Leydig Cells in Immunocastrated Polish Landrace Pig Testis: Differentiation Status and Steroid Enzyme Expression Status
Abstract
1. Introduction
2. Results
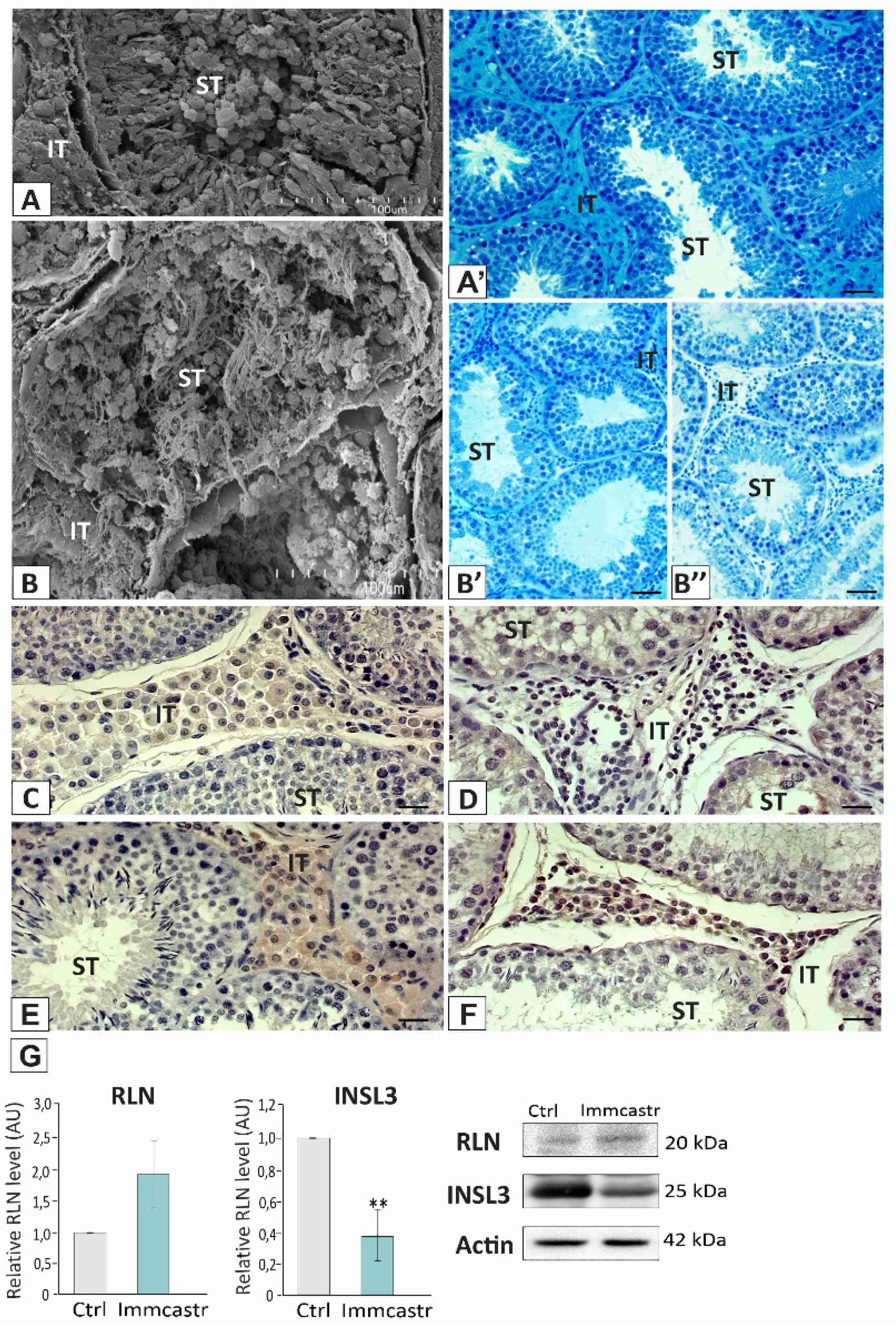
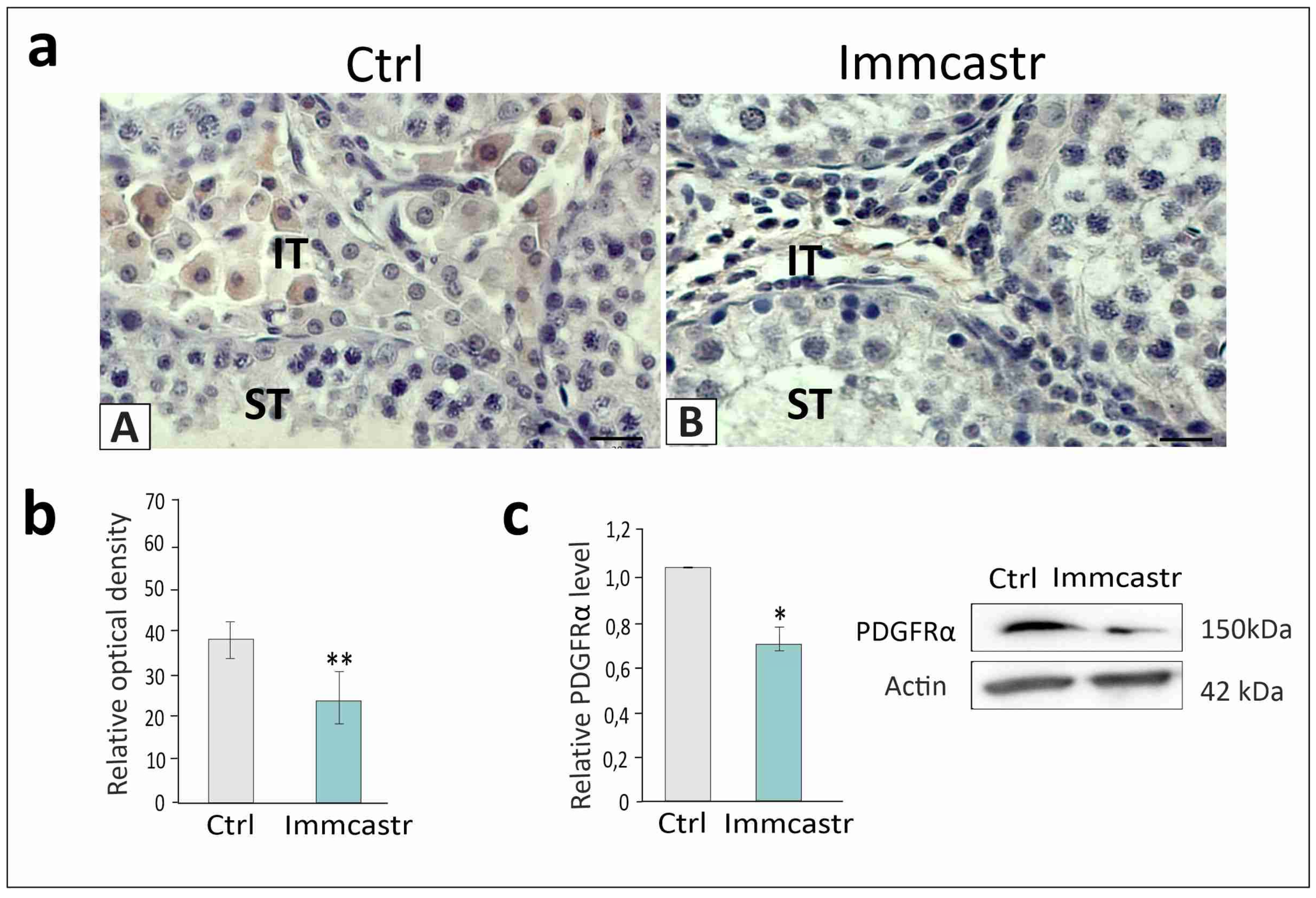
2.1. Topography, Morphology of the Testis, and RLN, INSL3, and PDGFRα Localization and Expression
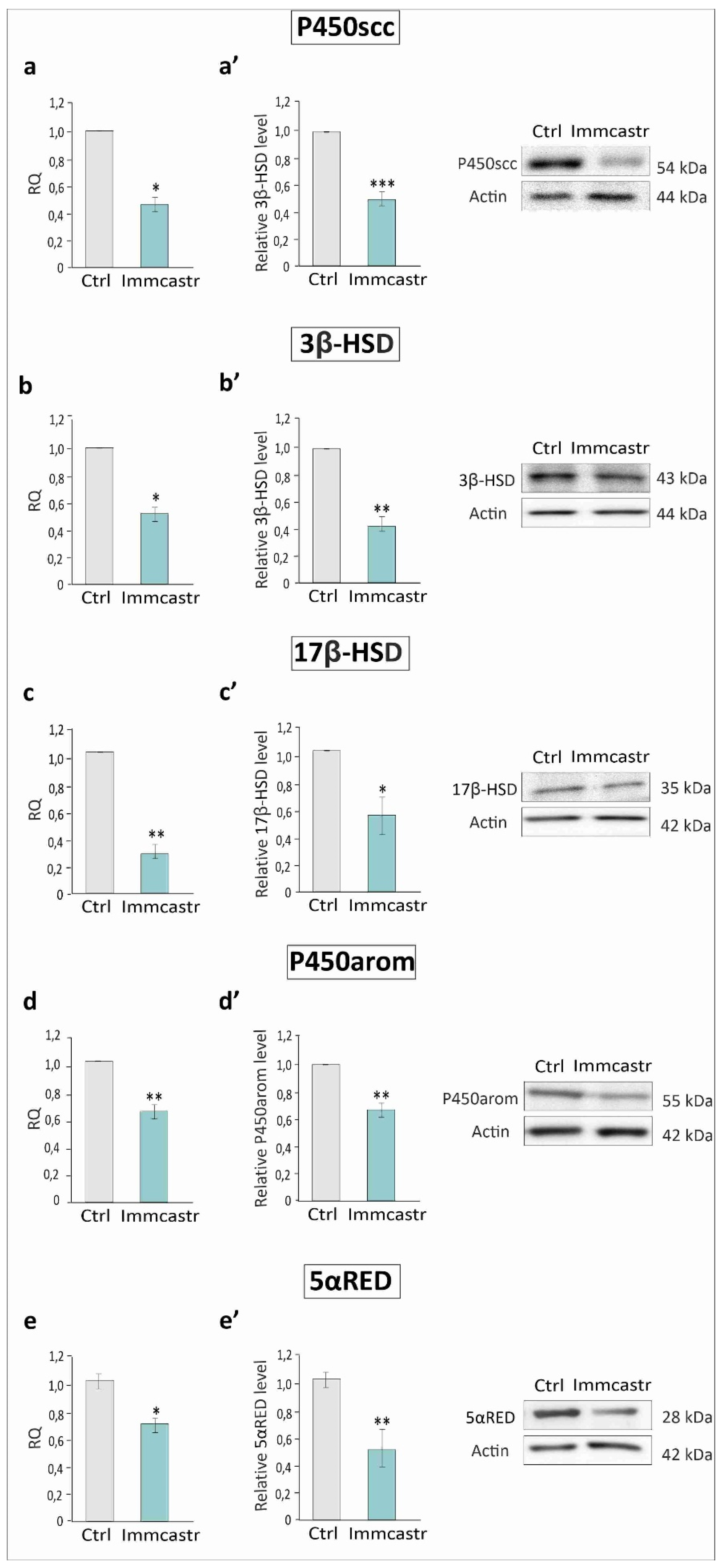
2.2. mRNA and Protein Steroidogenic Enzyme Expression
2.3. Steroidogenic Enzyme Expression and Localization
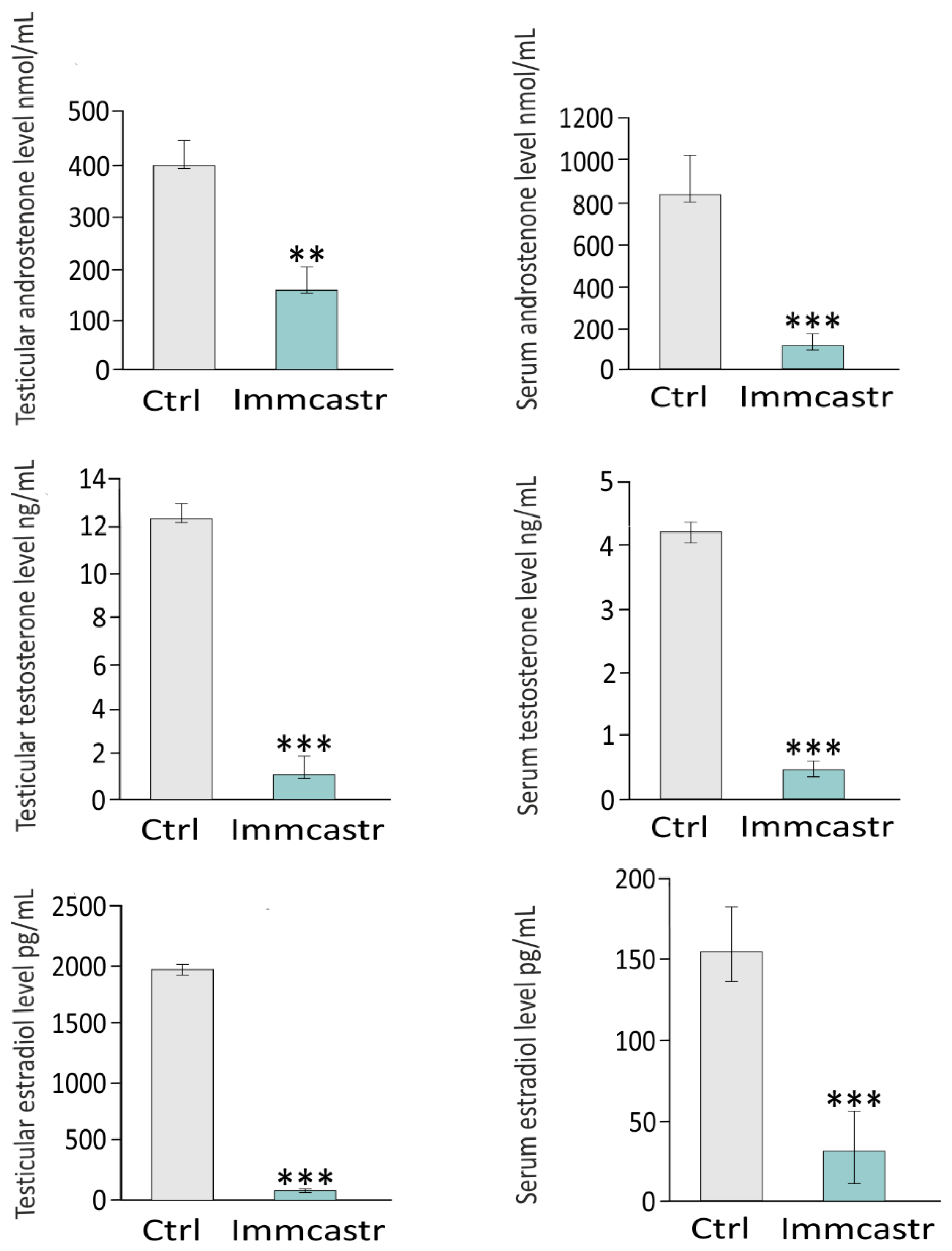
2.4. Testicular and Serum Androstenone, Testosterone, and Estradiol Levels
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Testis Topography and Morphology
4.3. Quantitative RT-PCR
4.4. Western Blot
4.5. Immunohistochemistry
4.6. Androstenone, Testosterone, and Estradiol Concentration Measurement
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugué, C.; Prunier, A.; Mercat, M.J.; Monziols, M.; Blanchet, B.; Larzul, C. Genetic determinism of boar taint and relationship with growth traits, meat quality and lesions. Animal 2020, 14, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Bone, C.; Cameron, J. Pork Production with Entire Males: Directions for Control of Boar Taint. Animals 2020, 16, 1665. [Google Scholar] [CrossRef] [PubMed]
- von Borell, E.V.; Bonneau, M.; Holinger, M.; Prunier, A.; Stefanski, V.; Zöls, S.; Weiler, U. Welfare Aspects of Raising Entire Male Pigs and Immunocastrates. Animals 2020, 17, 2140. [Google Scholar] [CrossRef] [PubMed]
- Millet, S.; Gielkens, K.; De Brabander, D.; Janssens, G.P. Considerations on the performance of immunocastrated male pigs. Animal 2011, 5, 1119–1123. [Google Scholar] [CrossRef]
- Zoels, S.; Reiter, S.; Ritzmann, M.; Weiß, C.; Numberger, J.; Schütz, A.; Lindner, P.; Stefanski, V.; Weiler, U. Influences of Immunocastration on Endocrine Parameters, Growth Performance and Carcass Quality, as Well as on Boar Taint and Penile Injuries. Animals 2020, 21, 346. [Google Scholar] [CrossRef]
- Kubale, V.; Batorek, N.; Skrlep, M.; Prunier, A.; Bonneau, M.; Fazarinc, G.; Candek-Potokar, M. Steroid hormones, boar taint compounds, and reproductive organs in pigs according to the delay between immunocastration and slaughter. Theriogenology 2013, 1, 69–80. [Google Scholar] [CrossRef]
- Srisuwatanasagul, K.; Srisuwatanasagul, S.; Roongsitthichai, A. Expressions of cytochrome P450 aromatase and anti-Müllerian hormone in testes of fattening pigs by the timing of the first vaccination for immunocastration. Reprod. Domest. Anim. 2021, 56, 400–407. [Google Scholar] [CrossRef]
- Huber, L.; Squires, E.J.; Mandell, I.B.; de Lange, C.F.M. Age at castration (surgical or immunological) impacts carcass characteristics and meat quality of male pigs. Animal 2018, 12, 648–656. [Google Scholar] [CrossRef]
- Pérez-Ciria, L.; Miana-Mena, F.J.; López-Mendoza, M.C.; Álvarez-Rodríguez, J.; Latorre, M.A. Influence of Immunocastration and Diet on Meat and Fat Quality of Heavy Female and Male Pigs. Animals 2021, 24, 3355. [Google Scholar] [CrossRef]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef]
- Poklukar, K.; Čandek-Potokar, M.; Vrecl, M.; Batorek-Lukač, N.; Fazarinc, G.; Kress, K.; Weiler, U.; Stefanski, V.; Škrlep, M. The effect of immunocastration on adipose tissue deposition and composition in pigs. Animal 2021, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Yunes, M.C.; Teixeira, D.L.; von Keyserlingk, M.A.G.; Hötzel, M.J. Is gene editing an acceptable alternative to castration in pigs? PLoS ONE 2019, 24, e0218176. [Google Scholar] [CrossRef] [PubMed]
- Baes, C.; Mattei, S.; Luther, H.; Ampuero, S.; Sidler, X.; Bee, G.; Spring, P.; Hofer, A. A performance test for boar taint compounds in live boars. Animal 2013, 7, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Squires, E.J. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 3, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Yi, D.; Teketay, W.; Jing, H.; Sohail, A.; Liu, G.; Jiang, X. Recent advances in immunocastration in sheep and goat and its animal welfare benefits: A review. J. Integr. Agric. 2022, 22, 299–309. [Google Scholar] [CrossRef]
- Kurtz, S.; Petersen, B. Pre-determination of sex in pigs by application of CRISPR/Cas system for genome editing. Theriogenology 2019, 137, 67–74. [Google Scholar] [CrossRef]
- Sato, M.; Miyoshi, K.; Kawaguchi, H.; Inada, E.; Saitoh, I.; Tanimoto, A. Recent Advance in Genome Editing-Based Gene Modification in Pigs. In Theriogenology; IntechOpen: London, UK, 2019. [Google Scholar]
- Grindflek, E.; Meuwissen, T.H.E.; Aasmundstad, T.; Hamland, H.; Hansen, M.H.S.; Nome, T.; Kent, M.; Torjesen, P.; Lien, S. Revealing genetic relationships between compounds affecting boar taint and reproduction in pigs 1. J. Anim. Sci. 2012, 89, 680–692. [Google Scholar] [CrossRef][Green Version]
- Ivell, R.; Wade, J.D.; Anand-Ivell, R. INSL3 as a biomarker of Leydig cell functionality. Biol. Reprod. 2013, 13, 147. [Google Scholar] [CrossRef]
- Mendis-Handagama, S.M.; Ariyaratne, H.B. Differentiation of the adult Leydig cell population in the postnatal testis. Biol. Reprod. 2001, 65, 660–671. [Google Scholar] [CrossRef]
- Griswold, S.L.; Behringer, R.R. Fetal Leydig cell origin and development. Sex. Dev. 2009, 3, 1–15. [Google Scholar] [CrossRef]
- Shima, Y. Development of fetal and adult Leydig cells. Reprod. Med. Biol. 2019, 2, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratne, H.B.; Mendis-Handagama, C. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol. Reprod. 2000, 62, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Kaur, J.; Ge, R.S.; Cooke, P.S.; Yao, H.H. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J. 2013, 27, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. [Updated 2017 Jan 11]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Stratakis, C.A.; D’Trence, D.L.; Wilson, D.P. Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Haeussler, S.; Wagner, A.; Welter, H.; Claus, R. Changes of testicular aromatase expression during fetal development in male pigs (Sus scrofa). Reproduction 2007, 133, 323–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotula-Balak, M.; Hejmej, A.; Kopera, I.; Lydka, M.; Bilinska, B. Prenatal and neonatal exposure to flutamide affects function of Leydig cells in adult boar. Domest. Anim. Endocrinol. 2012, 42, 142–154. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Robic, A.; Faraut, T.; Feve, K.; Djebali, S.; Prunier, A.; Larzul, C.; Liaubet, L. Correlation Networks Provide New Insights into the Architecture of Testicular Steroid Pathways in Pigs. Genes 2021, 9, 551. [Google Scholar] [CrossRef]
- Gorowska, E.; Zarzycka, M.; Chojnacka, K.; Bilinska, B.; Hejmej, A. Postnatal exposure to flutamide affects CDH1 and CTNNB1 gene expression in adult pig epididymis and prostate and alters metabolism of testosterone. Andrology 2014, 2, 186–197. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ⁵-Δ⁴ isomerase: A review. Int. J. Mol. Sci. 2013, 2, 17926–17942. [Google Scholar] [CrossRef]
- Aluwé, M.; Heyrman, E.; Almeida, J.M.; Babol, J.; Battacone, G.; Čítek, J.; Font, I.; Furnols, M.; Getya, A.; Karolyi, D.; et al. Exploratory Survey on European Consumer and Stakeholder Attitudes towards Alternatives for Surgical Castration of Piglets. Animals 2020, 10, 1758, Erratum in Animals 2020, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Aluwé, M.; Tuyttens, F.A.M.; Millet, S. Field experience with surgical castration with anaesthesia, analgesia, immunocastration and production of entire male pigs: Performance, carcass traits and boar taint prevalence. Animal 2015, 9, 500–508. [Google Scholar] [PubMed]
- Huber-Eicher, B.; Spring, P. Attitudes of Swiss consumers towards meat from entire or immunocastrated boars: A representative survey. Res. Vet. Sci. 2008, 85, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Lagerkvist, C.J. Swedish consumer preferences for animal welfare and biotech: A choice experiment. AgBioForum 2006, 9, 51–58. [Google Scholar]
- Tuyttens, F.A.M.; Vanhonacker, F.; Langendries, K.; Aluwé, M.; Millet, S.; Bekaert, K.; Verbeke, W. Effect of information provisioning on attitude toward surgical castration of male piglets and alternative strategies for avoiding boar taint. Res. Vet. Sci. 2011, 91, 327–332. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Verbeke, W. Consumer response to the possible use of vaccine method to control boar taint v. Physical piglet castration with anaesthesia: A quantitative study in four European countries. Animal 2011, 5, 1107–1118. [Google Scholar]
- Edwards, S.A.; Oliver, M.A.; Ouedraogo, A.; Gonzalez, J.; Gil, M.; Fredriksen, B.; von Borell, E.; Baumgartner, J.; Giershing, M.; Jaeggin, N.; et al. PIGCAS, Attitudes, Practices and State of the Art Regarding Piglet Castration in Europe. Report on Evaluation of Research and Other Information. 2009. Available online: http://w3.rennes.inra.fr/pigcas (accessed on 12 October 2013).
- Velarde, A. Agonistic behaviour. In On Farm Monitoring of Pig Welfare; Velarde, A., Geers, R., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2007; pp. 53–56. [Google Scholar]
- Wesoly, R.; Weiler, U. Nutritional Influences on Skatole Formation and Skatole Metabolism in the Pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Pawlicki, P.; Galuszka, A.; Pardyak, L.; Tuz, R.; Dubniewicz, K.; Skrzypczak-Wiercioch, A.; Rak, A.; Dawid, M.; Tarasiuk, K. Vaccination against gonadoliberin with Improvac influences adiponectin and leptin regulation in testes of Landrace pigs. Vet. Med. Sci. Pract. submitted.
- Martin, B.; Gabris-Weber, B.A.; Reddy, R.; Romero, G.; Chattopadhyay, A.; Salama, G. Relaxin reverses inflammatory and immune signals in aged hearts. PLoS ONE 2018, 18, e0190935. [Google Scholar]
- Stojanovic, S.; Uscebrka, G.; Zikic, D.; Stukelj, M. Histological and Morphometric Examination of the Testes of Boars and Male Pigs Immunocastrated with Improvac®. Acta Sci. Vet. 2007, 45, 1488. [Google Scholar]
- Molenaar, G.J.; Lugard-Kok, C.; Meloen, R.H.; Oonk, R.B.; de Koning, J.; Wessing, C.J.G. Lesions in the hypothalamus after active immunisation against GnRH in the pig. J. Neuroimmunol. 1993, 48, 1–11. [Google Scholar] [CrossRef]
- Kopera, I.; Tuz, R.; Hejmej, A.; Schwarz, T.; Koczanowski, J.; Bilińska, B. Immunolocalization of androgen receptor in the boar epididymis: The effect of GnRH agonist deslorelin. Reprod. Domest. Anim. 2009, 44, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kopera, I.; Tuz, R.; Kotula-Balak, M.; Schwarz, T.; Koczanowski, J.; Bilinska, B. Morphofunctional alterations in testicular cells of deslorelin-treated boars: An immunohistochemical study. J. Exp. Zool. A Ecol. Genet. Physiol. 2008, 309, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, D.; Burger, D.; Gremmes, S.; Szunyog, K.; Röthemeier, S.; Sieme, H. Active immunisation against GnRH as treatment for unilateral granulosa theca cell tumour in mares. Equine Vet. J. 2021, 53, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Adham, I.M.; Emmen, J.M.; Engel, W. The role of the testicular factor INSL3 in establishing the gonadal position. Mol. Cell Endocrinol. 2000, 160, 11–16. [Google Scholar] [CrossRef]
- Zimmermann, S.; Steding, G.; Emmen, J.M.; Brinkmann, A.O.; Nayernia, K.; Holstein, A.F.; Engel, W.; Adham, I.M. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999, 13, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.S.; Dong, Q.; Sottas, C.M.; Papadopoulos, V.; Zirkin, B.R.; Hardy, M.P. In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. USA 2006, 103, 2719–2724. [Google Scholar] [CrossRef]
- Mariani, S.; Basciani, S.; Arizzi, M.; Spera, G.; Gnessi, L. PDGF and the testis. Trends Endocrinol. Metab. 2002, 13, 11–17. [Google Scholar] [CrossRef]
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 6, 800–810. [Google Scholar] [CrossRef]
- Hatano, O.; Takakusu, A.; Nomura, M.; Morohashi, K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1996, 1, 663–671. [Google Scholar] [CrossRef]
- Mack, S.O.; Garrett, W.M.; Guthrie, H.D. Absence of correlation between in situ expression of cytochrome P450 17α-hydroxylase/lyase and 3β-hydroxysteroid dehydrogenase/(Delta5-4) isomerase messenger ribonucleic acids and steroidogenesis during pubertal development in the rat testis. J. Steroid. Biochem. Mol. Biol. 2000, 73, 19–28. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.T.; Chen, X.Y.; Wu, Q.Y.; Yang, L.Q.; Xu, L.; Zhang, Y.; Qazi, I.H.; Zhou, G.B.; Zeng, C.J.; et al. Improvac immunocastration affects the development of thigh muscles but not pectoral muscles in male chickens. Poult. Sci. 2019, 1, 6034–6045. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Rydhmer, L.; Andersson, H.K.; Chen, G.; Lowagie, S.; Andersson, K.; Lundström, K. Long-term effect of vaccination against gonadotropin-releasing hormone, using Improvac TM, on hormonal profile and behaviour of male pigs. Anim. Reprod. Sci. 2008, 108, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cue, R.-A.; Lundström, K.; Wood, J.H.; Doran, O. Regulation of cytochrome P450 2A6 protein expression by skatole, indole and testicular steroids in primary cultured porcine hepatocytes. Drug Metab. Disposit. 2008, 36, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Andersson, H.K.; Chen, G.; Andersson, K.; Madej, A.; Lundstrom, K. Effect of a gonadotropin-releasing hormone vaccine (Improvac) on steroid hormones, boar taint compounds and performance in entire male pigs. Reprod. Domest. Anim. 2008, 43, 351–359. [Google Scholar] [CrossRef]
- Rydhmer, L.; Lundström, K.; Andersson, K. Immunocastration reduces aggressive and sexual behavior in male pigs. Animal. 2010, 4, 965–997. [Google Scholar] [CrossRef]
- Rottner, S.; Claus, R. Return of testicular function after vaccination of boars against GnRH: Consequences on testes histology. Animal 2009, 3, 1279–1286. [Google Scholar] [CrossRef]
- Brunius, C.; Zamaratskaia, G.; Andersson, K.; Chen, G.; Norrby, M.; Madej, A.; Lundström, K. Early immunocastration of male pigs with Improvac—effect on boar taint, hormones and reproductive organs. Vaccine 2011, 29, 9514–9520. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Duliban, M.; Pawlicki, P.; Tuz, R.; Bilinska, B.; Płachno, B.J.; Arent, Z.J.; Krakowska, I.; Tarasiuk, K. The meaning of non-classical estrogen receptors and peroxisome proliferator-activated receptor for boar Leydig cell of immature testis. Acta Histochem. 2020, 122, 151526. [Google Scholar] [CrossRef]
- Pardyak, L.; Kaminska, A.; Galas, J.; Ptak, A.; Bilinska, B.; Kotula-Balak, M. Primary and tumor mouse Leydig cells exposed to polychlorinated naphthalenes mixture: Effect on estrogen related-receptors expression, intracellular calcium level and sex hormones secretion. Tissue Cell. 2016, 48, 432–441. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlicki, P.; Galuszka, A.; Pardyak, L.; Tuz, R.; Płachno, B.J.; Malopolska, M.; Dubniewicz, K.; Yang, P.; Kotula-Balak, M.; Tarasiuk, K. Leydig Cells in Immunocastrated Polish Landrace Pig Testis: Differentiation Status and Steroid Enzyme Expression Status. Int. J. Mol. Sci. 2022, 23, 6120. https://doi.org/10.3390/ijms23116120
Pawlicki P, Galuszka A, Pardyak L, Tuz R, Płachno BJ, Malopolska M, Dubniewicz K, Yang P, Kotula-Balak M, Tarasiuk K. Leydig Cells in Immunocastrated Polish Landrace Pig Testis: Differentiation Status and Steroid Enzyme Expression Status. International Journal of Molecular Sciences. 2022; 23(11):6120. https://doi.org/10.3390/ijms23116120
Chicago/Turabian StylePawlicki, Piotr, Anna Galuszka, Laura Pardyak, Ryszard Tuz, Bartosz J. Płachno, Martyna Malopolska, Klaudia Dubniewicz, Ping Yang, Malgorzata Kotula-Balak, and Kazimierz Tarasiuk. 2022. "Leydig Cells in Immunocastrated Polish Landrace Pig Testis: Differentiation Status and Steroid Enzyme Expression Status" International Journal of Molecular Sciences 23, no. 11: 6120. https://doi.org/10.3390/ijms23116120
APA StylePawlicki, P., Galuszka, A., Pardyak, L., Tuz, R., Płachno, B. J., Malopolska, M., Dubniewicz, K., Yang, P., Kotula-Balak, M., & Tarasiuk, K. (2022). Leydig Cells in Immunocastrated Polish Landrace Pig Testis: Differentiation Status and Steroid Enzyme Expression Status. International Journal of Molecular Sciences, 23(11), 6120. https://doi.org/10.3390/ijms23116120








