Skin Microbiota in Atopic Dermatitis
Abstract
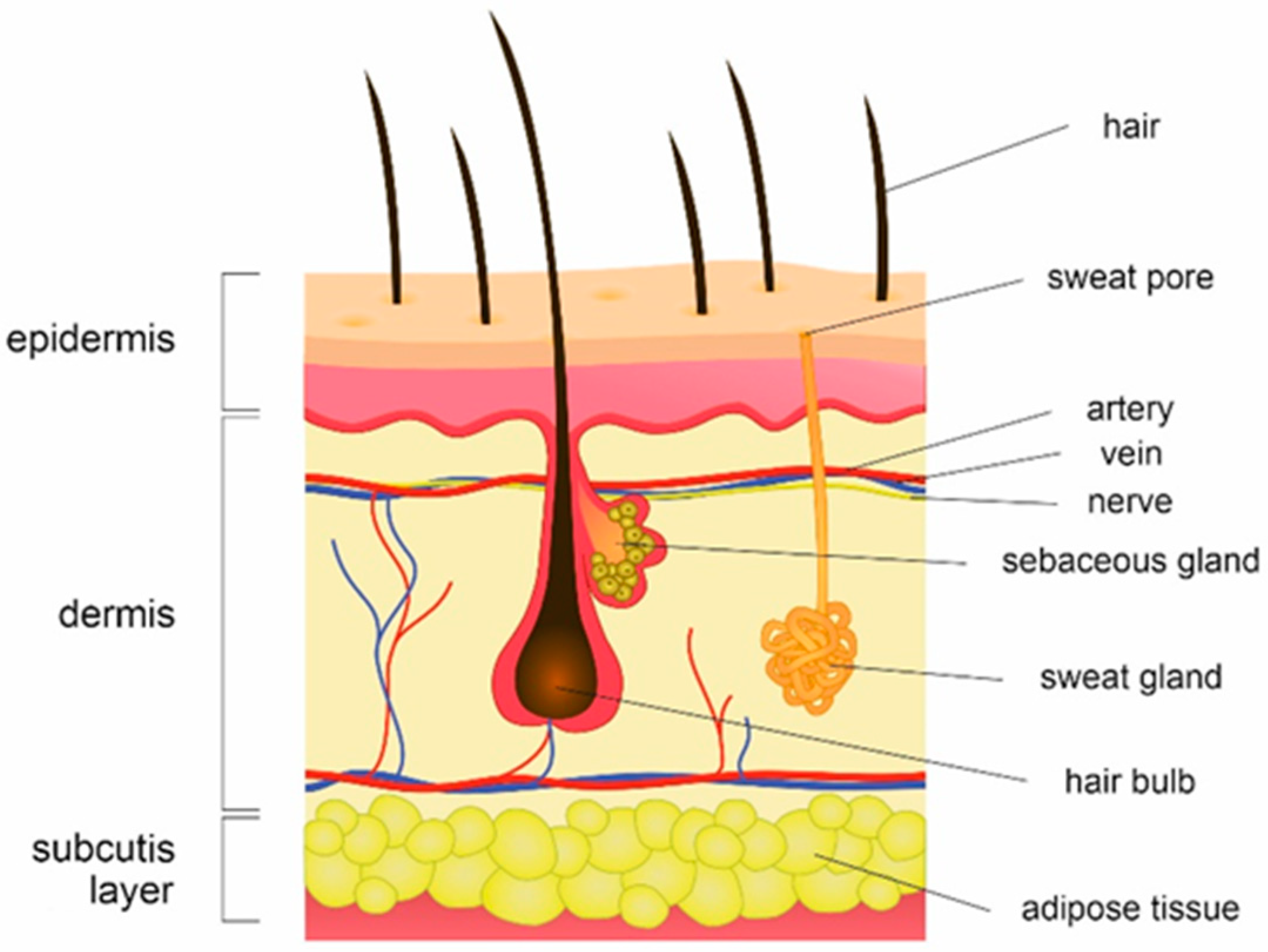
:1. Human Skin Microbiota
2. Atopic Dermatitis
3. Pathogenesis of Atopic Dermatitis
3.1. Epithelial Barrier Dysfunction and Immune Dysregulation
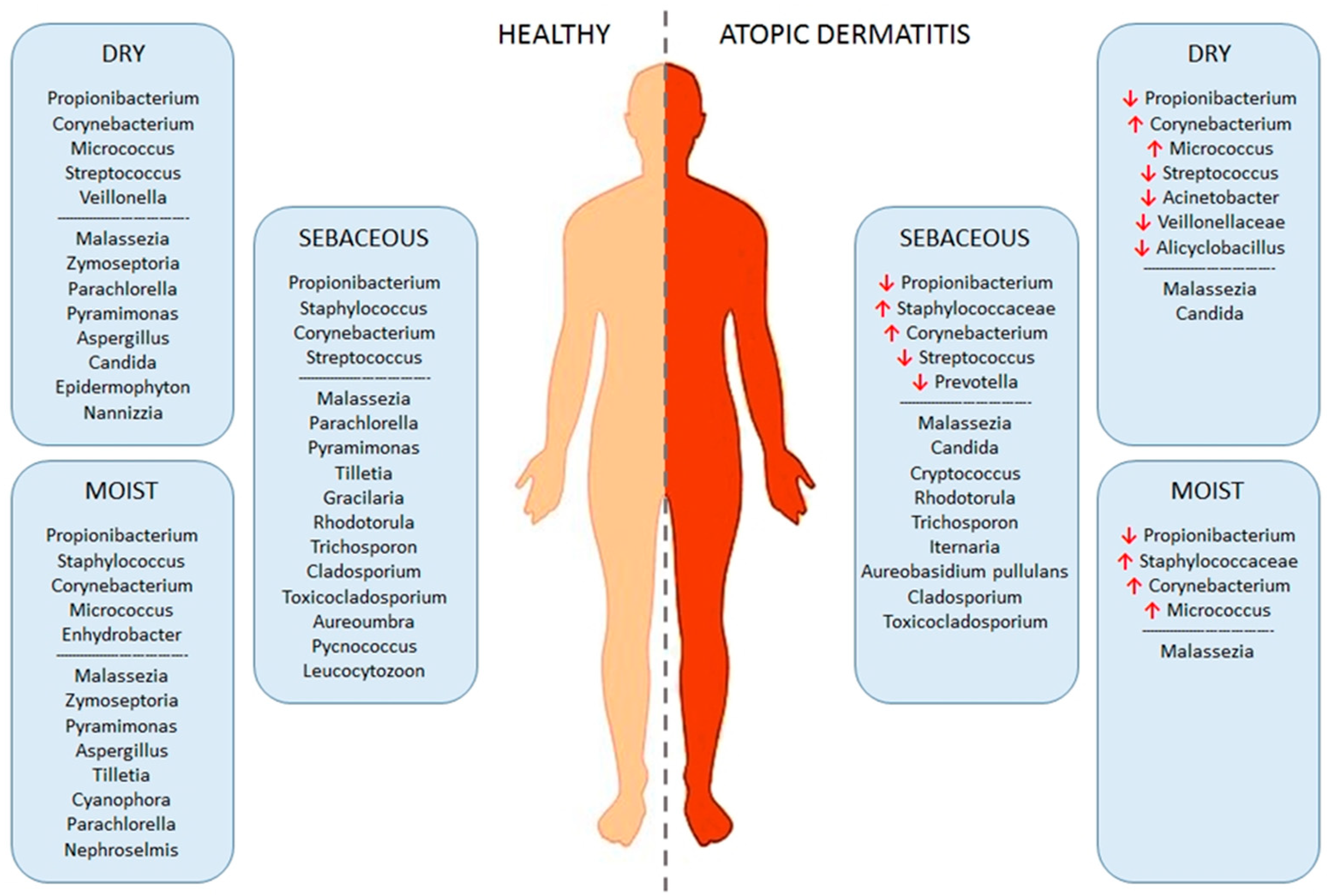
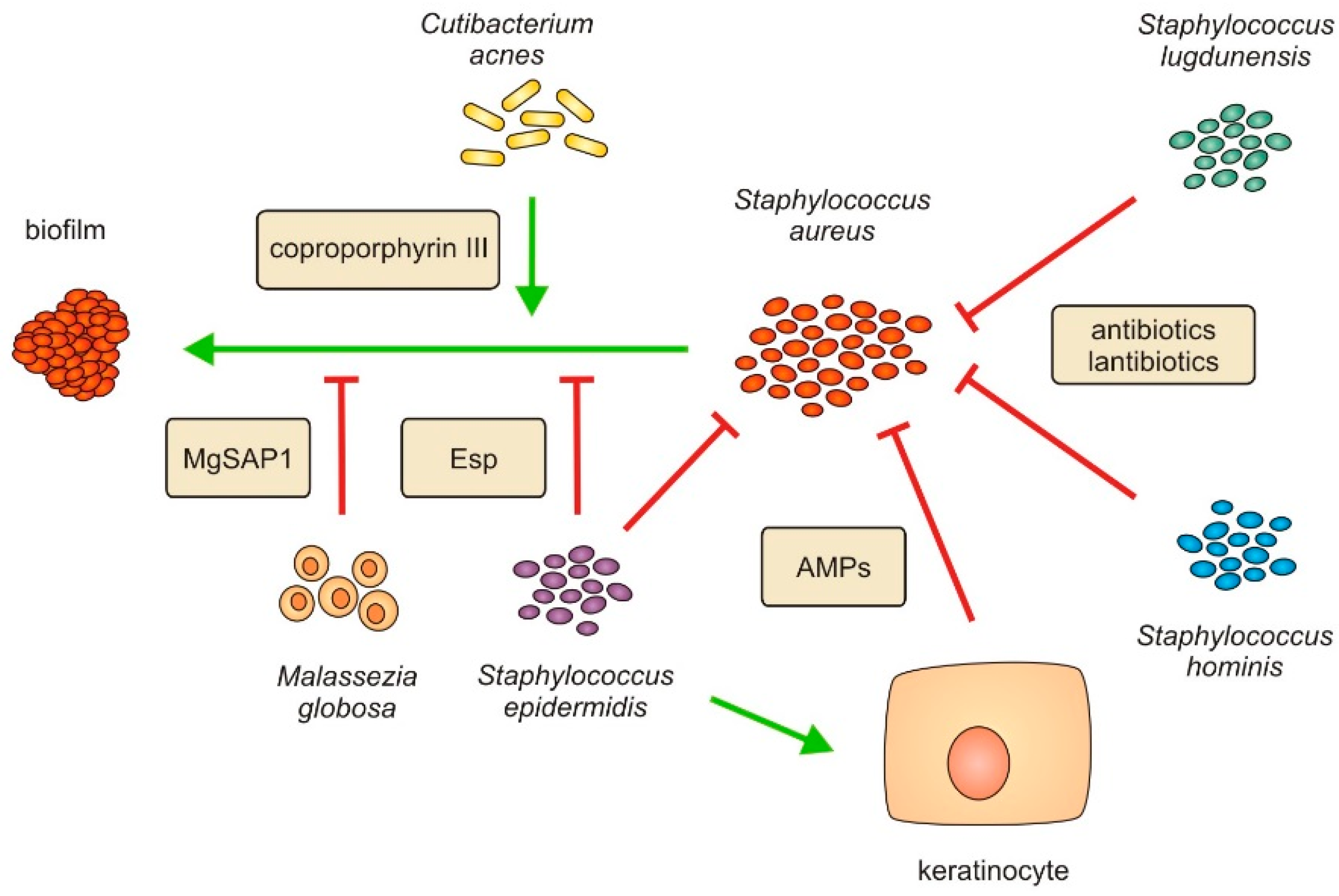
3.2. Dysbiosis of Skin Microbiota
4. Treatment of Atopic Dermatitis
5. Probiotics and Prebiotics in Treatment of Atopic Dermatitis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Scharschmidt, T.C.; Fischbach, M.A. What Lives On Our Skin: Ecology, Genomics and Therapeutic Opportunities Of the Skin Microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SanMiguel, A.; Grice, E.A. Interactions between host factors and the skin microbiome. Cell Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Timm, C.M.; Loomis, K.; Stone, W.; Mehoke, T.; Brensinger, B.; Pellicore, M.; Staniczenko, P.P.A.; Charles, C.; Nayak, S.; Karig, D.K. Isolation and characterization of diverse microbial representatives from the human skin microbiome. Microbiome 2020, 8, 58. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Program, N.C.S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Byrd, A.L.; Park, M.; Program, N.C.S.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Prohic, A.; Sadikovic, T.J.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef]
- Abdillah, A.; Khelaifia, S.; Raoult, D.; Bittar, F.; Ranque, S. Comparison of Three Skin Sampling Methods and Two Media for Culturing Malassezia Yeast. J. Fungi 2020, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Tanaka, T.; Tajima, M.; Tsuboi, R.; Nishikawa, A.; Sugita, T. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol. Immunol. 2011, 55, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Rafat, Z.; Hashemi, S.J.; Ahamdikia, K.; Daie Ghazvini, R.; Bazvandi, F. Study of skin and nail Candida species as a normal flora based on age groups in healthy persons in Tehran-Iran. J. Mycol. Med. 2017, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguiere, A.; Manuguerra, J.C.; et al. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 2015, 6, e01578-15. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Bangayan, N.J.; Curd, E.; Taylor, P.A.; Gallo, R.L.; Leung, D.Y.M.; Li, H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.H.; Deming, C.; Kennedy, E.A.; Conlan, S.; Polley, E.C.; Ng, W.I.; Program, N.C.S.; Segre, J.A.; Kong, H.H. Diverse Human Skin Fungal Communities in Children Converge in Adulthood. J. Investig. Dermatol. 2016, 136, 2356–2363. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Jurakic Toncic, R.; Jakasa, I.; Sun, Y.; Hurault, G.; Ljubojevic Hadzavdic, S.; Tanaka, R.J.; Pavicic, B.; Balic, A.; Zuzul, K.; Petkovic, M.; et al. Stratum corneum markers of innate and T helper cell-related immunity and their relation to the disease severity in Croatian patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1186–1196. [Google Scholar] [CrossRef]
- Hulpusch, C.; Tremmel, K.; Hammel, G.; Bhattacharyya, M.; de Tomassi, A.; Nussbaumer, T.; Neumann, A.U.; Reiger, M.; Traidl-Hoffmann, C. Skin pH-dependent Staphylococcus aureus abundance as predictor for increasing atopic dermatitis severity. Allergy 2020, 75, 2888–2898. [Google Scholar] [CrossRef]
- Rehbinder, E.M.; Advocaat Endre, K.M.; Lodrup Carlsen, K.C.; Asarnoj, A.; Stensby Bains, K.E.; Berents, T.L.; Carlsen, K.H.; Gudmundsdottir, H.K.; Haugen, G.; Hedlin, G.; et al. Predicting Skin Barrier Dysfunction and Atopic Dermatitis in Early Infancy. J. Allergy Clin. Immunol. Pract. 2020, 8, 664–673.e5. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, D.; Sjöberg, O.; Foucard, T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy 2000, 55, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112 (Suppl. S6), S118–S127. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, J.; Oh, M.H.; Zhu, Z. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol. Res. 2011, 3, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Hanifin, J.; Boguniewicz, M.; Eichenfield, L.F.; Spergel, J.M.; Dakovic, R.; Paller, A.S. Study of the Atopic March: Development of Atopic Comorbidities. Pediatr. Dermatol. 2016, 33, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schappi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139 (Suppl. S4), S58–S64. [Google Scholar] [CrossRef] [Green Version]
- Williamson, S.; Merritt, J.; De Benedetto, A. Atopic dermatitis in the elderly: A review of clinical and pathophysiological hallmarks. Br. J. Dermatol. 2020, 182, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Tanei, R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020, 37, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic-Dermatitis. Acta Derm. Venereol. 1980, 60, 44–47. [Google Scholar]
- Andersen, R.M.; Thyssen, J.P.; Maibach, H.I. Qualitative vs. quantitative atopic dermatitis criteria-in historical and present perspectives. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C.; Burney, P.G.; Hay, R.J.; Archer, C.B.; Shipley, M.J.; Hunter, J.J.; Bingham, E.A.; Finlay, A.Y.; Pembroke, A.C.; Graham-Brown, R.A.; et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br. J. Dermatol. 1994, 131, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.A.; Hajar, T.; Hanifin, J.M.; Simpson, E.L. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: An interpretability study. Br. J. Dermatol. 2015, 172, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 1708–1710.e1. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, J.I.; Lei, D.; Yousaf, M.; Janmohamed, S.R.; Vakharia, P.P.; Chopra, R.; Chavda, R.; Gabriel, S.; Patel, K.R.; Singam, V.; et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br. J. Dermatol. 2021, 184, 888–895. [Google Scholar] [CrossRef]
- Torres, T.; Ferreira, E.O.; Goncalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef]
- Weidinger, S.; Illig, T.; Baurecht, H.; Irvine, A.D.; Rodriguez, E.; Diaz-Lacava, A.; Klopp, N.; Wagenpfeil, S.; Zhao, Y.; Liao, H.; et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J. Allergy Clin. Immunol. 2006, 118, 214–219. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nagao, K.; Kubo, A.; Hata, T.; Shimizu, A.; Mizuno, H.; Yamada, T.; Amagai, M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 2012, 129, 1538–1546.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Heitmiller, K.D.; Czarnowicki, T. An Update on the Pathophysiology of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Mehta, H.; Bissonnette, R.; Sarfati, M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J. Dermatol. Sci. 2017, 88, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Yang, T.; Wu, Z.; Zhong, J.; Huang, Y.; Huang, T.; Zheng, E. Differentiation of T-helper cells in distinct phases of atopic dermatitis involves Th1/Th2 and Th17/Treg. Eur. J. Inflamm. 2017, 15, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Novak, N.; Bieber, T.; Hoffmann, M.; Folster-Holst, R.; Homey, B.; Werfel, T.; Sager, A.; Zuberbier, T. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 925–931.e4. [Google Scholar] [CrossRef]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Program, N.C.S.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Nakatsuji, T.; Gallo, R.L. Staphylococcus aureus: Master Manipulator of the Skin. Cell Host Microbe 2017, 22, 579–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [Green Version]
- Totte, J.E.; van der Feltz, W.T.; Hennekam, M.; van Belkum, A.; van Zuuren, E.J.; Pasmans, S.G. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: A systematic review and meta-analysis. Br. J. Dermatol. 2016, 175, 687–695. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Fleury, O.M.; McAleer, M.A.; Feuillie, C.; Formosa-Dague, C.; Sansevere, E.; Bennett, D.E.; Towell, A.M.; McLean, W.H.I.; Kezic, S.; Robinson, D.A.; et al. Clumping Factor B Promotes Adherence of Staphylococcus aureus to Corneocytes in Atopic Dermatitis. Infect. Immun. 2017, 85, e00994-16. [Google Scholar] [CrossRef] [Green Version]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y.M. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.R.; Kotol, P.; Boguniewicz, M.; Taylor, P.; Paik, A.; Jackson, M.; Nguyen, M.; Kabigting, F.; Miller, J.; Gerber, M.; et al. History of eczema herpeticum is associated with the inability to induce human beta-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br. J. Dermatol. 2010, 163, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Feuillie, C.; Vitry, P.; McAleer, M.A.; Kezic, S.; Irvine, A.D.; Geoghegan, J.A.; Dufrene, Y.F. Adhesion of Staphylococcus aureus to Corneocytes from Atopic Dermatitis Patients Is Controlled by Natural Moisturizing Factor Levels. mBio 2018, 9, e01184-18. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Chen, T.H.; Two, A.M.; Chun, K.A.; Narala, S.; Geha, R.S.; Hata, T.R.; Gallo, R.L. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J. Investig. Dermatol. 2016, 136, 2192–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.R.; Nakatsuji, T.; Sanford, J.A.; Vrbanac, A.F.; Gallo, R.L. Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes. J. Investig. Dermatol. 2017, 137, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hon, K.L.; Tsang, Y.C.; Pong, N.H.; Leung, T.F.; Ip, M. Exploring Staphylococcus epidermidis in atopic eczema: Friend or foe? Clin. Exp. Dermatol. 2016, 41, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, C.W.; Lai, Y.K.; Liu, Y.T.; Gallo, R.L.; Huang, C.M. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: Immunization targeting beta-hemolysin and CAMP factor. J. Invest. Dermatol. 2011, 131, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollenberg, M.S.; Claesen, J.; Escapa, I.F.; Aldridge, K.L.; Fischbach, M.A.; Lemon, K.P. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 2014, 5, e01286-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, D.; Nawrot, U. Contribution of Malassezia spp. to the development of atopic dermatitis. Mycoses 2019, 62, 588–596. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Hata, T.R.; Tong, Y.; Cheng, J.Y.; Shafiq, F.; Butcher, A.M.; Salem, S.S.; Brinton, S.L.; Rudman Spergel, A.K.; Johnson, K.; et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat. Med. 2021, 27, 700–709. [Google Scholar] [CrossRef]
- Ramsey, M.M.; Freire, M.O.; Gabrilska, R.A.; Rumbaugh, K.P.; Lemon, K.P. Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Front. Microbiol. 2016, 7, 1230. [Google Scholar] [CrossRef] [Green Version]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Goh, B.N.; Teh, W.K.; Jiang, Z.; Goh, J.P.Z.; Goh, A.; Wu, G.; Hoon, S.S.; Raida, M.; Camattari, A.; et al. Skin Commensal Malassezia globosa Secreted Protease Attenuates Staphylococcus aureus Biofilm Formation. J. Investig. Dermatol. 2018, 138, 1137–1145. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.W.; Jamil, A.; Safian, N.; Md Nor, N.; Muhammad, N.; Saharudin, N.L. A randomized half-body, double blind, controlled trial on the effects of a pH-modified moisturizer vs. standard moisturizer in mild to moderate atopic dermatitis. An. Bras. Dermatol. 2020, 95, 320–325. [Google Scholar] [CrossRef]
- Flores, G.E.; Seite, S.; Henley, J.B.; Martin, R.; Zelenkova, H.; Aguilar, L.; Fierer, N. Microbiome of Affected and Unaffected Skin of Patients With Atopic Dermatitis Before and After Emollient Treatment. J. Drugs Dermatol. 2014, 13, 1365–1372. [Google Scholar]
- Drucker, A.M.; Eyerich, K.; de Bruin-Weller, M.S.; Thyssen, J.P.; Spuls, P.I.; Irvine, A.D.; Girolomoni, G.; Dhar, S.; Flohr, C.; Murrell, D.F.; et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br. J. Dermatol. 2018, 178, 768–775. [Google Scholar] [CrossRef]
- Norris, D.A. Mechanisms of action of topical therapies and the rationale for combination therapy. J. Am. Acad. Dermatol. 2005, 53 (Suppl. S1), S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Paller, A.S.; Irvine, A.D.; Sidbury, R.; Eichenfield, L.F.; Werfel, T.; Bieber, T. Topical therapy of atopic dermatitis with a focus on pimecrolimus. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Wongpiyabovorn, J.; Soonthornchai, W.; Wilantho, A.; Palasuk, M.; Payungporn, S.; Sodsai, P.; Poomipak, W.; Weschawalit, S.; Ruchusatsawat, K.; Baillie, G.S.; et al. Effect of tacrolimus on skin microbiome in atopic dermatitis. Allergy 2019, 74, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Bessa, G.R.; Quinto, V.P.; Machado, D.C.; Lipnharski, C.; Weber, M.B.; Bonamigo, R.R.; D’Azevedo, P.A. Staphylococcus aureus resistance to topical antimicrobials in atopic dermatitis. An. Bras. Dermatol. 2016, 91, 604–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Choi, J.Y.; Shin, J.W.; Huh, C.H.; Park, K.C.; Du, M.H.; Yoon, S.; Na, J.I. Changes in Lesional and Non-lesional Skin Microbiome During Treatment of Atopic Dermatitis. Acta Derm. Venereol. 2019, 99, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 850–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Jang, Y.J.; Han, D.H.; Jeon, K.; Lee, C.; Han, H.S.; Ko, G. Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes 2020, 12, 1819156. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lim, S.K.; Jang, J.Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus sakei WIKIM30 Ameliorates Atopic Dermatitis-Like Skin Lesions by Inducing Regulatory T Cells and Altering Gut Microbiota Structure in Mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Yoon, J.M.; Kim, Y.H.; Jeong, D.G.; Park, S.; Kang, D.J. Therapeutic effect of tyndallized Lactobacillus rhamnosus IDCC 3201 on atopic dermatitis mediated by down-regulation of immunoglobulin E in NC/Nga mice. Microbiol. Immunol. 2016, 60, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Lee, S.H.; Kwon, Y.M.; Adhikari, B.; Kim, J.A.; Yu, D.Y.; Kim, G.I.; Lim, J.M.; Kim, S.H.; Lee, S.S.; et al. Oral Administration of beta-Glucan and Lactobacillus plantarum Alleviates Atopic Dermatitis-Like Symptoms. J. Microbiol. Biotechnol. 2019, 29, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Benfeldt, E.; Nielsen, S.D.; Michaelsen, K.F.; Jeppesen, D.L.; Valerius, N.H.; Paerregaard, A. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 2003, 111, 389–395. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Stanley, T.V.; Mitchell, E.A.; Barthow, C.; Fitzharris, P.; Purdie, G.; Siebers, R.; Black, P.N.; Crane, J. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: Does it also reduce atopic sensitization? Clin. Exp. Allergy 2013, 43, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wu, W.F.; Hung, C.W.; Ku, M.S.; Liao, P.F.; Sun, H.L.; Lu, K.H.; Sheu, J.N.; Lue, K.H. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J. Microbiol. Immunol. Infect. 2017, 50, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef]
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897. [Google Scholar] [CrossRef]
- Niccoli, A.A.; Artesi, A.L.; Candio, F.; Ceccarelli, S.; Cozzali, R.; Ferraro, L.; Fiumana, D.; Mencacci, M.; Morlupo, M.; Pazzelli, P.; et al. Preliminary Results on Clinical Effects of Probiotic Lactobacillus salivarius LS01 in Children Affected by Atopic Dermatitis. J. Clin. Gastroenterol. 2014, 48, S34–S36. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ebata, T.; Hirooka, J.; Hosoya, R.; Inoue, N.; Itami, S.; Tsuji, K.; Yaginuma, T.; Muramatsu, K.; Nakamura, A.; et al. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Ann. Allergy Asthma Immunol. 2014, 113, 209–216.e7. [Google Scholar] [CrossRef]
- Navarro-Lopez, V.; Ramirez-Bosca, A.; Ramon-Vidal, D.; Ruzafa-Costas, B.; Genoves-Martinez, S.; Chenoll-Cuadros, E.; Carrion-Gutierrez, M.; Horga de la Parte, J.; Prieto-Merino, D.; Codoner-Cortes, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients With Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lise, M.; Mayer, I.; Silveira, M. Use of probiotics in atopic dermatitis. Rev. Assoc. Med. Bras. 2018, 64, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Ah, Y.M.; Yu, Y.M.; Choi, K.H.; Shin, W.G.; Lee, J.Y. Effects of probiotics for the treatment of atopic dermatitis: A meta-analysis of randomized controlled trials. Ann. Allergy Asthma Immunol. 2014, 113, 217–226. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [Green Version]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef] [Green Version]
- Blanchet-Rethore, S.; Bourdes, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin. Cosmet Investig. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzio, L.; Centi, C.; Cinque, B.; Masci, S.; Giuliani, M.; Arcieri, A.; Zicari, L.; De Simone, C.; Cifone, M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp. Dermatol. 2003, 12, 615–620. [Google Scholar] [CrossRef]
- Gueniche, A.; Knaudt, B.; Schuck, E.; Volz, T.; Bastien, P.; Martin, R.; Rocken, M.; Breton, L.; Biedermann, T. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Br. J. Dermatol. 2008, 159, 1357–1363. [Google Scholar] [CrossRef]
- Chang, Y.S.; Trivedi, M.K.; Jha, A.; Lin, Y.F.; Dimaano, L.; Garcia-Romero, M.T. Synbiotics for Prevention and Treatment of Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials. JAMA Pediatr. 2016, 170, 236–242. [Google Scholar] [CrossRef]
- Passeron, T.; Lacour, J.P.; Fontas, E.; Ortonne, J.P. Prebiotics and synbiotics: Two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy 2006, 61, 431–437. [Google Scholar] [CrossRef]
- Aldaghi, M.; Tehrani, H.; Karrabi, M.; Abadi, F.S.; Sahebkar, M. The effect of multistrain synbiotic and vitamin D3 supplements on the severity of atopic dermatitis among infants under 1 year of age: A double-blind, randomized clinical trial study. J. Dermatol. Treat. 2020. [Google Scholar] [CrossRef]
- Pharmabiotic Research Institute. Available online: https://www.pharmabiotic.org/#mmps (accessed on 17 March 2022).
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L.S.; Fester, R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; MIT Press: Cambridge, MA, USA, 1991; 454p. [Google Scholar]
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the Hologenome Concept Right: An Eco-Evolutionary Framework for Hosts and Their Microbiomes. mSystems 2016, 1, e00028-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study, Year | Study Type | Bacterial Strain | Administration | Animal Model | Outcome Summary |
|---|---|---|---|---|---|
| Kim et al., 2020 [93] | mouse model | L. paracasei KBL382 | oral | NC/Nga mice DFE- and DNCB-induced AD | modulation of the immune response and gut microbiota |
| Kwon et al., 2018 [94] | mouse model | L. sakei WIKIM30 | oral | BALB/c mice DNCB-induced AD | stimulation of Treg cell generation and suppression of TH2 inflammatory response, restoring the balance of gut microbiota |
| Lee et al., 2016 [95] | mouse model | L. rhamnosus IDCC 3201 tyndallizate (RHT3201) | oral | NC/Nga mice DFE-induced AD | less severe AD symptoms in comparison to controls, dose-dependent reductions in dermatitis scores |
| Kim et al., 2019 [96] | rat/mouse models | L. plantarum LM1004 | oral | Sprague-Dawley rats, ddY mice histamine-induced AD DNFB-induced AD | inhibition of TH2 cell responses and activation of Treg immunoregulatory functions, increase of relative abundance of butyrate-generating microorganisms in the gut |
| Study, Year | Study Type | Bacterial Strain | Administration | Participants (Age) | Outcome Summary |
| Rosenfeldt et al., 2003 [97] | human | lyophilized L. rhamnosus 19070-2 and L. reuteri DSM 122460 | oral | children (1–13 y) | moderate improvement in the clinical severity of eczema |
| Wickens et al., 2012 [98] | human | L. rhamnosus | oral | infants | reduced eczema prevalence |
| Wickens et al., 2013 [99] | human | L. rhamnosus | oral | children (<6 y) | significantly reduced cumulative eczema prevalence, decrease in SCORAD values and atopic sensitization |
| Wu et al., 2015 [100] | human | L. rhamnosus | oral | children (4–48 mos.) | decrease of SCORAD values and disease intensity |
| Han et al., 2012 [101] | human | L. plantarum CJLP133 | oral | children (12 mos.–13 y) | decrease of SCORAD values, IFN-γ and IL-4 |
| Weston et al., 2005 [102] | human | L. fermentum VRI-033 PCC | oral | children (6–18 mos.) | change in AD severity compared to placebo-treated individuals |
| Niccoli et al., 2014 [103] | human | L. salivarious LS01 | oral | children | decrease of SCORAD values and significant improvement in itching intensity, both therapy benefits persisting after suspension of treatment |
| Matsumoto et al., 2014 [104] | human | B. animalis subsp. lactis LKM512 | oral | adults | alleviated itch in AD patients and considerably improved the quality-of-life scores |
| Navarro-Lopez et al., 2018 [105] | human | B. lactis CECT 8145, B. longum CECT 7347, and L. casei CECT 9104 | oral | children (4–17 y) | decrease of SCORAD values in patients with moderate AD |
| Lise et al., 2018 [106] | human | B. lactis HN019, L. acidophilus NCFM, L. rhamnosus HN001 and L. paracasei LPC-37 | oral | children | evident response in treating severe AD with significant change in AD severity scores |
| Kim et al., 2014 [107] | human | Lactobacillus and Bifidobacterium species | oral | children and adults (1 mo.–65 y) | decrease of SCORAD values |
| Nakatsuji et al., 2017 [108] | human | topical application of commensal skin bacteria | topical | adults | protective effect against pathogen species (reduced S. aureus colonization due to selective AMPs secreted by commensal CoNS), improvement of clinical symptoms and decreased inflammation |
| Nakatsuji et al., 2021 [80] | human | S. hominis A9 (ShA9) | topical | adults | fewer adverse events associated with AD, inhibited expression of mRNA for psmα |
| Myles et al., 2018 [109] | human | R. mucosa | topical | children and adults | significant decrease in SCORAD and pruritus, reduction in disease severity and no adverse effects or complications |
| Blanchet-Rethoré et al., 2017 [110] | human | heat-treated L. johnsonii | topical | adults | clinical improvement of AD symptoms in patients with moderate AD |
| Di Marzio et al., 2003 [111] | human | sonicated S. thermophilus | topical | adults | significant improvement in skin barrier integrity, erythema, scaling and pruritus |
| Gueniche et al., 2008 [112] | human | lysate of V. filiformis | topical | children and adults | clinical improvement in patients with AD, decreased SCORAD values and pruritus |
| Chang et al., 2016 [113] | human | multiple strains of bacteria | topical | children (>1 y) | decrease of SCORAD values |
| Passeron et al., 2006 [114] | human | L. rhamnosus Lcr35 plus prebiotics | topical | children (>2 y) | improved AD symptoms and decreased SCORAD values |
| Aldaghi et al., 2020 [115] | human | L. rhamnosus, L. euteri and B. infantis or vitamin D3 | topical | infants | significantly decreased SCORAD values |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrestak, D.; Matijašić, M.; Čipčić Paljetak, H.; Ledić Drvar, D.; Ljubojević Hadžavdić, S.; Perić, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. https://doi.org/10.3390/ijms23073503
Hrestak D, Matijašić M, Čipčić Paljetak H, Ledić Drvar D, Ljubojević Hadžavdić S, Perić M. Skin Microbiota in Atopic Dermatitis. International Journal of Molecular Sciences. 2022; 23(7):3503. https://doi.org/10.3390/ijms23073503
Chicago/Turabian StyleHrestak, Dora, Mario Matijašić, Hana Čipčić Paljetak, Daniela Ledić Drvar, Suzana Ljubojević Hadžavdić, and Mihaela Perić. 2022. "Skin Microbiota in Atopic Dermatitis" International Journal of Molecular Sciences 23, no. 7: 3503. https://doi.org/10.3390/ijms23073503
APA StyleHrestak, D., Matijašić, M., Čipčić Paljetak, H., Ledić Drvar, D., Ljubojević Hadžavdić, S., & Perić, M. (2022). Skin Microbiota in Atopic Dermatitis. International Journal of Molecular Sciences, 23(7), 3503. https://doi.org/10.3390/ijms23073503






