Abstract
The current obesity pandemic has been expanding in both developing and developed countries. This suggests that the factors contributing to this condition need to be reconsidered since some new factors are arising as etiological causes of this disease. Moreover, recent clinical and experimental findings have shown an association between the progress of obesity and some infections, and the functions of adipose tissues, which involve cell metabolism and adipokine release, among others. Furthermore, it has recently been reported that adipocytes could either be reservoirs for these pathogens or play an active role in this process. In addition, there is abundant evidence indicating that during obesity, the immune system is exacerbated, suggesting an increased susceptibility of the patient to the development of several forms of illness or death. Thus, there could be a relationship between infection as a trigger for an increase in adipose cells and the impact on the metabolism that contributes to the development of obesity. In this review, we describe the findings concerning the role of adipose tissue as a mediator in the immune response as well as the possible role of adipocytes as infection targets, with both roles constituting a possible cause of obesity.
1. Introduction
We are currently facing an obesity epidemic, as obesity is becoming a growing health problem worldwide and has a complex and multifactorial etiology [1]. According to the World Health Organization (WHO), obesity and overweight are defined as excessive fat accumulation that leads to higher morbidity rates for various health problems [2].
Data from several databases focused on the obesity research field indicate that susceptibility to infections and obesity are related. These databases have also identified adipocytes as key components in the crosstalk between microorganisms, obesity, and immune cells [3], thus opening up a new landscape in this research field and prompting the emergence of a new “infectobesity theory”. This theory is sustained by multiple lines of evidence: Firstly, many reports have described how infections by pathogens alter the architecture of adipose tissue due to inflammation or pathogen persistence [4]. Additionally, there is evidence that supports the role of infected adipocytes in obesity, since some pathogens are able to dysregulate components (i.e., signaling pathways) of adipose tissues and participate in the development of obesity [1,4]. This theory maintains that some microorganisms are capable of inducing the obesogenic process [5,6], with adenovirus 36 being one of the most studied pathogens associated with obesity.
Adipose tissue is a specialized connective tissue emerging as a critical metabolic organ that regulates energy homeostasis in the body [7,8]. Its main function is to store energy in the form of neutral lipids (fat) [8]. However, growing evidence now indicates that adipose tissue can sense and integrate mixed signals, such as signals of a hormonal, metabolic, inflammatory, and even neuronal origin [9,10,11]. Adipose tissue consists of lipid-rich cells called adipocytes and can be divided into white, pink, beige, and brown adipose tissue. White adipose tissue is crucial in the host’s metabolism due to its capacity to store and release fatty acids, which supply fuel to the organism during fasting periods; on the other hand, brown adipose tissue helps to transform energy into heat to regulate body temperature by thermogenesis [7,8]. Nevertheless, excessive adipose tissue is associated with several chronic diseases [12].
Adipocytes have been related to different pathologies such as diabetes and metabolic syndrome [13,14]. Likewise, adipocytes have recently been described as natural targets of several infections [15] that trigger the alteration of the normal metabolic response. Moreover, obesity has been considered as a state of low-grade inflammation due to an excess of cytokines and adipokines, which modify glucose and insulin metabolism [12] and a combination thereof, thus leading to insulin resistance and metabolic syndrome.
A comprehensive understanding of the relationship between adipocytes, cellular metabolism, infections, and immune responses may further bring insight into how the specific responses to pathogens can act as etiological factors in obesity. In this review, we will focus on how adipocytes’ immunological mediators can respond to infections whilst also contributing to obesity.
2. The Role of Adipose Tissue in the Immune Response
2.1. Adipose Tissue’s Composition
Adipose tissue is a dynamic organ distributed throughout the body [16]. For many years, adipose tissue (AT) was considered an inert mass; however, the recognition of its importance has been increasing [8]. Adipose tissue is currently regarded as a critical endocrine system (organ) that releases cytokines, chemokines, and adipokines, including adiponectin, leptin, Tumor Necrosis Factor-α (TNF-α), Interleukin (IL)-1β, IL-6, and others that are crucial in the regulation of energy and immune responses [8].
Adipose tissue is organized into discrete depots throughout the body [17,18]. Brown and beige adipocytes represent a small proportion of the total adipose tissue [18], with white adipose tissue (WAT) being the most abundant form as it is found in almost every area of the body. In fact, white adipocytes change their phenotype during pregnancy and lactation (transdifferentiation), developing into pink adipocytes [19,20]. The major WAT depots can be broadly classified by location, as either subcutaneous (under the skin) or visceral and as visceral/omental (located intra-abdominally, adjacent to internal organs) [17,18].
Although adipocytes account for most of the volume of AT, they only make up about 50% of the cellular content [16]. Other cell types, including endothelial cells, fibroblasts, and immune cells, are present in this tissue [17]. Macrophages and T lymphocytes are the principal immune cells infiltrating adipose tissue, and one of their functions is to secrete proinflammatory mediators [21,22]. In more detail, visceral AT, in contrast to subcutaneous AT, tends to have a higher content of macrophages, regulatory T cells, natural-killer T cells, and eosinophils [16], suggesting that it could be a potential immunologic effector site; however, more studies need to be conducted to position adipose tissue as a new immune site.
2.2. Adipokines
Adipokines are a family of hormones and cytokines that are secreted by adipose tissue and can modulate diverse cellular responses in immune cells, such as cell migration [23] or even the cellular subpopulations of T cells [24]. Among these proteins, leptin and adiponectin are the principal regulators of the adipocytes, and it is for this reason that we will focus on them [25].
2.2.1. Leptin
Leptin is secreted by adipocytes in response to changes in a person’s nutritional status. Leptin is a 167-amino-acid product of the human leptin gene. It was identified for the first time via a defect in the gene responsible for the obesity syndrome in leptin-deficient (ob/ob) mice [26,27]. Leptin is mainly secreted by white adipose tissue, and its levels are positively correlated with the amount of body fat [27].
The main function of leptin is to modulate energy homeostasis. To achieve this, its signals are focused on the central nervous system (CNS) and peripheral organs, promoting satiety and decreasing food intake and metabolism. However, leptin can also act peripherally to increase glucose utilization by skeletal muscles and to decrease gluconeogenesis in the liver. The most significant role of leptin is its neuroendocrine function, mediated through pathways that overlap with insulin, as recently shown in both animal models and humans [25,26,28].
Leptin mediates its effects by binding to specific leptin receptors (ObRs) expressed in the brain and peripheral tissues [27]. The binding of leptin to the ObR receptor activates several signal transduction pathways, including Janus Kinase (JAK)-Signal Transducer and Activator of Transcription 3 (STAT3), as well as Phosphatidylinositol 3 Kinase (PI3K) and Mitogen-Activated Protein Kinases (MAPK) [25,27].
Leptin also activates the AMP-activated Protein Kinase (AMPK) pathway, promotes fatty acid oxidation by inactivating acetyl-coenzyme A (CoA) carboxylase, and inhibits lipogenesis. In mouse models, lipogenic transcription factors, cholesterol biosynthesis, plasma, and tissue lipids are also regulated by leptin [28].
In addition to its regulatory function in the CNS, leptin is a proinflammatory adipokine that plays a major role in innate and adaptive immunity. Leptin protects T lymphocytes from apoptosis and regulates their proliferation and activation. Leptin also influences the cytokine production of T lymphocytes, generally by switching the phenotype toward a TH1 response [29,30,31].
In monocytes, leptin also influences activation, phagocytosis, and cytokine production (TNF-α, IL-1, IL-6, IL-8, and IL-18) and cell proliferation. In turn, leptin expression is also elevated by proinflammatory cytokines such as TNF-α and IL-1, indicating a bidirectional interaction between leptin and inflammation [25,26,27,32].
2.2.2. Adiponectin
Adiponectin is the most abundant adipokine secreted by adipocytes. It circulates at the highest levels (in the microgram per milliliter range versus the nanogram per milliliter range for leptin). This adipokine is best known for its role in the regulation of insulin sensitivity [25].
The central functions of adiponectin are orchestrated via AMPK signaling. In subjects with increased body masses, the downregulation of adiponectin has been detected, and adiponectin levels have been shown to be inversely correlated with glucose intolerance and type 2 diabetes. In this context, adiponectin appears to promote beta-cell function and survival [32]. Moreover, adiponectin enhances insulin secretion by stimulating both the expression of the insulin gene and the exocytosis of insulin granules [33]. In general, obese individuals release less adiponectin than non-obese people [34]. Adiponectin increases insulin sensitivity by stimulating insulin receptors via tyrosine phosphorylation and by the activation of AMPK, which leads to fatty acid oxidation [35]. Additionally, adiponectin can decrease (Reactive Oxygen Species) ROS production and inhibit the activation of MAPK, leading to the inhibition of cell proliferation [36].
Adiponectin presents specific anti-inflammatory properties through the inhibition of IL-6 production, through the induction of anti-inflammatory cytokines such as IL-10 or an IL-1-receptor antagonist, and through a reduction in ICAM-1 and VCAM-1. In fact, older ICAM-1-deficient mice, or even those lacking CD11b/CD18 (one of the main ligands for ICAM-1), have shown an increase in obesity [37]. Additionally, adiponectin levels are decreased in obese and type 2 diabetic subjects and negatively correlate with visceral mass [32].
2.3. Adipocyte Mass and Function and Their Relationship with the Immune Cells
From an immunologic point of view, obesity is considered as a state of low-grade inflammation of adipose tissue, which leads to the chronic activation of the immune system that alters diverse cell processes such as insulin resistance [12] and even diabetes. Additionally, obesity is associated with alterations in the function and number of immune cells; for example, increases in phagocytosis [38] and oxidative bursts [39] in monocytes and granulocytes and an increase in the number of leukocyte cells have been detected [40] (Figure 1). Studies on germ-free mice showed that these mice had 40% lower fat mass than mice in conventional conditions [41,42], suggesting that signals arising from the microbiome and immune response are vital for adipose tissue architecture and homeostasis.
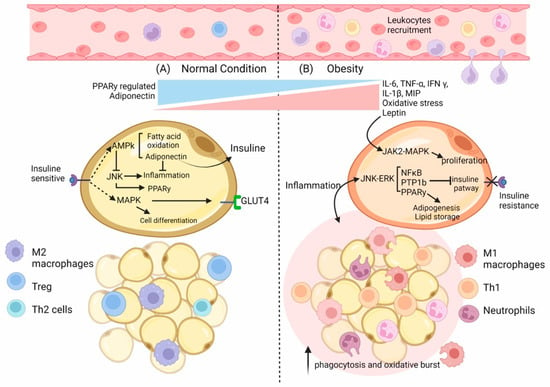
Figure 1.
Environment state and adipocyte’s changes in normal vs. obese conditions. (A) In normal conditions in the adipose tissue (AT), M2 macrophages are distributed evenly throughout the tissue. Lean AT also contains CD4+ T cells—TH2 and Treg cells—and preadipocytes that contribute to the anti-inflammatory and insulin-sensitive state. As a result, adipokines such as adiponectin are increased, whereas leptin content is decreased. In adipocytes, the regulation of differentiation and proliferation occurs by activating the insulin receptor (IR), which also controls the incorporation of glucose. Moreover, IR regulates the activation of AMPK in an energy state-dependent manner, promoting β-oxidation and adiponectin expression, resulting in the control of inflammation. (B) In obese AT, specific immune cells such as macrophages increase their number by local proliferation and contribute to secreting pro-inflammatory cytokines and ROS. In addition, the content of other immune cells also changes with an increase in Neutrophils, CD4+ Th1, and CD8+ T cell numbers, a reduction in Treg cell as well as an increase in the number of preadipocytes. As a result, the production of pro-inflammatory adipokines such as TNF-α, IL-6, IL-1β, and MCP1 increases; consequently, there is an increased immune cell infiltration and a polarization of the phenotype. Furthermore, in adipocytes, the augmented inflammatory state disrupts the regulation of signaling pathways such as JNK-ERK, which involves the inhibition of the IR by PTPB1, the up-regulation of PPAR-γ, and the synthesis of lipids as well as of the inflammatory NFkB pathway, which is also enhanced by overexpressed Leptin in an obese condition.
The immune cells present in AT can also be affected by obesity; for example, adipose tissue macrophages from obese animals have more cells expressing I-Ab (mouse class II MHC molecule) that are implicated in antigen presentation as well as in the induction of T-cell proliferation [43], which is indicative of their essential role in immune regulation. Additionally, a study conducted in 2019 demonstrated that a high-fat diet (HFD) induced adiponectin expression. This led to a reduction in Interferon (IFN)-γ and IL-17-positive CD4+ T cells and dampened the differentiation of naïve T cells into Th1 cells and Th17 cells. They concluded that adiponectin reduces Th17 cell differentiation and restrains glycolysis in an AMPK-dependent fashion [24].
Interestingly, in obese subjects, the increased activity of Nuclear Factor Kappa B (NFkB) [44,45], a master regulator of proinflammatory cytokines [46], has been shown to lead to a decreased level of the NFkB inhibitor, IκBα [47]. This nuclear factor modulates the expression of IL-6, TNF-α [46], and MIP [48]; these cytokines are closely associated with obesity [49,50] and pathogen responses [51,52,53]. In fact, in obese subjects, the elevation of pro-inflammatory cytokines such as TNF-α or IL-6 has been noted [49,54]. Remarkably, the IL-6 secreted by adipocytes could reach up to 30% of the total IL-6 found in serum [55,56], suggesting that this tissue plays a vital role in modulating the immune response. The relationship between inflammation and obesity has been controversial. On the one hand, several studies have mentioned that inflammatory markers may be associated with weight gain, especially in middle-aged adults [57]. By contrast, some reports have noted a negative correlation between cytokine levels (IL-6, adiponectin, and TNF-α) and obesity [58], suggesting that inflammation is a consequence of obesity rather than a contributing factor [56].
Other signaling pathways related to obesity, infection, and immunity are MAPKs (especially C-Jun N-terminal Kinases (JNK)) and JAK–STAT [59,60,61]. Although JNK is one of the most addressed signal transducers in metabolic processes such as obesity and insulin resistance [62], JNK is mainly activated by growth factors and cytokines [63]. Additionally, in several tissues of obese subjects, inflammatory signals that activate JNK have been observed. The functional consequences of this include an increase in NFkB and Activator Protein 1 (AP-1) signaling, leading to more inflammatory signals (IL-1β, TNF-α, and IL-6) [64] that alter insulin sensitivity and later culminate in aggravated insulin resistance. Moreover, these proinflammatory signals modulate the Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ), controlling adiponectin expression and, later, the proinflammatory status [64].
JNK can modulate pathways other than NFkB, AP-1, and PPAR-γ. It can also activate the PTP1b (Protein-Tyrosine Phosphatase 1B) signaling pathway to regulate the insulin pathway in diverse cells and tissues [64]. For example, the expression and activation levels of PTP1b are increased in obese subjects, blocking insulin pathways. In contrast, PTP1b knockout mice show resistance to weight gain and have an increased insulin sensitivity even with a high-fat diet [64].
The importance of JNK in insulin resistance has been addressed in different ways; for example, with gene knockout technology, the deletion of JNK1 in adipocytes has been found to inhibit insulin resistance [65], whereas the lack of JNK2 was found to “boost” the sensitivity to insulin at a cellular level [66]. JNK signaling pathways are divergent; first, the lack of JNK1 leads to a decrease in the phosphorylation of IRS1 (Insulin Receptor Substrate 1), enhancing the insulin signaling pathway, which leads to a rise in cell sensitivity to insulin. Second, the absence or inhibition of JNK2 suppresses the expression and function of NFkB and AP-1, which lessens insulin resistance [67].
PPAR-γ is a key factor in adipocyte differentiation, lipid metabolism, atherosclerosis, insulin resistance, and inflammatory response [29]. The activation of this molecule inhibits the expression of inflammatory-related genes such as those encoding Nitric Oxide Synthase (NOS), metalloproteinases, ILs, and cytokines in general [29], which contribute to insulin resistance.
It has been noted that TNF-α can block the transcriptional activity of PPAR-γ via Extracellular signal-Regulated Kinase (ERK) and JNK pathways [30]. This PPAR-γ downregulation impacts the expression of adiponectin, decreasing its levels and altering the modulation of insulin sensitivity in target cells. For example, fat cells that do not express PPAR-γ present lower levels of adiponectin, a reduced ability to store fat as well as decreased insulin sensitivity [68].
Finally, the JAK–STAT and MAPK signaling pathways are also altered in obesity [59,60,61]. The leptin receptor has been shown to activate JAK–STAT signaling pathways, mainly JAK2–STAT3, inducing the transcription of target genes [31,69]. It is worth mentioning that JAK2 can phosphorylate IRS1 and subsequently attract PI3K, thus activating downstream pathways [69]. On the other hand, JAK2 could also trigger the MAPK pathway, especially ERK1/2, in response to insulin, leading to the expression of several target genes such as c-fos and egr-1 [70], which participate in cell proliferation and differentiation [71]. In fact, the cardiomyocytes of C57bl6 mice stimulated with leptin showed the phosphorylation of STAT3 after 15 min of treatment [72]. Additionally, P38 MAPK is phosphorylated in response to leptin [59,72].
In general, diverse signaling pathways are activated by either microorganisms or immune signals that affect adipocyte homeostasis; this convergence highlights the infectobesity hypothesis as a new research field.
2.4. IFN Response
As previously mentioned, one of the most altered signaling pathways is JAK–STAT, with one of the most important downstream effectors being IFNs. Type I and type II IFNs are molecules formed in response to viral infections or cancer invasion and are fundamental to the initiation and regulation of the immune response. In general, IFNs directly induce antiviral responses within infected cells and are more critical for surrounding cells through the upregulation of molecules that can antagonize virus replication (e.g., IFN-stimulated effector genes (ISGs) that limit virus replication and spreading [73]). Moreover, as previously mentioned, several viruses have been related to obesity. Thus, IFNs—especially antiviral IFNs—appear to be pivotal actors in regulating adipogenesis, lipid metabolism, and membrane composition [74]. These phenomena are achieved by the repression of fatty acid and cholesterol synthesis and by controlling upstream mediators of the AMPK and the Mechanistic Target of Rapamycin (mTOR) complex [74]. In more detail, mTOR regulates IFN expression, and IFN regulates mTOR effectors [75], which leads to a mutual relationship.
On the other hand, obesity can drive changes in immune response such as by producing an attenuated and prolonged IFN response that would later result in antiviral inefficacy. One of the most closely related molecules is leptin. Leptin could upregulate the Suppressor of Cytokine Signaling 3 (SOCS3) [76] (the expression levels of SOCS3 are higher in obese patients than in non-obese subjects) [77]; this change in SOCS3 expression impairs type I IFN response (via JAK–STAT) as well as other functions in various immune cells. For example, the IFN-α/β production and altered signaling in respiratory epithelial cells and macrophages in obese patients as compared to those in non-obese individuals [78] were found to cause an increase in inflammatory responses that resulted in infectobesity.
Additionally, long-chain fatty acids can induce type I IFN responses in hepatocytes and macrophages. At the same time, a high-fat diet stimulates type I IFN-regulated genes in hepatocytes [79]. One of these genes is Interferon-Response Factor 3 (IRF3), the expression of which is positively associated with insulin sensitivity and negatively associated with type 2 diabetes in human adipose tissue. In the preadipocytes of mice, a lack of IRF3 induces an increase in PPAR-γ [80] and its proadipogenic genes, resulting in high adipogenesis and increased effects on adipocyte functionality. Furthermore, IRF3−/− mice develop obesity, insulin resistance, glucose intolerance, and finally, type 2 diabetes due to an increase in proinflammatory macrophages (M1) and the concomitant loss of IL-10 in adipose tissue [79].
Additionally, IFN-γ and IFN-β regulate ceramide metabolism [74]; the levels of ceramide are considered as a biomarker of insulin resistance and obesity. It has been demonstrated that the lack of IFN-γ in mice (C57BL6) modestly increases insulin sensitivity, thus decreasing adipocyte size and regulating cytokine release. It has been suggested that IFN-γ is a pivotal molecule in regulating inflammation and glucose [81]. In 2009, McGillicuddy et al. demonstrated that IFN-γ downregulates the insulin receptor, IRS-1, and Glucose Transporter 4 (GLUT4), in addition to causing the loss of adipocyte triglyceride storage and a reduction in PPAR-γ, adiponectin, perilipin, and fatty acid synthase, among proteins. Additionally, they noted that the treatment with IFN-γ affected the differentiation of preadipocytes into mature adipocytes [81]. Moreover, obese IFN-γ−/− mice showed a reduced expression of inflammatory genes (TNF-α and MCP1) in adipose tissue, which led to a decrease in local inflammation and an improvement in glucose tolerance [82].
Type I IFN is closely related to obesity; for example, an IFN-β1 overexpression model exhibited an inhibition of body weight gain independently of food intake [83]. Moreover, IFNs enhance glucose uptake in diverse cell types (MEFs and human pDCs) and increase ATP production (TCA cycle), glycolysis, and oxidative phosphorylation in immune cells, cancer cell lines, and keratinocytes [74].
The dysregulation of the IFN–obesity axis may result in the potential increase in a host’s susceptibility to viral infections, such as co-infections and even opportunistic infections in overweight patients [74].
The tissue-specific response induced by IFN has been previously described; for example, the deletion of IFNαr1 in hepatocytes exacerbated the steatosis and inflammation generated by a methionine- and choline-deficient diet. Additionally, a lack of this gene in adipose tissue was found to worsen the metabolic dysregulation induced by a high-fat diet, as indicated by weight gain, insulin resistance, and impaired glucose tolerance. By contrast, its deletion in intestinal epithelial or myeloid cells does not affect susceptibility to metabolic disease [79].
3. Adipocytes as Infection Targets and Their Relationship with Infectobesity
As mentioned previously, adipose tissue plays a vital role in immunity and the inflammation mediated by infiltrated or resident immune cells or by humoral factors such as cytokines or adipokines. Furthermore, several reports have established that obesity is a state of prolonged inflammation. This leads to the hypothesis that chronic inflammation due to an infection (infectobesity) in adipose tissue can cause obesity. Infectobesity is a phenomenon that has been characterized in diverse viral infections, and those pathogens can infect the adipocytes. Interestingly, viruses are not the only pathogens that can infect adipose tissue components; in recent years, it has been described how different pathogens can infect and alter lipid metabolism in the cell.
Diverse signaling pathways in adipose tissue are affected in response to infection, with the PI3K–AKT–mTOR axis being one of the most altered. This signaling pathway is involved in the control of nutrients, cellular proliferation, growth, survival, and mobility [84,85]. In the case of infections, PI3K–AKT–mTOR is a target for the modulation of expression or the state of activation. For example, Trypanosome cruzi (T. cruzi) infection increases PI3K expression and AKT activation in 3T3-L1 adipocytes [86], linking T. cruzi infection with the insulin–IGF-1 receptor cascade, and later with insulin resistance.
Additionally, leptin is essential for negatively modulating the immune response against infection; for example, the absence of leptin in ob/ob mice leads to a higher formation of granulomas in mice infected with mycobacteria. Moreover, an increase in leptin receptors has been described in Human Immunodeficiency Virus (HIV) infections [26]. Additionally, active tuberculosis is correlated with weight loss, cachexia, and low serum concentrations of leptin, which in turn suppresses lymphocyte stimulation and the secretion of Th1 cytokines such as IL-2, IFN-γ, and TNF-α [26].
Therefore, the relationship between adipocyte infection and the development of obesity can be a relevant point, and it is necessary to gain a better understanding of adipocytes’ participation in the factors that trigger weight gain. Hence, the next section focuses on diverse pathogens that have demonstrated tropism toward adipocytes, some of which are arising as new candidates for adipocyte dysregulation.
3.1. Adenovirus-36
Adenoviruses are the best characterized pathogens that have been associated with the development of obesity. Clinical data, meta-analysis, and serologic studies support the idea that the serotype of adenovirus-36 (Ad-36) is the only one related to the property of triggering obesity in humans [87,88]. The main research models to understand the mechanisms utilized by Ad-36 to promote obesity have mainly been focused on animals infected with Ad-36 as compared with noninfected animals [89,90]. The biochemical markers in serum that are associated with an altered lipid profile and obesity (cholesterol, triglycerides, and glucose) are interesting because their detection during Ad-36 infection is decreased as compared to that in controls and even animals subjected to high-calorie diets. This suggests a process involving the metabolic regulation of these molecules in adipose tissues [91,92].
The idea that a respiratory virus has tropism toward adipose tissue is complex. However, experimental evidence in animals infected with Ad-36 showed that the virus could spread to peripheral organs, including the spleen, kidney, liver, and brain, but had tropism toward adipose tissues. A finding that strongly supports this tropism was the identification of viral DNA in human adipose tissue samples, thus suggesting the adipogenic potential of this pathogen [93]. In humans, serological studies have been contradictory in proposing a correlation between obesity and antibodies against Ad-36, whereas other studies have identified the null relationship between these two events [94,95,96].
The key to the adipogenic process induced by Ad-36 is the direct infection of adipose cells, which induces an increase in adipocyte cell size (hypertrophy) and number (hyperplasia) both in vitro and in vivo [97,98,99]. In addition, several studies have shown the differentiation and proliferation of adipocytes in human primary cells, human mesenchymal cells, and muscle cells. In this context, the cellular models widely used to study this adipogenesis are the mouse fibroblast 3T3-L1 preadipocytes, which are differentiated from adipocytes under the stimulus of insulin and dexamethasone [100,101]. Interestingly, Ad-36 triggers the differentiation of adipocytes without any additional stimulus by inducing the expression of adipogenesis-related genes. The upregulated genes observed were those involved in the increase in the intracellular levels of triglycerides (LPL), molecules such as glycerol-3-phosphate dehydrogenase, PPAR-γ, and CCAAT/binding enhancer proteins α (C/EBP-α) and β (C/EBP-β) [101]. In fact, PPAR-γ is considered the master regulator of the adipogenic process, and the increase in these cellular markers strongly suggests the modulation of the differentiation events in mature adipocytes after infection by Ad-36. Furthermore, the infection generates a reduction in the synthesis and secretion of leptin of up to 52%, the accumulation and de novo synthesis of fatty acids, and an increase in glucose uptake of up to 93%. Thus, the authors of [100,101] pointed out that these findings may contribute to the adipogenic effect mediated by Ad-36 infection.
The viral protein E4Orf1 has been identified as the principal protein responsible for affecting the molecular pathways that are involved in adipogenesis during Ad-36 infection. This protein possesses a PDZ domain-binding motif (PBM) in the carboxyl-terminal region [102]. This domain lets E4orf1 interact with several cellular proteins involved in regulating growth and metabolism. One of them is PI3K, which, via the stimulation of the insulin receptor, leads to the translocation of GLUT4 to the plasma membrane in adipocytes and thus to the uptake of glucose [103,104]. In addition, another study demonstrated that the E4ORF1 protein of Ad-36 is responsible for adipogenesis and the proliferation of adipocytes in 3T3-L1 cells and human adipocyte stem cells (hASCs), highlighting this protein for its ability to upregulate PI3K [105]. The molecular mechanism by which E4ORF1 activates PI3K forms a ternary complex with the Drosophila disc-large protein (Dlg1), recruits the kinase at the membrane, and promotes its activation. At later stages, PI3K activates AKT to promote an increase in cellular metabolism, survival, and even oncogenic transformation [106] (Figure 2A).
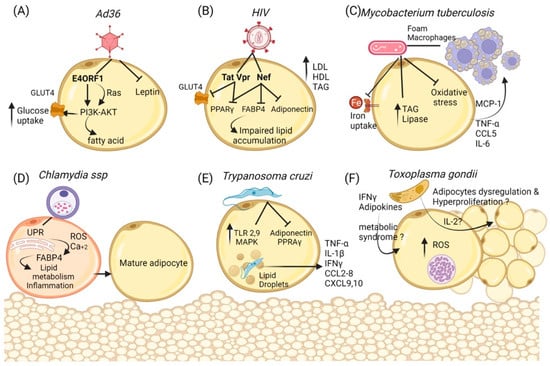
Figure 2.
Cellular and metabolic changes in adipocytes during infections. (A) Ad36 regulates the activation of Phosphoinositide 3-kinase (PI3K) by the recruitment of P13K to the membrane, PI3K-AKT leads to cellular glucose uptake by the glucose transporter Glut4 and could induce the de novo synthesis of fatty acids despite the down-regulation of the Insulin Receptor. Moreover, the inhibition of Leptin may increase lipid accumulation and the prevalence of obesity. (B) HIV proteins Vpr, Tat, and Nef participate as repressors of PPAR-γ and its target, such as GLUT4, besides Vpr expression, thus accelerating lipolysis and insulin resistance as well as preventing the maturation of adipocytes. (C) Mycobacterium tuberculosis can use adipose tissue as a reservoir and as macrophages, inducing lipogenic metabolism that can accumulate lipids (foam macrophages). Furthermore, adipocytes regulate inflammation by reducing oxidative stress in a decreased iron uptake manner, but augmenting pro-inflammatory cytokines and chemokines that recruit cells similar to macrophages. Additionally, the synthesis of triacylglycerol and lipase is increased to dispose of free fatty acids. (D) Chlamydia ssp infection increases the unfolded protein response and the guides to ROS production and Ca++ release; this could lead to FABP4 activity in adipocytes. Moreover, the expression of adipogenic markers is accompanied by lipid droplet accumulation, suggesting their transformation into differentiated adipocytes. (E) Trypanosoma cruzi infection dysregulates some proteins in the adipocyte, reducing adiponectin and PPAR- γ, but also signal molecules that stimulate inflammation such as Toll-like receptors (TLR-2 and -9). Additionally, cultured adipocytes presented a higher activation of the MAPK pathway in cultured adipocytes. Cytokines (TNF-α, IL-1β, IFN-γ) and chemokines (CCL2-CCL8 and CCL12), as well as the levels of CXCL9 and CXCL10, were markedly elevated in the adipose tissue of acutely infected mice. (F) Toxoplasma gondii bradyzoites use the adipose tissue as reservoirs; the infection with T gondii can induce oxidative stress and the subsequent inflammatory response. In addition, several cytokines, such as IL-2, could affect the adipocyte tissue and alter their metabolism or promote cell proliferation and hypertrophy as well the presence of Interferon-gamma and adiponectin chemerin in the serum, thus encouraging the development of the metabolic syndrome.
All this implies that Ad-36 infection leads to adipogenesis in mice; however, in humans, this hypothesis needs further research. Although the majority of the papers describe Ad-36 as the main actor in weight gain by prompting adipocyte hypertrophy, the complexity of this phenomenon based on the relationship of immune cells, adipocytes, and Ad-36 is not fully understood; a network involving these three components as a possible cause for obesity may exist.
3.2. Human Immunodeficiency Virus
Another pathogen that can infect adipocytes is the HIV. This infection is mainly characterized by massive CD4+ T cell depletion in the intestinal mucosa, which then affects blood and lymphoid CD4+ T cells, thus causing sustained systemic immune activation and inflammation [107]. In addition, people infected with HIV suffer from metabolic alterations (including dyslipidemia, insulin resistance, and lipodystrophy). The HIV lipodystrophy syndrome was initially considered an adverse reaction to the use of Antiretroviral drugs (ARVs). However, these pathologies have been observed in untreated patients, indicating that the virus directly impacts metabolism [108]. The metabolic disturbances associated with HIV lipodystrophy include insulin resistance, an elevation in Low-Density Lipoprotein (LDL)-C and triglyceride levels, and a decrease in High-Density Lipoprotein (HDL)-C levels [109].
Early approaches demonstrated how leptin levels were higher and adiponectin levels were lower in patients with HIV lipodystrophy syndrome, which was manifested by central fat accumulation and peripheral fat loss [109].
Subsequent evidence suggested that HIV affected the adipose tissue, and this led to the theory that adipose tissue may constitute a viral reservoir. The first approach was carried out in 2002 using lysates of whole adipose tissue samples from twenty-three antiretroviral-treated HIV patients. However, only two patients were found to be positive for HIV RNA [110]. Afterward, in 2015, using more suitable techniques, HIV was found in 11 samples from adipose tissue of HIV-infected patients, and HIV RNA was detected in the stromal vascular fraction (SVF) of the AT using ultrasensitive PCR [107]. In a different study, HIV RNA was detectable in the AT-SVF cells from other adipose depots (visceral, subcutaneous, or deep neck) of all five HIV patients studied [111].
In this context, it was investigated whether adipocytes were permissive to HIV, or whether HIV affected adipose tissue metabolism. As mentioned previously, some lipodystrophy is present in these patients. Damouche et al. found that the simian immunodeficiency virus (SIV), which infects macaques, could lead to elevated immune activation and inflammation of adipose tissue. Both residents’ CD4+ T cells and macrophages were infected, and this led to further investigation into HIV-infected patients. The results showed the replication of an HIV component in ex vivo CD4+ T cells sorted from adipose tissue that came from six “aviremic” patients. Those results confirmed that adipose tissue constituted a viral reservoir, even in ART-suppressed patients [107].
Later, it was described that some viral proteins of HIV—such as Viral protein R (Vpr), the Trans-activator of the Transcription protein (Tat), and the Negative regulatory Factor (Nef)—could mediate changes in adipocyte function through the alteration of the expression of adiponectin, LPL, GLUT4, and PPAR-γ [112,113] (Figure 2B). This correlates with the lipodystrophy syndrome observed in some patients.
In animal models, with the transgenic overexpression of viral proteins, Vpr was shown to block preadipocyte differentiation and the PPAR-γ-expression of the glucocorticoid receptor in adipocytes and was found to inhibit PPAR-γ in hepatocytes [114].
Additionally, a different study conducted using human samples from HIV-infected and HIV-negative women reported that HIV altered fat architecture. This study found that HIV patients presented a higher number of smaller adipocytes and increased fibrosis characterized by compact and thick fibrils of collagen invading the intercellular area. Further investigation showed that an increase in the mRNA and protein levels of collagen 1 α2 induces collagen expression in adipocytes, which causes fibrosis. Moreover, the presence of lentiviral proteins such as Nef was associated with a decrease in cellular lipid accumulation and in the expression and protein level of the adipogenic markers PPAR-γ and fatty acid-binding protein 4 (FABP4) [115]. Altogether, this suggests that HIV is a pathogen capable of using adipose tissue as a reservoir, thus infecting T CD4 cells present in the adipose tissue, which, in the end, alters the metabolism of these patients.
3.3. Mycobacterium tuberculosis
In the case of bacteria, some reports also associate the presence of bacteria in adipose tissue with dysregulation and obesity.
Mycobacterium tuberculosis (Mtb) causes tuberculosis, which is one of the top infectious diseases in humans worldwide. According to the WHO, a quarter of the world’s population has been infected with this pathogen. The primary tissue infected by Mtb is the lung; however, it has been described that during a latent infection, when the symptoms of the disease are absent but the bacteria are present, Mtb can stay in extrapulmonary cells that function as reservoirs.
Among the Mtb reservoirs are adipocytes [116]. This tropism has been thoroughly described in mouse models. In addition, several studies found the presence of Mtb in the adipose tissue after infection with the use of aerosol doses or by using different infection routes such as intravenous or intranasal routes. In this case, Mtb was identified in visceral adipose tissue, in both the adipose fraction and in the SVF (macrophages) between 14 and 28 days post-infection, indicating that adipose cells and leukocytes present in fat tissue harbor Mtb [117,118]. In addition, Mtb can infect adipocytes by binding through scavenger receptors in both mice and humans [119].
Mycobacteria remain in a dormant state in adipocytes [120] and need fatty acids from the host to survive [120]. Once in the cells, Mtb dysregulates all the cell metabolisms. For example, it has been well-described how Mtb modifies lipid and glucose metabolism at the cellular level by differentiating macrophages into foamy cells [121].
In order to obtain free fatty acids during active growth in vitro and in vivo, Mtb upregulates neutral lipid stores, some genes related to the accumulation of lipid bodies, and genes related to triacylglycerol synthases and lipases [120] (Figure 2C). Additional evidence identified that Mtb infection in 3T3-L1 induced the downregulation of genes involved in de novo fatty acid synthesis and the upregulation of genes related to triglyceride biosynthesis [122]. Aside from affecting lipid metabolism, mice afflicted with low doses of aerosol infection also presented an inflammation process in visceral and brown adipose tissues due to higher macrophage recruitment [123].
Interestingly, the link between Mtb infection and the immune response in adipose tissue has been evaluated and was found to affect the production of pro-inflammatory cytokines (TNF-α, IL-6, MCP-1) and leptin, which induces metabolic dysfunction in obesity [117,118]. It was also observed that these infected adipocytes secreted more CCL5; this increase is associated with hypertrophy. Furthermore, after eight weeks of infection, the mice showed an increase in hypertrophy as well as an increase in hyperplasic cells [123]. The alteration of cytokines was also observed in rabbits infected with Mtb, and an increase in the levels of proinflammatory mediators such as leptin, TNF-α, IL-6, and PPAR-γ was found. All of this could lead to dyslipidemia [116].
Although the majority of the functional tests have been conducted in mice, there has been evidence of the presence and influence of Mtb in humans. For example, in 2006, Mtb was found in the biopsy of the adipose tissue of an infected patient. Additionally, the researchers found mycobacteria in the adipose tissue of patients who died of other causes, suggesting that the infection was persistent. Later on, the Mtb’s presence in adipocytes was shown in vitro and in adipose tissue biopsies from TB patients [123].
Overall, it is important to consider Mtb infection as a subjacent cause of obesity, as it could remain dormant for years in adipose tissue, altering cell metabolism, triggering potential low-grade inflammation, and later, an increase in obesity.
3.4. Chlamydia ssp.
The principal pathogen associated with urogenital infections and sexual transmission is the intra-cellular obligate bacterium Chlamydia ssp. [124]. Some evidence to establish the seroprevalences in obese humans showed a critical response to IgG and IgA for Chlamydia pneumoniae. As for the other pathogens that were previously described, an increase in circulating lipid levels has been observed in samples from Chlamydia-seropositive individuals, suggesting a connection with the possible development of obesity. The vital role of lipids as intracellular nutrients for the proliferation of Chlamydia has been documented [125,126]. A current in vitro study has shown the ability of Chlamydia to infect preadipocytes and promote their differentiation to adipocytes in the 3T3-L1 cell line, suggesting the possibility that Chlamydia can induce infectobesity [127]. The animal models support the idea that Chlamydia activates lipid pathways during infection. Mouse adipocytes infected with Chlamydia develop a stress response of the endoplasmic reticulum that culminates in an overproduction of reactive oxygen species and the accumulation of cytoplasmic calcium. These signals have been associated with the production and secretion of molecules such as FABP4 [128,129] (Figure 2D). FABP4 is a cellular factor that regulates fatty acid mobilization and activates pathways associated with inflammation and metabolisms. Several reports suggest the use of this protein as a marker of obesity [130]. The link between infection with Chlamydia and this pathway could be a rearrangement of the cellular distribution of lipids to help the intracellular proliferation of bacteria; however, more studies are necessary to support this idea.
3.5. Trypanosoma cruzi
It has also been described how some parasites are capable of infecting adipocytes, which is the case of T. cruzi. This pathogen is the etiological agent of Chagas disease, a zoonotic pathology that can be transmitted to humans by blood-sucking triatomine bugs and is mainly found in rural regions in America [131,132]. One of the most affected tissues in Chagas disease is the heart, which triggers inflammatory cardiomyopathy [133].
The lifecycle of T. cruzi occurs between an infected triatomine insects (vector) and a human (host) and consists of three different stages [131,132]. This parasite infects several cell types such as macrophages, fibroblasts, nerve cells, and muscle cells [132]. However, in recent years, the ability of T. cruzi to infect adipose tissue has been described together with its relationship with metabolic alterations [15]. During chronic infection, adipocytes may be considered crucial long-term reservoirs for T. cruzi [15].
Experimental reports have described how hyperglycemia could be related to higher parasitaemia and mortality in T. cruzi-infected mice [134]. In the case of in vitro experiments, it has been demonstrated that 3T3-L1 cells can be efficiently infected with T. cruzi, which modifies their metabolic and proteomic response. For example, T. cruzi-infected adipocytes switch the expression levels of adipocyte-specific or -enriched proteins, with adipokines being one of the main altered proteins. Then, T. cruzi infection guides adipokines to a unique metabolic signature [15]. Additionally, electron micrographs of T. cruzi-infected cells showed a significant part with intracellular parasites around the lipid droplets, which are considered master regulators of lipid and energy homeostasis [135].
During acute infection, glucose levels in all the T. cruzi-infected mice were below those measured in the controls. However, an oral glucose tolerance test indicated a relatively normal ability to clear ingested glucose despite the high degree of inflammation associated with this infection [134]. Moreover, it was observed during murine acute infection that the levels of adiponectin were diminished in mice infected with T. cruzi. Furthermore, the expression of PPAR-y was also low. In the same mice model, it was found that the changes in lipid regulators were associated with calcium control, as the Ca2þ/calcineurin/NFAT cascade was upregulated in adipocytes, thus correlating with the decrease of adiponectin in adipocytes infected with T. cruzi [136].
In mice, not only adipokines were dysregulated in the presence of T. cruzi, but so were signaling molecules stimulating inflammation such as Toll-like receptors (TLR-2 and -9). Additionally, cultured adipocytes presented a higher activation of the MAPK pathway [86]. Cytokines (TNF-α, IL-1β, and IFN-γ) and chemokines (CCL2, CCL4, CCL5, CCL6, CCL7, CCL8, and CCL12), as well as the levels of CXCL9 and CXCL10, were markedly elevated in the adipose tissue of acutely infected mice. The levels of IL-1a, IL-1b, IL-17b, and IL-18 were also increased [134,137] (Figure 2E). The infection also affected the amount of lipids in adipose tissue, with a 15% reduction in lipid content and a 25% loss of lipids in the adipose tissue being observed [137].
Continuing with the mouse model, in the case of chronic infection, it has been reported that the loss of fat cells in T. cruzi-infected mice produces an increase in cardiac lipid load and a deregulated cardiac lipid metabolism, which leads to mitochondrial oxidative stress and endoplasmic reticulum stress. In addition, the loss of fat cells has been found to increase cardiac parasite load during acute infection and to alter immune signaling in the hearts of infected mice during chronic infection [138]. Altogether, these results suggest that adipose tissue (fat tissue) and the liver play important roles in maintaining and regulating cardiac lipid metabolism.
In humans, a higher incidence of diabetes in Chagas-infected people has also been described. This relationship may be due to the reduction of insulin in infected people. Additionally, it is worth mentioning that the infection of adipocytes produces an increase in proinflammatory cytokines and chemokines and a decrease in adiponectin and PPAR-γ (negative regulators of inflammation). Diverse reports indicate that the lack of adiponectin produces a cardiomyopathic phenotype. It is possible that T. cruzi infection affects the heart via the lack of adiponectin during Chagas pathogenicity [15].
It is well-known that T. cruzi persists in adipose tissue, and that it is capable of altering the homeostasis of the adipose tissue by regulating lipolysis and inflammation, thus promoting weight gain and supporting the infectobesity theory.
3.6. Toxoplasma gondii
Toxoplasma gondii (T. gondii), which is an intracellular protozoan that infects humans and domesticated and wild warm-blooded animals, is widely distributed around the world [139]. T. gondii infection is acquired by the ingestion of oocytes in contaminated water or vegetables, or by “fed” cysts in infected meat [139]. This parasite could pass through the intestinal epithelial barrier and multiply in the parasitophorous vacuole inside of various cell types [140]. The cells that can be infected by this parasite include dendritic cells, natural killers, monocytes, and macrophages [141,142]. This parasite can even cross the placental barrier and infect the fetus [143]. At the same time, the slow form of T. gondii (bradyzoites) can invade several tissues such as the heart, skeletal muscle, lung, brain [144,145], or adipose tissue. T. gondii has recently been described as a potential target in obesity research due to its implication in inflammation and its participation in the damage of pancreatic beta cells [146]. Those phenomena are related to metabolic syndrome. Additionally, the prevalence of metabolic syndrome was higher in patients who belonged to the T gondii-seropositive group than a T. gondii-seronegative group [147].
Experimental data show that T. gondii infection is related to a variation in weight gain; rats that were infected with T. gondii presented weight gain after 30 days. This was followed by a decrease in their weight in the following months. These weight variations were associated with behavioral changes due to the presence of T. gondii cysts in the brain, which possibly altered appetite regulation and hypothalamic function [148,149]. A study conducted on male Swiss Webster mice infected with T. gondii indicated that all infected mice presented a variation in weight, with an initial loss of 20–30%, and where half of these animals regained the lost weight or even more; however, another portion of these animals retained their low body weight [150]. Additionally, some authors mentioned that all these effects on weight variation may be influenced by the strain For example, it has been reported that two different strains of T. gondii had opposite effects on body weight [149,151].
In the case of human reports, there are some controversial studies. Thiodleifsson et al. reported a negative correlation between T. gondii and obesity; in this report, the authors did not find any correlation between the antibodies against T. gondii in overweight and non-overweight people [152]. However, Reeves et al. found a positive correlation between being overweight and T. gondii seroprevalence in adults (18–80 years) [149]. The variation in the results between these two investigations could be explained by the age range among the patients used by each independent study. Whereas the range in Reeves’ study was wider, Thiodleifsson excluded people older than 44 years old in their study.
These kinds of studies help to deepen our understanding of the relationship between obesity and infection. In addition, in the case of T. gondii, the presence of cytokines or immune molecules has recently been described. The presence of T. gondii leading to the release of IL-2 and other pro-inflammatory cytokines [153] could explain the weight variations present in T. gondii-infected people, i.e., they could be due to the presence of proinflammatory cytokines that induce adipocyte dysregulation [154]. The dysregulation of adipocytes then leads to metabolic syndrome due to an increase in the number of adipocytes and in insulin receptor sensitivity [154,155,156]. Additionally, adipocytes produce reactive oxygen species in response to inflammation and infections, including T. gondii infection [157,158,159,160] (Figure 2F). In general, the crosstalk between host immunity and parasites determines the dissemination of parasites and disease severity. However, further investigations are needed in order to fully understand the relationship between T. gondii and adipocytes and their association with overweight in rats and humans.
Altogether, the relationship between the previously described pathogens (viruses, bacteria, and parasites) and overweight demonstrates that adipocytes should be understood as cells associated with immunity. All of this is not strictly restricted to the pathogens mentioned here; we only focused on those for which more studies are to be conducted in humans. However, there are more pathogens associated with this theory in different animal models, and new ones that are capable of infecting human adipocytes are arising. Lastly, as this is a very complex field, and a detailed study of the pathogens needs to be conducted.
4. Gut Microbiota and Its Relationship with Obesity
In recent years, a natural component of our organism (the microbiota) has been considered as a principal regulator in different physiological processes. The microbiota is a community of microorganisms within a particular niche, whereas the consortium of all different microorganisms and their genomes is known as the microbiome [161,162]. Although bacteria have been identified within the main microorganisms that constitute this ecosystem, the participation of other biological entities such as archaea, eukaryotes (fungi), and viruses (bacteriophages) has begun to be considered [163,164,165]. Recent studies have revealed that these non-bacterial microbial populations are also dynamic communities, interacting with one another and playing a vital role in host wellbeing [165]. The gut microbiota has a wide distribution in our body, with a weight of 1–2 kg. In addition, the genetic information in this biological community is 10 times greater than the human genome [166]. Among the main functions of the gut microbiota is protecting and maintaining the intestinal mucosa through a symbiotic relationship with the host; additionally, it plays important roles such as supporting the immune development and providing protective, structural, and metabolic functions essential for the human body [165]. This intimate relationship allows microorganisms to contribute to physiological homeostasis, and abnormalities in the microbiota participate in the development of various diseases [167]. In this sense, it has been proven that there is an essential connection between metabolic disorders (including obesity) and the gut microbiota by showing that there is a regulation of fat storage, thus increasing energy collection and regulating the formation of substrates for lipid storage [166,168,169].
Studies to understand the relationship between the microbiota and obesity have identified a different microbial diversity in the obese population, with a higher proportion of Firmicutes bacteria and a low amount of Bacteroidetes compared to the microbiota of people with healthy weights [168] (Figure 3A). The main microbes identified in the microbiota of the obese population include Clostridium innocuum, Eubacterium dolichum, Catenibacterium mitsuokai, Lactobacillus reuteri, Lactobacillus sakei, and Actinobacteria, while Archaea such as Methanobrevibacter smithii have also been identified, albeit in lower numbers [169,170]. Therefore, it has been strongly suggested that a high proportion of the metabolic products and other biomolecules produced by these bacterial consortia could be responsible for the molecular mechanisms that link obesity and the microbiota [171].
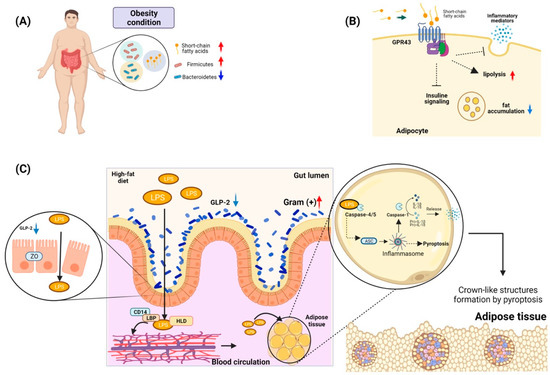
Figure 3.
Gut microbiota and its role in obesity. (A) Phylum of bacteria related to the development of obesity in individuals. Under conditions of obesity, bacteria belonging to the phylum Firmicutes are increased, while Bacteroidetes are decreased. These bacteria have been related to the presence of short-chain fatty acids. (B) Some G protein-coupled receptors abundant in adipocytes, such as GPR43, may play a role in the modulation of the immune response. Activation of GPR43 can promote lipolysis and decrease fatty acid accumulation. (C) Antigens from the bacterial microbiota of the gut in conditions of obesity, such as LPS, can cross the epithelial barrier of the gut due to the rupture of tight junctions such as zona occludens (ZO), which is related to the decrease in the expression of GLP-2. This LPS can travel through the circulation by forming a complex of HLD plus LBP and CD14. This complex gives it hydrosoluble characteristics, allowing it to reach the adipose tissue to be absorbed by adipocytes. Once inside the adipocyte, it is recognized by Caspases 4/5, which trigger the activation of the inflammasome. Then, it could activate either the maturation of proinflammatory molecules such as IL-1B and IL-18 or activate pyroptosis. The pyroptosis present in the adipose tissue can trigger the formation of crown-like structures, which are composed of immune system cells such as macrophages and adipose tissue cells and cells in pyroptosis. These structures have been described as an essential element in insulin resistance and in the development of chronic inflammation in obese individuals.
It has been documented that the abundance of Bacteroidetes in the intestinal microbiota has a direct positive relationship with the concentrations of short-chain fatty acids (propionate, butyrate, and acetate) in feces; these are associated with fermentation processes or the absorption of carbohydrates from the diet [172]. Moreover, these metabolites play a role in cellular signaling mediated by interactions with G-protein-coupled receptors, which have been reported to be widely distributed in adipocytes [173]. Interestingly, the receptor GPR43, when interacting with a short-chain fatty acid, participates in the modulation of the immune response by reducing the release of inflammatory mediators and increasing hypothalamic sensitivity to leptin. In addition, in adipose tissue, the activation of GPR43 could suppress insulin signaling, culminating in the inhibition of fat accumulation and activating lipolysis in adipocytes (Figure 3B) [174]. Finally, animal models deficient in the expression of GPR43 that were subjected to a diet high in carbohydrates and fats developed less body mass and higher lean mass than wild-type animals [175].
Another microbiota product that may be a factor related to obesity is lipopolysaccharide (LPS), an amphipathic molecule mainly consisting of an O-antigen, a core region, and a lipophilic lipid (lipid A) that has toxic effects and immunomodulatory functions [176]. Studies with animals subjected to high-fat diets have identified an increase in the presence of Gram-positive bacteria such as Bifidobacterium in the intestinal gut and high levels of LPS in the plasma of these animals. Additionally, a mechanism for the presence of LPS in the circulation has been evidenced to be associated with a decrease in Bifidobacterium, which is related to the low production of a proglucagon-derived peptide in the intestine with trophic action (GLP-2) [177]. This molecule is produced by the L cells in the intestinal mucosa, participates in intestinal growth, and functions as a barrier. Its decrease promotes the lowering of the expression of tight-junction proteins such as zona occludens proteins, which promotes an increase in intestinal permeability and thus facilitates the translocation of LPS to the circulation [177]. However, for the LPS to regulate adipose tissues, it needs to be transported in the serum to these areas. It has been identified that there is a primary interaction of 90% of LPS with lipoproteins [178]. One of the proteins with the most significant capacity to bind to LPS is high-density lipoprotein (HDL). In addition, it has been observed that LPS also binds rapidly to soluble CD14 or to LPS-binding proteins (LBPs), which are located within HDL and thereby transport the LPS to fat tissue (Figure 3C). Finally, this complex, HDL–sCD14–LB–LPS, may be internalized by the adipocytes and macrophages [179,180].
To better understand the possible role of LPS as a stimulus in adipocytes, several hypotheses are being investigated. One of them is related to a possible effect of pyroptosis generation and inflammation mediated by inflammasome activation [181]. In animal models, it has been identified that intracellular LPS can activate caspase-11, whose homolog in humans is caspase-4/5 [182]. Furthermore, it has been described that this non-canonical pathway can serve as a sensor of the innate response independent of the effect of LPS recognition by TLR-4 [183]. Evidence has suggested that there is an LPS-binding site within the CARD domain of caspase-11 (caspase-4/5), and it has also been identified that LPS binding promotes caspase oligomerization, promoting NLRP-3-mediated assembly by ASC. Therefore, it is possible that NLRP-3 activates caspase-1, which leads to the activation of pro-IL-1b and pro-lL-18, and finally, to the secretion of these proinflammatory cytokines. However, LPS-activated caspase-4/5/11 can also directly induce pyroptosis [184].
Interestingly, it has been identified that in the adipose tissue of obese people, there are cellular structures made up of adipocytes, macrophages, and the remnants of adipocytes that have undergone pyroptosis. These crown-like structures can hold macrophages in adipose tissue, thereby initiating insulin resistance [185]. The hypothesis on the role of LPS in the generation of pyroptosis in adipocytes is associated with the possible aggregation of LPS in the adipocyte membrane and its interaction with pro-caspases-4, 5 and 11; through the CARD domains, the LPS can associate and mediate the oligomerization and activation of caspases, culminating in the activation of pyroptotic pathways [181].
The emerging relationship between obesity and the microbiota opens a new and interesting landscape for investigation, given that the microbiota is not widely considered from a pathogenic perspective; a slight imbalance could lead to obesity. For this reason, further research could help to elucidate the association between microbiota stimulation, immunity, and infectobesity.
5. Conclusions
The immediate solution for managing obesity in the population is the promotion of weight control and weight loss. However, the multifactorial etiology that triggers this condition in human health is complex. It involves new points that must be addressed, such as the possible interaction between some infections and the development of obesity. In this respect, the evidence shows that adipocytes in some infections have been correlated with a trigger for obesity. These cells could function as a potential target for infection, allowing pathogens to access this tissue and generate different events that lead to the activation of lipogenic pathways, adipocyte differentiation, and the proliferation process. Additionally, the possibility that adipocytes could function as reservoirs for these pathogens could be another mechanism by which infections in the obese population exhibit persistent behavior and produce more severe outcomes.
Interestingly, a link between obesity and infections is the intermediary role of the immune response of the host. It is clear that the notion that adipocytes function simply as energy reservoirs is incorrect. These cells have great plasticity, allowing them to sense inflammatory signals and produce molecules that function as immune stimulators. The infectobesity hypothesis should consider future interrogatives to help elucidate how the immune response regulates inflammatory processes within the adipocyte under infection conditions. These signals could produce a favorable cellular or organism environment, which may hamper the control of the infection or trigger mechanisms that generate a type of persistence in these tissues, thus favoring an increase in fat cells and initiating the obesity process. It is necessary to extend and address this interaction.
Author Contributions
Conceptualization, M.L.-J., O.L.-O. and N.C.M.-C.; writing—original draft preparation, O.L.-O., N.C.M.-C., V.J.C.-H., L.D.G.-G. and M.L.-J.; writing—review and editing, A.C.H.-R., M.R.-V., H.A.-R. and L.C.-B.; visualization, L.D.G.-G.; supervision, M.L.-J.; funding acquisition, M.L.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from Instituto Nacional de Perinatologia “Isidro Espinosa de lo Reyes” (2017-2-81) to M.L.-J.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
V.J.C.-H. and L.D.G.-G. received a fellowship from CONACYT. Additionally, O.L.-O., A.C.H.-R., M.R.-V, L.C.-B. and M.L.-J. acknowledge their membership in the National System of Research (SNI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tarantino, G.; Citro, V.; Cataldi, M. Findings from Studies Are Congruent with Obesity Having a Viral Origin, but What about Obesity-Related NAFLD? Viruses 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 April 2022).
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Berge, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Dhurandhar, N.V. Infectobesity: Obesity of infectious origin. Adv. Food Nutr. Res. 2007, 52, 61–102. [Google Scholar] [CrossRef] [PubMed]
- Catalan, V.; Aviles-Olmos, I.; Rodriguez, A.; Becerril, S.; Fernandez-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gomez-Ambrosi, J.; Fruhbeck, G. Time to Consider the “Exposome Hypothesis” in the Development of the Obesity Pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. (Lausanne) 2016, 7, 30. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The role of estrogen in adipose tissue metabolism: Insights into glucose homeostasis regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef]
- Cousin, B.; Casteilla, L.; Laharrague, P.; Luche, E.; Lorsignol, A.; Cuminetti, V.; Paupert, J. Immuno-metabolism and adipose tissue: The key role of hematopoietic stem cells. Biochimie 2016, 124, 21–26. [Google Scholar] [CrossRef]
- Chi, J.; Wu, Z.; Choi, C.H.J.; Nguyen, L.; Tegegne, S.; Ackerman, S.E.; Crane, A.; Marchildon, F.; Tessier-Lavigne, M.; Cohen, P. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab. 2018, 27, 226–236.e223. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.W.; Scherer, P.E. Minireview: The adipocyte—At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Benrick, A.; Chanclon, B.; Micallef, P.; Wu, Y.; Hadi, L.; Shelton, J.M.; Stener-Victorin, E.; Wernstedt Asterholm, I. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proc. Natl. Acad. Sci. USA 2017, 114, E7187–E7196. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Nagajyothi; Mukherjee, S.; de Almeida, C.J.; Jelicks, L.A.; Schubert, W.; Lin, Y.; Jayabalan, D.S.; Zhao, D.; Braunstein, V.L.; et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J. Biol. Chem. 2005, 280, 24085–24094. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Correa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 2019, 8, 662. [Google Scholar] [CrossRef]
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Gadgil, A.; Duncan, S.R. Role of T-lymphocytes and pro-inflammatory mediators in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 531–541. [Google Scholar] [CrossRef]
- Ouedraogo, R.; Gong, Y.; Berzins, B.; Wu, X.; Mahadev, K.; Hough, K.; Chan, L.; Goldstein, B.J.; Scalia, R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Investig. 2007, 117, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Surendar, J.; Frohberger, S.J.; Karunakaran, I.; Schmitt, V.; Stamminger, W.; Neumann, A.L.; Wilhelm, C.; Hoerauf, A.; Hubner, M.P. Adiponectin Limits IFN-gamma and IL-17 Producing CD4 T Cells in Obesity by Restraining Cell Intrinsic Glycolysis. Front. Immunol. 2019, 10, 2555. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919, quiz 920. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Tsoukas, M.A.; Farr, O.M.; Mantzoros, C.S. Leptin in congenital and HIV-associated lipodystrophy. Metabolism 2015, 64, 47–59. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef]
- Ye, J. Regulation of PPARgamma function by TNF-alpha. Biochem. Biophys. Res. Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, S.J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell. Signal. 2010, 22, 1350–1362. [Google Scholar] [CrossRef]
- Hornung, F.; Rogal, J.; Loskill, P.; Loffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Dong, Z.M.; Gutierrez-Ramos, J.C.; Coxon, A.; Mayadas, T.N.; Wagner, D.D. A new class of obesity genes encodes leukocyte adhesion receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 7526–7530. [Google Scholar] [CrossRef]
- Pires-Lapa, M.A.; Tamura, E.K.; Salustiano, E.M.; Markus, R.P. Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. J. Pineal Res. 2013, 55, 240–246. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, X.J.; Ma, Q.H.; Xu, Y.; Chen, X.; Wang, P.; Pan, C.W. Leukocyte related parameters in older adults with metabolically healthy and unhealthy overweight or obesity. Sci. Rep. 2021, 11, 4652. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gerard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Cho, K.W.; Delproposto, J.L.; Oatmen, K.E.; Geletka, L.M.; Martinez-Santibanez, G.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013, 62, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Tantiwong, P.; Shanmugasundaram, K.; Monroy, A.; Ghosh, S.; Li, M.; DeFronzo, R.A.; Cersosimo, E.; Sriwijitkamol, A.; Mohan, S.; Musi, N. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E794–E801. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.A.; Anderson-Baucum, E.K.; Kennedy, A.J.; Webb, C.D.; Yull, F.E.; Hasty, A.H. Activation of NF-kappaB drives the enhanced survival of adipose tissue macrophages in an obesogenic environment. Mol. Metab. 2015, 4, 665–677. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Yuan, M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int. J. Obes. 2003, 27 (Suppl. 3), S49–S52. [Google Scholar] [CrossRef]
- Achyut, B.R.; Angara, K.; Jain, M.; Borin, T.F.; Rashid, M.H.; Iskander, A.S.M.; Ara, R.; Kolhe, R.; Howard, S.; Venugopal, N.; et al. Canonical NFkappaB signaling in myeloid cells is required for the glioblastoma growth. Sci. Rep. 2017, 7, 13754. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 120–122. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Szretter, K.J.; Gangappa, S.; Lu, X.; Smith, C.; Shieh, W.J.; Zaki, S.R.; Sambhara, S.; Tumpey, T.M.; Katz, J.M. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 2007, 81, 2736–2744. [Google Scholar] [CrossRef]
- Albrecht, L.J.; Tauber, S.C.; Merres, J.; Kress, E.; Stope, M.B.; Jansen, S.; Pufe, T.; Brandenburg, L.O. Lack of Proinflammatory Cytokine Interleukin-6 or Tumor Necrosis Factor Receptor-1 Results in a Failure of the Innate Immune Response after Bacterial Meningitis. Mediat. Inflamm. 2016, 2016, 7678542. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Vanier, G.; Al-Numani, D.; Lacouture, S.; Olivier, M.; Gottschalk, M. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol. Med. Microbiol. 2006, 47, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Fasshauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Harkins, J.M.; Moustaid-Moussa, N.; Chung, Y.J.; Penner, K.M.; Pestka, J.J.; North, C.M.; Claycombe, K.J. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J. Nutr. 2004, 134, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Chambless, L.E.; Folsom, A.R.; Carpenter, M.; Heiss, G. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults—The ARIC study. Atherosclerosis Risk in Communities. Obes. Res. 2000, 8, 279–286. [Google Scholar] [CrossRef]
- Tuomisto, K.; Jousilahti, P.; Havulinna, A.S.; Borodulin, K.; Mannisto, S.; Salomaa, V. Role of inflammation markers in the prediction of weight gain and development of obesity in adults—A prospective study. Metab. Open 2019, 3, 100016. [Google Scholar] [CrossRef]
- Yang, R.; Barouch, L.A. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 2007, 101, 545–559. [Google Scholar] [CrossRef]
- Richard, A.J.; Stephens, J.M. The role of JAK-STAT signaling in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 431–439. [Google Scholar] [CrossRef]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.; Stephens, L.R.; Hawkins, P.T.; Saklatvala, J. Synergistic activation of JNK/SAPK by interleukin-1 and platelet-derived growth factor is independent of Rac and Cdc42. Biochem. J. 1999, 338 Pt 2, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The Role of JNk Signaling Pathway in Obesity-Driven Insulin Resistance. Diabetes Metab. Syndr. Obes. 2020, 13, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wilcox, D.M.; Haasch, D.L.; Jung, P.M.; Nguyen, P.T.; Voorbach, M.J.; Doktor, S.; Brodjian, S.; Bush, E.N.; Lin, E.; et al. Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J. Biol. Chem. 2007, 282, 22765–22774. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Gorgun, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Shen, J.; Yang, T.; Xu, Y.; Luo, Y.; Zhong, X.; Shi, L.; Hu, T.; Guo, T.; Nie, Y.; Luo, F.; et al. delta-Tocotrienol, Isolated from Rice Bran, Exerts an Anti-Inflammatory Effect via MAPKs and PPARs Signaling Pathways in Lipopolysaccharide-Stimulated Macrophages. Int. J. Mol. Sci. 2018, 19, 3022. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, H.; Du, M.; Wu, X. The effect of apelin-13 on pancreatic islet beta cell mass and myocardial fatty acid and glucose metabolism of experimental type 2 diabetic rats. Peptides 2019, 114, 1–7. [Google Scholar] [CrossRef]
- Mullen, M.; Gonzalez-Perez, R.R. Leptin-Induced JAK/STAT Signaling and Cancer Growth. Vaccines 2016, 4, 26. [Google Scholar] [CrossRef]
- Gong, T.W.; Meyer, D.J.; Liao, J.; Hodge, C.L.; Campbell, G.S.; Wang, X.; Billestrup, N.; Carter-Su, C.; Schwartz, J. Regulation of glucose transport and c-fos and egr-1 expression in cells with mutated or endogenous growth hormone receptors. Endocrinology 1998, 139, 1863–1871. [Google Scholar] [CrossRef][Green Version]
- Min, I.M.; Pietramaggiori, G.; Kim, F.S.; Passegue, E.; Stevenson, K.E.; Wagers, A.J. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2008, 2, 380–391. [Google Scholar] [CrossRef]
- Leifheit-Nestler, M.; Wagner, N.M.; Gogiraju, R.; Didie, M.; Konstantinides, S.; Hasenfuss, G.; Schafer, K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Li, M.M.H. All about the RNA: Interferon-Stimulated Genes that Interfere with Viral RNA Processes. Front. Immunol. 2020, 11, 605024. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jennings, J.; Gong, Y.; Sang, Y. Viral Infections and Interferons in the Development of Obesity. Biomolecules 2019, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Miller, L.C.; Blecha, F.; Sang, Y. Reduction of infection by inhibiting mTOR pathway is associated with reversed repression of type I interferon by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2017, 98, 1316–1328. [Google Scholar] [CrossRef][Green Version]
- Inagaki-Ohara, K.; Mayuzumi, H.; Kato, S.; Minokoshi, Y.; Otsubo, T.; Kawamura, Y.I.; Dohi, T.; Matsuzaki, G.; Yoshimura, A. Enhancement of leptin receptor signaling by SOCS3 deficiency induces development of gastric tumors in mice. Oncogene 2014, 33, 74–84. [Google Scholar] [CrossRef]
- Wunderlich, C.M.; Hovelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT 2013, 2, e23878. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Wieser, V.; Adolph, T.E.; Grander, C.; Grabherr, F.; Enrich, B.; Moser, P.; Moschen, A.R.; Kaser, S.; Tilg, H. Adipose type I interferon signalling protects against metabolic dysfunction. Gut 2018, 67, 157–165. [Google Scholar] [CrossRef]
- Kumari, M.; Wang, X.; Lantier, L.; Lyubetskaya, A.; Eguchi, J.; Kang, S.; Tenen, D.; Roh, H.C.; Kong, X.; Kazak, L.; et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Investig. 2016, 126, 2839–2854. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Winters, B.R.; Diggs, B.S.; Zhu, X.; Marks, D.L. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism 2012, 61, 1152–1161. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Alsaggar, M.; Mills, M.; Liu, D. Interferon beta overexpression attenuates adipose tissue inflammation and high-fat diet-induced obesity and maintains glucose homeostasis. Gene Ther. 2017, 24, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Guan, K.L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef]
- Nagajyothi, F.; Desruisseaux, M.S.; Thiruvur, N.; Weiss, L.M.; Braunstein, V.L.; Albanese, C.; Teixeira, M.M.; de Almeida, C.J.; Lisanti, M.P.; Scherer, P.E.; et al. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity 2008, 16, 1992–1997. [Google Scholar] [CrossRef]
- Trovato, G.M.; Martines, G.F.; Garozzo, A.; Tonzuso, A.; Timpanaro, R.; Pirri, C.; Trovato, F.M.; Catalano, D. Ad36 adipogenic adenovirus in human non-alcoholic fatty liver disease. Liver Int. 2010, 30, 184–190. [Google Scholar] [CrossRef]
- Parra-Rojas, I.; Del Moral-Hernandez, O.; Salgado-Bernabe, A.B.; Guzman-Guzman, I.P.; Salgado-Goytia, L.; Munoz-Valle, J.F. Adenovirus-36 seropositivity and its relation with obesity and metabolic profile in children. Int. J. Endocrinol. 2013, 2013, 463194. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Israel, B.A.; Kolesar, J.M.; Mayhew, G.F.; Cook, M.E.; Atkinson, R.L. Increased adiposity in animals due to a human virus. Int. J. Obes. 2000, 24, 989–996. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Whigham, L.D.; Abbott, D.H.; Schultz-Darken, N.J.; Israel, B.A.; Bradley, S.M.; Kemnitz, J.W.; Allison, D.B.; Atkinson, R.L. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J. Nutr. 2002, 132, 3155–3160. [Google Scholar] [CrossRef]
- Kapila, M.; Khosla, P.; Dhurandhar, N.V. Novel short-term effects of adenovirus Ad-36 on hamster lipoproteins. Int. J. Obes. 2004, 28, 1521–1527. [Google Scholar] [CrossRef]
- Na, H.N.; Park, S.; Jeon, H.J.; Kim, H.B.; Nam, J.H. Reduction of adenovirus 36-induced obesity and inflammation by mulberry extract. Microbiol. Immunol. 2014, 58, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Loiler, S.; Dhurandhar, N.V. Acute effect of infection by adipogenic human adenovirus Ad36. Arch. Virol. 2008, 153, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Broderick, M.P.; Hansen, C.J.; Irvine, M.; Metzgar, D.; Campbell, K.; Baker, C.; Russell, K.L. Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among US military personnel. Int. J. Obes. 2010, 34, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Pollock, N.K.; Laing, E.M.; Warden, S.J.; Hill Gallant, K.M.; Hausman, D.B.; Tripp, R.A.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M.; et al. Association of adenovirus 36 infection with adiposity and inflammatory-related markers in children. J. Clin. Endocrinol. Metab. 2014, 99, 3240–3246. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Lee, I.; Shin, H.J.; He, J. Human adenovirus-36 antibody status is associated with obesity in children. Int. J. Pediatr. Obes. 2010, 5, 157–160. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Bailey, D.; Thomas, D. Interaction of obesity and infections. Obes. Rev. 2015, 16, 1017–1029. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Dhurandhar, E.J.; Ingram, D.K.; Vaughan, K.; Mattison, J.A. Natural infection of human adenovirus 36 in rhesus monkeys is associated with a reduction in fasting glucose 36. J. Diabetes 2014, 6, 614–616. [Google Scholar] [CrossRef]
- Rathod, M.; Vangipuram, S.D.; Krishnan, B.; Heydari, A.R.; Holland, T.C.; Dhurandhar, N.V. Viral mRNA expression but not DNA replication is required for lipogenic effect of human adenovirus Ad-36 in preadipocytes. Int. J. Obes. 2007, 31, 78–86. [Google Scholar] [CrossRef]
- Vangipuram, S.D.; Sheele, J.; Atkinson, R.L.; Holland, T.C.; Dhurandhar, N.V. A human adenovirus enhances preadipocyte differentiation. Obes. Res. 2004, 12, 770–777. [Google Scholar] [CrossRef]
- Vangipuram, S.D.; Yu, M.; Tian, J.; Stanhope, K.L.; Pasarica, M.; Havel, P.J.; Heydari, A.R.; Dhurandhar, N.V. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int. J. Obes. 2007, 31, 87–96. [Google Scholar] [CrossRef]
- Frese, K.K.; Lee, S.S.; Thomas, D.L.; Latorre, I.J.; Weiss, R.S.; Glaunsinger, B.A.; Javier, R.T. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene 2003, 22, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P.; Corvera, S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 1999, 274, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Yamanaka, H.; Fukuoka, T.; Dai, Y.; Obata, K.; Mashimo, T.; Noguchi, K. Expression of HGF and cMet in the peripheral nervous system of adult rats following sciatic nerve injury. Neuroreport 2001, 12, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.M.; Fusinski, K.A.; Rathod, M.A.; Loiler, S.A.; Pasarica, M.; Shaw, M.K.; Kilroy, G.; Sutton, G.M.; McAllister, E.J.; Mashtalir, N.; et al. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. Int. J. Obes. 2008, 32, 397–406. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Dubuisson, O.; Mashtalir, N.; Krishnapuram, R.; Hegde, V.; Dhurandhar, N.V. E4orf1: A novel ligand that improves glucose disposal in cell culture. PLoS ONE 2011, 6, e23394. [Google Scholar] [CrossRef]
- Damouche, A.; Lazure, T.; Avettand-Fenoel, V.; Huot, N.; Dejucq-Rainsford, N.; Satie, A.P.; Melard, A.; David, L.; Gommet, C.; Ghosn, J.; et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015, 11, e1005153. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Barrail-Tran, A.; Lagathu, C.; Capeau, J.; Desjardins, D.; Le Grand, R.; Damouche, A.; Bereziat, V.; Lambotte, O. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front. Microbiol. 2019, 10, 2837. [Google Scholar] [CrossRef]
- Kosmiski, L.; Kuritzkes, D.; Lichtenstein, K.; Eckel, R. Adipocyte-derived hormone levels in HIV lipodystrophy. Antivir. Ther. 2003, 8, 9–15. [Google Scholar] [CrossRef]
- Dupin, N.; Buffet, M.; Marcelin, A.G.; Lamotte, C.; Gorin, I.; Ait-Arkoub, Z.; Treluyer, J.M.; Bui, P.; Calvez, V.; Peytavin, G. HIV and antiretroviral drug distribution in plasma and fat tissue of HIV-infected patients with lipodystrophy. AIDS 2002, 16, 2419–2424. [Google Scholar] [CrossRef]
- Couturier, J.; Suliburk, J.W.; Brown, J.M.; Luke, D.J.; Agarwal, N.; Yu, X.; Nguyen, C.; Iyer, D.; Kozinetz, C.A.; Overbeek, P.A.; et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015, 29, 667–674. [Google Scholar] [CrossRef]
- Agarwal, N.; Iyer, D.; Patel, S.G.; Sekhar, R.V.; Phillips, T.M.; Schubert, U.; Oplt, T.; Buras, E.D.; Samson, S.L.; Couturier, J.; et al. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci. Transl. Med. 2013, 5, 213ra164. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Lewis, D.E. HIV Persistence in Adipose Tissue Reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Iyer, D.; Gabbi, C.; Saha, P.; Patel, S.G.; Mo, Q.; Chang, B.; Goswami, B.; Schubert, U.; Kopp, J.B.; et al. HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRalpha and PPARalpha dysregulation: Implications for HIV-specific pathogenesis of NAFLD. Sci. Rep. 2017, 7, 13362. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Feve, B.; Lambotte, O.; et al. Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, J.P.; Vinnard, C.; Subbian, S.; Nagajyothi, J.F. Effect of Mycobacterium tuberculosis infection on adipocyte physiology. Microbes Infect. 2018, 20, 81–88. [Google Scholar] [CrossRef]
- Beigier-Bompadre, M.; Montagna, G.N.; Kuhl, A.A.; Lozza, L.; Weiner, J., 3rd; Kupz, A.; Vogelzang, A.; Mollenkopf, H.J.; Lowe, D.; Bandermann, S.; et al. Mycobacterium tuberculosis infection modulates adipose tissue biology. PLoS Pathog. 2017, 13, e1006676. [Google Scholar] [CrossRef]
- Agarwal, P.; Khan, S.R.; Verma, S.C.; Beg, M.; Singh, K.; Mitra, K.; Gaikwad, A.N.; Akhtar, M.S.; Krishnan, M.Y. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes Infect. 2014, 16, 571–580. [Google Scholar] [CrossRef]
- Neyrolles, O.; Hernandez-Pando, R.; Pietri-Rouxel, F.; Fornes, P.; Tailleux, L.; Barrios Payan, J.A.; Pivert, E.; Bordat, Y.; Aguilar, D.; Prevost, M.C.; et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS ONE 2006, 1, e43. [Google Scholar] [CrossRef]
- Rastogi, S.; Agarwal, P.; Krishnan, M.Y. Use of an adipocyte model to study the transcriptional adaptation of Mycobacterium tuberculosis to store and degrade host fat. Int. J. Mycobacteriology 2016, 5, 92–98. [Google Scholar] [CrossRef]
- D’Avila, H.; Melo, R.C.; Parreira, G.G.; Werneck-Barroso, E.; Castro-Faria-Neto, H.C.; Bozza, P.T. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: Intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 2006, 176, 3087–3097. [Google Scholar] [CrossRef]
- Nandy, A.; Mondal, A.K.; Pandey, R.; Arumugam, P.; Dawa, S.; Jaisinghani, N.; Rao, V.; Dash, D.; Gandotra, S. Adipocyte Model of Mycobacterium tuberculosis Infection Reveals Differential Availability of Iron to Bacilli in the Lipid-Rich Caseous Environment. Infect. Immun. 2018, 86, e00041-18. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Cheng, C.Y.; Ketheesan, N.; Cullen, A.; Tang, Y.; Lum, J.; West, K.; Poidinger, M.; Guertin, D.A.; Singhal, A.; et al. mTORC2/Akt activation in adipocytes is required for adipose tissue inflammation in tuberculosis. EBioMedicine 2019, 45, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef] [PubMed]
- Jaworowska, A.; Bazylak, G. Chlamydophila pneumoniae antibodies may be independently associated with increased BMI and percentage of body fat among women. Int. J. Obes. 2011, 35, 1225–1232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rantala, A.; Lajunen, T.; Juvonen, R.; Bloigu, A.; Paldanius, M.; Silvennoinen-Kassinen, S.; Peitso, A.; Vainio, O.; Leinonen, M.; Saikku, P. Chlamydia pneumoniae infection is associated with elevated body mass index in young men. Epidemiol. Infect. 2010, 138, 1267–1273. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Zigangirova, N.A.; Kapotina, L.N.; Fedina, E.D.; Kyle, N.H. Chlamydia trachomatis promotes 3T3 cell differentiation into adipocytes. Adv. Clin. Exp. Med. 2014, 23, 511–516. [Google Scholar] [CrossRef]
- Goodall, K.J.; Nguyen, A.; McKenzie, C.; Eckle, S.B.G.; Sullivan, L.C.; Andrews, D.M. The murine CD94/NKG2 ligand, Qa-1(b), is a high-affinity, functional ligand for the CD8alphaalpha homodimer. J. Biol. Chem. 2020, 295, 3239–3246. [Google Scholar] [CrossRef]
- Walenna, N.F.; Kurihara, Y.; Chou, B.; Ishii, K.; Soejima, T.; Itoh, R.; Shimizu, A.; Ichinohe, T.; Hiromatsu, K. Chlamydia pneumoniae exploits adipocyte lipid chaperone FABP4 to facilitate fat mobilization and intracellular growth in murine adipocytes. Biochem. Biophys. Res. Commun. 2018, 495, 353–359. [Google Scholar] [CrossRef]
- Engeli, S.; Utz, W.; Haufe, S.; Lamounier-Zepter, V.; Pofahl, M.; Traber, J.; Janke, J.; Luft, F.C.; Boschmann, M.; Schulz-Menger, J.; et al. Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart 2013, 99, 944–948. [Google Scholar] [CrossRef]
- Steverding, D. The history of Chagas disease. Parasites Vectors 2014, 7, 317. [Google Scholar] [CrossRef]
- Urdaneta-Morales, S. Chagas’ disease: An emergent urban zoonosis. The caracas valley (Venezuela) as an epidemiological model. Front. Public Health 2014, 2, 265. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverria, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, F.; Desruisseaux, M.S.; Jelicks, L.A.; Machado, F.S.; Chua, S.; Scherer, P.E.; Tanowitz, H.B. Perspectives on adipose tissue, chagas disease and implications for the metabolic syndrome. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 824324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.B.; Villar, S.R.; Toneatto, J.; Pacini, M.F.; Marquez, J.; D’Attilio, L.; Bottasso, O.A.; Piwien-Pilipuk, G.; Perez, A.R. Immune response triggered by Trypanosoma cruzi infection strikes adipose tissue homeostasis altering lipid storage, enzyme profile and adipokine expression. Med. Microbiol. Immunol. 2019, 208, 651–666. [Google Scholar] [CrossRef]
- Santamaria, M.H.; Rios, L.D.; Corral, R.S. Trypanosoma cruzi down-regulates adiponectin expression in mouse adipocytes via the NFAT signaling pathway. Microbes Infect. 2021, 23, 104757. [Google Scholar] [CrossRef]
- Nagajyothi, F.; Desruisseaux, M.S.; Machado, F.S.; Upadhya, R.; Zhao, D.; Schwartz, G.J.; Teixeira, M.M.; Albanese, C.; Lisanti, M.P.; Chua, S.C., Jr.; et al. Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain). J. Infect. Dis. 2012, 205, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, K.; Ayyappan, J.P.; Oswal, N.; Weiss, L.M.; Scherer, P.E.; Nagajyothi, J.F. Fat tissue regulates the pathogenesis and severity of cardiomyopathy in murine chagas disease. PLoS Negl. Trop. Dis. 2021, 15, e0008964. [Google Scholar] [CrossRef]
- Tian, Y.M.; Dai, F.Y.; Huang, S.Y.; Deng, Z.H.; Duan, G.; Zhou, D.H.; Yang, J.F.; Weng, Y.B.; Zhu, X.Q.; Zou, F.C. First report of Toxoplasma gondii seroprevalence in peafowls in Yunnan Province, Southwestern China. Parasites Vectors 2012, 5, 205. [Google Scholar] [CrossRef]
- Feustel, S.M.; Meissner, M.; Liesenfeld, O. Toxoplasma gondii and the blood-brain barrier. Virulence 2012, 3, 182–192. [Google Scholar] [CrossRef]
- Persson, C.M.; Lambert, H.; Vutova, P.P.; Dellacasa-Lindberg, I.; Nederby, J.; Yagita, H.; Ljunggren, H.G.; Grandien, A.; Barragan, A.; Chambers, B.J. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infect. Immun. 2009, 77, 970–976. [Google Scholar] [CrossRef]
- Courret, N.; Darche, S.; Sonigo, P.; Milon, G.; Buzoni-Gatel, D.; Tardieux, I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 2006, 107, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.R.; Zeldovich, V.B.; Poukchanski, A.; Boothroyd, J.C.; Bakardjiev, A.I. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 2012, 80, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.S.; Cavanaugh, E.N. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N. Engl. J. Med. 1965, 273, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Di Cristina, M.; Marocco, D.; Galizi, R.; Proietti, C.; Spaccapelo, R.; Crisanti, A. Temporal and spatial distribution of Toxoplasma gondii differentiation into Bradyzoites and tissue cyst formation in vivo. Infect. Immun. 2008, 76, 3491–3501. [Google Scholar] [CrossRef]
- Shirbazou, S.; Delpisheh, A.; Mokhetari, R.; Tavakoli, G. Serologic Detection of Anti Toxoplasma gondii Infection in Diabetic Patients. Iran. Red Crescent Med. J. 2013, 15, 701–703. [Google Scholar] [CrossRef]
- Salem, D.A.; Salem, N.A.; Hendawy, S.R. Association between Toxoplasma gondii infection and metabolic syndrome in obese adolescents: A possible immune-metabolic link. Parasitol. Int. 2021, 83, 102343. [Google Scholar] [CrossRef]
- Hermes-Uliana, C.; Pereira-Severi, L.S.; Luerdes, R.B.; Franco, C.L.; da Silva, A.V.; Araujo, E.J.; Sant’Ana Dde, M. Chronic infection with Toxoplasma gondii causes myenteric neuroplasticity of the jejunum in rats. Auton. Neurosci. 2011, 160, 3–8. [Google Scholar] [CrossRef]
- Reeves, G.M.; Mazaheri, S.; Snitker, S.; Langenberg, P.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Okusaga, O.; Groer, M.W.; et al. A Positive Association between T. gondii Seropositivity and Obesity. Front. Public Health 2013, 1, 73. [Google Scholar] [CrossRef]
- Picard, F.; Arsenijevic, D.; Richard, D.; Deshaies, Y. Responses of adipose and muscle lipoprotein lipase to chronic infection and subsequent acute lipopolysaccharide challenge. Clin. Vaccine Immunol. 2002, 9, 771–776. [Google Scholar] [CrossRef]
- Kannan, G.; Moldovan, K.; Xiao, J.C.; Yolken, R.H.; Jones-Brando, L.; Pletnikov, M.V. Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol 2010, 57, 151–155. [Google Scholar] [CrossRef]
- Thjodleifsson, B.; Olafsson, I.; Gislason, D.; Gislason, T.; Jogi, R.; Janson, C. Infections and obesity: A multinational epidemiological study. Scand. J. Infect. Dis. 2008, 40, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Melchor, S.J.; Ewald, S.E. Disease Tolerance in Toxoplasma Infection. Front. Cell. Infect. Microbiol. 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Bluher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef]
- Zhuang, H.; Yao, C.; Zhao, X.; Chen, X.; Yang, Y.; Huang, S.; Pan, L.; Du, A.; Yang, Y. DNA double-strand breaks in the Toxoplasma gondii-infected cells by the action of reactive oxygen species. Parasites Vectors 2020, 13, 490. [Google Scholar] [CrossRef]
- Wang, Z.J.; Yu, S.M.; Gao, J.M.; Zhang, P.; Hide, G.; Yamamoto, M.; Lai, D.H.; Lun, Z.R. High resistance to Toxoplasma gondii infection in inducible nitric oxide synthase knockout rats. iScience 2021, 24, 103280. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Q.R.; Du, Y.; Yang, X.; Fan, J.M.; Dong, Z.M. Toxicity of derivatives from semicarbazide-sensitive amine oxidase-mediated deamination of methylamine against Toxoplasma gondii after infection of differentiated 3T3-L1 cells. Toxicol. Vit. 2010, 24, 809–814. [Google Scholar] [CrossRef]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host-microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Runge, S.; Rosshart, S.P. The Mammalian Metaorganism: A Holistic View on How Microbes of All Kingdoms and Niches Shape Local and Systemic Immunity. Front. Immunol. 2021, 12, 702378. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the Human Gut: The “Known Unknown” of the Microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Kapitan, M.; Niemiec, M.J.; Steimle, A.; Frick, J.S.; Jacobsen, I.D. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. Curr. Top Microbiol. Immunol. 2019, 422, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Matijasic, M.; Mestrovic, T.; Paljetak, H.C.; Peric, M.; Baresic, A.; Verbanac, D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Admyre, T.; Goransson, M.; Marley, A.E.; Smith, D.M.; Oscarsson, J.; Bohlooly, Y.M. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E211–E220. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, A.C.; Castanho, M.A.; Martins-Silva, J.; Saldanha, C. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. Chembiochem 2003, 4, 96–100. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Kallio, K.A.; Buhlin, K.; Jauhiainen, M.; Keva, R.; Tuomainen, A.M.; Klinge, B.; Gustafsson, A.; Pussinen, P.J. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun. 2008, 14, 247–253. [Google Scholar] [CrossRef]
- Levels, J.H.; Abraham, P.R.; van den Ende, A.; van Deventer, S.J. Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 2001, 69, 2821–2828. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.P.; Zhao, H.; Ackerman, A.L. Considerations for Bedside Urologic Procedures in Patients with Severe Acute Respiratory Syndrome Coronavirus-2. Urology 2020, 142, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Shao, F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015, 32, 78–83. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).