Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications
Abstract
1. Background
1.1. The Plasminogen Activation System and Its Regulation
1.2. The Complexity and the Diversity of Platelets
1.3. Platelets as Balance between Thrombus Formation and Fibrinolysis: An Emerging Concept
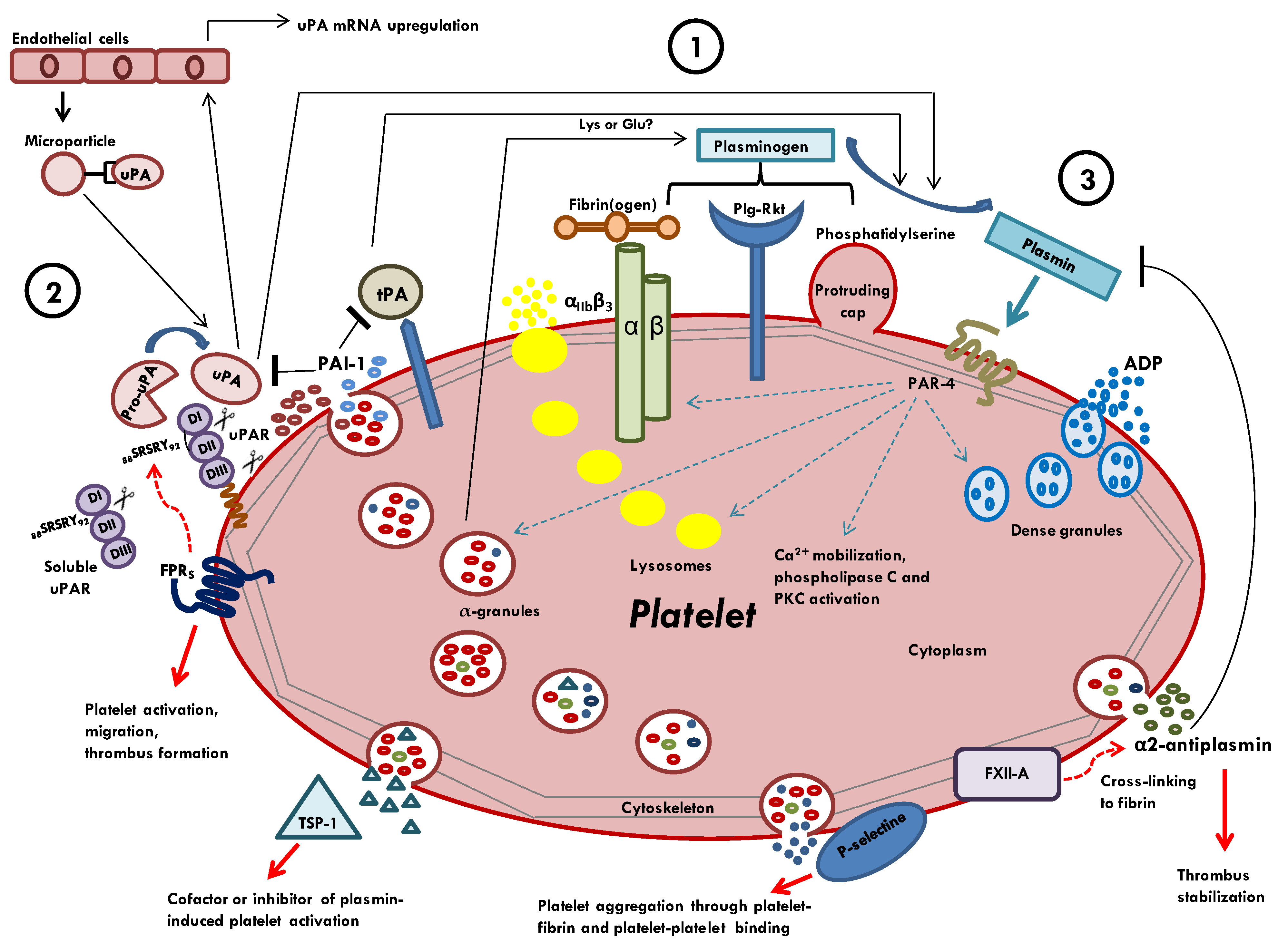
2. Molecular Connections between Platelets and Components of Plasminogen Activation System
2.1. Plasminogen as Zymogen Form
2.2. Plasmin
2.3. Tissue-Type Plasminogen Activator
2.4. Urokinase Plasminogen Activator
2.5. High-Affinity uPA Receptor (uPAR)
2.6. Plasminogen Activator Inhibitors (PAI-1 and PAI-2)
2.7. Alpha 2-Antiplasmin
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Marshall, J.M.; Cederholm-Williams, S.A. Plasminogen: A structural review. Blood Coagul. Fibrinolysis 1992, 3, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Abu-Ssaydeh, D.; Whisstock, J.C. New insights into the structure and function of the plasminogen/plasmin system. Curr. Opin. Struct. Biol. 2013, 23, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.J.; Ploplis, V.A. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 2005, 93, 647–654. [Google Scholar]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol. Life Sci. 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Aĭsina, R.B.; Mukhametova, L.I. Structure and functions of plasminogen/plasmin system. Bioorg. Khim. 2014, 40, 642–657. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 12, 24. [Google Scholar] [CrossRef]
- Lin, H.; Xu, L.; Yu, S.; Hong, W.; Huang, M.; Xu, P. Therapeutics targeting the fibrinolytic system. Exp. Mol. Med. 2020, 52, 367–379. [Google Scholar] [CrossRef]
- Schaller, J.; Gerber, S.S. The plasmin–antiplasmin system: Structural and functional aspects. Cell Mol. Life Sci. 2010, 68, 785–801. [Google Scholar] [CrossRef]
- Petersen, L.C.; Lund, L.R.; Nielsen, L.S.; Danø, K.; Skriver, L. One-chain urokinase-type plasminogen activator from human sarcoma cells is a proenzyme with little or no intrinsic activity. J. Biol. Chem. 1988, 263, 11189–11195. [Google Scholar] [CrossRef]
- Li Santi, A.; Napolitano, F.; Montuori, N.; Ragno, P. The Urokinase Receptor: A Multifunctional Receptor in Cancer Cell Biology. Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4111. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Di Spigna, G.; Vargas, M.; Iacovazzo, C.; Pinchera, B.; Spalletti Cernia, D.; Ricciardone, M.; Covelli, B.; Servillo, G.; Gentile, I.; et al. Soluble Urokinase Receptor as a Promising Marker for Early Prediction of Outcome in COVID-19 Hospitalized Patients. J. Clin. Med. 2021, 10, 4914. [Google Scholar] [CrossRef] [PubMed]
- Medcalf, R.L. What drives “fibrinolysis”? Hamostaseologie 2015, 35, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.; Philips, M.; Selmer, J.; Lecander, I.; Astedt, B. Kinetics of inhibition of tissue-type and urokinase-type plasminogen activator by plasminogen-activator inhibitor type 1 and type 2. Eur. J. Biochem. 1988, 175, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 3670. [Google Scholar] [CrossRef]
- Li, W.Y.; Chong, S.S.; Huang, E.Y.; Tuan, T.L. Plasminogen activator/plasmin system: A major player in wound healing? Wound Repair Regen 2003, 11, 239–247. [Google Scholar] [CrossRef]
- Baker, S.K.; Strickland, S. A critical role for plasminogen in inflammation. J. Exp. Med. 2020, 217, e20191865. [Google Scholar] [CrossRef]
- Danø, K.; Behrendt, N.; Høyer-Hansen, G.; Johnsen, M.; Lund, L.R.; Ploug, M.; Rømer, J. Plasminogen activation and cancer. Thromb. Haemost. 2005, 93, 676–681. [Google Scholar]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Napolitano, F.; Montuori, N.; Postiglione, L.; Selleri, C.; de Paulis, A. The Urokinase/Urokinase Receptor System in Mast Cells: Effects of its Functional Interaction with fMLF Receptors. Transl. Med. UniSa 2016, 15, 34–41. [Google Scholar]
- Napolitano, F.; Montuori, N. The Role of the Plasminogen Activation System in Angioedema: Novel Insights on the Pathogenesis. J. Clin. Med. 2021, 10, 518. [Google Scholar] [CrossRef]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef]
- Maynard, D.M.; Heijnen, H.F.G.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic analysis of platelet alpha-granules using mass spectrometry. J. Thromb. Haemost. 2007, 5, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, S.; Flaumenhaft, R. Advances in platelet granule biology. Curr. Opin. Hematol. 2013, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pr. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef]
- Eisinger, F.; Patzelt, J.; Langer, H.F. The Platelet Response to Tissue Injury. Front. Med. 2018, 5, 317. [Google Scholar] [CrossRef]
- Fong, K.P.; Barry, C.; Tran, A.N.; Traxler, E.A.; Wannemacher, K.M.; Tang, H.Y.; Speicher, K.D.; Blair, I.A.; Speicher, D.W.; Grosser, T.; et al. Deciphering the human platelet sheddome. Blood 2011, 117, e15–e26. [Google Scholar] [CrossRef]
- Stalker, T.J.; Welsh, J.D.; Brass, L.F. Shaping the platelet response to vascular injury. Curr. Opin. Hematol. 2014, 21, 410–417. [Google Scholar] [CrossRef]
- Munnix, I.C.A.; Cosemans, J.M.E.M.; Auger, J.M.; Heemskerk, J.W.M. Platelet response heterogeneity in thrombus formation. Thromb. Haemost. 2009, 102, 1149–1156. [Google Scholar] [PubMed]
- Kunitada, S.; FitzGerald, G.A.; Fitzgerald, D.J. Inhibition of clot lysis and decreased binding of tissue-type plasminogen activator as a consequence of clot retraction. Blood 1992, 79, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Sumi, Y.; Miura, O.; Hirosawa, S. Human alpha 2-plasmin inhibitor. Methods Enzym. 1993, 223, 185–197. [Google Scholar]
- Whyte, C.S.; Mitchell, J.L.; Mutch, N.J. Platelet-Mediated Modulation of Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sano, H.; Mochizuki, L.; Honkura, N.; Urano, T. Activated platelet-based inhibition of fibrinolysis via thrombin-activatable fibrinolysis inhibitor activation system. Blood Adv. 2020, 4, 5501–5511. [Google Scholar] [CrossRef]
- Colucci, M.; Semeraro, N.; Semeraro, F. Platelets and Fibrinolysis. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N., Lopez, J., Page, C., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef]
- Veljkovic, D.K.; Rivard, G.E.; Diamandis, M.; Blavignac, J.; Cramer-Bordé, E.M.; Hayward, C.P. Increased expression of urokinase plasminogen activator in Quebec platelet disorder is linked to megakaryocyte differentiation. Blood 2009, 113, 1535–1542. [Google Scholar] [CrossRef]
- Miles, L.A.; Plow, E.F. Binding and activation of plasminogen on the platelet surface. J. Biol. Chem. 1985, 260, 4303–4311. [Google Scholar] [CrossRef]
- Miles, L.A.; Dahlberg, C.M.; Plow, E.F. The cell-binding domains of plasminogen and their function in plasma. J. Biol. Chem. 1988, 263, 11928–11934. [Google Scholar] [CrossRef]
- Adelman, B.; Rizk, A.; Hanners, E. Plasminogen interactions with platelets in plasma. Blood 1988, 5, 1530–1535. [Google Scholar] [CrossRef]
- Loscalzo, J.; Pasche, B.; Ouimet, H.; Freedman, J.E. Platelets and plasminogen activation. Thromb Haemost 1995, 74, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Baeten, K.M.; Richard, M.C.; Kanse, S.M.; Mutch, N.J.; Degen, J.L.; Booth, N.A. Activation of single-chain urokinase-type plasminogen activator by platelet-associated plasminogen: A mechanism for stimulation of fibrinolysis by platelets. J. Thromb. Haemost. 2010, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Dejouvencel, T.; Doeuvre, L.; Lacroix, R.; Plawinski, L.; Dignat-George, F.; Lijnen, H.R.; Anglés-Cano, E. Fibrinolytic cross-talk: A new mechanism for plasmin formation. Blood 2010, 115, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.S.; Swieringa, F.; Mastenbroek, T.G.; Lionikiene, A.S.; Lancé, M.D.; van der Meijden, P.E.J.; Heemskerk, J.W.M.; Mutch, N.J. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood 2015, 125, 2568–2578. [Google Scholar] [CrossRef]
- Whyte, C.S.; Morrow, G.B.; Baik, N.; Booth, N.A.; Jalal, M.M.; Parmer, R.J.; Miles, L.A.; Mutch, N.J. Exposure of plasminogen and a novel plasminogen receptor, Plg-RKT, on activated human and murine platelets. Blood 2021, 137, 248–257. [Google Scholar] [CrossRef]
- Van der Vorm, L.N.; Remijn, J.A.; de Laat, B.; Huskens, D. Effects of Plasmin on von Willebrand Factor and Platelets: A Narrative Review. TH Open 2018, 2, e218–e228. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Senyi, A.F.; Gillies, P. Plasmin-induced platelet aggregation and platelet release reaction. Effects on hemostasis. J. Clin. Investig. 1973, 52, 1647–1659. [Google Scholar] [CrossRef]
- Ishii-Watabe, A.; Uchida, E.; Mizuguchi, H.; Hayakawa, T. On the mechanism of plasmin-induced platelet aggregation. Implications of the dual role of granule ADP. Biochem. Pharm. 2000, 59, 1345–1355. [Google Scholar] [CrossRef]
- Quinton, T.M.; Kim, S.; Derian, C.K.; Jin, J.; Kunapuli, S.P. Plasmin-mediated activation of platelets occurs by cleavage of protease-activated receptor 4. J. Biol. Chem. 2004, 279, 18434–18439. [Google Scholar] [CrossRef]
- Shigeta, O.; Kojima, H.; Jikuya, T.; Terada, Y.; Atsumi, N.; Sakakibara, Y.; Nagasawa, T.; Mitsui, T. Aprotinin inhibits plasmin-induced platelet activation during cardiopulmonary bypass. Circulation 1997, 96, 569–574. [Google Scholar] [CrossRef]
- Pielsticker, C.; Brodde, M.F.; Raum, L.; Jurk, K.; Kehrel, B.E. Plasmin-Induced Activation of Human Platelets Is Modulated by Thrombospondin-1, Bona Fide Misfolded Proteins and Thiol Isomerases. Int J. Mol. Sci. 2020, 21, 8851. [Google Scholar] [CrossRef] [PubMed]
- Blockmans, D.; Deckmyn, H.; Hove, L.V.; Vermylen, J. The effect of plasmin on platelet function. Platelets 1996, 7, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.I.; Adelman, B. Plasmin inhibition of platelet function and of arachidonic acid metabolism. J. Clin. Invest. 1985, 75, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Gouin, I.; Lecompte, T.; Morel, M.C.; Lebrazi, J.; Modderman, P.W.; Kaplan, C.; Samama, M.M. In vitro effect of plasmin on human platelet function in plasma. Inhibition of aggregation caused by fibrinogenolysis. Circulation 1992, 85, 935–941. [Google Scholar] [CrossRef]
- Vaughan, D.E.; Mendelsohn, M.E.; Declerck, P.J.; Van Houtte, E.; Collen, D.; Loscalzo, J. Characterization of the binding of human tissue-type plasminogen activator to platelets. J. Biol. Chem. 1989, 264, 15869–15874. [Google Scholar] [CrossRef]
- Brisson-Jeanneau, C.; Nelles, L.; Rouer, E.; Sultan, Y.; Benarous, R. Tissue-plasminogen activator RNA detected in megakaryocytes by in situ hybridization and biotinylated probe. Histochemistry 1990, 95, 23–26. [Google Scholar] [CrossRef]
- Wang, D.L.; Pan, Y.T.; Wang, J.J.; Cheng, C.H.; Liu, C.Y. Demonstration of a functionally active tPA-like plasminogen activator in human platelets. Thromb. Haemost. 1994, 71, 493–498. [Google Scholar] [CrossRef]
- Stamler, J.S.; Simon, D.I.; Jaraki, O.; Osborne, J.A.; Francis, S.; Mullins, M.; Singel, D.; Loscalzo, J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc. Natl. Acad. Sci. USA 1992, 89, 8087–8091. [Google Scholar] [CrossRef]
- Collet, J.P.; Montalescot, G.; Lesty, C.; Weisel, J.W. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ. Res. 2002, 90, 428–434. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Hansen, K.C.; Dzieciatkowska, M.; Chapman, M.P.; Sauaia, A.; West, B.; Banerjee, A.; Silliman, C.C. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: Evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock 2015, 43, 39–46. [Google Scholar] [CrossRef]
- Diamandis, M.; Veljkovic, D.K.; Maurer-Spurej, E.; Rivard, G.E.; Hayward, C.P. Quebec platelet disorder: Features, pathogenesis and treatment. Blood Coagul. Fibrinolysis 2008, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gurewich, V.; Johnstone, M.; Loza, J.P.; Pannell, R. Pro-urokinase and prekallikrein are both associated with platelets. Implications for the intrinsic pathway of fibrinolysis and for therapeutic thrombolysis. FEBS Lett. 1993, 318, 317–321. [Google Scholar] [CrossRef]
- Loza, J.P.; Gurewich, V.; Johnstone, M.; Pannell, R. Platelet-bound prekallikrein promotes pro-urokinase-induced clot lysis: A mechanism for targeting the factor XII dependent intrinsic pathway of fibrinolysis. Thromb. Haemost. 1994, 71, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Gurewich, V. Fragment E-2 from fibrin substantially enhances pro-urokinase-induced Glu-plasminogen activation. A kinetic study using the plasmin-resistant mutant pro-urokinase Ala-158-rpro-UK. Biochemistry 1992, 31, 6311–6317. [Google Scholar] [CrossRef]
- Fleury, V.; Lijnen, H.R.; Anglés-Cano, E. Mechanism of the enhanced intrinsic activity of single-chain urokinase-type plasminogen activator during ongoing fibrinolysis. J. Biol. Chem. 1993, 268, 18554–18559. [Google Scholar] [CrossRef]
- Pannell, R.; Gurewich, V. Pro-urokinase: A study of its stability in plasma and of a mechanism for its selective fibrinolytic effect. Blood 1986, 67, 1215–1223. [Google Scholar] [CrossRef]
- Kahr, W.H.; Zheng, S.; Sheth, P.M.; Pai, M.; Cowie, A.; Bouchard, M.; Podor, T.J.; Rivard, G.E.; Hayward, C.P. Platelets from patients with the Quebec platelet disorder contain and secrete abnormal amounts of urokinase-type plasminogen activator. Blood 2001, 98, 257–265. [Google Scholar] [CrossRef]
- Hayward, C.P.M.; Liang, M.; Tasneem, S.; Soomro, A.; Waye, J.S.; Paterson, A.D.; Rivard, G.E.; Wilson, M.D. The duplication mutation of Quebec platelet disorder dysregulates PLAU, but not C10orf55, selectively increasing production of normal PLAU transcripts by megakaryocytes but not granulocytes. PLoS ONE 2017, 12, e0173991. [Google Scholar] [CrossRef]
- Camoin-Jau, L.; Pannell, R.; Anfosso, F.; Bardin, N.; Sabatier, F.; Sampol, J.; Gurewich, V.; Dignat-George, F. Platelet associated u-PA up-regulates u-PA synthesis by endothelial cells. Thromb. Haemost. 2002, 88, 517–523. [Google Scholar] [CrossRef]
- Jurasz, P.; Santos-Martinez, M.J.; Radomska, A.; Radomski, M.W. Generation of platelet angiostatin mediated by urokinase plasminogen activator: Effects on angiogenesis. J. Thromb. Haemost. 2006, 4, 1095–1106. [Google Scholar] [CrossRef]
- Piguet, P.F.; Vesin, C.; Donati, Y.; Tacchini-Cottier, F.; Belin, D.; Barazzone, C. Urokinase receptor (uPAR, CD87) is a platelet receptor important for kinetics and TNF-induced endothelial adhesion in mice. Circulation 1999, 99, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Sloand, E.M.; Pfannes, L.; Scheinberg, P.; More, K.; Wu, C.O.; Horne, M.; Young, N.S. Increased soluble urokinase plasminogen activator receptor (suPAR) is associated with thrombosis and inhibition of plasmin generation in paroxysmal nocturnal hemoglobinuria (PNH) patients. Exp. Hematol. 2008, 36, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.W.; Napolitano, F.; Pesapane, A.; Mascolo, M.; Staibano, S.; Matucci-Cerinic, M.; Guiducci, S.; Ragno, P.; di Spigna, G.; Postiglione, L.; et al. Upregulation of the N-formyl Peptide receptors in scleroderma fibroblasts fosters the switch to myofibroblasts. J. Immunol 2015, 194, 5161–5173. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Rossi, F.W.; Pesapane, A.; Varricchio, S.; Ilardi, G.; Mascolo, M.; Staibano, S.; Lavecchia, A.; Ragno, P.; Selleri, C.; et al. N-Formyl Peptide Receptors Induce Radical Oxygen Production in Fibroblasts Derived From Systemic Sclerosis by Interacting With a Cleaved Form of Urokinase Receptor. Front. Immunol. 2018, 9, 574. [Google Scholar] [CrossRef]
- Czapiga, M.; Gao, J.L.; Kirk, A.; Lekstrom-Himes, J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp. Hematol. 2005, 33, 73–84. [Google Scholar] [CrossRef]
- Salamah, M.F.; Ravishankar, D.; Kodji, X.; Moraes, L.A.; Williams, H.F.; Vallance, T.M.; Albadawi, D.A.; Vaiyapuri, R.; Watson, K.; Gibbins, J.M.; et al. The endogenous antimicrobial cathelicidin LL37 induces platelet activation and augments thrombus formation. Blood Adv. 2018, 2, 2973–2985. [Google Scholar] [CrossRef]
- Brogren, H.; Karlsson, L.; Andersson, M.; Wang, L.; Erlinge, D.; Jern, S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood 2004, 104, 3943–3948. [Google Scholar] [CrossRef]
- Birdane, A.; Haznedaroglu, I.C.; Bavbek, N.; Kosar, A.; Buyukasik, Y.; Ozcebe, O.; Dündar, S.V.; Kirazli, S. The plasma levels of prostanoids and plasminogen activator inhibitor-1 in primary and secondary thrombocytosis. Clin. Appl. Thromb. Hemost. 2005, 11, 197–201. [Google Scholar] [CrossRef]
- Brogren, H.; Wallmark, K.; Deinum, J.; Karlsson, L.; Jern, S. Platelets retain high levels of active plasminogen activator inhibitor 1. PLoS ONE 2011, 6, e26762. [Google Scholar] [CrossRef]
- Brogren, H.; Sihlbom, C.; Wallmark, K.; Lönn, M.; Deinum, J.; Karlsson, L.; Jern, S. Heterogeneous glycosylation patterns of human PAI-1 may reveal its cellular origin. Thromb. Res. 2008, 122, 271–281. [Google Scholar] [CrossRef]
- Braaten, J.V.; Handt, S.; Jerome, W.G.; Kirkpatrick, J.; Lewis, J.C.; Hantgan, R.R. Regulation of fibrinolysis by platelet-released plasminogen activator inhibitor 1: Light scattering and ultrastructural examination of lysis of a model platelet-fibrin thrombus. Blood 1993, 81, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, K.; Urano, T.; Kozima, Y.; Takada, Y.; Takada, A. The potential role of platelet PAl-1 in t-PA mediated clot lysis of platelet rich plasma. Thromb. Res. 1993, 71, 289–300. [Google Scholar] [CrossRef][Green Version]
- Wiman, B. Plasminogen activator inhibitor 1 (PAI-1) in plasma: Its role in thrombotic disease. Thromb. Haemost. 1995, 74, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Podor, T.J.; Singh, D.; Chindemi, P.; Foulon, D.M.; McKelvie, R.; Weitz, J.I.; Austin, R.; Boudreau, G.; Davies, R. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J. Biol. Chem. 2002, 277, 7529–7539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Carmeliet, P.; Fay, W.P. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation 1999, 99, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.A.; Bennett, B.; Croll, A.M.; Brown, P.A.; Booth, N.A. Proteins of the fibrinolytic system in human thrombi. Thromb. Haemost. 1996, 75, 127–133. [Google Scholar] [CrossRef]
- Huebner, B.R.; Moore, E.E.; Moore, H.B.; Stettler, G.R.; Nunns, G.R.; Lawson, P.; Sauaia, A.; Kelher, M.; Banerjee, A.; Silliman, C.C. Thrombin Provokes Degranulation of Platelet α-Granules Leading to the Release of Active Plasminogen Activator Inhibitor-1 (PAI-1). Shock 2018, 50, 671–676. [Google Scholar] [CrossRef]
- Chaurasia, S.N.; Kushwaha, G.; Kulkarni, P.P.; Mallick, R.L.; Latheef, N.A.; Mishra, J.K.; Dash, D. Platelet HIF-2α promotes thrombogenicity through PAI-1 synthesis and extracellular vesicle release. Haematologica 2019, 104, 2482–2492. [Google Scholar] [CrossRef]
- Alfano, D.; Gorrasi, A.; Li Santi, A.; Ricci, P.; Montuori, N.; Selleri, C.; Ragno, P. Urokinase receptor and CXCR4 are regulated by common microRNAs in leukaemia cells. J. Cell Mol. Med. 2015, 19, 2262–2272. [Google Scholar] [CrossRef]
- Jokl, R.; Klein, R.L.; Lopes-Virella, M.F.; Colwell, J.A. Release of platelet plasminogen activator inhibitor 1 in whole blood is increased in patients with type II diabetes. Diabetes Care 1995, 18, 1150–1155. [Google Scholar] [CrossRef]
- Luo, M.; Li, R.; Ren, M.; Chen, N.; Deng, X.; Tan, X.; Li, Y.; Zeng, M.; Yang, Y.; Wan, Q.; et al. Hyperglycaemia-induced reciprocal changes in miR-30c and PAI-1 expression in platelets. Sci Rep. 2016, 6, 36687. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, M.; Tamponi, G.; Gallo, E.; Stella, S.; Schinco, P.C.; Pannocchia, A.; Pileri, A. Fibrinolytic imbalance in essential thrombocythemia: Role of platelets. Haemostasis 1993, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schroder, W.A.; Le, T.T.; Gardner, J.; Andrews, R.K.; Gardiner, E.E.; Callaway, L.; Suhrbier, A. SerpinB2 deficiency in mice reduces bleeding times via dysregulated platelet activation. Platelets 2019, 30, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Miles, L.A.; Collen, D. Platelet alpha 2-antiplasmin. Methods Enzymol. 1989, 169, 296–300. [Google Scholar]
- Mitchell, J.L.; Lionikiene, A.S.; Fraser, S.R.; Whyte, C.S.; Booth, N.A.; Mutch, N.J. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood 2014, 124, 3982–3990. [Google Scholar] [CrossRef]
- Takei, M.; Matsuno, H.; Okada, K.; Ueshima, S.; Matsuo, O.; Kozawa, O. Lack of alpha 2-antiplasmin enhances ADP induced platelet micro-aggregation through the presence of excess active plasmin in mice. J. Thromb. Thrombolysis 2002, 14, 205–211. [Google Scholar] [CrossRef]
- Brzoska, T.; Tanaka-Murakami, A.; Suzuki, Y.; Sano, H.; Kanayama, N.; Urano, T. Endogenously generated plasmin at the vascular wall injury site amplifies lysine binding site-dependent plasminogen accumulation in microthrombi. PLoS ONE 2015, 10, e0122196. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, F.; Montuori, N. Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications. Int. J. Mol. Sci. 2022, 23, 6065. https://doi.org/10.3390/ijms23116065
Napolitano F, Montuori N. Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications. International Journal of Molecular Sciences. 2022; 23(11):6065. https://doi.org/10.3390/ijms23116065
Chicago/Turabian StyleNapolitano, Filomena, and Nunzia Montuori. 2022. "Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications" International Journal of Molecular Sciences 23, no. 11: 6065. https://doi.org/10.3390/ijms23116065
APA StyleNapolitano, F., & Montuori, N. (2022). Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications. International Journal of Molecular Sciences, 23(11), 6065. https://doi.org/10.3390/ijms23116065






