermatt, H.J.; Stewart, C.M.; Schaffner, T.; Wang, Q.; Lim, C.K.; Bonnett, R.; Althaus, U. Photodynamic therapy with m-tetrahydroxyphenylchlorin in vivo: Optimization of the therapeutic index. Int. J. Cancer 1993, 55, 245–249. [Google Scholar] [CrossRef]
- Kessel, D.; Reiners, J.J. Enhanced efficacy of photodynamic therapy via a sequential targeting protocol. Photochem. Photobiol. 2014, 90, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, I.; Obaid, G.; Bano, S.; Hasan, T.; Kessel, D. Photodynamic therapy: Promoting in vitro efficacy of photodynamic therapy by liposomal formulations of a photosensitizing agent. Lasers Surg. Med. 2018, 50, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pogue, B.; Hoopes, P.J.; Hasan, T. Vascular and Cellular Targeting for Photodynamic Therapy. Crit. Rev. Eukaryot. Gene Expr. 2006, 16, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Critical PDT Theory. Photochem. Photobiol. 2022; in press. [Google Scholar]
- Lilge, L.; Wu, J.; Xu, Y.; Manalac, A.; Molehuis, D.; Schwiegelshohn, F.; Vesselov, L.; Embree, W.; Nesbit, M.; Betz, V.; et al. Minimal required PDT light dosimetry for nonmuscle invasive bladder cancer. J. Biomed. Opt. 2020, 25, 1–13. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Guo, Y.; Yuan, H.; Cui, T.; Yao, S.; Jin, S.; Fan, H.; Wang, C.; Xie, R.; et al. Golgi apparatus-targeted aggregation-induced emission luminogens for effective cancer photodynamic therapy. Nat. Commun. 2022, 3, 2179. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jeeves, W.P.; Lowe, D.M. In vivo and Post Mortem Measurements of the Attenuation Spectra of Light in Mammalian Tissues. Photochem. Photobiol. 1985, 42, 153–162. [Google Scholar] [CrossRef]
- Price, M.; Kessel, D. On the use of fluorescence probes for detecting reactive oxygen and nitrogen species associated with photodynamic therapy. J. Biomed. Opt. 2010, 15, 051605. [Google Scholar] [CrossRef] [Green Version]
- Setsukinai, K.-I.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of Novel Fluorescence Probes That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [Green Version]
- Cincotta, L.; Szeto, D.; Lampros, E.; Hasan, T.; Cincotta, A.H. Benzophenothiazine and benzoporphyrin derivative combina-tion phototherapy effectively eradicates large murine sarcomas. Photochem. Photobiol. 1996, 63, 229–237. [Google Scholar] [CrossRef]
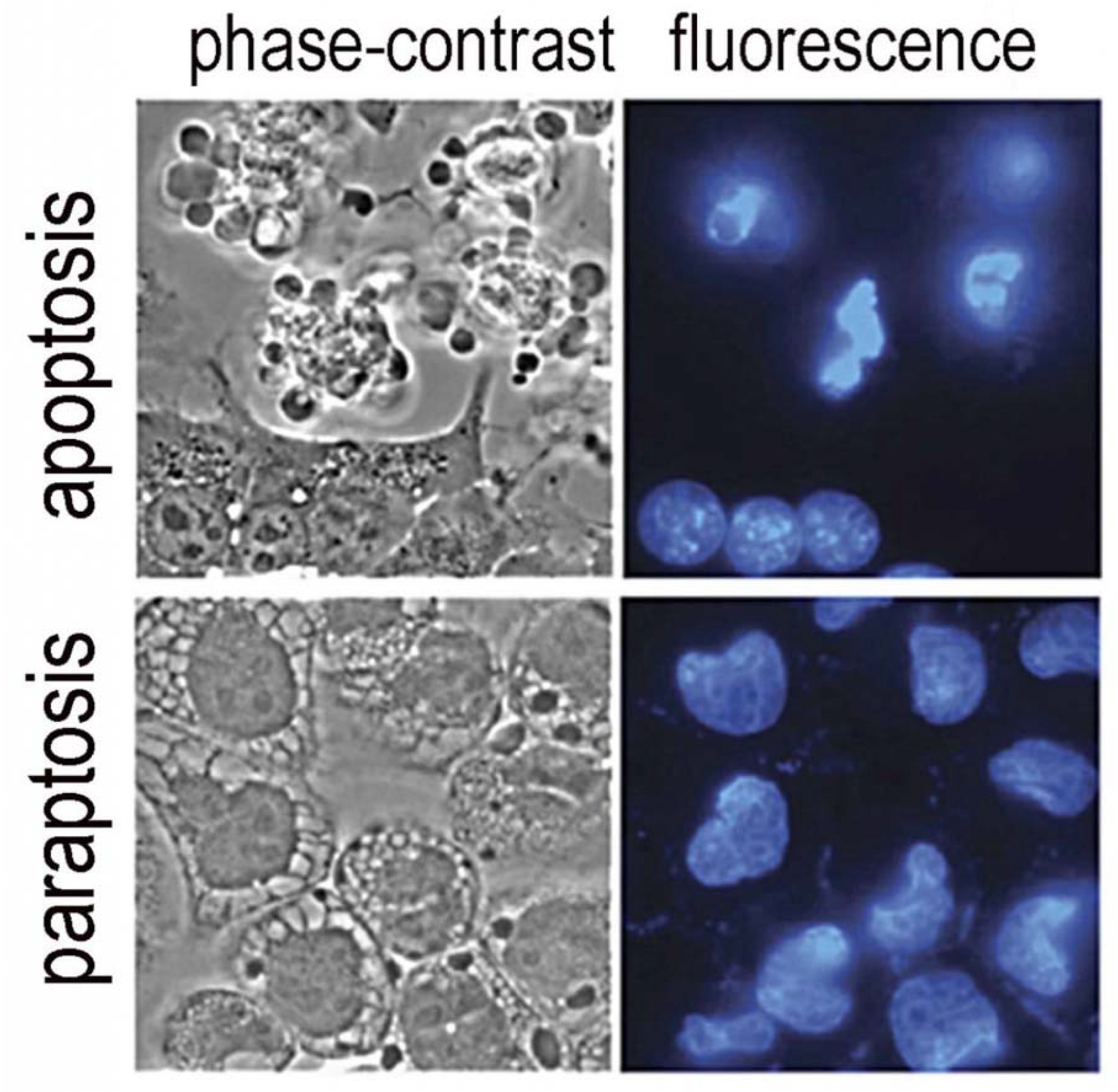
- Kessel, D. Photodynamic therapy: Apoptosis, paraptosis and beyond. Apoptosis 2020, 25, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Perozzo, R.; Schmid, I.; Ziemiecki, A.; Schaffner, T.; Scapozza, L.; Brunner, T.; Simon, H.-U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006, 8, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Nath, S.; Obaid, G.; Ruhi, M.K.; Moore, K.; Bano, S.; Kessel, D.; Hasan, T. A combination of Visudyne and a li-pid-anchored liposomal formulation of benzoporphyrin derivative enhances photodynamic therapy efficacy in a 3D Model for ovarian cancer. Photochem. Photobiol. 2019, 95, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessel, D.; Cho, W.J.; Rakowski, J.; Kim, H.E.; Kim, H.C. Characteristics of an Impaired PDT Response. Photochem. Photobiol. 2021, 97, 836–840. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Squire, J.; Guirguis, M.; Saha, D.; Hoyt, K.; Wang, K.K.-H.; Agarwal, V.; Obaid, G. Deep-Tissue Activation of Photonanomedicines: An Update and Clinical Perspectives. Cancers 2022, 14, 2004. [Google Scholar] [CrossRef]
- Biel, M.A. Photodynamic therapy in head and neck cancer. Curr. Oncol. Rep. 2002, 4, 87–96. [Google Scholar] [CrossRef]
- Tanha, K.; Pashazadeh, A.M.; Pogue, B.W. Review of biomedical Čerenkov luminescence imaging applications. Biomed. Opt. Express 2015, 6, 3053–3065. [Google Scholar] [CrossRef] [Green Version]
- Sorrin, A.J.; Liu, C.; Cicalo, J.; Reader, J.; Najafali, D.; Zhang, Y.; Roque, D.M.; Huang, H.-C. Photodynamic Priming Improves the Anti-Migratory Activity of Prostaglandin E Receptor 4 Antagonist in Cancer Cells In Vitro. Cancers 2021, 13, 5259. [Google Scholar] [CrossRef]
- Overchuk, M.; Harmatys, K.M.; Sindhwani, S.; Rajora, M.A.; Koebel, A.; Charron, D.M.; Syed, A.M.; Chen, J.; Pomper, M.G.; Wilson, B.C.; et al. Subtherapeutic Photodynamic Treatment Facilitates Tumor Nanomedicine Delivery and Overcomes Desmoplasia. Nano Lett. 2020, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Rizvi, I.; Liu, J.; Anbil, S.; Kalra, A.; Lee, H.; Baglo, Y.; Paz, N.; Hayden, D.; Pereira, S.; et al. Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res. 2017, 78, 558–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fingar, V.H. Vascular Effects of Photodynamic Therapy. J. Clin. Laser Med. Surg. 1996, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Krammer, B. Vascular effects of photodynamic therapy. Anticancer Res. 2002, 21, 4271–4277. [Google Scholar]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessel, D. Critical PDT Theory III: Events at the Molecular and Cellular Level. Int. J. Mol. Sci. 2022, 23, 6195. https://doi.org/10.3390/ijms23116195
Kessel D. Critical PDT Theory III: Events at the Molecular and Cellular Level. International Journal of Molecular Sciences. 2022; 23(11):6195. https://doi.org/10.3390/ijms23116195
Chicago/Turabian StyleKessel, David. 2022. "Critical PDT Theory III: Events at the Molecular and Cellular Level" International Journal of Molecular Sciences 23, no. 11: 6195. https://doi.org/10.3390/ijms23116195
APA StyleKessel, D. (2022). Critical PDT Theory III: Events at the Molecular and Cellular Level. International Journal of Molecular Sciences, 23(11), 6195. https://doi.org/10.3390/ijms23116195





