Ethyl Hydroxyethyl Cellulose—A Biocompatible Polymer Carrier in Blood
Abstract
1. Introduction
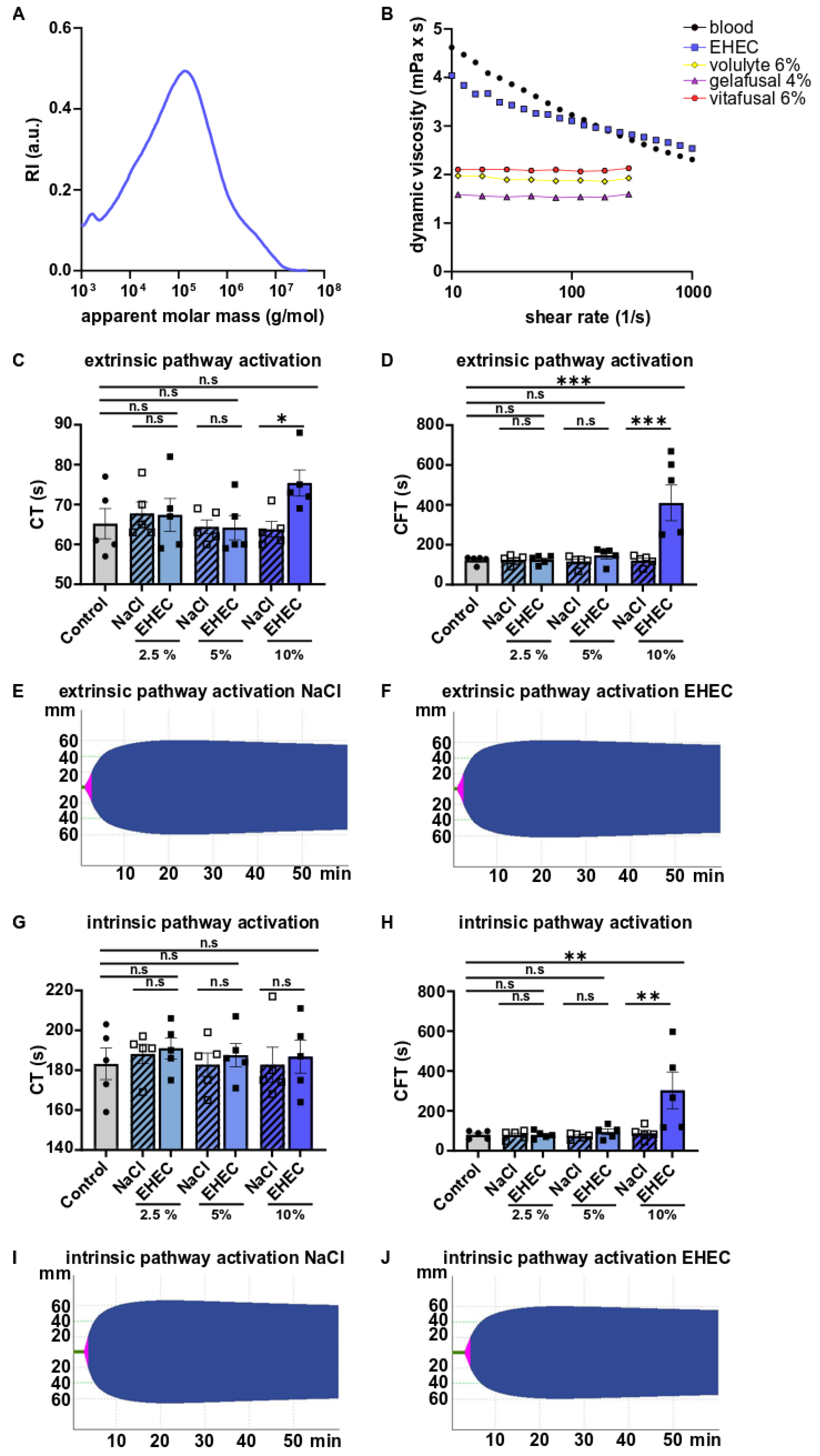
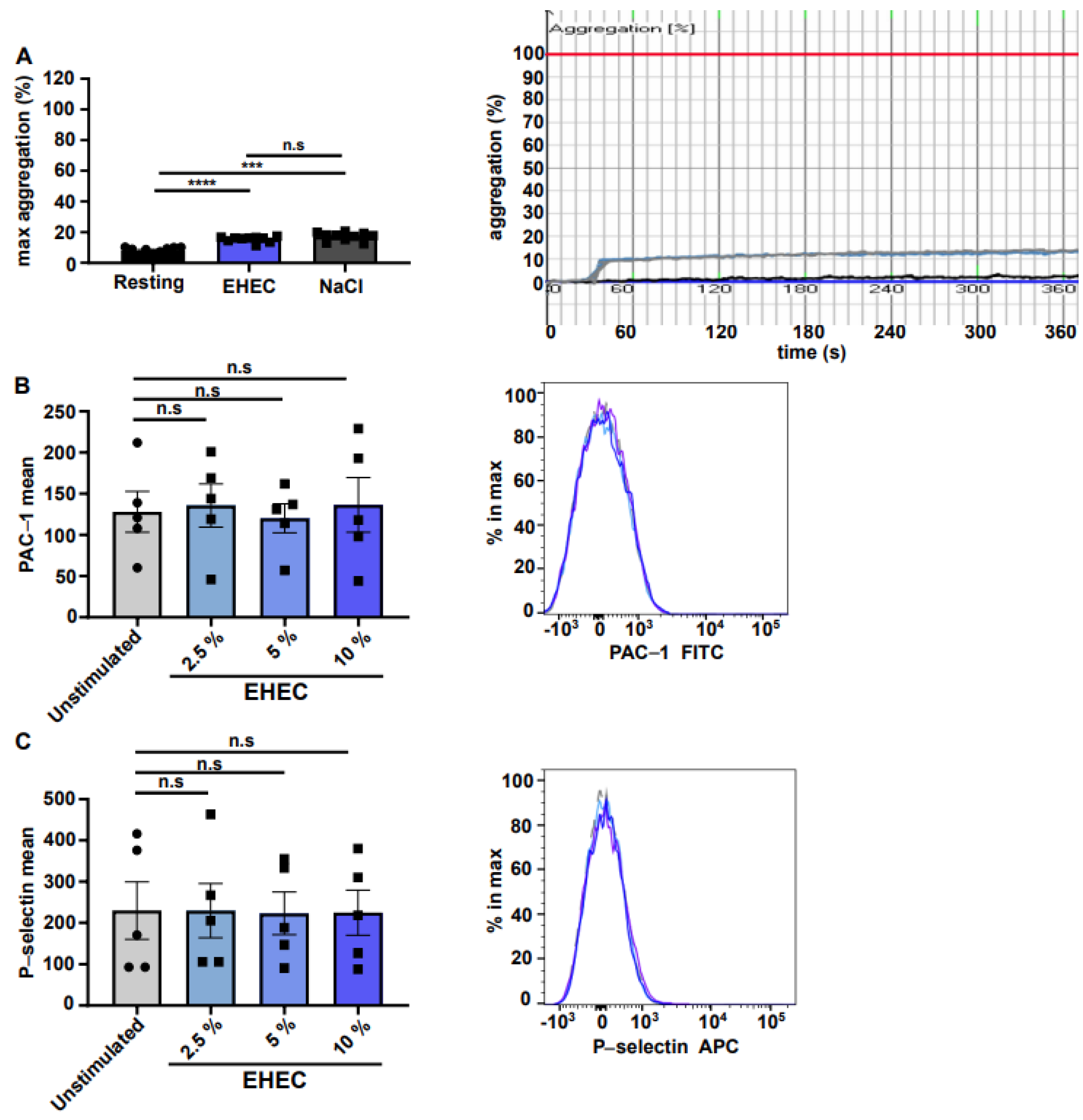
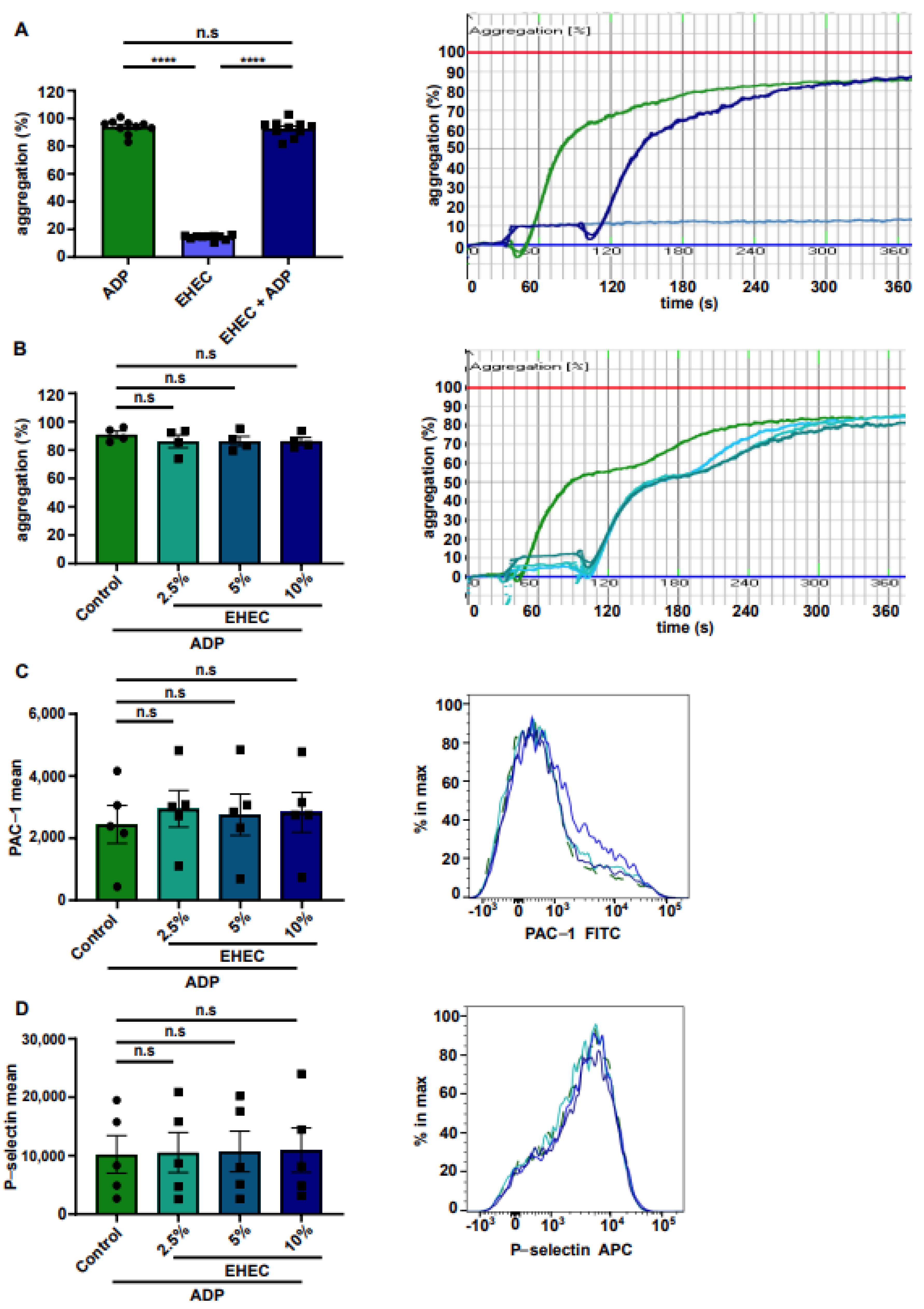
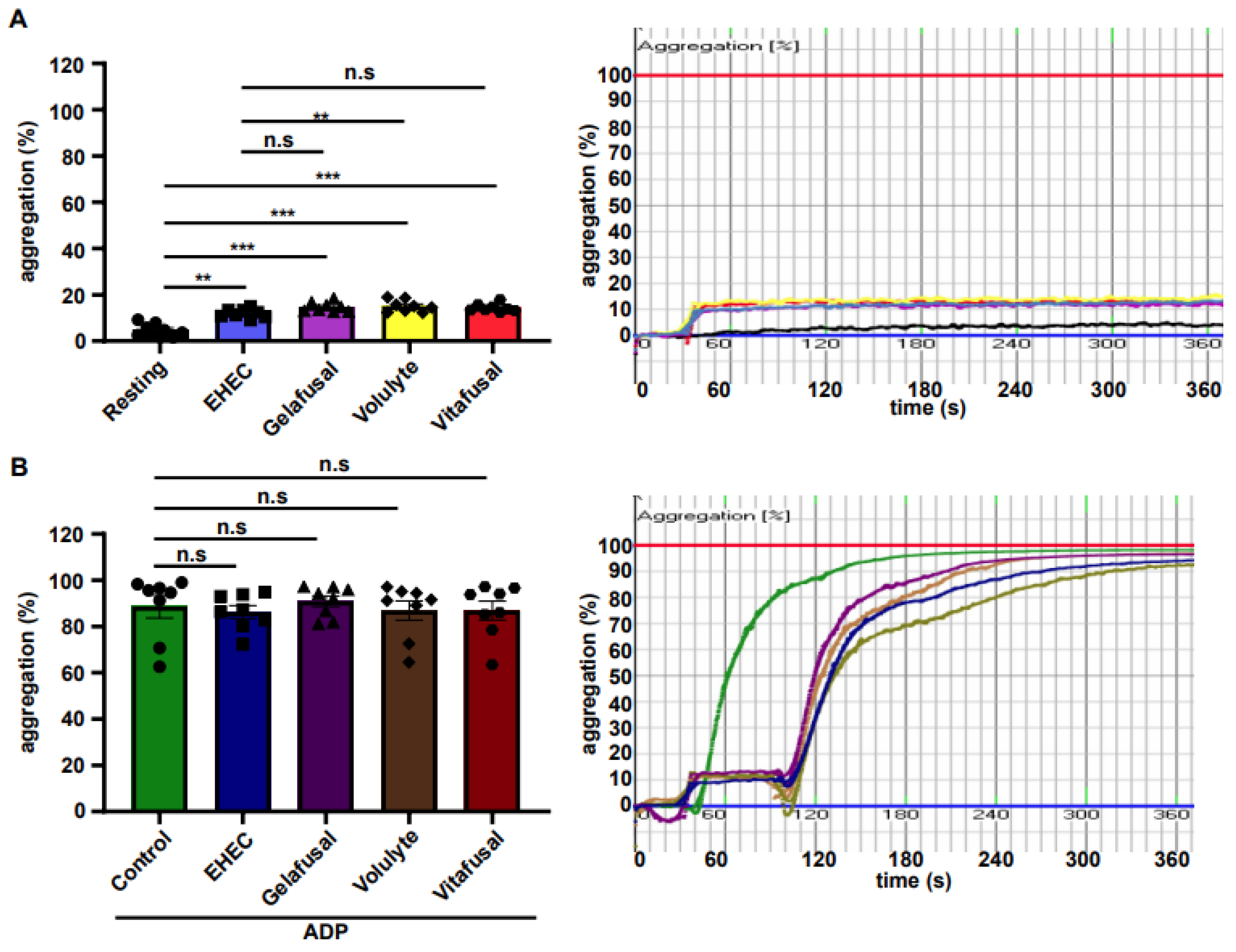
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethyl Hydroxyethyl Cellulose (EHEC)
4.2. Size-Exclusion Chromatography (SEC)
4.3. Rheology Studies
4.4. Blood Collection
4.5. Rotational Thromboelastometry (ROTEM)
4.6. Aggregometry
4.7. Flow Cytometry
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mittnacht, U.; Hartmann, H.; Hein, S.; Oliveira, H.; Dong, M.; Pego, A.P.; Kjems, J.; Howard, K.A.; Schlosshauer, B. Chitosan/siRNA nanoparticles biofunctionalize nerve implants and enable neurite outgrowth. Nano Lett. 2010, 10, 3033–3939. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Leber, N.; Klefenz, A.; Choteschovsky, N.; Zentel, R.; Nuhn, L.; Schuppan, D. In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles. Cells 2020, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, L.; Zhao, L. Silica nanoparticle-based dual-responsive nanoprodrug system for liver cancer therapy. Exp. Med. 2017, 14, 2071–2077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.; Zhang, S.; Ma, J.; Fan, L.; Yin, C.; Lin, G.; Li, Q. Double loaded self-decomposable SiO2 nanoparticles for sustained drug release. Nanoscale 2015, 7, 16389–16398. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lewis, D.R.; Petersen, L.K.; Moghe, P.V.; Uhrich, E.K. Amphiphilic macromolecule nanoassemblies suppress smooth muscle cell proliferation and platelet adhesion. Biomaterials 2016, 84, 219–229. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, L.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Docter, D.; Distler, U.; Storck, W.; Kuharev, J.; Wünsch, D.; Hahlbrock, A.; Knauer, S.K.; Tenzer, S.; Stauber, R.H. Quantitative profiling of the protein coronas that form around nanoparticles. Nat. Protoc. 2014, 9, 2030–2044. [Google Scholar] [CrossRef]
- Konduru, N.V.; Murdaugh, K.M.; Swami, A.; Jimenez, R.J.; Donaghey, T.C.; Demokritou, P.; Brain, J.D.; Molina, R.M. Surface modification of zinc oxide nanoparticles with amorphous silica alters their fate in the circulation. Nanotoxicology 2016, 10, 720–727. [Google Scholar] [CrossRef]
- Baiyan Sui, X.L.; Sun, J. Blood-brain barrier dysfunction induced by silica NPs in vitro and in vivo: Involvement of oxidative stress and Rho-kinase/JNK signaling pathways. Biomaterials 2017, 121, 64–82. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Dias, A.; Werner, M.; Ward, K.R.; Fleuryd, J.B.; Baulin, V.A. High-throughput 3D visualization of nanoparticles attached to the surface of red blood cells. Nanoscale 2019, 11, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martinez, M.J.; Inkielewicz-Stepniak, I.; Medina, C.; Rahme, K.; D’Arcy, D.M.; Fox, D.; Holmes, J.D.; Zhang, H.; Radomski, M.W. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int. J. Nanomed. 2012, 7, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles aggregate human platelets: Potential implications for vascular homeostasis. Int. J. Nanomed. 2012, 7, 631–639. [Google Scholar]
- Santos-Martinez, M.J.; Tomaszewski, K.A.; Medina, C.; Bazou, D.; Gilmer, J.F.; Radomski, M.W. Pharmacological characterization of nanoparticle-induced platelet microaggregation using quartz crystal microbalance with dissipation: Comparison with light aggregometry. Int. J. Nanomed. 2015, 10, 5107–5119. [Google Scholar]
- Soddu, L.; Trinh, D.N.; Dunne, E.; Kenny, D.; Bernardini, G.; Kokalari, I.; Marucco, A.; Monopoli, M.P.; Fenoglio, I. Identification of physicochemical properties that modulate nanoparticle aggregation in blood. Beilstein J. Nanotechnol. 2020, 11, 550–567. [Google Scholar] [CrossRef]
- Besheer, A.; Hause, G.; Kressler, J.; Mäder, K. Hydrophobically modified hydroxyethyl starch: Synthesis, characterization, and aqueous self-assembly into nano-sized polymeric micelles and vesicles. Biomacromolecules 2007, 8, 359–367. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Liu, J.; Maleck, W.H.; Mattinger, C.; Bergler, W.F. The effect of In vitro hemodilution with gelatin, dextran, hydroxyethyl starch, or Ringer’s solution on Thrombelastograph. Anesth. Analg. 2000, 90, 795–800. [Google Scholar]
- Farrales, F.B.; Belcher, C.; Summers, T.; Bayer, W.L. Effect of hydroxyethyl starch on platelet function following granulocyte collection using the continuous flow cell separator. Transfusion 1977, 17, 635–637. [Google Scholar] [CrossRef]
- Klose, R.; Feldmann, U.; Hoecker, P. Effect of 10% hydroxyethyl starch 200/0.5 and 10% dextran 40 on the flow property and coagulation of whole blood In Vivo. Infus. Klin. Ernahr. 1987, 14 (Suppl. S2), 37–42. [Google Scholar]
- Schaden, E.; Wetzel, L.; Kozek-Langenacker, S.; Thaler, U.; Scharbert, G. Effect of the carrier solution for hydroxyethyl starch on platelet aggregation and clot formaton. Br. J. Anaesth. 2012, 109, 572–577. [Google Scholar] [CrossRef][Green Version]
- Görlinger, K.; Jambor, C.; Dirkmann, D.; Dusse, F.; Hanke, A.; Adamzik, M.; Hartmann, M.; Philipp, S.; Weber, A.A.; Rahe-Meyer, N. Platelet function analysis with point-of-care methods. Herz 2008, 33, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Schulte, V.; Brockhoff, G.; Bier, U.; Zirngibl, H.; Nieswandt, B. Flow cytometric detection of activated mouse alphaIIbbeta3 with a novel monoclonal antibody. Cytometry 2002, 48, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vlastos, G.A.; Lerche, D.; Koch, B. The superposition of steady on oscillatory shear and its effect on the viscoelasticity of human blood and a blood-like model fluid. Biorheology 1997, 34, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Karoutsos, S.; Nathan, N.; Lahrimi, A.; Grouille, D.; Feiss, P.; Cox, D.J. Thrombelastogram reveals hypercoagulability after administration of gelatin solution. Br. J. Anaesth. 1999, 82, 175–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapiotis, S.; Quehenberger, P.; Eichler, H.G.; Schwarzinger, I.; Pärtan, C.; Schneider, B.; Lechner, K.; Speiser, W. Effect of hydroxyethyl starch on the activity of blood coagulation and fibrinolysis in healthy volunteers: Comparison with albumin. Crit. Care Med. 1994, 22, 606–612. [Google Scholar] [CrossRef]
- Egli, G.A.; Zollinger, A.; Seifert, B.; Popovic, D.; Pasch, T.; Spahn, D.R. Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br. J. Anaesth. 1997, 78, 684–689. [Google Scholar] [CrossRef]
- Nielsen, V.G. A Hydroxyethyl starch enhances fibrinolysis in human plasma by diminishing alpha2-antiplasmin-plasmin interactions. Blood Coagul. Fibrinolysis 2007, 18, 647–656. [Google Scholar] [CrossRef]
- Blaicher, A.M.; Reiter, W.J.; Blaicher, W.; Kettner, S.C.; Felfernig, M.; Grabner, C.M.; Zimpfer, M. The effect of hydroxyethyl starch on platelet aggregation in vitro. Anesth. Analg. 1998, 86, 1318–1321. [Google Scholar] [CrossRef]
- De Jonge, E.; Levi, M.; Berends, F.; van der Ende, A.E.; ten Cate, J.W.; Stoutenbeek, C.P. Impaired haemostasis by intravenous administration of a gelatin-based plasma expander in human subjects. Thromb. Haemost. 1998, 79, 286–290. [Google Scholar]
- Tabuchi, N.; de Haan, J.; Gallandat Huet, R.C.; Boonstra, P.W.; van Oeveren, W. Gelatin use impairs platelet adhesion during cardiac surgery. Thromb. Haemost. 1995, 74, 14471451. [Google Scholar] [CrossRef]
- Scherlund, M.; Brodin, A.; Malmsten, M. Nonionic Cellulose Ethers as Potential Drug Delivery Systems for Periodontal Anesthesia. J. Colloid Interface Sci. 2000, 229, 365–374. [Google Scholar] [CrossRef] [PubMed]
| Gelafusal® | Volulyte® | Vitafusal® | |||
|---|---|---|---|---|---|
| w (g) in 1000 mL | Compound | w (g) in 1000 mL | Compound | w (g) in 1000 mL | Compound |
| 40.000 | Gelatine polysuccinate | 60.000 | Hydroxyethyl starch | 60.000 | Poly(O-2-hydroxyethyl) starch |
| 3.675 | Sodium acetate trihydrate | 4.630 | Sodium acetate trihydrate | 3.700 | Sodium acetate trihydrate |
| 4.590 | Sodium chloride | 6.020 | Sodium chloride | 6.000 | Sodium chloride |
| 0.403 | Potassium chloride | 0.300 | Potassium chloride | 0.400 | Potassium chloride |
| 0.133 | Calcium chloride dihydrate | 0.300 | Magnesium chloride hexahydrate | 0.134 | Calcium chloride dihydrate |
| 0.203 | Magnesium chloride hexahydrate | 0.200 | Magnesium chloride hexahydrate | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckelt, A.; Wichmann, F.; Bayer, F.; Eckelt, J.; Groß, J.; Opatz, T.; Jurk, K.; Reinhardt, C.; Kiouptsi, K. Ethyl Hydroxyethyl Cellulose—A Biocompatible Polymer Carrier in Blood. Int. J. Mol. Sci. 2022, 23, 6432. https://doi.org/10.3390/ijms23126432
Eckelt A, Wichmann F, Bayer F, Eckelt J, Groß J, Opatz T, Jurk K, Reinhardt C, Kiouptsi K. Ethyl Hydroxyethyl Cellulose—A Biocompatible Polymer Carrier in Blood. International Journal of Molecular Sciences. 2022; 23(12):6432. https://doi.org/10.3390/ijms23126432
Chicago/Turabian StyleEckelt, Anja, Franziska Wichmann, Franziska Bayer, John Eckelt, Jonathan Groß, Till Opatz, Kerstin Jurk, Christoph Reinhardt, and Klytaimnistra Kiouptsi. 2022. "Ethyl Hydroxyethyl Cellulose—A Biocompatible Polymer Carrier in Blood" International Journal of Molecular Sciences 23, no. 12: 6432. https://doi.org/10.3390/ijms23126432
APA StyleEckelt, A., Wichmann, F., Bayer, F., Eckelt, J., Groß, J., Opatz, T., Jurk, K., Reinhardt, C., & Kiouptsi, K. (2022). Ethyl Hydroxyethyl Cellulose—A Biocompatible Polymer Carrier in Blood. International Journal of Molecular Sciences, 23(12), 6432. https://doi.org/10.3390/ijms23126432







