Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities
Abstract
:1. Introduction
2. Molecular Mechanisms of Inflammation and Oxidative Stress
3. Lignans with Antioxidant and Anti-Inflammatory Action
| Lignan | Source | Model/Assay | Target | Concentration | Ref. |
|---|---|---|---|---|---|
| Aryltetralinstructure group | |||||
| (−)-Isoguaiacin | Machilusthunbergii Sieb, et Zucc. | CCl4-induced hepatotoxicity | ↓GPT level | 50–100 μM | [24] |
| ↓MDA content, ↑GSH/GSSG level, ↑SOD1, ↑CAT | 50 μM | ||||
| (+)-Isolariciresinol | Riesling wine | TEAC assay | radical scavenging capacity | 2.5 mmol Trolox/mmol | [25] |
| Ephedra viridis | DCFH assay in HL-60 cells | ↓iROS level | IC50 21 μg/mL | [26] | |
| Euterpe oleracea Mart. | HO assay | HO• scavenging | IC50 0.68 ± 0.02 μg/mL | [27] | |
| DPPH assay | DPPH radical scavenging | IC50 37.4 ± 0.9 μg/mL | |||
| (±)-Isolariciresinol | Synthetic | DPPH assay | DPPH radical scavenging | IC50 53.0 μM | [28] |
| (−)-Isolariciresinol 5-methoxy-9-β-D-xylopyranoside | Saracaasoca (Roxb.) De Wilde | DPPH assay | DPPH radical scavenging | IC50 44 μM | [29] |
| (+)-Isolariciresinol 3a-O-β-D-glucopyranoside | Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 45.7 ± 1.5 μM | [30] |
| FRAP assay | ferric-reducing potentiality | 33 mmol Fe2+/g | |||
| H2O2-induced L02 cells cytotoxicity | ↓iROS level | 5 μM | |||
| Isolariciresinol-9’-O-α-L-arabinofuranoside | Pinus massoniana Lamb. | H2O2-induced HUVECs cytotoxicity | ↑PI3K, ↑p-Akt, ↑p-Bad, ↓Bax | 31.3–125 μg/mL | [31] |
| Lyoniresinol | Berberis vulgaris Linn. | HO assay | HO• scavenging | IC50 1.4 ± 0.12 μg/mL | [32] |
| Viscum album Linn. | ABTS assay | ABTS radical scavenging | 10–100 μM | [33] | |
| DPPH assay | DPPH radical scavenging | ||||
| Glu-treated HT22 cells | ↓iROS level | 25 μM | |||
| (+)-Lyoniresinol-3α-O-β-glucopyranoside | Strychnosvanprukii | DPPH assay | DPPH radical scavenging | IC50 31.2 μM | [34] |
| Sauchinone | Synthetic | AngII-induced mesangial cells | ↓iROS level | 1 μM | [35] |
| Dibenzocyclooctadiene structure group | |||||
| Gomisin A | Synthetic | ZnPP/high glucose-injured MC3T3 E1 cells | ↓iROS level, ↑SOD, ↑HO-1 | 1–10 μM | [36] |
| Gomisin N | Synthetic | HeLa cells | ↑iROS level | 100 μM | [37] |
| ethanol-treated HepG2 cells | ↓iROS level, ↑GSH/GSSG level, ↑CAT, ↑SOD, ↑GPx ↑SIRT1/AMPK, ↓CYP2E1 | 50–100 μM | [38] | ||
| Schisandrin A | Schisandra chinensis Baill. | CCl4-treated HepG2 cells | ↓TBARS level, ↓iROS level | 50 μM | [39] |
| Synthetic | LPS-stimulated RAW 264.7 macrophages | ↓iROS level ↓Keap1, ↑Nrf2, ↑HO-1 | 200 μM | [40] | |
| H2O2-induced C2C12 cell cytotoxicity | ↓iROS level, ↑AMPK, ↑Bcl-2/Bax | 200 μM | [41] | ||
| DON-induced cytotoxicity in HT-29 cells | ↓iROS level, ↓TBARS level, ↓CAT, ↓SOD, ↓GPx, ↑Nrf2, ↑HO-1, ↑GST, ↑GSH/GSSG level | 2.5–10 μM | [42] | ||
| RANKL-induced osteoclast differentiation model | ↓iROS level, ↑Nrf2, ↑HO-1, ↑CAT ↓TRAF6, ↓Nox1 | 50–200 μM | [43] | ||
| Schisandrin B | Schisandra chinensis (Turcz.) Baill. | CCl4-treated HepG2 cells | ↓TBARS level | 50 μM | [39] |
| ↓iROS level | 10–50 μM | ||||
| ↑CYP3A4 expression and activity | 50 μM | ||||
| Synthetic | PQ-induced PC12 cells cytotoxicity | ↓iROS level, ↑GSH/GSSG level | 15 μM | [44] | |
| solar-irradiated BJ human fibroblast | ↓iROS level, ↓MMP, ↑GSH/GSSG level | 25–75 μM | [45] | ||
| intact lymphocytes | ↑iROS level, ↓GSH/GSSG level, ↑Nrf2, ↑HO-1, ↑TR, ↑GCLC | 25–50 μM | [46] | ||
| H2O2-induced PC12 cells cytotoxicity | ↓iROS level, ↓MDA content, ↑SOD | 2.5–10 μM | [47] | ||
| ↑Bcl-2/Bax, ↑p-Akt/Akt | 10 μM | ||||
| CsA-induced cytotoxicity in HK-2 cells | ↓iROS level, ↑GSH/GSSG level, ↑Nrf2, ↑HO-1, ↑NQO1, ↑GCLM ↑Bcl-2/Bax | 2.5–10 μM | [48] | ||
| tBHP-induced HaCaT cell injury | ↓iROS level, ↑Nrf2, ↑HO-1, ↑SOD, ↑GPx, ↑CAT, ↑p-AMPK, ↑p-Akt, ↑p-Erk1/2, ↑p-JNK, ↑p-p38 | 2.5–10 μM | [49] | ||
| H/R-induced H9c2 cell injury | ↓iROS level, ↑SOD, ↑GPx, ↑Nrf2, ↑NQO-1, ↑HO-1, ↓Keap1, ↑AMPK | 20 μM | [50,51] | ||
| Schisandrin C | Synthetic | solar-irradiated BJ human fibroblast | ↓iROS level, ↓MMP, ↑GSH/GSSG level | 25–75 μM | [45] |
| LPS-stimulated HDPCs | ↓iROS level, ↑SOD ↑HO-1, ↑PGC-1α, ↑Nrf2, ↑p-Akt | 10–20 μM | [52] | ||
| Schisantherin A | Schisandra chinensis (Turcz.) Baill. | CCl4-treated HepG2 cells | ↓TBARS level | 50 μM | [39] |
| ↓iROS level | 2–50 μM | ||||
| Schisandra sphenanthera | H/R-induced HK-2 cells | ↓iROS level, ↑SOD, ↑MDA content ↑Bcl2/Bax, ↑PI3K/AKT | 5–20 μM | [53] | |
| LPS-stimulated BV-2 microglial cells | ↓iROS level, ↑HO-1, ↑NQO-1 | 2.5–50 μM | [54] | ||
| ↑Nrf2 | 50 μM | ||||
| Synthetic | LPS-stimulated NRK-52E cells | ↑γGCS, ↑Nrf2 | 25–50 μM | [55] | |
| Dibenzylbutane structure group | |||||
| (–)-Carinol | Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 20.2 μM | [56] |
| Synthetic | DPPH assay | DPPH radical scavenging | IC50 4.4 μg/mL | [57] | |
| XOD assay | ↓xanthine oxidase enzyme | IC50 219.4 μg/mL | |||
| Meso-dihydroguaiaretic acid | Machilusthunbergii Sieb, et Zucc. | CCl4-induced hepatotoxicity | ↓GPT level | 10–100 μM | [24] |
| ↓MDA content, ↑GSH/GSSG level, ↑SOD1, ↑CAT | 50 μM | ||||
| Machilusphilippinensis Merr. | fMLF-activated human neutrophils | ↓O2•– level | IC50 0.78 ± 0.17 μM | [58] | |
| ↓iROS level | IC50 0.79 ± 0.26 μM | ||||
| ↓p-ERK, ↓p-JNK, ↓p-Akt | 10 μM | ||||
| MMK-1-activated human neutrophils | ↓O2•– level | IC50 1.17 ± 0.64 μM | |||
| PMA-activated human neutrophils | ↓iROS level | IC50 3.57 ± 3.93 μM | |||
| ABTS assay | ABTS radical scavenging | 1–10 μM | |||
| DPPH assay | DPPH radical scavenging | ||||
| ORAC assay | ROS scavenging | ||||
| XOD assay | superoxide anion scavenging | ||||
| Nordihydroguaiaretic acid | Larrea tridentate | DCFH assay in HL-60 cells | ↓iROS level | IC50 0.7 μg/mL | [59] |
| Synthetic | FL5.12 cells | ↑p-ERK1/2, ↑p-JNK, ↑p-p38 | 20 μM | [60] | |
| HOCl assay | hypochlorous acid scavenging | IC50 622 ± 42 μM | [61] | ||
| O2•– assay | superoxide anion scavenging | IC50 15 ± 1 μM | |||
| OH assay | OH radical scavenging | IC50 0.15 ± 0.02 μM | |||
| 1O2 assay | singlet oxygen scavenging | IC50 151 ± 20 μM | |||
| ONOO assay | ONOO anion scavenging | IC50 4 ± 0.94 μM | |||
| H2O2/3-NP-induced CGNs neurotoxicity | ↑Nrf2, ↑HO-1 | 20 μM | [62] | ||
| OH assay | OH radical scavenging | 10 μM | [63] | ||
| TPA-treated mouse model | ↑GPx, ↑GR, ↑GST, ↑GSH/GSSG level, ↑SOD, ↑CAT | 15–25 μM | [64] | ||
| H2O2-induced LLC-PK1/MEFs cells cytotoxicity | ↓iROS level, ↑Nrf2, ↑HO-1 ↑p-Akt, ↑p-ERK1/2, ↑p-p38, ↑p-JNK, ↑p-GSK-3 | 15 μM | [65] | ||
| IAA/H2O2-induced cytotoxicity in MN and THP-1 cells | ↓iROS level, ↑GSH/GSSG level ↑CD33 | 20 μM | [66] | ||
| Daoy cells | ↑GSH/GSSG level | 75 μM | [67] | ||
| (–)-Secoisolariciresinol | Taxus yunnanensis | DPPH assay | DPPH radical scavenging | IC50 28.9 μM | [68] |
| Araucaria angustifolia | rat liver microsomes | ↓lipid peroxidation activity | IC50 0.1–0.15 μM | [69] | |
| O2•– assay | superoxide anion scavenging | IC50 4.8 nM | |||
| ROO assay | peroxyl radicals scavenging | SF 3.1–4.0 mole/mole | |||
| Piceaabies | DPPH assay | DPPH radical scavenging | IC50 9.0 ± 1.0 μM | [70] | |
| Linum usitatissimum Linn. | L-α-phosphatidylcholine liposome/pBR322 plasmid DNA | AAPH radical scavenging | 50–100 μM | [71] | |
| DPPH radical scavenging | 25–200 μM | ||||
| Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 26.2 μM | [56] | |
| Secoisolariciresinol-7-hydroxyl | Piceaabies | DPPH assay | DPPH radical scavenging | IC50 12.7 ± 1.5 μM | [70] |
| Secoisolariciresinol diglucoside | Linum usitatissimum Linn. | L-α-phosphatidylcholine liposome/pBR322 plasmid DNA | AAPH radical scavenging | 10–100 μM | [71] |
| DPPH radical scavenging | 25–200 μM | ||||
| Synthetic | DPPH assay | DPPH radical scavenging | IC50 78.9 ± 0.29 μg/mL | [72] | |
| Linum usitatissimum Linn. | iron treated H9c2 cells | ↓iROS level, ↑SOD, ↑Bcl-2/Bax ↓MMP-2, ↓MMP-9, ↓FOXO3a, ↓p70S6K1, ↑AMPK | 500 μM | [73] | |
| Synthetic (LGM2605) | asbestos-exposed MFs | ↓iROS level, ↓MDA content, ↓8-isoP, ↑Nrf2, ↑NQO-1, ↑HO-1, ↑GST, ↑TR, ↓nitrate/nitrite ratio | 50–100 μM | [74,75] | |
| LPS-stimulated AC16 cells | ↓iROS level | 50 μM | [76] | ||
| Dibenzylbutyrolactone structure group | |||||
| Arctigenin | Synthetic | glutamate-treated rat cortical cells | ↓iROS level | IC50 33.2 μM | [77] |
| LPS-treated Raw264.7 cells | ↓iROS level | 5–50 μM | [78] | ||
| Arctium lappa Linn. | glucose-starved A549 cells | ↓iROS level | 10 μM | [79] | |
| H2O2-treated L6 cells | ↑Nrf2, ↑SOD, ↑GR, ↑GPx, ↑Trx1, ↑UCP2, ↑p-AMPK, ↑p-p53, ↑p21, ↑PGC-1α, ↑PPARα | 1–20 μM | [80] | ||
| Synthetic | MDA-MB-231 cells | ↑iROS level, ↓GSH/GSSG level, ↑Nox, ↑p-p38, ↑p-ATF-2, ↓Bcl-2 | 5 μM | [81] | |
| H2O2-treated astrocytes | ↓iROS level | 10–20 μM | [82] | ||
| intact astrocytes | ↑HO-1, ↑Nrf2, ↑c-Jun, ↑p-Akt | ||||
| TGF-β1-induced HK-2 cells | ↓iROS level, ↓Nox ↓p-Akt, ↓p-ERK1/2, ↓p-IκBα | 0.5–1 μM | [83] | ||
| Arctium lappa Linn. | DPPH assay | DPPH radical scavenging | IC50 31.47 ± 2.33 μM | [84] | |
| H2DCF-DA assay | ↓iROS level | 10–100 μM | |||
| Synthetic | OA-treated WRL68 hepatocytes | ↓MDA content ↑p-PI3K, ↑p-Akt, ↑p-AMPK | 50 μM | [85] | |
| Hep G2 cells | ↑iROS level, ↓GSH/GSSG level | 5–100 μM | [86] | ||
| ↑p-p38, ↑p-JNK | 20 μM | ||||
| OGD-injured H9c2 cardiomyocytes | ↓iROS level, ↓MDA content, ↑SOD ↑AMPK/SIRT1 | 50–200 μM | [87] | ||
| silica-injured RAW 264.7 macrophages | ↓iROS level | 1 μM | [88] | ||
| Hinokinin | Synthetic | antioxidant assay | inhibition of H2O2 produced by Trypanosoma cruzi mitochondria | IC50 17.84 μM | [89] |
| Matairesinol | Cedrus deodara | DPPH assay | DPPH radical scavenging | IC50 33.24 ± 0.47 μM | [90] |
| Piceaabies | rat liver microsomes | ↓lipid peroxidation activity | IC50 0.28 μM | [69] | |
| O2•– assay | superoxide anion scavenging | IC50 40 nM | |||
| ROO assay | peroxyl radicals scavenging | SF 1.0 mole/mole | |||
| DPPH assay | DPPH radical scavenging | IC50 14.0 ± 0.0 μM | [70] | ||
| Arctium lappa | DPPH assay | DPPH radical scavenging | IC50 14.95 ± 0.38 μM | [84] | |
| H2DCF-DA assay | ↓iROS level | 100 μM | |||
| Synthetic | DPPH assay | DPPH radical scavenging | 20 μM | [91] | |
| O2•– assay | superoxide anion scavenging | ||||
| hypoxia-induced HeLa cells | ↓miROS levels ↓HIF-1α, ↓VEGF | 10–50 μM | [92] | ||
| LPS-stimulated NSC-34 neurons and BV2 microglia | ↓MDA content, ↑SOD, ↑CAT, ↑GPx, ↑Nrf2, ↑HO-1, ↑AMPK | 5–20 μM | [93] | ||
| Matairesinol-7′-hydroxyl | Piceaabies | rat liver microsomes | ↓lipid peroxidation activity | IC50 0.15–0.18 μM | [69] |
| O2•– assay | superoxide anion scavenging | IC50 217 nM | |||
| ROO assay | peroxyl radicals scavenging | SF 2.1–2.7 mole/mole | |||
| DPPH assay | DPPH radical scavenging | IC50 15.7 ± 0.6 μM | [70] | ||
| DPPH assay | DPPH radical scavenging | IC50 20.0 ± 0.1 μM | [94] | ||
| (+)-Nortrachelogenin | Wikstroemia indica | DPPH assay | DPPH radical scavenging | IC50 90.1 μM | [95] |
| (–)-Nortrachelogenin | Cedrus deodara | DPPH assay | DPPH radical scavenging | IC50 36.79 ± 1.69 μM | [90] |
| Pinus contorta | rat liver microsomes | ↓lipid peroxidation activity | IC50 0.14–0.19 μM | [69] | |
| O2•– assay | superoxide anion scavenging | IC50 1.4 nM | |||
| ROO assay | peroxyl radicals scavenging | SF 2.0–2.2 mole/mole | |||
| Piceaabies | DPPH assay | DPPH radical scavenging | IC50 17.7 ± 1.5 μM | [70] | |
| Carissa carandas Linn. | DPPH assay | DPPH radical scavenging | IC50 30.2 μM | [96] | |
| Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 35.8 μM | [56] | |
| Galactites elegans | DPPH assay | DPPH radical scavenging | IC50 38.6 ± 2.7 μM | [97] | |
| BHP-treated Jurkat cells | peroxyl radicals scavenging | 50 μM | |||
| Furanoid structure group | |||||
| (+)-Lariciresinol | Abies balsamea | rat liver microsomes | ↓lipid peroxidation activity | IC50 0.17–0.35 μM | [69] |
| O2•– assay | superoxide anion scavenging | IC50 35 nM | |||
| ROO assay | peroxyl radicals scavenging | SF 1.0–2.6 mole/mole | |||
| Hemerocallis fulva | LUVs assay | ↓lipid peroxidation activity | 50 μg/mL | [98] | |
| Piceaabies | DPPH assay | DPPH radical scavenging | IC50 10.7 ± 1.2 μM | [70] | |
| Ephedra viridis | DCFH assay in HL-60 cells | ↓iROS level | IC50 17.7 μg/mL | [26] | |
| Euterpe oleracea Mart. | HO assay | HO• scavenging | IC50 0.70 ± 0.13 μg/mL | [27] | |
| DPPH assay | DPPH radical scavenging | IC50 22.4 ± 3.0 μg/mL | |||
| Rubia philippinensis | ABTS assay | ABTS radical scavenging | 12.5–50 μM | [99] | |
| DPPH assay | DPPH radical scavenging | ||||
| HO assay | HO• scavenging | 1.5–6 μM | |||
| ORAC assay | ↓ROO•-induced oxidation | ||||
| CUPRAC assay | cupric-reducing potentiality | 6.25–50 μM | |||
| FRAP assay | ferric-reducing potentiality | ||||
| AAPH-treated RAW 264.7 cells | ↓iROS level | 12.5–50 μM | |||
| RAW 264.7 cells | ↑Nrf2, ↑SOD1, ↑CAT, ↑GPx, ↑HO-1, ↑NQO1, ↑GCLc, ↑GCLm ↑p-p38, ↑p-ERK1/2 | ||||
| Nectandrin B | Myristica fragrans | DPPH assay | DPPH radical scavenging | 5–50 μg/mL | [100] |
| old HDFs | ↓p-AMPK, ↑p-PI3K, ↑p-Akt, ↓p-ERK1/2, ↓p-p38 | 10–20 μg/mL | |||
| H2O2/palmitic acid-treated old HDFs | ↓iROS level, ↑SOD1,2 | ||||
| (−)-Olivil | Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 18.1 μM | [56] |
| Taxiresinol | Taxus yunnanensis | DPPH assay | DPPH radical scavenging | IC50 18.4 μM | [68] |
| Furofuranoid structure group | |||||
| 4-ketopinoresinol | Coixlachryma-jobi Linn. var. ma-yuen Stapf | DPPH assay | DPPH radical scavenging | IC50 52.7 ± 4.6 μg/mL | [101] |
| H2O2-induced HSC-3 cell cytotoxicity | ↓iROS level | 12.5–50 μM | [102] | ||
| ↑GSH/GSSG level | 50 μM | ||||
| ↑Nrf2 | 6.25–50 μM | ||||
| ↑HO-1, ↑AKR1C1-3, ↑ABCC2, ↑GR, ↑GCLC, ↑GCLM, ↑TR, ↑ABCC5, ↑PI3K/Akt | 25 μM | ||||
| Galactites elegans | DPPH assay | DPPH radical scavenging | IC50 143.3 ± 13.1 μM | [97] | |
| BHP-treated Jurkat cells | peroxyl radicals scavenging | 50 μM | |||
| Dendranlignan A | Dendranthema morifolium (Ramat.) | LPS-induced H9c2 cells | ↓iROS level | 10 μM | [103] |
| Isoeucommin A | Eucommia ulmoides Oliv. | high-glucose-stimulated HRMCs | ↓MDA content, ↑SOD,↑p-GSK-3β, ↑Nrf2, ↑HO-1 | 62.5–125 μM | [104] |
| Koreanaside A | Forsythia koreana | ORAC assay | ↓ROO•-induced oxidation | 25 μg/mL | [105] |
| MOVAS cells | ↓VCAM-1 | ||||
| Pinoresinol | Forsythia suspensa (Thunb.) | Cu2+-induced LDL lipid peroxidation | ↓lipid peroxidation activity | IC50 1.39 μM | [106] |
| Eucalyptus globulus Labill | rat liver microsomes | ↓lipid peroxidation activity | IC50 7.9 μg/mL | [107] | |
| Piceaabies | DPPH assay | DPPH radical scavenging | IC50 17.7 ± 0.6 μM | [70] | |
| Euterpe oleracea Mart. | DPPH assay | DPPH radical scavenging | IC50 34.7 ± 5.0 μg/mL | [27] | |
| HO assay | HO• scavenging | IC50 1.8 ± 0.2 μg/mL | |||
| Carissa spinarum Linn. | DPPH assay | DPPH radical scavenging | IC50 43.4 μM | [56] | |
| Forsythia koreana | ORAC assay | ↓ROO•-induced oxidation | 25 μg/mL | [105] | |
| Galactites elegans | DPPH assay | DPPH radical scavenging | IC50 50.8 ± 3.1 μM | [97] | |
| Cinnamon | intact Beas-2B cells | ↑Nrf2, ↑NQO1, ↑γ-GCS | 25 μM | [108] | |
| As(III)-induced Beas-2B cells injury | ↓iROS level, ↑GSH/GSSG level | ||||
| Pinoresinol diglucoside | Eucommia ulmoides | oxLDL-induced HUVECs cytotoxicity | ↓iROS level, ↓MDA content, ↑SOD | 1 μM | [109] |
| Sesamin | Sesamum indicum Linn. | oxLDL-induced HUVECs cytotoxicity | ↓iROS level, ↑SOD1 ↑Bcl-2/Bax level | 12.5–100 μM | [110] |
| Synthetic | KA-induced PC12 and BV-2 cells | ↓iROS level, ↓MDA content | 0.1–2 μM | [111] | |
| Sesamum indicum Linn. | dexamethasone-treated osteoblasts | ↓iROS level, ↑Bcl-2/Bax, ↑p-Akt | 5–20 μM | [112] | |
| H2O2-induced Caco-2 cell cytotoxicity | ↓iROS, ↑GSH/GSSG level, ↓MDA content, ↑SOD, ↑Nrf2, ↓Keap1, ↑HO-1, ↑NQO1, ↑GCLC, ↑GCLM, ↑GR, ↑p-AKT, ↑p-ERK1/2 | 20–80 μM | [113] | ||
| H2O2-induced SH-SY5Y cell cytotoxicity | ↓iROS level, ↑SOD2, ↑CAT ↑FoxO3a, ↑SIRT1, ↑SIRT3 | 1 μM | [114] | ||
| Syringaresinol | Coixlachryma-jobi Linn. var. ma-yuen Stapf | DPPH assay | DPPH radical scavenging | IC50 24.6 ± 3.1 μg/mL | [101] |
| Euterpe oleracea Mart. | DPPH assay | DPPH radical scavenging | IC50 29.7 ± 2.0 μg/mL | [27] | |
| HO assay | HO• scavenging | IC50 0.40 ± 0.13 μg/mL | |||
| Panax ginseng C.A. Meyer | H/R-induced H9c2 cells | ↓iROS level, ↑MnSOD, ↑CAT, ↑LC3, ↑Bcl-2/Bax, ↓HIF-1, ↑FoxO3a, ↓BNIP3, ↓cCYC, ↑mCYC | 25 μM | [115] | |
| Sargentodoxa cuneata | high glucose-injured NRVMs | ↑Nrf2, ↑NQO-1, ↑HO-1, ↓Keap1, ↑SOD, ↑Bcl-2/Bax | 50–100 μM | [116] | |
3.1. Arylnaphthalene Skeletons
Sevanol
3.2. Aryltetralin Skeletons
3.2.1. Isoguaiacin
3.2.2. Isolariciresinol and Isolariciresinol Glucoconjugates
3.2.3. Lyoniresinol and Its Derivatives
3.2.4. Podophyllotoxin
3.2.5. Sauchinone
| Lignan | Source | Model | Target | Dose, Road | Ref. | |
|---|---|---|---|---|---|---|
| Dibenzocyclooctadiene structure group | ||||||
| Gomisin A | Schisandra chinensis Baill. | CCl4-induced hepatotoxicity | ↓MDA content, ↑SOD | 50–100 mg/kg of rat, i.p. | [127] | |
| Gomisin N | Synthetic | ethanol-injured model | ↓iROS, ↑GSH/GSSG, ↑CAT, ↑SOD, ↑GPx, ↑SIRT1/AMPK, ↓CYP2E1 | 5–20 mg/kg of mice, p.o. | [38] | |
| Schisandrin A | Synthetic | ovariectomy-induced osteoporosis | ↓iROS level, ↑Nrf2 | 100 mg/kg of mice, i.p. | [43] | |
| Schisandrin B | Schisandra chinensis (Turcz.) Baill. | I/R injury model | ↑GSH/GSSG level | 1.2 mmol/kg of rat, e.v.p. | [128] | |
| Synthetic | CCl4-induced hepatotoxicity | ↑mtGSH/GSSG level, ↓mtMDA content, ↑GR, ↑GST, ↑GPx | 2 mmol/kg of mice, p.o. | [129,130] | ||
| ethanol-injured model | ↓iROS, ↑GSH/GSSG level, ↑α-TOC, ↓MDA content, ↑GR, ↑GST, ↑MnSOD, ↑GPx | 10 mg/kg of rat, i.g. | [131] | |||
| Aβ-infused model | inhibition of ROO•-induced oxidation, ↑ORAC, ↑GSH/GSSG level, ↓MDA content, ↑SOD | 25–50 mg/kg of rat, p.o. | [132] | |||
| TSCI model | ↑SOD | 50 mg/kg of rat, p.o. | [133] | |||
| I/R injury model | ↓MDA content, ↑SOD | 80 mg/kg of rat, p.o. | [134] | |||
| STZ-induced diabetic model | ↓iROS level, ↑Nrf2 ↑Bcl-2/Bax | 20 mg/kg of mice, p.o. | [135] | |||
| acute stress-induced anxiety | ↓iROS level, ↓Keap1, ↑Nrf2, ↑SOD, ↑GSH/GSSG level | 30–60 mg/kg of mice, p.o. | [136] | |||
| pirarubicin-induced cardiotoxicity | ↑SOD2, ↑CAT ↑Bcl-2/Bax | 50 mg/kg of rat, diet | [137] | |||
| Schisandrin C | Synthetic | Ang II-induced endothelial deficit model | ↓iROS level ↑Nrf2, ↑NQO-1, ↑HO-1, ↓Keap1 | 10 mg/kg of mice, i.g. | [138] | |
| Schisantherin A | Schisandra chinensis (Turcz.) Baill. | Aβ-infused model | ↓MDA content, ↑GSH/GSSG level, ↑SOD, ↑GPx | 0.1 mg/kg of mice, i.c.v. | [139] | |
| chronic fatigue/D-galactose-induced LMI model | ↑GSH/GSSG level, ↓MDA content, ↓Keap1, ↑Nrf2, ↑HO-1, ↑SOD, ↑CAT, ↑Bcl-2/Bax | 2.5–5 mg/kg of mice, i.g. | [140,141] | |||
| Synthetic | MCAO/R-induced brain injury | ↓MDA level, ↑SOD, ↑Trx, ↑PRDx, ↓NOX4 | 5–10 mg/kg of rat, i.g. | [142] | ||
| Dibenzylbutane structure group | ||||||
| Nordihydroguaiaretic acid | Synthetic | ozone-induced lung injury | ↓tyrosine nitration level | 20 mg/kg of rat, Alzet osmotic pumps | [61] | |
| K2Cr2O7-induced renal injury | ↓NAG, ↑GPx | 17 mg/kg of rat, mini-osmotic pumps | [143] | |||
| Larrea tridentata | ALIOS-fed model | ↑GPx4, ↑PRDx3, ↑PPARα | 2.5 g/kg of mice, diet | [144] | ||
| Secoisolariciresinol diglucoside | Linum usitatissimum Linn. | metabolic syndrome model | ↓TBARS, ↓iROS, ↑GSH/GSSG level, ↑SOD, ↑CAT, ↑GPx | 20 mg/kg of rat, p.o. | [145] | |
| Synthetic | CCl4-induced hepato- and nephrotoxicity | ↓MDA content, ↑CAT, ↑SOD, ↑POX, ↓LPO | 12.5–25 mg/kg of rat, p.o. | [72] | ||
| MCT-induced heart failure | ↓iROS level, ↑SOD, ↑CAT, ↑GPx | 25 mg/kg of rat, p.o. | [146] | |||
| Synthetic (LGM2605) | CLP-induced sepsis | ↓iROS level | 100 mg/kg of mice, i.p. | [76] | ||
| NRC painful model | ↓8-OHG | 200 mg/kg of rat, s.c. | [147] | |||
| Linum usitatissimum Linn. | CdCl2-injured model | ↑SOD, ↑CAT, ↑GPx, ↑GR | 10 mg/kg of rat, s.c. | [148] | ||
| Synthetic | BaP-injured model | ↑GSH/GSSG, ↓MDA, ↑SOD, ↑CAT ↓p-p38, ↓p-ERK, ↑MKP-1, ↓miR-101A | 100 mg/kg of mice, i.g. | [149] | ||
| aging ovaries | ↓iROS level | 7–70 mg/kg of mice, i.g. | [150] | |||
| Dibenzylbutyrolactone structure group | ||||||
| Arctigenin | Arctium lappa Linn. | WFST model | ↑Nrf2, ↑SOD, ↑GR, ↑GPx, ↑Trx1, ↑UCP2, ↑p-AMPK, ↑p-p53, ↑p21, ↑PGC-1α, ↑PPARα | 15 mg/kg of rat, i.p. | [80] | |
| ethanol-induced gastric ulcer | ↓MDA content, ↑SOD | 0.05–0.45 mg/kg of rat, p.o. | [151] | |||
| Synthetic | JEV-infected model | ↓ROS level, ↑SOD1 | 10 mg/kg of mice, i.p. | [152] | ||
| LPS-injured model | ↑GSH/GSSG level, ↓MDA content, ↑SOD, ↑CAT, ↑HO-1 | 50 mg/kg of mice, i.p. | [153] | |||
| I/R injury model | ↓MDA content, ↑SOD, ↑GPx ↑Nox1, ↑Trx1, ↑Nrf2 | 50–200 mg/kg of rat, i.g. | [154] | |||
| AMI model | ↓MDA content, ↑SOD, ↑GPx, ↑CAT, ↑HO-1 | 100–200 μmol/kg of rat | [155] | |||
| Hep G2 xenograft model | ↑p-p38, ↑p-JNK,↑Bax,↑TNF-α | 20 mg/kg of mice, s.c. | [86] | |||
| BLM-induced skin fibrosis | ↑GSH/GSSG level, ↓MDA content, ↑SOD, ↑Nrf2, ↑HO-1 | 3 mg/kg of mice, i.p. | [156] | |||
| I/R injury model | ↓iROS level, ↓MDA content, ↑SOD ↑AMPK/SIRT1 | 100 μmol/kg of rat, i.p. | [87] | |||
| cadmium-intoxicated model | ↑GSH/GSSG, ↓8-oxo-dG level, ↓MDA, ↑GSR, ↑GCL, ↑GPx, ↑CAT ↑Nrf2, ↑HO-1, ↑NQO1 | 80 mg/kg of rat, i.g. | [157] | |||
| Hinokinin | Synthetic | HFD/STZ-induced type 2 diabetes | ↓MDA, ↑SOD, ↑CAT, ↑GPx, ↑GST, ↑HO-1, ↑Nrf2, ↓Keap-1 | 20–40 mg/kg of mice, p.o. | [158] | |
| Matairesinol | Synthetic | CLP-induced sepsis | ↓MDA content, ↑SOD, ↑CAT, ↑GPx, ↑Nrf2, ↑HO-1, ↑AMPK | 5–20 mg/kg of rat, p.o. | [93] | |
| Furofuranoid structure group | ||||||
| Fargesin | Synthetic | I/R injury model | ↓MDA content, ↓ROS level, ↑SOD, ↑GPx, ↑CAT | 15 μmol/kg of rat, i.v. | [159] | |
| Isoeucommin A | Eucommia ulmoides Oliv. | H2O2-injured RTECs | ↑SOD, ↑HO-1, ↑Nrf2 | 31.25–125 μM | [104] | |
| ↑GSH/GSSG level | 62.5–125 μM | |||||
| ↓MDA content | 125 μM | |||||
| STZ-induced diabetic nephropathy | ↓MDA content, ↑GSH/GSSG level | 2.5–10 mg/kg of rat, i.v. | ||||
| ↑SOD | 5–10 mg/kg of rat, i.v. | |||||
| Pinoresinol diglucoside | Synthetic | Aβ-infused model | ↓iROS level, ↓MDA content, ↑SOD, ↑CAT, ↑Nrf2, ↑HO-1 ↑Bcl-2/Bax | 5–10 mg/kg of mice, i.g. | [160] | |
| MCAO model | ↓iROS level, ↓MDA content, ↑GSH/GSSG level, ↑SOD, ↑GPx, ↑Nrf2, ↑NQO-1, ↑HO-1 | 5–10 mg/kg of mice, i.v. | [161] | |||
| Sesamin | Synthetic | STZ-induced diabetes | ↓MDA content, ↑SOD | 20 mg/kg of rat, p.o. | [162] | |
| nickel-induced hepatotoxicity | ↓iROS, ↓TBARS, ↑GSH/GSSG level, ↓8-OHdG, ↑SOD, ↑CAT, ↑GPx ↑PI3K/AKT, ↑Bcl-2/Bax | 60–120 mg/kg of mice, p.o. | [163] | |||
| CCl4-induced hepatotoxicity | ↓iROS, ↓TBARS level ↓p-JNK, ↓p-c-Jun, ↓cCYC, ↓Bax, ↓Bak, ↓Bcl-2 | 60–120 mg/kg of mice, p.o. | [164] | |||
| fluoride-exposed model | ↓iROS, ↓TBARS, ↑GSH/GSSG level, ↑SOD, ↑CAT, ↑GPx, ↑GST ↓p-JNK, ↓p-c-Jun, ↑Bcl-2/Bax | 0.5–1 g/kg of carp, diet | [165] | |||
| Sesamum indicum Linn. | DOX-treated model | ↓iROS level, ↓MDA content ↑SOD, ↑CAT, ↑GPx | 20–40 mg/kg of rat, i.g. | [166] | ||
| 6-OHDA model | ↓iROS level, ↓MDA content, ↑SOD | 20 mg/kg of rat, p.o. | [167] | |||
| LPS-treated model | ↑SOD, ↓MDA content | 10 mg/kg of rat, p.o. | [168] | |||
| LPS-treated model | ↑GSH/GSSG level, ↓MDA content ↑SOD, ↑CAT, ↑Nrf2 | 100 mg/kg of mice, p.o. | [169] | |||
| DSS-induced colitis | ↓iROS level, ↑GSH/GSSG level, ↓MDA content, ↑SOD, ↑Nrf2, ↓Keap1, ↑HO-1, ↑NQO1, ↑GCLC, ↑GCLM, ↑GR, ↑p-AKT, ↑p-ERK1/2 | 50–100 mg/kg of mice, i.g. | [113] | |||
| cisplatin-injured model | ↓MDA content, ↑SOD, ↑Nrf2 ↓nitrate/nitrite ratio | 5 mg/kg of rat, p.o. | [170] | |||
| adult Drosophila | ↑Nrf2/Cnc | 2 mg/mL, diet | [171] | |||
| Syringaresinol | Panax ginseng C.A. Meyer | Sod1–/– double-mutant model | ↓iROS level, ↓8-isoprostane level ↓FoxO3a, ↓MMP-2 | 50 mg/kg of mice, p.o. | [172] | |
| Sargentodoxa cuneata | STZ-induced diabetes | ↑Nrf2, ↑NQO-1, ↑HO-1, ↓Keap1, ↑SOD, ↑Bcl-2/Bax | 25 mg/kg of mice, p.o. | [116] | ||
3.3. Dibenzocyclooctadiene Skeletons
3.3.1. Gomisins
3.3.2. Schisandrins
3.3.3. Schisantherin A
| Lignan | Source | In Vitro Model | Target | Concentration | Ref. |
|---|---|---|---|---|---|
| Arylnaphthalene structure group | |||||
| Sevanol | Thymus armeniacus | HEO of X. laevis | ↓hASIC3 | IC50 353 ± 23 μM | [119] |
| ↓rASIC1a | IC50 2.2 ± 0.6 mM | ||||
| Synthetic | HEO of X. laevis | ↓rASIC3 | IC50 175 ± 18 μM | [120,121] | |
| ↓rASIC1a | IC50 227.5 ± 37.4 μM | ||||
| RA-treated SH-SY5Y cells | ↓hASIC1a | 300 μM | [118] | ||
| Aryltetralin structure group | |||||
| (+)-Isolariciresinol 3a-O-β-D-glucopyranoside | Carissa spinarum Linn. | COX-2 assay | ↓COX-2 | IC50 0.3 μM | [30] |
| Sauchinone | Saururus chinensis | LPS-stimulated RAW264.7 | ↓NO production | IC50 4.08 μM | [124] |
| ↓iNOS, ↓TNF-α, ↓COX-2 | 1–30 μM | [125] | |||
| Synthetic | AngII-induced mesangial cells | ↓TGF-β, | 0.1–1 μM | [35] | |
| ↓NLRP3, ↓ICAM-1, ↓MCP-1, ↓IL-1β, ↓NF-κB p65 | 1 μM | ||||
| Dibenzocyclooctadiene structure group | |||||
| Schisandrin A | Schisandra chinensis (Turcz.) Baill. | LPS-stimulated RAW 264.7 macrophages | ↓NO level, ↓iNOS, ↓PGE2, ↓COX-2, ↓NF-κB, ↑IκBα, ↓p-JNK, ↓p-p38 MAPK | 25–100 μM | [173] |
| Synthetic | LPS-stimulated RAW 264.7 macrophages | ↓iNOS, ↓COX-2, ↓TNF-α, ↓IL-1β, ↑IκB-α, ↓p-JNK, ↓p-p38 MAPK, ↓p-ERK, ↓p-PI3K, ↓p-Akt | 200 μM | [40] | |
| DON-induced cytotoxicity in HT-29 cells | ↓PGE2, ↓COX-2, ↓NF-κB, ↓IL8, ↓p-p38, ↓p-ERK | 2.5–10 μM | [42] | ||
| RANKL-induced osteoclast differentiation | ↓PGE2, ↓COX-2, ↓NF-κB, ↓IL8, ↓p-p38, ↓p-ERK | 50–200 μM | [43] | ||
| Schisandrin B | Synthetic | Con A-induced lymphocytes | ↓NF-κB, ↓p-MEK, ↓p-p38, ↓p-ERK, ↓p-JNK, ↑IκBα, ↓IL-2, ↓IL-4, ↓IL-6, ↓IFN-γ | 25–50 μM | [46] |
| Ang II/TNF-α/ROS-induced HUVECs | ↓NF-κB, TNF-α, ↓p-Smad2/3, ↓vimentin, ↓α-SMA, ↓Snail/slug, ↓TGF-β, ↓Twist, ↑VE-cadherin | 10 μM | [176] | ||
| TH17 cell differentiation | ↓p-STAT3 | 1 μM | [178] | ||
| H/R-induced H9c2 cell injury | ↓IL-1β, ↓TNF-α, ↓IL-6, ↓IL-8, ↓TGF-β, ↑IL-10 | 20 μM | [50,51] | ||
| LPS+ATP-treated intestinal epithelial cells | ↓TNF-α, ↓IL-6, ↓IL-18, ↓IL-1β, ↓NLRP3, ↑p-AMPK | 40 μM | [177] | ||
| Schisandrin C | Synthetic | LPS-stimulated HDPCs | ↓NO level, ↓p-ERK1/2, ↓p-SAPK/JNK, ↓p-p38, ↓NF-κB | 10–20 μM | [52] |
| Schisantherin A | Schisandra chinensis (Turcz.) Baill. | H/R-induced HK-2 cells | ↓TNF-α, ↓IL-1β, ↓IL-6 | 5–20 μM | [53] |
| LPS-stimulated BV-2 microglial cells | ↓NF-κB, ↓IKK, ↑IκB, ↓TNF-α, ↓IL-6, ↓IL-1β, ↑IL-10 ↓iNOS, ↓COX-2 ↓p-p38, ↑p-ERK, ↓p-JNK, ↓p-Akt | 50 μM | [54] | ||
| Synthetic | LPS-stimulated NRK-52E cells | ↓NF-κB, ↓TNF-α, ↓Rantes | 25–50 μM | [55] | |
| Dibenzylbutane structure group | |||||
| Nordihydroguaiaretic acid | Synthetic | IL-1β-induced PC12 cells | ↓APP secretion and processing | 10 μM | [183] |
| IFN-γ- induced rat brain astrocytes/C6 cells | ↓IRF-1, ↓IP-10 ↓p-STAT1, ↓p-STAT3,↓p-↓JAK2 | 5–20 μM | [184] | ||
| RANKL-induced bone marrow-derived macrophage/RAW-D cells | ↓osteoclast differentiation, ↓RANKL-induced signal cascade ↓NFATc1, ↓p-ERK | 1–10 μM | [185] | ||
| Secoisolariciresinol diglucoside | Linum usitatissimum Linn. | iron treated H9c2 cells | ↓TNF-α, ↑IL-10 | 500 μM | [73] |
| CdCl2-injured model | ↓MPO, ↓NO level | 10 mg/kg of rat | [148] | ||
| Synthetic (LGM2605) | asbestos-exposed MFs | ↓iNOS, ↓IL-1β, ↓IL-6, ↓IL-18, ↓TNFα | 50–100 μM | [74,75] | |
| Dibenzylbutyrolactone structure group | |||||
| Arctigenin | Forsythia fructus | pro-inflammatory enzyme assays | ↓PLA2, ↓COX-1, ↓COX-2, ↓5-LOX | 100 μM | [186] |
| Arctium lappa Linn. | bone marrow-derived MDSCs | ↑Arg-1, ↑iNOS | 10–20 μM | [187] | |
| Synthetic | LPS-treated Raw264.7 cells | ↓iNOS, ↓p-STAT, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓p-JAK2 | 5–50 μM | [78] | |
| TGF-β1-induced HK-2 cells | ↓NF-κB p65, ↓MCP-1 | 0.5–1 μM | [83] | ||
| OA-treated WRL68 hepatocytes | ↓ICAM-1, ↓IL-1β, ↓IL-6, ↓IL-7, ↓IL-8, ↓TNFα | 50 μM | [85] | ||
| LPS-treated RAW264.7 cells | ↓TNF-α, ↓IFN-γ, ↓IL-17, ↓IL-1β, ↓CXCL10, ↑TGF-β1, ↑IL-4 | 10–100 μM | [188] | ||
| LPS-treated RAW264.7 cells | ↓TNF-α, ↓IFN-γ, ↓IL-17, ↓IL-1β, ↓CXCL10, ↑TGF-β1, ↑IL-4 | 10–100 μM | [188] | ||
| IL-1β–stimulated human chondrocytes | ↓TNF-α, ↓COX-2, ↓iNOS, ↓IL-6, ↓PGE2, ↓NO, ↑IκBα, ↓p65, ↓PI3K, ↓Akt | 10–50 μM | [189] | ||
| scintillation proximity assay | ↓PDE4 | IC50 3.76 ± 0.28 μM | [190] | ||
| LPS-stimulated human PBMCs | ↓TNF-α | IC50 35.18 ± 6.01 μM | |||
| LPS-treated RAW264.7 cells | ↓TNF-α, ↑p-CREB, ↓PDE4 | 100 μM | |||
| OGD-injured H9c2 cardiomyocytes | ↓NF-κB, ↑IKBα, ↓TNF-α, ↓IL-1β, ↓IL-6 | 50–200 μM | [87] | ||
| silica-injured RAW 264.7 macrophages | ↓iNOS, ↓Arg-1, ↓TLR-4, ↓NLRP3, ↓TGF-β | 1 μM | [88] | ||
| Hinokinin | Aristolochia indica L. | LPS-stimulated THP-1 cells | ↓IL-6 | 20.5 ± 0.5 μM | [191] |
| ↓TNF-α | 77.5 ± 27.5 μM | ||||
| Matairesinol | Synthetic | naive CD4+ T cells | ↓p-p38, ↓p-ERK, ↓ROR-γt | 20 μM | [192] |
| LPS-stimulatedNSC-34neurons andBV2microglia | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓IFN-γ, ↓IL-8, ↓MCP1, ↓MAPK, ↓JNK, ↓NF-κB | 5–20 μM | [93] | ||
| Matairesinol-7′-hydroxyl | Piceaabies | TNF-α-induced HAEC | ↓ICAM-1, ↓VCAM-1, ↓monocyte adhesion | 0.1–100 μM | [193] |
| ↓p-NF-κB | 10–100 μM | ||||
| ↓p-ERK | 100 μM | ||||
| Nortrachelogenin | Synthetic | LPS-stimulated J774 macrophages | ↓PGE2, ↓NO, ↓iNOS | 1–30 μM | [194] |
| ↓MCP-1, ↓IL-6 | 3–30 μM | ||||
| ↓mPGES-1 | 30 μM | ||||
| Furanoid structure group | |||||
| (−)-Olivil | Osmanthus fragrans var. aurantiacus | LPS-activated RAW264.7 cells | ↓NO level | IC50 85.6 ± 1.49 μM | [195] |
| Taxiresinol | Osmanthus fragrans var. aurantiacus | LPS-activated RAW264.7 cells | ↓NO level | IC50 58.1 ± 1.42 μM | [195] |
| Perovskiaatriplicifolia Benth | RBL-1 leukemia cells | ↓leukotriene C4 release | IC50 3.4 ± 0.09 μM | [196] | |
| Furofuranoid structure group | |||||
| Dendranlignan A | Dendranthema morifolium (Ramat.) | LPS-induced H9c2 cells | ↓TNF-α, ↓IL-6, ↓IFN-γ ↓p-cJUN, ↓p-P65, ↓p-IRF3 | 10 μM | [103] |
| (+)-Diayangambin | Piper fimbriulatum | human mononuclear cells | ↓proliferation | 1.5 μM | [197] |
| LPS-stimulated RAW264.7 macrophages | ↓PGE2 | 10 μM | |||
| Fargesin | Magnolia fargesii | PMA-stimulated THP-1 | ↓iNOS, ↓COX-2, ↓IL-1β, ↓TNF-α, ↓AP-1, ↓NF-κB, ↓JNK | 5–20 μM | [198] |
| Magnolia sp. | LPS-stimulated RAW264.7 | ↓iNOS, ↓COX-2, ↓NF-κB | 25 μM | [199] | |
| Koreanaside A | Forsythia koreana | LPS-stimulated RAW 264.7 macrophages | ↓iNOS, ↓COX-2, ↓IL-6, ↓TNF-α, ↓p-IκBα, ↓p-TAK1 | 20–80 μM | [200] |
| ↓AP-1, ↓p-c-Fos, ↓p-p65, ↓NF-κB, ↓p-IKKα/β, ↓p-STAT1, ↓p-STAT3, ↓p-JAK1, ↓p-JAK2 | 40–80 μM | ||||
| Phillygenin | Forsythia koreana | RAW 264.7 cells | ↓PGE2, ↓NO, ↓iNOS, ↓NF-κB | 1–100 μM | [201] |
| Pinoresinol | Synthetic | IL-1β-stimulated Caco-2 cells | ↓PGE2, ↓MCP-1, ↓NF-κB | 50–100 μM | [202] |
| ↓IL-6 | 10–100 μM | ||||
| Pinoresinol diglucoside | Eucommia ulmoides | oxLDL-induced HUVEC cytotoxicity | ↓eNOS, ↓p-p38MAPK, ↓p-NF-κB p65 | 1 μM | [109] |
| Sesamin | Sesamum indicum Linn. | oxLDL-induced HUVECs cytotoxicity | ↓NF-κB, ↓IL-8 | 12.5–100 μM | [110] |
| FPR-transfected ETFR cells, THP1 cells | ↓cell migration, ↓NF-κB activation, ↓ERK1/2 phosphorylation | 6.25–50 μM | [203] | ||
| KA-induced PC12 and BV-2 cells | ↓ERK1/2, ↓p38 MAPK, ↓COX-2 | 10–50 μM | [111] | ||
| RPMC | ↓histamine release | 25–100 μM | [204] | ||
| HMC-1 | ↓TNF-α, ↓IL-6, ↓p38 MAPK, ↓NF-κB | ||||
| Synthetic | RLE-6TN and L2 cells | ↑A20, ↑TAX1BP1 | 10 μM | [205] | |
| epi-Sesamin | Asarum siebodlii | HUVEC | ↓EPCR shedding | 1–10 μM | [206] |
| Syringaresinol | Perovskiaatriplicifolia Benth | RBL-1 leukemia cells | ↓leukotriene C4 release | IC50 7.9 ± 0.04 μM | [196] |
| Rubia philippinensis | LPS-stimulated RAW 264.7 cells | ↓iNOS, ↓COX-2, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓PGE2, ↓ERK1/2, ↓JNK, ↓p38 MAPK | 25, 50, 100 μM | [207] | |
| High glucose-treated NRVM | ↓TNF-α, ↓IL-6, ↓IL-1β, ↓TGF-β, ↓p-Smad2/3 | 50, 100 μM | [116] | ||
| LPS+ ATP-treated H9c2 cells | ↓IL-1β, ↓IL-18, ↑SIRT1 expression, ↓NLRP3 inflammasome activation | 100 μM | [208] | ||
| Albiziae cortex | BV2 microglia cells | ↓TNF-α, ↓IL-6, ↓IL-1β, ↓COX-2, ↓NO, ↑M2 phenotype, ↓NF-κB | 25, 50, 100 μM | [209] | |
3.4. Dibenzylbutane Skeletons
3.4.1. Nordihydroguaiaretic Acid (NDGA) and Its Derivatives
3.4.2. Meso-Dihydroguaiaretic Acid
3.4.3. Secoisolariciresinol and Its Derivatives
| Lignan | Source | Model | Target | Dose, Road | Ref. |
|---|---|---|---|---|---|
| Arylnaphthalene structure group | |||||
| Sevanol | Thymus armeniacus | CFA-induced thermal hyperalgesia | ↑withdrawal latency of inflamed hind paw | 1–10 mg/kg of mice, i.v. | [119] |
| Synthetic | CFA-induced paw edema | ↓paw edema | 0.1–1 mg/kg of mice, i.m., i.n., p.o. | [120,121] | |
| Aryltetralin structure group | |||||
| Podophyllotoxin | Synthetic (G-003M) | TGR-exposed model | ↑survival, ↓NO, ↓IL-6, ↓TNF-α, ↓TGF-β1 | 5 mg/kg of mice, i.m. | [122] |
| Synthetic, conjugated with PAA dendrimer | HCC-induced model | ↓IL-6, ↓NF-κB, ↓α-SMA, ↓TGF-β | 10, 20 mg/kg of mice, p.o. | [123] | |
| Sauchinone | Saururus chinensis | OVA-induced asthma model | ↓neutrophil, lymphocyte, eosinophil infiltration in BALF, ↓IL-5, ↓IL-13, ↓Th2 cell development | 10, 100 mg/kg of mice, i.p. | [126] |
| Dibenzocyclooctadiene structure group | |||||
| Gomisin A | Schisandra chinensis Baill. | CCl4-induced hepatotoxicity | ↓TNF-α, ↓IL-1β, ↓iNOS, ↓NF-κB, ↓p-IκB | 50–100 mg/kg of rat, i.p. | [127] |
| Gomisin N | Schisandra chinensis Baill. | ethanol-induced liver injury | ↓NF-κB p65, ↑IκB, ↓TNF-α, ↓IL-6, ↓MCP-1 | 5–20 mg/kg of mice, p.o. | [38] |
| Schisandrin A | Schisandra chinensis (Turcz.) Baill. | LPS-treated model | ↓NO level | 100–200 mg/kg of mice, i.p. | [173] |
| carrageenan-induced paw edema | ↓paw edema volume | ||||
| xylene-induced ear edema | ↓ear edema degree | 25–50 mg/kg of mice, p.o. | [174] | ||
| carrageenan-induced paw edema | ↓paw edema volume ↓TNF-α, ↓IL-1β, ↓MPO, ↓p-p65NF-κB, ↓p-IκB, ↓TLR4 | 25–50 mg/kg of mice, p.o. | |||
| Schisandrin B | Synthetic | Con A-induced lymphocytes | ↓IL-2, ↓IL-4, ↓IL-6, ↓IFN-γ | 80 mg/kg of mice, i.p. | [46] |
| myocardial infarction model | ↓in left ventricular end-systolic and end-diastolic diameter, ↓heart weight/body weight ratio, ↓infarct size, ↓NF-κB, ↓TGF-β1, ↓TNF-α | 80 mg/kg of mice, i.g. | [179] | ||
| Aβ-infused model | ↓COX-2, ↓iNOS, ↓TNF-α, ↓IL-1β, ↓IL-6 | 25–50 mg/kg of rat, i.g. | [132] | ||
| I/R injury model | ↓IL-1β, ↓TNF-α, ↓p-p38MAPK, ↓p-ERK1/2, ↓NF-κB p65 | 80 mg/kg of rat, p.o. | [134] | ||
| TSCI model | ↓NF-κB p65, ↓TNF-α | 50 mg/kg of rat, p.o. | [133] | ||
| IL-1β-induced rat chondrocytes | ↓IL-6, ↓iNOS, ↓MMP3, ↓MMP13, ↓NF-κB, ↓MAPK | 50 μM, i.a. | [180] | ||
| STZ-induced diabetes | ↑IκBα, ↓VCAM-1, ↓TNF-α | 20 mg/kg of mice, p.o. | [135] | ||
| Ang II-induced vascular injury model | ↓α-SMA, ↓p-Smad2/3, ↑VE-cadherin | 20 mg/kg of mice, p.o. | [176] | ||
| DSS induced colitis | ↓TNF-α, ↓IL-6, ↓IL-18, ↓IL-1β, ↓NLRP3, ↑p-AMPK | 10 mg/kg of mice, i.p. | [177] | ||
| Schisantherin A | Synthetic | MCAO/R-induced brain injury model | ↓IL-1β, ↓IL-6, ↓p-IκBα, ↓NF-κB, ↓p-ERK, ↓p-JNK, ↓p-p38, ↓TLR4, ↓C5aR1 | 5–10 mg/kg of rat, i.g. | [142] |
| LPS-induced acute kidney injury | ↓accumulation neutrophils and T-lymphocytes, ↓NF-κB, ↓TNF-α, ↓Rantes | 40 mg/kg of mice, i.p. | [55] | ||
| Dibenzylbutane structure group | |||||
| Meso-dihydroguaiaretic acid | Machilusphilippinensis Merr. | LPS-induced ARDS model | ↓MPO, ↓4-HNE, ↓elastase accumulation | 30 mg/kg of mice, i.p. | [58] |
| Nordihydroguaiaretic acid | Synthetic | TPA-treated model | ↓LPO level, ↓XOD, ↓MPO | 15–25 μM, shaved area of dorsal skin | [64] |
| spinal cord injury | ↓MPO, ↓TNF-α, ↓IL-1β | 30 mg/kg of rat, i.p. | [211] | ||
| leptin-deficient (ob/ob) mice | ↑PPARα, ↑p-AMPK ↑fatty acid oxidation pathway | 0.83 g/kg, 2.5 g/kg, diet | [212] | ||
| CLP-induced sepsis | ↓lung edema, ↓lactate, ↓blood urea nitrogen, ↓histologic lung injury | 20 mg/kg of rat, i.p. | [213] | ||
| Secoisolariciresinol diglucoside | Synthetic | CLP-induced sepsis | ↓p-IκBα, ↓NF-κΒ | 100 mg/kg of mice, i.p. | [76] |
| BaP-injured model | ↓MPO, ↓NO level, ↓TNF-α, ↓IL-6, ↓IL-1β, ↓NF-κB | 100 mg/kg of mice, i.g. | [149] | ||
| Dibenzylbutyrolactone structure group | |||||
| Arctigenin | Forsythia fructus | OVA-induced asthma model | ↓PDE | 10–100 μM | [186] |
| 48/80-induced RPMCs | ↓histamine release | 10 μM | |||
| IgE-rich mouse serum-induced PCA skin model | ↓amount of Evans blue leakage | 15–45 mg/kg of rat, p.o. | |||
| anti-rat rabbit serum antibody-induced RCA skin model | ↓skin edema | ||||
| SRBC-induced Arthus reaction model | ↓footpad thickness, ↓hemolysis tier, ↓hemagglutinin titer, ↓plaque-forming cells | 15–45 mg/kg of mice, p.o. | |||
| SRBC-induced DTH model | ↓footpad thickness, ↓rosette-forming cells | ||||
| DNFB/PC-induced contact dermatitis | ↓ear edema | 0.1–1 mg/ear of mice | |||
| Arctium lappa Linn. | LPS-/PGN-stimulated peritoneal macrophages | ↓IL-6, ↓TNF-α, ↓IL-1β, ↑IL-10, ↑CD204, ↓p-PI3K, ↓p-Akt, ↓p-p65, ↓p-IKKβ | 10–20 μM | [216] | |
| LPS-/PGN-induced model | ↓TNF-α, ↓IL-1β | 5 mg/kg of mice, i.p. | |||
| TNBS-induced colitic model | ↓IL-6, ↓TNF-α, ↓IL-1β, ↓MPO, ↑IL-10, ↓p-PI3K, ↓p-Akt, ↓p-p65 | 30–60 mg/kg of mice, p.o. | |||
| acetic acid-induced chronic ulcer model | ↓TNF-α, ↓IL-6, ↑IL-10, ↓CRP | 0.05–0.45 mg/kg of rat, p.o. | [151] | ||
| LPS-induced acute inflammation model | ↓CD86, ↓IL-6, ↓IL-12, ↓TNF-α, ↓IL-1β, ↑IL-10, ↑G-MDSCs, ↓M-MDSCs, ↓IRF8, ↑miR-127-5p, ↓M1 macrophage polarization, ↑Arg-1, ↑iNOS | 50 mg/kg of mice, i.p. | [187] | ||
| Synthetic | JEV-infected model | ↓iNOS, ↓TNF-α, ↓IFN-γ, ↓MCP-1, ↓IL-6, ↓p-p38 MAPK, ↓p-c-Jun ↓p-ERK-1/2, ↑p-Akt | 10 mg/kg of mice, i.p. | [152] | |
| LPS-injured model | ↓nitrate/nitrite ratio, ↓iNOS, ↓TNF-α, ↓IL-6, ↓MIP-2, ↓p-ERK1/2, ↓p-JNK, ↓p-p38 | 50 mg/kg of mice, i.p. | [153] | ||
| EAE model | ↓IFN-γ, ↓T-bet, ↓IL-17, ↓ROR-γt, ↓Th1, ↓Th17 | 5–10 mg/kg of mice, i.p. | [217] | ||
| ConA-induced acute hepatitis | ↑IL-4, ↓F4/80, ↓CD49b, ↓CD4 T cells | 5–10 mg/kg of mice, i.p. | [188] | ||
| AMI model | ↓iNOS, ↓COX-2, ↓IL-1β, ↓IL-6, ↓p-ERK1/2 | 100–200 μmol/kg of rat | [155] | ||
| BLM-induced skin fibrosis model | ↓TGF-β1, ↓IL-1β, ↓IL-4, ↓IL-6, ↓TNF-α, ↓MCP-1 | 3 mg/kg of mice, i.p. | [156] | ||
| DMM model | ↓cartilage erosion, ↓hypocellularity, ↓proteoglycan loss | 30 mg/kg of mice, p.o. | [189] | ||
| imiquimod-induced murine psoriasis model | ↑p-CREB, ↑cAMP, ↑IL-10, ↓TNF-α, IFN-γ, ↓COX-2, ↓iNOS, ↓IL-2, ↓IL-6, ↓IL-12, ↓IL-17, ↓IL-22, ↓IL-23, ↓IL-27 | 5% cream | [190] | ||
| silicosis model | ↓TGF-β, ↓TLR-4 | 30–60 mg/kg of rat, i.g. | [88] | ||
| cadmium-intoxicated model | ↓NF-κB p65, ↓TNF-α, ↓IL-1β | 80 mg/kg of rat, i.g. | [157] | ||
| Hinokinin | Synthetic | high-fat diet/STZ-induced type 2 diabetic | ↓TLR 4, ↓MYD88, ↓NF-κB p65, ↑IKBα, ↓TNF-α, ↓IL-1β, ↓p38, ↓ERK 1/2, ↓JNK, ↓MEK | 20–40 mg/kg of mice, p.o. | [158] |
| Matairesinol | Synthetic | IRBP/CFA-induced EAU model | ↓T17 cells, ↓IL-17A, ↓IL-17F, ↓IL-21, ↓GM-CSF, ↓IRF-4, ↓Hif1, ↓Batf, ↓ROR-γt, ↓TNF-α | 1 mg/kg of mice, i.p. | [192] |
| CLP-induced sepsis | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓IFN-γ, ↓IL- 8, ↓MCP1, ↓MAPK, ↓JNK, ↓NF-κB | 5–20 mg/kg of rat, p.o. | [93] | ||
| Nortrachelogenin | Synthetic | carrageenan-induced paw edema | ↓paw edema volume | 100 mg/kg of mice, i.p. | [194] |
| Furanoid structure group | |||||
| Nectandrin B | Guaiacum officinale L. | IL-1β-treated rat hepatocytes | ↓NO level | IC50 43.4 μM | [218] |
| Taxiresinol | Taxus baccata Linn. | carrageenan-induced paw edema | ↓paw edema volume | 100 mg/kg of mice, p.o. | [219] |
| Furofuranoid structure group | |||||
| Acanthoside B | Salicornia europaea Linn. | Amnesic AD-like model | ↓iNOS, ↓COX-2, ↓TNF-α, ↓IL-1β, ↓IL-6, ↑IL-10 | 10, 20 mg/kg of mice, p.o. | [220] |
| (+)-Diayangambin | Piper fimbriulatum | carrageenan-induced paw edema | ↓paw volume, ↓prostaglandin E2 | 40 mg/kg of mice, p.o. | [197] |
| Fargesin | Magnolia sp. | DSS-induced colitis | ↓inflammatory infiltration, ↓MPO, ↓TNF-α, ↓NO, ↑IκBα, ↓NF-κB | 50 mg/kg of mice, p.o. | [199] |
| Synthetic | ApoE−/− model | ↓macrophage infiltration, ↑M2 phenotype polarization, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓MCP-1, ↑IL-10 | 50 mg/kg of mice, p.o. | [221] | |
| Isoeucommin A | Eucommia ulmoides Oliv. | STZ-induced diabetic nephropathy | ↓immune infiltration, ↓TNF-α, ↓IL-1β, ↓IL-6 | 2.5–10 mg/kg of rat, i.v. | [104] |
| Koreanaside A | Forsythia koreana | DSS-induced acute colitis | ↓iNOS, ↓COX-2, ↓IL-6, ↓TNF-α ↓p-c-Fos, ↓p-p65, ↓p-STAT1, ↓p-STAT3 | 5–20 mg/kg of mice, i.p. | [200] |
| Phillygenin | Forsythia koreana | carrageenan-induced paw edema | ↓paw volume | 12.5–100 mg/kg of mice, i.p. | [201] |
| Forsythia fructus | CCl4-induced liver fibrosis | ↓LPS, ↓MIP-1, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓immune infiltration | 20, 40 mg/kg of mice, i.g. | [222] | |
| Pinoresinol diglucoside | Synthetic | Aβ-infused model | ↓TLR4, ↓NF-κB p65, ↓TNF-α, ↓IL-1β | 5–10 mg/kg of mice, i.g. | [160] |
| MCAO model | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓p-IKKβ, ↓p-IkBα, ↑cNF-κB p65, ↓p-p65 | 5–10 mg/kg of mice, i.v. | [161] | ||
| Sesamin | Sesamum indicum Linn. | fMLF-induced inflammation in a murine air-pouch model | ↓leukocyte infiltration | 12 mg/kg of mice, i.p. | [203] |
| PCA model | ↓PCA reaction | 50–200 mg/kg of rat, p.o. | [204] | ||
| LPS-treated model | ↓TNF-α, ↓MCP-1, ↓IL-1β | 10 mg/kg of rat, p.o. | [168] | ||
| LPS-treated model | ↓NF-κB, ↓TLR4, ↓Cox2, ↓TNF-α, ↓IL-6 | 100 mg/kg of mice, p.o. | [169] | ||
| DSS-induced colitis model | ↓IL-6, ↓IL-1β, ↓TNF-α | 50–100 mg/kg of mice, i.g. | [113] | ||
| cisplatin-injured model | ↓TNF-α, ↓IL-1β, ↓TGF-β1, ↓MPO | 5 mg/kg of rat, p.o. | [170] | ||
| Synthetic | CCl4-induced hepatotoxicity model | ↓TNF-α | 60–120 mg/kg of mice, p.o. | [164] | |
| carrageenan-induced lung inflammation | ↑A20, ↑TAX1BP1, ↓IL-6, ↓IL-8, ↓IL-1β, ↓TNF-α, ↓MIP-2, ↓MPO, ↓β-glucuronidase, ↓p-p65, ↓TRAF6 | 50–100 mg/kg of rat, p.o. | [205] | ||
| fluoride-exposed model | ↓TNF-α | 0.5–1 g/kg of carp, diet | [165] | ||
| Syringaresinol | Rubia philippinensis | carrageenan-induced paw edema model | ↓paw edema volume | 50 mg/kg of mice, p.o. | [207] |
| CLP-induced sepsis | ↓TNF-α, ↓IL-6, ↓IL-18, ↓IL-1β | 50 mg/kg of mice, p.o. | [208] | ||
| STZ-induced type 1 diabetic model | ↓macrophage, monocyte, neutrophil infiltration in myocardium, ↓TNF-α, ↓IL-6, ↓IL-1β | 25 mg/kg of mice, p.o. | [116] | ||
| Albiziae cortex | LPS-treated model | ↓IL-6, ↓IL-1β, ↓TNF-α, ↓COX-2, ↓iNOS, ↓microglia activation | 60 mg/kg of mice, p.o. | [209] | |
3.5. Dibenzylbutyrolactone and Dibenzylbutyrolactol Skeletons
3.5.1. Arctigenin
3.5.2. Carissanol
3.5.3. Hinokinin
3.5.4. Matairesinol and Its Derivatives
3.5.5. Nortrachelogenin
3.6. Furanoid Skeletons
3.6.1. Lariciresinol
3.6.2. Nectandrin B
3.6.3. Olivil
3.6.4. Taxiresinol
3.7. Furofuran Skeletons
3.7.1. Acanthoside B
3.7.2. Dendranlignan A
3.7.3. Diayangambin
3.7.4. Fargesin
3.7.5. Isoeucommin A
3.7.6. Koreanaside A
3.7.7. Phylligenin
3.7.8. Pinoresinol and Its Derivatives
3.7.9. Sesamin
3.7.10. Syringaresinol
4. Main Approaches for Lignan Synthesis
4.1. Oxidative Dimerization
4.2. Classical Cyclization and Non-Phenolic Oxidative Dimerization
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–539. ISBN 9780470741689. [Google Scholar]
- Lee, K.H.; Xiao, Z. Lignans in treatment of cancer and other diseases. Phytochem. Rev. 2003, 2, 341–362. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M.; Crane, C.R. Exosomes, DAMPs and miRNA: Features of Stress Physiology and Immune Homeostasis. Trends Immunol. 2017, 38, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M. The IκB kinase (IKK) and NF-κB: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.-Y.; Bach, F.H. Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [Green Version]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, H.; Xue, B.; Wu, Y.; Wang, J.; Yin, Z.; Luo, L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm. Res. 2006, 55, 504–510. [Google Scholar] [CrossRef]
- Vinogradov, A.D.; Grivennikova, V.G. Oxidation of NADH and ROS production by respiratory complex i. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 863–871. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St.-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Aquilano, K.; Baldelli, S.; Pagliei, B.; Cannata, S.M.; Rotilio, G.; Ciriolo, M.R. P53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid. Redox Signal. 2013, 18, 386–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.U.; Kang, S.Y.; Park, H.Y.; Sung, S.H.; Lee, E.J.; Kim, S.Y.; Kim, Y.C. Antioxidant Lignans from Machilusthunbergii Protect CCl4-injured Primary Cultures of Rat Hepatocytes. J. Pharm. Pharmacol. 2000, 52, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef]
- Pullela, S.V.; Takamatsu, S.; Khan, S.I.; Khan, I.A. Isolation of lignans and biological activity studies of Ephedra viridis. Planta Med. 2005, 71, 789–791. [Google Scholar] [CrossRef]
- Chin, Y.W.; Chai, H.B.; Keller, W.J.; Kinghorn, A.D. Lignans and other constituents of the fruits of Euterpe oleracea (Açai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef]
- Sampei, M.; Arai, M.A.; Ishibashi, M. Total syntheses of schizandriside, saracoside and (±)-isolariciresinol with antioxidant activities. J. Nat. Med. 2018, 72, 651–654. [Google Scholar] [CrossRef]
- Sadhu, S.K.; Khatun, A.; Phattanawasin, P.; Ohtsuki, T.; Ishibashi, M. Lignan glycosides and flavonoids from Saracaasoca with antioxidant activity. J. Nat. Med. 2007, 61, 480–482. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Muema, F.W.; Kimutai, F.; Chen, G.; Guo, M. Phenolic compounds from carissa spinarum are characterized by their antioxidant, anti-inflammatory and hepatoprotective activities. Antioxidants 2021, 10, 652. [Google Scholar] [CrossRef]
- Liu, L.T.; Liang, L.; Wang, W.; Yan, C.Q.; Zhang, J.; Xiao, Y.C.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Bian, J.J.; et al. Isolariciresinol-9’-O-α-L-arabinofuranoside protects against hydrogen peroxide-induced apoptosis of human umbilical vein endothelial cells via a PI3K/Akt/Bad-dependent pathway. Mol. Med. Rep. 2018, 17, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Tomosaka, H.; Chin, Y.W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kinghorn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry). Phyther. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, E.J.; Son, Y.K.; Yeo, J.H.; Song, K.S. Enhanced anti-oxidative effect of fermented Korean mistletoe is originated from an increase in the contents of caffeic acid and lyoniresinol. Food Funct. 2016, 7, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Thongphasuk, P.; Suttisri, R.; Bavovada, R.; Verpoorte, R. Antioxidant lignan glucosides from Strychnosvanprukii. Fitoterapia 2004, 75, 623–628. [Google Scholar] [CrossRef]
- Yoon, J.J.; Lee, H.K.; Kim, H.Y.; Han, B.H.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Sauchinone protects renal mesangial cell dysfunction against angiotensin ii by improving renal fibrosis and inflammation. Int. J. Mol. Sci. 2020, 21, 7003. [Google Scholar] [CrossRef] [PubMed]
- Takanche, J.S.; Kim, J.-E.; Han, S.-H.; Yi, H.-K. Effect of gomisin A on osteoblast differentiation in high glucose-mediated oxidative stress. Phytomedicine 2020, 66, 153107. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Waiwut, P.; Saiki, I.; Shimada, Y.; Sakurai, H. Gomisin N enhances TRAIL-induced apoptosis via reactive oxygen species-mediated up-regulation of death receptors 4 and 5. Int. J. Oncol. 2012, 40, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, A.; Jung, D.; Kim, J.-H.; Lee, H.; Jung, M. Gomisin N Alleviates Ethanol-Induced Liver Injury through Ameliorating Lipid Metabolism and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2601. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hao, H.; Wang, H.; Guo, C.; Kang, A.; Wang, G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J. Ethnopharmacol. 2014, 155, 213–221. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed. Pharmacother. 2018, 106, 902–909. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 19173. [Google Scholar] [CrossRef]
- Ni, S.; Qian, Z.; Yuan, Y.; Li, D.; Zhong, Z.; Ghorbani, F.; Zhang, X.; Zhang, F.; Zhang, Z.; Liu, Z.; et al. Schisandrin A restrains osteoclastogenesis by inhibiting reactive oxygen species and activating Nrf2 signalling. Cell Prolif. 2020, 53, e12882. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Ming Ko, K. (−)Schisandrin B ameliorates paraquat-induced oxidative stress by suppressing glutathione depletion and enhancing glutathione recovery in differentiated PC12 cells. BioFactors 2011, 37, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Lam, P.Y.; Yan, C.W.; Ko, K.M. Schisandrin B protects against solar irradiation-induced oxidative injury in BJ human fibroblasts. Fitoterapia 2011, 82, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Bhilwade, H.N.; Konishi, T.; Sandur, S.K. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic. Biol. Med. 2012, 53, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.-P.; Li, H.; Yu, C.-R.; Yu, C.-Y.; Jing, S.; Sun, H.-X.; Wang, C.-M.; Fan, X.-T.; Chen, J.-G.; Wang, S. Schisandrin B protects PC12 cells against oxidative stress of neurodegenerative diseases. Neuroreport 2015, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Luo, Z.; Wu, C.; Lai, S.; Wei, H.; Li, T.; Wang, Q.; Yu, Y. Attenuation of cyclosporine A induced nephrotoxicity by schisandrin B through suppression of oxidative stress, apoptosis and autophagy. Int. Immunopharmacol. 2017, 52, 15–23. [Google Scholar] [CrossRef]
- Ding, M.; Shu, P.; Gao, S.; Wang, F.; Gao, Y.; Chen, Y.; Deng, W.; He, G.; Hu, Z.; Li, T. Schisandrin B protects human keratinocyte-derived HaCaT cells from tert-butyl hydroperoxide-induced oxidative damage through activating the Nrf2 signaling pathway. Int. J. Mol. Med. 2018, 42, 3571–3581. [Google Scholar] [CrossRef] [Green Version]
- Chiu, P.Y.; Chen, N.; Leong, P.K.; Leung, H.Y.; Ko, K.M. Schisandrin B elicits a glutathione antioxidant response and protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway in H9c2 cells. Mol. Cell. Biochem. 2011, 350, 237–250. [Google Scholar] [CrossRef]
- Zhao, B.; Li, G.-P.; Peng, J.-J.; Ren, L.-H.; Lei, L.-C.; Ye, H.-M.; Wang, Z.-Y.; Zhao, S. Schizandrin B attenuates hypoxia/reoxygenation injury in H9c2 cells by activating the AMPK/Nrf2 signaling pathway. Exp. Ther. Med. 2021, 21, 220. [Google Scholar] [CrossRef]
- Takanche, J.S.; Lee, Y.-H.; Kim, J.-S.; Kim, J.-E.; Han, S.-H.; Lee, S.-W.; Yi, H.-K. Anti-inflammatory and antioxidant properties of Schisandrin C promote mitochondrial biogenesis in human dental pulp cells. Int. Endod. J. 2018, 51, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, X. Schisantherin A protects renal tubular epithelial cells from hypoxia/reoxygenation injury through the activation of PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2018, 32, e22160. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, T.; Zhou, H.; Zhang, C.; Feng, Y.; Tang, F.; Hoi, M.P.M.; He, C.; Zheng, Y.; Lee, S.M.Y. Schisantherin a attenuates neuroinflammation in activated microglia: Role of Nrf2 activation through ERK phosphorylation. Cell. Physiol. Biochem. 2018, 47, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Yang, Y.; Xu, D.; Tao, S.; Li, J. Schisantherin A attenuates sepsis-induced acute kidney injury by suppressing inflammation via regulating the NRF2 pathway. Life Sci. 2020, 258, 118161. [Google Scholar] [CrossRef] [PubMed]
- Wangteeraprasert, R.; Lipipun, V.; Gunaratnam, M.; Neidle, S.; Gibbons, S.; Likhitwitayawuid, K. Bioactive compounds from Carissa spinarum. Phyther. Res. 2012, 26, 1496–1499. [Google Scholar] [CrossRef]
- Suryadevara, P.K.; Tatipaka, H.B.; Vidadala, R.S.R.; Tiwari, A.K.; Rao, J.M.; Babu, K.S. Novel C-9, 9’-O-acyl Esters of (-)-Carinol as Free-radical Scavengers and Xanthine Oxidase Enzyme Inhibitors: Synthesis and Biological Evaluation. Med. Chem. 2013, 9, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Chen, Y.L.; Wu, Y.H.; Chen, I.S.; Chang, H.S.; Wang, Y.H.; Chang, S.H.; Wu, Y.H.; Kao, T.I.; Yu, H.P.; et al. Meso-Dihydroguaiaretic Acid Ameliorates Acute Respiratory Distress Syndrome through Inhibiting Neutrophilic Inflammation and Scavenging Free Radical. Antioxidants 2022, 11, 123. [Google Scholar] [CrossRef]
- Abou-Gazar, H.; Bedir, E.; Takamatsu, S.; Ferreira, D.; Khan, I.A. Antioxidant lignans from Larrea tridentata. Phytochemistry 2004, 65, 2499–2505. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Kehrer, J.P. Oxidative stress-driven mechanisms of nordihydroguaiaretic acid-induced apoptosis in FL5.12 cells. Toxicol. Appl. Pharmacol. 2006, 214, 230–236. [Google Scholar] [CrossRef]
- Floriano-Sánchez, E.; Villanueva, C.; Noel Medina-Campos, O.; Rocha, D.; Javier Sánchez-González, D.; Cárdenas-Rodríguez, N.; Pedraza-Chaverrí, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic. Res. 2006, 40, 523–533. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Espada, S.; Orozco-Ibarra, M.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci. Lett. 2008, 447, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Macías-Ruvalcaba, N.A.; Medina Campos, O.N.; Pedraza-Chaverri, J. Mechanism of the OH Radical Scavenging Activity of Nordihydroguaiaretic Acid: A Combined Theoretical and Experimental Study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef]
- Rahman, S.; Ansari, R.A.; Rehman, H.; Parvez, S.; Raisuddin, S. Nordihydroguaiaretic Acid from Creosote Bush (Larrea tridentata) Mitigates 12- O -Tetradecanoylphorbol-13-Acetate-Induced Inflammatory and Oxidative Stress Responses of Tumor Promotion Cascade in Mouse Skin. Evid. Based Complement. Altern. Med. 2011, 2011, 734785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, A.I.; Medina-Campos, O.N.; Rada, P.; Zúñiga-Toalá, A.; López-Gazcón, A.; Espada, S.; Pedraza-Chaverri, J.; Cuadrado, A. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free Radic. Biol. Med. 2012, 52, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Beltrán, S.; Pedraza-Chaverri, J.; Gonzalez-Reyes, S.; Hernández-Sánchez, F.; Juarez-Figueroa, U.E.; Gonzalez, Y.; Bobadilla, K.; Torres, M. Nordihydroguaiaretic Acid Attenuates the Oxidative Stress-Induced Decrease of CD33 Expression in Human Monocytes. Oxid. Med. Cell. Longev. 2013, 2013, 375893. [Google Scholar] [CrossRef]
- Rojas-Ochoa, A.; Córdova, E.J.; Carrillo-García, A.; Lizano, M.; Pedraza-Chaverri, J.; Patiño, N.; Cruz-Gregorio, A.; Osnaya, N. The polyphenols α-mangostin and nordihydroguaiaretic acid induce oxidative stress, cell cycle arrest, and apoptosis in a cellular model of medulloblastoma. Molecules 2021, 26, 7230. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Nguyen, N.T.; Awale, S.; Nobukawa, T.; Kadota, S. DPPH Radical Scavenging and Nitric Oxide Inhibitory Activities of the Constituents from the Wood of Taxus yunnanensis. Planta Med. 2003, 69, 500–505. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.T.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.E.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant Activity of Knotwood Extractives and Phenolic Compounds of Selected Tree Species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinoldiglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Moree, S.S.; Rajesha, J. Investigation of in vitro and in vivo antioxidant potential of secoisolariciresinoldiglucoside. Mol. Cell. Biochem. 2013, 373, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Puukila, S.; Bryan, S.; Laakso, A.; Abdel-Malak, J.; Gurney, C.; Agostino, A.; Belló-Klein, A.; Prasad, K.; Khaper, N. SecoisolariciresinolDiglucoside Abrogates Oxidative Stress-Induced Damage in Cardiac Iron Overload Condition. PLoS ONE 2015, 10, e0122852. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Velalopoulou, A.; Albelda, S.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan SecoisolariciresinolDiglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrofesa, R.A.; Chatterjee, S.; Park, K.; Arguiri, E.; Albelda, S.M.; Christofidou-Solomidou, M. Synthetic lignan secoisolariciresinoldiglucoside (LGM2605) reduces asbestos-induced cytotoxicity in an Nrf2-dependent and -independent manner. Antioxidants 2018, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Kokkinaki, D.; Hoffman, M.; Kalliora, C.; Kyriazis, I.D.; Maning, J.; Lucchese, A.M.; Shanmughapriya, S.; Tomar, D.; Park, J.Y.; Wang, H.; et al. Chemically synthesized Secoisolariciresinoldiglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 232–245. [Google Scholar] [CrossRef]
- Jang, Y.P.; Kim, S.R.; Choi, Y.H.; Kim, J.; Kim, S.G.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J. Neurosci. Res. 2002, 68, 233–240. [Google Scholar] [CrossRef]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef]
- Gu, Y.; Qi, C.; Sun, X.; Ma, X.; Zhang, H.; Hu, L.; Yuan, J.; Yu, Q. Arctigenin preferentially induces tumor cell death under glucose deprivation by inhibiting cellular energy metabolism. Biochem. Pharmacol. 2012, 84, 468–476. [Google Scholar] [CrossRef]
- Wu, R.; Sun, Y.; Zhou, T.; Zhu, Z.; Zhuang, J.; Tang, X.; Chen, J.; Hu, L.; Shen, X. Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol. Sin. 2014, 35, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-J.; Kuo, P.-L.; Hsu, Y.-C.; Huang, Y.-F.; Tsai, E.-M.; Hsu, Y.-L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic. Biol. Med. 2014, 67, 159–170. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Park, J.-S.; Kim, D.-H.; Kim, H.-S. Arctigenin Increases Hemeoxygenase-1 Gene Expression by Modulating PI3K/AKT Signaling Pathway in Rat Primary Astrocytes. Biomol. Ther. 2014, 22, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Wang, J.; Zhu, D.; Zhang, X.; Pan, R.; Wang, R. Arctigenin suppresses transforming growth factor-β1-induced expression of monocyte chemoattractant protein-1 and the subsequent epithelial–mesenchymal transition through reactive oxygen species-dependent ERK/NF-κB signaling pathway in renal tubular epithe. Free Radic. Res. 2015, 49, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Lin, J.-A.; Yao, H.-Y.; Hsu, A.-C.; Tai, Y.-T.; Chen, J.-T.; Hsieh, M.-C.; Shen, T.-L.; Hsu, R.-Y.; Wu, H.-T.; et al. Arctigenin protects against steatosis in WRL68 hepatocytes through activation of phosphoinositide 3-kinase/protein kinase B and AMP-activated protein kinase pathways. Nutr. Res. 2018, 52, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, H.; Zhang, S.; Dai, W.; Zhang, Y.; Hong, L.; Chen, F.; Cao, J. Activation of reactive oxygen species-mediated mitogen-activated protein kinases pathway regulates both extrinsic and intrinsic apoptosis induced by arctigenin in Hep G2. J. Pharm. Pharmacol. 2019, 72, 29–43. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhou, Y.; Chen, T.; Lei, J.-C.; Jiang, X.-J. AMPK/SIRT1 Pathway is Involved in Arctigenin-Mediated Protective Effects Against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2021, 11, 2351. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Dou, P.; Zhang, X.; Ran, X.; Liu, L.; Dou, D. The Ameliorative Effects of Arctiin and Arctigenin on the Oxidative Injury of Lung Induced by Silica via TLR-4/NLRP3/TGF-β Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Medola, J.F.; Cintra, V.P.; e Silva, P.P.; Royo, V.; da Silva, R.; Saraiva, J.; de Albuquerque, S.; Bastos, J.K.; e Silva, M.L.A.; Tavares, D.C. (−)-Hinokinin causes antigenotoxicity but not genotoxicity in peripheral blood of Wistar rats. Food Chem. Toxicol. 2007, 45, 638–642. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Srinivas, P.V.; Praveen Kumar, S.; Madhusudana Rao, J. Free radical scavenging active components from Cedrus deodara. J. Agric. Food Chem. 2001, 49, 4642–4645. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sugahara, T.; Nakashima, Y.; Okada, A.; Akiyama, K.; Kishida, T.; Maruyama, M.; Masuda, T. Radical and superoxide scavenging activities of matairesinol and oxidized matairesinol. Biosci. Biotechnol. Biochem. 2006, 70, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Kim, K.H.; Jung, H.J.; Kwon, H.J. Matairesinol inhibits angiogenesis via suppression of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2012, 421, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Y.; Li, Q. Matairesinol exerts anti-inflammatory and antioxidant effects in sepsis-mediated brain injury by repressing the MAPK and NF-κB pathways through up-regulating AMPK. Aging 2021, 13, 23780–23795. [Google Scholar] [CrossRef] [PubMed]
- Moraux, T.; Dumarçay, S.; Gérardin, P.; Gérardin-Charbonnier, C. Derivatives of the Lignan 7′-Hydroxymatairesinol with Antioxidant Properties and Enhanced Lipophilicity. J. Nat. Prod. 2017, 80, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Unehara, N.; Kitanaka, S. Lignans from the Roots of Wikstroemia indica and their DPPH radical scavenging and nitric oxide inhibitory activities. Chem. Pharm. Bull. 2005, 53, 1348–1351. [Google Scholar] [CrossRef] [Green Version]
- Wangteeraprasert, R.; Likhitwitayawuid, K. Lignans and a sesquiterpene glucoside from Carissa carandas stem. Helv. Chim. Acta 2009, 92, 1217–1223. [Google Scholar] [CrossRef]
- Tebboub, O.; Cotugno, R.; Oke-Altuntas, F.; Bouheroum, M.; Demirtas, Í.; D’Ambola, M.; Malafronte, N.; Vassallo, A. Antioxidant potential of herbal preparations and components from galactites elegans (All.) nyman ex soldano. Evid. Based Complement. Altern. Med. 2018, 2018, 9294358. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cichewicz, R.H.; Nair, M.G. Lipid peroxidation inhibitory compounds from daylily (Hemerocallis fulva) leaves. Life Sci. 2004, 75, 753–763. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.J.; Yang, K.E.; Oh, W.K.; Lee, S.I.; Hwang, I.H.; Ban, K.T.; Yoo, H.S.; Choi, J.S.; Yeo, E.J.; Jang, I.S. Nectandrin B-mediated activation of the AMPK pathway prevents cellular senescence in human diploid fibroblasts by reducing intracellular ROS levels. Aging 2019, 11, 3731–3749. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chiang, W.; Liu, G.P.; Chien, Y.L.; Chang, J.Y.; Lee, C.K.; Lo, J.M.; Huang, S.L.; Shih, M.C.; Kuo, Y.H. 2,2′-Diphenyl-1-picrylhydrazyl radical-scavenging active components from adlay (Coixlachryma-jobi L. var. ma-yuen Stapf) hulls. J. Agric. Food Chem. 2002, 50, 5850–5855. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chen, Y.-T.; Huang, Y.-W.; Tsai, H.-J.; Kuo, C.-C. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic. Biol. Med. 2012, 52, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, M.; Chen, Y.; Zhang, J.; Cao, Y.; Zhang, B.; Feng, W.; Zheng, X.; Yu, Z. A new bisepoxylignandendranlignan A isolated from Chrysanthemum Flower inhibits the production of inflammatory mediators via the TLR4 pathway in LPS-induced H9c2 cardiomyocytes. Arch. Biochem. Biophys. 2020, 690, 108506. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ouyang, D.S.; Liu, Q. Isoeucommin A attenuates kidney injury in diabetic nephropathy through the Nrf2/HO-1 pathway. FEBS Open Bio 2021, 11, 2350–2363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Jang, S.A.; Seo, K.H.; Gwag, J.E.; Kim, H.G.; Ko, J.H.; Ji, S.A.; Kang, S.C.; Lee, D.Y.; Baek, N.I. New Lignans from the Flower of Forsythia koreana and Their Suppression Effect on VCAM-1 Expression in MOVAS Cells. Chem. Biodivers. 2018, 15, e1800026. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chen, H.-Y.; Shiao, M.-S.; Lin, Y.-L.; Kuo, Y.-H.; Ou, J.-C. Inhibition of Low Density Lipoprotein Oxidation by Tetrahydrofurofuran Lignans from Forsythia suspensa and Magnolia coco. Planta Med. 1999, 65, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Lee, I.K.; Kim, J.P.; Chung, S.H.; Shim, G.S.; Yoo, I.D. Lipid peroxidation inhibitory activity of some constituents isolated from the stem bark of Eucalyptus globulus. Arch. Pharm. Res. 2000, 23, 147–150. [Google Scholar] [CrossRef]
- Li, A.L.; Li, G.H.; Li, Y.R.; Wu, X.Y.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Lignan and flavonoid support the prevention of cinnamon against oxidative stress related diseases. Phytomedicine 2019, 53, 143–153. [Google Scholar] [CrossRef]
- Yao, J.; Zou, Z.; Wang, X.; Ji, X.; Yang, J. PinoresinolDiglucoside Alleviates oxLDL-Induced Dysfunction in Human Umbilical Vein Endothelial Cells. Evid. Based Complement. Altern. Med. 2016, 2016, 3124519. [Google Scholar] [CrossRef]
- Lee, W.-J.; Ou, H.-C.; Wu, C.-M.; Lee, I.-T.; Lin, S.-Y.; Lin, L.-Y.; Tsai, K.-L.; Lee, S.-D.; Sheu, W.H.-H. Sesamin mitigates inflammation and oxidative stress in endothelial cells exposed to oxidized low-density lipoprotein. J. Agric. Food Chem. 2009, 57, 11406–11417. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Hou, C.-W.; Yao, P.-W.; Wu, S.-P.; Peng, Y.-F.; Shen, M.-L.; Lin, C.-H.; Chao, Y.-Y.; Chang, M.-H.; Jeng, K.-C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflamm. 2011, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Zhou, J.-L.; Fang, H.-S.; Nie, Z.-G.; Chen, S.; Peng, H. Sesamin Protects the Femoral Head from Osteonecrosis by Inhibiting ROS-Induced Osteoblast Apoptosis in Rat Model. Front. Physiol. 2018, 9, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxid. Med. Cell. Longev. 2019, 2019, 2432416. [Google Scholar] [CrossRef]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and sesamol attenuate H2O2-induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr. Neurosci. 2021, 24, 90–101. [Google Scholar] [CrossRef]
- Cho, S.; Cho, M.; Kim, J.; Kaeberlein, M.; Lee, S.J.; Suh, Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget 2015, 6, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, L.; Feng, L.; Yang, J.; Li, Y.; An, J.; Li, D.; Xu, Y.; Gao, Y.; Li, J.; et al. Syringaresinol Protects against Type 1 Diabetic Cardiomyopathy by Alleviating Inflammation Responses, Cardiac Fibrosis, and Oxidative Stress. Mol. Nutr. Food Res. 2020, 64, 2000231. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Koshelev, S.G.; Belozerova, O.A.; Kublitski, V.S.; Andreev, Y.A.; Grishin, E.V.; Kozlov, S.A. Biological Activity of Sevanol and Its Analogues 1. Russ. J. Bioorganic Chem. 2015, 41, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskii, A.P.; Osmakov, D.I.; Koshelev, S.G.; Lubova, K.I.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology 2022, 11, 167. [Google Scholar] [CrossRef]
- Dubinnyi, M.A.; Osmakov, D.I.; Koshelev, S.G.; Kozlov, S.A.; Andreev, Y.A.; Zakaryan, N.A.; Dyachenko, I.A.; Bondarenko, D.A.; Arseniev, A.S.; Grishin, E.V. Lignan from Thyme Possesses Inhibitory Effect on ASIC3 Channel Current. J. Biol. Chem. 2012, 287, 32993–33000. [Google Scholar] [CrossRef] [Green Version]
- Andreev, Y.; Osmakov, D.; Koshelev, S.; Maleeva, E.; Logashina, Y.; Palikov, V.; Palikova, Y.; Dyachenko, I.; Kozlov, S. Analgesic Activity of Acid-Sensing Ion Channel 3 (ASIC3) Inhibitors: Sea Anemones Peptides Ugr9-1 and APETx2 versus Low Molecular Weight Compounds. Mar. Drugs 2018, 16, 500. [Google Scholar] [CrossRef] [Green Version]
- Belozerova, O.A.; Osmakov, D.I.; Vladimirov, A.; Koshelev, S.G.; Chugunov, A.O.; Andreev, Y.A.; Palikov, V.A.; Palikova, Y.A.; Shaykhutdinova, E.R.; Gvozd, A.N.; et al. Sevanol and Its Analogues: Chemical Synthesis, Biological Effects and Molecular Docking. Pharmaceuticals 2020, 13, 163. [Google Scholar] [CrossRef]
- Verma, S.; Kalita, B.; Bajaj, S.; Prakash, H.; Singh, A.K.; Gupta, M.L. A combination of podophyllotoxin and rutin alleviates radiation-induced pneumonitis and fibrosis through modulation of lung inflammation in mice. Front. Immunol. 2017, 8, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Mehak; Chhimwal, J.; Patial, V.; Sk, U.H. Dendrimer-conjugated podophyllotoxin suppresses DENA-induced HCC progression by modulation of inflammatory and fibrogenic factors. Toxicol. Res. 2019, 8, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.Y.; Lee, J.H.; Jung, H.S.; Kim, K.S.; Nam, J.B.; Hong, Y.S.; Paik, S.G.; Lee, J.J. Sauchinone, a Lignan from Saururus chinensis, Suppresses iNOS Expression through the Inhibition of Transactivation Activity of ReIA of NF-κB. Planta Med. 2003, 69, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Sung, S.H.; Kim, Y.C.; Kim, S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-α and COX-2 expression by sauchinone effects on I-κBα phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003, 139, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Min, H.J.; Won, H.Y.; Kim, Y.C.; Sung, S.H.; Byun, M.R.; Hwang, J.H.; Hong, J.H.; Hwang, E.S. Suppression of Th2-driven, allergen-induced airway inflammation by sauchinone. Biochem. Biophys. Res. Commun. 2009, 385, 204–209. [Google Scholar] [CrossRef]
- Teraoka, R.; Shimada, T.; Aburada, M. The Molecular Mechanisms of the Hepatoprotective Effect of Gomisin A against Oxidative Stress and Inflammatory Response in Rats with Carbon Tetrachloride-Induced Acute Liver Injury. Biol. Pharm. Bull. 2012, 35, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Yim, T.K.; Ko, K.M. Schisandrin B protects against myocardial ischemia-reperfusion injury by enhancing myocardial glutathione antioxidant status. Mol. Cell. Biochem. 1999, 196, 151–156. [Google Scholar] [CrossRef]
- Ip, S.P.; Ko, K.M. The crucial antioxidant action of schisandrin B in protecting against carbon tetrachloride hepatotoxicity in mice: A comparative study with butylated hydroxytoluene. Biochem. Pharmacol. 1996, 52, 1687–1693. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Tang, M.H.; Mak, D.H.F.; Poon, M.K.T.; Ko, K.M. Hepatoprotective mechanism of schisandrin B: Role of mitochondrial glutathione antioxidant status and heat shock proteins. Free Radic. Biol. Med. 2003, 35, 368–380. [Google Scholar] [CrossRef]
- Lam, P.Y.; Chiu, P.Y.; Leung, H.Y.; Chen, N.; Leong, P.K.; Ko, K.M. Schisandrin B co-treatment ameliorates the impairment on mitochondrial antioxidant status in various tissues of long-term ethanol treated rats. Fitoterapia 2010, 81, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Arumugam, S.; Mizuno, M.; Nawa, H.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T.D. Schisandrin B ameliorates ICV-infused amyloid β induced oxidative stress and neuronal dysfunction through inhibiting RAGE/NF-κB/MAPK and Up-regulating HSP/beclin expression. PLoS ONE 2015, 10, e0142483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, D.Q.; Hu, Z.M.; Huo, H.J.; Yang, X.J.; Han, D.; Xing, W.H.; Zhao, Y.; Qiu, Q.H. Schisandrin B attenuates the inflammatory response, oxidative stress and apoptosis induced by traumatic spinal cord injury via inhibition of p53 signaling in adult rats. Mol. Med. Rep. 2017, 16, 533–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Cai, C.; Zhou, A.; Zhao, X.; Xiang, Y.; Zeng, C. Schisandrin B Prevents Hind Limb from Ischemia-Reperfusion-Induced Oxidative Stress and Inflammation via MAPK/NF-κB Pathways in Rats. Biomed Res. Int. 2017, 2017, 4237973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, Z.; Feng, Z.; Xu, Z.; Zhuang, F.; Zheng, X.; Li, X.; Qian, J.; Liang, G. Schisandrin B alleviates diabetic nephropathy through suppressing excessive inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2019, 508, 243–249. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Yao, L.; Li, M.; Tang, M. Schisandrin B alleviates acute oxidative stress via modulation of the Nrf2/Keap1-mediated antioxidant pathway. Appl. Physiol. Nutr. Metab. 2019, 44, 1–6. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, J.; Feng, R.; Pu, P.; Wen, L. Reducing oxidative stress may be important for treating pirarubicin-induced cardiotoxicity with schisandrin B. Exp. Ther. Med. 2021, 23, 68. [Google Scholar] [CrossRef]
- Han, J.; Shi, X.; Du, Y.; Shi, F.; Zhang, B.; Zheng, Z.; Xu, J.; Jiang, L. Schisandrin C targets Keap1 and attenuates oxidative stress by activating Nrf2 pathway in Ang II-challenged vascular endothelium. Phyther. Res. 2019, 33, 779–790. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Xu, X.; Mao, X.; Liu, Z.; Li, H.; Guo, L.; Bi, K.; Jia, Y. Schisantherin A recovers Aβ-induced neurodegeneration with cognitive decline in mice. Physiol. Behav. 2014, 132, 10–16. [Google Scholar] [CrossRef]
- Liu, C.; Sun, W.; Li, N.; Gao, J.; Yu, C.; Wang, C.; Sun, J.; Jing, S.; Chen, J.; Li, H. Schisantherin A Improves Learning and Memory of Mice with D-Galactose-Induced Learning and Memory Impairment Through Its Antioxidation and Regulation of p19/p53/p21/Cyclin D1/CDK4/RB Gene Expressions. J. Med. Food 2018, 21, 678–688. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Liu, J.; Yuan, L.; Liu, J.; Wang, C.; Sun, J.; Chen, J.; Jing, S.; Li, H. Schisantherin A improves learning and memory abilities partly through regulating the Nrf2/Keap1/ARE signaling pathway in chronic fatigue mice. Exp. Ther. Med. 2021, 21, 385. [Google Scholar] [CrossRef]
- Shi, Y.W.; Zhang, X.C.; Chen, C.; Tang, M.; Wang, Z.W.; Liang, X.M.; Ding, F.; Wang, C.P. Schisantherin A attenuates ischemia/reperfusion-induced neuronal injury in rats via regulation of TLR4 and C5aR1 signaling pathways. Brain. Behav. Immun. 2017, 66, 244–256. [Google Scholar] [CrossRef]
- Yam-Canul, P.; Chirino, Y.I.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Cruz, C.; Villanueva, C.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid attenuates potassium dichromate-induced oxidative stress and nephrotoxicity. Food Chem. Toxicol. 2008, 46, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.W.; Bittner, S.; Bittner, A.; Atwal, S.; Shen, W.J.; Inayathullah, M.; Rajada, J.; Nicolls, M.R.; Kraemer, F.B.; Azhar, S. Nordihydroguaiaretic acid, a lignan from larrea tridentata (Creosote bush), protects against American lifestyle-induced obesity syndrome diet-induced metabolic dysfunction in mice. J. Pharmacol. Exp. Ther. 2018, 365, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective Role of Flaxseed Oil and Flaxseed Lignan SecoisolariciresinolDiglucoside Against Oxidative Stress in Rats with Metabolic Syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Puukila, S.; Fernandes, R.O.; Türck, P.; Carraro, C.C.; Bonetto, J.H.P.; de Lima-Seolin, B.G.; da Rosa Araujo, A.S.; Belló-Klein, A.; Boreham, D.; Khaper, N. Secoisolariciresinoldiglucoside attenuates cardiac hypertrophy and oxidative stress in monocrotaline-induced right heart dysfunction. Mol. Cell. Biochem. 2017, 432, 33–39. [Google Scholar] [CrossRef]
- Kartha, S.; Weisshaar, C.L.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Winkelstein, B.A. Synthetic secoisolariciresinoldiglucoside attenuates established pain, oxidative stress and neuroinflammation in a rodent model of painful radiculopathy. Antioxidants 2020, 9, 1209. [Google Scholar] [CrossRef]
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Javaraiah, R. Secoisolariciresinoldiglucoside protects against cadmium-induced oxidative stress-mediated renal toxicity in rats. J. Trace Elem. Med. Biol. 2020, 61, 126552. [Google Scholar] [CrossRef]
- Ge, J.; Hao, R.; Rong, X.; Dou, Q.P.; Tan, X.; Li, G.; Li, F.; Li, D. Secoisolariciresinoldiglucoside mitigates benzo[a]pyrene-induced liver and kidney toxicity in mice via miR-101a/MKP-1-mediated p38 and ERK pathway. Food Chem. Toxicol. 2022, 159, 112733. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Wu, M.; Wei, J.; Sun, X.; Wang, A.; Hu, G.; Jia, J. SecoisolariciresinolDiglucoside Improves Ovarian Reserve in Aging Mouse by Inhibiting Oxidative Stress. Front. Mol. Biosci. 2022, 8, 806412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Miao, Y.; Su, Q.-Y.; Yao, J.-C.; Li, H.-H.; Zhang, G.-M. Gastroprotective effects of arctigenin of Arctium lappa L. on a rat model of gastric ulcers. Biomed. Rep. 2016, 5, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Swarup, V.; Ghosh, J.; Mishra, M.K.; Basu, A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J. Antimicrob. Chemother. 2008, 61, 679–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Jiang, Z.; He, B.; Liu, X. Arctigenin Protects against Lipopolysaccharide-Induced Pulmonary Oxidative Stress and Inflammation in a Mouse Model via Suppression of MAPK, HO-1, and iNOS Signaling. Inflammation 2015, 38, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, H.S.; Cao, Y.J.; Jiang, Z.A.; Li, Y.J.; Song, M.C.; Wang, Y.F.; Wang, Z.H.; Yang, R.; Jiang, Y.F.; et al. Arctigenin Attenuates Ischemia/Reperfusion Induced Ventricular Arrhythmias by Decreasing Oxidative Stress in Rats. Cell. Physiol. Biochem. 2018, 49, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y. Arctigenin exerts protective effects against myocardial infarction via regulation of iNOS, COX-2, ERK1/2 and HO-1 in rats. Mol. Med. Rep. 2018, 17, 4839–4845. [Google Scholar] [CrossRef]
- Jiang, L.; Deng, Y.; Li, W.; Lu, Y. Arctigenin suppresses fibroblast activity and extracellular matrix deposition in hypertrophic scarring by reducing inflammation and oxidative stress. Mol. Med. Rep. 2020, 22, 4783–4791. [Google Scholar] [CrossRef]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef]
- Lu, Q.; Zheng, R.; Zhu, P.; Bian, J.; Liu, Z.; Du, J. Hinokinin alleviates high fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2021, 137, 111361. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; Xue, H.; Yue, Y.; Zhang, W.; Li, X. Fargesin as a potential β1 adrenergic receptor antagonist protects the hearts against ischemia/reperfusion injury in rats via attenuating oxidative stress and apoptosis. Fitoterapia 2015, 105, 16–25. [Google Scholar] [CrossRef]
- Lei, S.; Wu, S.; Wang, G.; Li, B.; Liu, B.; Lei, X. Pinoresinoldiglucoside attenuates neuroinflammation, apoptosis and oxidative stress in a mice model with Alzheimer’s disease. Neuroreport 2021, 32, 259–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Yao, X.; Yi, J.; Feng, G. Pinoresinoldiglucoside alleviates ischemia/reperfusion-induced brain injury by modulating neuroinflammation and oxidative stress. Chem. Biol. Drug Des. 2021, 98, 986–996. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M.; Jalali Nadoushan, M.-R.; Vaez Mahdavi, M.-R.; Kalalian-Moghaddam, H.; Roghani-Dehkordi, F.; Dariani, S.; Raoufi, S. The sesame lignan sesamin attenuates vascular dysfunction in streptozotocin diabetic rats: Involvement of nitric oxide and oxidative stress. Eur. J. Pharmacol. 2013, 698, 316–321. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zheng, G.-H.; Ming, Q.-L.; Chao, C.; Sun, J.-M. Sesamin Protects Mouse Liver against Nickel-Induced Oxidative DNA Damage and Apoptosis by the PI3K-Akt Pathway. J. Agric. Food Chem. 2013, 61, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem. Toxicol. 2014, 64, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, J.; Xie, L.; Wang, J.; Feng, C.; Song, J. Protective properties of sesamin against fluoride-induced oxidative stress and apoptosis in kidney of carp (Cyprinus carpio) via JNK signaling pathway. Aquat. Toxicol. 2015, 167, 180–190. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Wang, L.; Zhang, G.; Su, S.; Zhang, R.; Zhang, J.; Li, A.; Shang, C.; Bi, B.; et al. Alleviation of doxorubicin–induced hepatorenal toxicities with sesamin via the suppression of oxidative stress. Hum. Exp. Toxicol. 2016, 35, 1183–1193. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Mansouri, M.; Ghalami, J.; Mokhtari, Z.; Roghani, M. Sesamin imparts neuroprotection against intrastriatal 6-hydroxydopamine toxicity by inhibition of astroglial activation, apoptosis, and oxidative stress. Biomed. Pharmacother. 2017, 88, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Yashaswini, P.S.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. In vivo modulation of LPS induced leukotrienes generation and oxidative stress by sesame lignans. J. Nutr. Biochem. 2017, 41, 151–157. [Google Scholar] [CrossRef]
- Rousta, A.-M.; Mirahmadi, S.-M.-S.; Shahmohammadi, A.; Nourabadi, D.; Khajevand-Khazaei, M.-R.; Baluchnejadmojarad, T.; Roghani, M. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacol. Immunotoxicol. 2018, 40, 423–429. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Ashique, M.; Manoj, P.; Sudhadevi, M.; Al Tobi, M.; Nemmar, A. Ameliorative effect of sesamin in cisplatin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020, 69, 61–72. [Google Scholar] [CrossRef]
- Le, T.D.; Inoue, Y.H. Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains. Antioxidants 2021, 10, 924. [Google Scholar] [CrossRef]
- Kim, J.; Toda, T.; Watanabe, K.; Shibuya, S.; Ozawa, Y.; Izuo, N.; Cho, S.; Seo, D.B.; Yokote, K.; Shimizu, T. Syringaresinol Reverses Age-Related Skin Atrophy by Suppressing FoxO3a-Mediated Matrix Metalloproteinase–2 Activation in Copper/Zinc Superoxide Dismutase–Deficient Mice. J. Investig. Dermatol. 2019, 139, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhu, W.; Yang, Z.; Song, X.; Xu, C.; Cui, Z.; Xiang, L. Evidence of anti-inflammatory activity of Schizandrin A in animal models of acute inflammation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Mak, D.H.F.; Poon, M.K.T.; Ko, K.M. Role of cytochrome P-450 in schisandrin B-induced antioxidant and heat shock responses in mouse liver. Life Sci. 2005, 77, 2887–2895. [Google Scholar] [CrossRef]
- You, S.; Qian, J.; Wu, G.; Qian, Y.; Wang, Z.; Chen, T.; Wang, J.; Huang, W.; Liang, G. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-κB activation. Phytomedicine 2019, 62, 152955. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Shen, C.; Wang, X.; Pu, Z.; Yin, Q. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging 2021, 13, 22193–22209. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, G.; Shen, Y.; Hu, S.; Lin, X.; Zhou, J.; Zhao, W.; Liu, J.; Wang, J.; Guo, J. Schisandrin B-mediated TH17 cell differentiation attenuates bowel inflammation. Pharmacol. Res. 2021, 166, 105459. [Google Scholar] [CrossRef]
- Chen, P.; Pang, S.; Yang, N.; Meng, H.; Liu, J.; Zhou, N.; Zhang, M.; Xu, Z.; Gao, W.; Chen, B.; et al. Beneficial effects of schisandrin B on the cardiac function in mice model of myocardial infarction. PLoS ONE 2013, 8, e79418. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; Ma, C.; Xu, K.; Xu, L.; He, Y.; Moqbel, S.A.A.; Hu, P.; Jiang, L.; Chen, W.; Bao, J.; et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways. Drug Des. Dev. Ther. 2018, 12, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Z.W.; Jin, H.; Hu, C.; He, Z.X.; Yu, Z.L.; Ko, K.M.; Yang, T.; Zhang, X.; Pan, S.Y.; et al. Schisandrin B inhibits cell growth and induces cellular apoptosis and autophagy in mouse hepatocytes and macrophages: Implications for its hepatotoxicity. Drug Des. Dev. Ther. 2015, 9, 2001–2027. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Pan, S.Y.; Zhou, S.F.; Wang, X.Y.; Sun, N.; Zhu, P.L.; Chu, Z.S.; Yu, Z.L.; Ko, K.M. Time and dose relationships between schisandrin B- and schisandrae fructus oil-induced hepatotoxicity and the associated elevations in hepatic and serum triglyceride levels in mice. Drug Des. Dev. Ther. 2014, 8, 1429–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, P.K.; Moore, A.N. Enhanced processing of APP induced by IL-1β can be reduced by indomethacin and nordihydroguaiaretic acid. Biochem. Biophys. Res. Commun. 1995, 208, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.B.; Ji, K.A.; You, H.J.; Kim, J.H.; Jou, I.; Joe, E.H. Nordihydroguaiaretic acid inhibits IFN-γ-induced STAT tyrosine phosphorylation in rat brain astrocytes. Biochem. Biophys. Res. Commun. 2005, 328, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Kukita, A.; Watanabe, T.; Takano, T.; Qu, P.; Sanematsu, K.; Ninomiya, Y.; Kukita, T. Nordihydroguaiaretic acid inhibition of NFATc1 suppresses osteoclastogenesis and arthritis bone destruction in rats. Lab. Investig. 2012, 92, 1777–1787. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kim, C.J. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I-IV allergic inflammation and pro-inflammatory enzymes. Arch. Pharm. Res. 2010, 33, 947–957. [Google Scholar] [CrossRef]
- Shi, H.; Dong, G.; Yan, F.; Zhang, H.; Li, C.; Ma, Q.; Zhang, J.; Ning, Z.; Li, Z.; Dai, J.; et al. Arctigenin Ameliorates Inflammation by Regulating Accumulation and Functional Activity of MDSCs in Endotoxin Shock. Inflammation 2018, 41, 2090–2100. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, H.; Yang, J.; Cheng, Y.; Wang, D.; Yang, F.; Li, Y.; Zhou, D.; Wang, Y.; Xue, Z.; et al. Arctigenin protects against liver injury from acute hepatitis by suppressing immune cells in mice. Biomed. Pharmacother. 2018, 102, 464–471. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, W.; Zhong, X.; Xu, J.; Huang, H.; Zheng, X.; Zhang, J.; Yang, S.; Shang, P.; Tang, Q.; et al. Arctigenin prevents the progression of osteoarthritis by targeting PI3K/Akt/NF-κB axis: In vitro and in vivo studies. J. Cell. Mol. Med. 2020, 24, 4183–4193. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Xiang, C.; Feng, C.; Fan, C.; Liu, M.; Lu, H.; Su, H.; Zhou, Y.; Qi, Q.; et al. Identification of phosphodiesterase-4 as the therapeutic target of arctigenin in alleviating psoriatic skin inflammation. J. Adv. Res. 2021, 33, 241–251. [Google Scholar] [CrossRef]
- Desai, D.C.; Jacob, J.; Almeida, A.; Kshirsagar, R.; Manju, S.L. Isolation, structural elucidation and anti-inflammatory activity of astragalin, (-)hinokinin, aristolactami and aristolochic acids (I & II) from Aristolochia indica. Nat. Prod. Res. 2014, 28, 1413–1417. [Google Scholar] [CrossRef]
- Li, X.; Gao, Q.; Yang, L.; Han, M.; Zhou, C.; Mu, H. Matairesinol ameliorates experimental autoimmune uveitis by suppression of IRBP-specific Th17 cells. J. Neuroimmunol. 2020, 345, 577286. [Google Scholar] [CrossRef] [PubMed]
- Spilioti, E.; Holmbom, B.; Papavassiliou, A.G.; Moutsatsou, P. Lignans 7-hydroxymatairesinol and 7-hydroxymatairesinol 2 exhibit anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2014, 58, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Leppänen, T.; Eräsalo, H.; Hämäläinen, M.; Nieminen, R.; Moilanen, E. Anti-inflammatory Effects of Nortrachelogenin in Murine J774 Macrophages and in Carrageenan-Induced Paw Edema Model in the Mouse. Planta Med. 2017, 83, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.G.; Lee, S.M.; Bang, M.H.; Park, H.J.; Lee, T.H.; Kim, Y.H.; Kim, J.Y.; Baek, N.I. Lignans from the flowers of osmanthus fragrans var. aurantiacus and their inhibition effect on NO production. Arch. Pharm. Res. 2011, 34, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Fozia; Waheed, A.; Tahir, N.B.; Rais, A.K. Anti-inflammatory constituents from Perovskiaatriplicifolia. Pharm. Biol. 2015, 53, 1628–1631. [Google Scholar] [CrossRef]
- De León, E.J.; Olmedo, D.A.; Solís, P.N.; Gupta, M.P.; Terencio, M.C. Diayangambin exerts immunosuppressive and anti-inflammatory effects in vitro and in vivo. Planta Med. 2002, 68, 1128–1131. [Google Scholar] [CrossRef]
- Pham, T.H.; Kim, M.S.; Le, M.Q.; Song, Y.S.; Bak, Y.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine 2017, 24, 96–103. [Google Scholar] [CrossRef]
- Yue, B.; Ren, Y.J.; Zhang, J.J.; Luo, X.P.; Yu, Z.L.; Ren, G.Y.; Sun, A.N.; Deng, C.; Wang, Z.T.; Dou, W. Anti-inflammatory effects of fargesin on chemically induced inflammatory bowel disease in mice. Molecules 2018, 23, 1380. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Shin, J.S.; Chung, K.S.; Lee, Y.G.; Baek, N.I.; Lee, K.T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-κB, and JAK/STAT Signaling. Cells 2019, 8, 1163. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Kim, H.P. Anti-inflammatory activity of phylligenin, a lignan from the fruits of Forsythia koreana, and its cellular mechanism of action. J. Ethnopharmacol. 2008, 118, 113–117. [Google Scholar] [CrossRef]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among plant lignans, pinoresinol has the strongest antiinammatory properties in human intestinal Caco-2 cells. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Hou, X.; Chen, J.; Xie, L.; Yang, L.; Le, Y. Sesamin inhibits bacterial formylpeptide-induced inflammatory responses in a murine air-pouch model and in THP-1 human monocytes. J. Nutr. 2010, 140, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Li, L.C.; Piao, H.M.; Zheng, M.Y.; Lin, Z.H.; Li, G.; Yan, G.H. Sesamin attenuates mast cell-mediated allergic responses by suppressing the activation of p38 and nuclear factor-κB. Mol. Med. Rep. 2016, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Sun, L.; Li, J.; Wang, Y.; Bai, J.; Wu, L.; Han, Q.; Yang, Z.; Li, L. Sesamin attenuates carrageenan-induced lung inflammation through upregulation of A20 and TAX1BP1 in rats. Int. Immunopharmacol. 2020, 88, 107009. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Lee, W.; Yoo, H.; Han, C.K.; Bae, J.S. Inhibitory effects of epi-sesamin on endothelial protein C receptor shedding in vitro and in vivo. Inflamm. Res. 2013, 62, 895–902. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Ju, M.K.; Majumder, R.; Shukla, S.; Huh, Y.S.; Na, M.K.; Lee, S.H.; Han, Y.K. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci. Rep. 2018, 8, 9216. [Google Scholar] [CrossRef]
- Wei, A.; Liu, J.; Li, D.; Lu, Y.; Yang, L.; Zhuo, Y.; Tian, W.; Cong, H. Syringaresinol attenuates sepsis-induced cardiac dysfunction by inhibiting inflammation and pyroptosis in mice. Eur. J. Pharmacol. 2021, 913, 174644. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Zhang, J.; Gao, H.; Yang, L.; Li, D.; Zhang, Q.; Wang, B.; Cui, L.; Wang, X. (−)-Syringaresinol suppressed LPS-induced microglia activation via downregulation of NF-κB p65 signaling and interaction with ERβ. Int. Immunopharmacol. 2021, 99, 107986. [Google Scholar] [CrossRef]
- Manda, G.; Rojo, A.I.; Martínez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic Acid: From Herbal Medicine to Clinical Development for Cancer and Chronic Diseases. Front. Pharmacol. 2020, 11, 151. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, X.Y.; Liu, J.M.; Song, Y.; Liu, T.T.; Chen, D. NDGA reduces secondary damage after spinal cord injury in rats via anti-inflammatory effects. Brain Res. 2013, 1516, 83–92. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, W.J.; Cortez, Y.; Kraemer, F.B.; Azhar, S. Nordihydroguaiaretic acid improves metabolic dysregulation and aberrant hepatic lipid metabolism in mice by both PPARα-dependent and -independent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G72–G86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubrow, M.E.; Margulies, S.S.; Yehya, N. Nordihydroguaiaretic acid reduces secondary organ injury in septic rats after cecal ligation and puncture. PLoS ONE 2020, 15, e0237613. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Zhao, D.; Meyers, R.O.; Kuester, R.K.; Timmermann, B.N.; Dorr, R.T. Nordihydroguaiaretic acid: Hepatotoxicity and detoxification in the mouse. Toxicon 2002, 40, 1701–1708. [Google Scholar] [CrossRef]
- Lü, J.M.; Nurko, J.; Weakley, S.M.; Jiang, J.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: An update. Med. Sci. Monit. 2010, 16, RA93–RA100. [Google Scholar] [PubMed]
- Hyam, S.R.; Lee, I.A.; Gu, W.; Kim, K.A.; Jeong, J.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur. J. Pharmacol. 2013, 708, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Zhang, K.; Xue, Z.; Li, Y.; Zhang, Z.; Zhang, L.; Gu, C.; Zhang, Q.; Hao, J.; et al. Arctigenin Suppress Th17 Cells and Ameliorates Experimental Autoimmune Encephalomyelitis Through AMPK and PPAR-γ/ROR-γt Signaling. Mol. Neurobiol. 2016, 53, 5356–5366. [Google Scholar] [CrossRef]
- Nakano, Y.; Nasu, M.; Kano, M.; Kameoka, H.; Okuyama, T.; Nishizawa, M.; Ikeya, Y. Lignans from guaiac resin decrease nitric oxide production in interleukin 1β-treated hepatocytes. J. Nat. Med. 2017, 71, 190–197. [Google Scholar] [CrossRef]
- Küpeli, E.; Erdemoǧlu, N.; Yeşilada, E.; Şener, B. Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J. Ethnopharmacol. 2003, 89, 265–270. [Google Scholar] [CrossRef]
- Karthivashan, G.; Kweon, M.H.; Park, S.Y.; Kim, J.S.; Kim, D.H.; Ganesan, P.; Choi, D.K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.H.; He, L.H.; Yu, X.H.; Zhao, Z.W.; Zou, J.; Wen, F.J.; Zhou, L.; Wan, X.J.; Tang, C.K. Fargesin alleviates atherosclerosis by promoting reverse cholesterol transport and reducing inflammatory response. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158633. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Fu, K.; Gong, L.H.; Zhang, Y.F.; Zhou, H.L.; Li, Y.X. Phillygenin Attenuates Carbon Tetrachloride-Induced Liver Fibrosis via Modulating Inflammation and Gut Microbiota. Front. Pharmacol. 2021, 12, 2537. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Ren, Y.S.; Gao, L.; Li, L.F.; Cui, L.J.; Li, B.; Li, X.; Yang, J.; Wang, M.Z.; Lv, Y.Y.; et al. 28-Day Oral Chronic Toxicity Study of Arctigenin in Rats. Front. Pharmacol. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.J.; Kumar, U.S.; Reddy, S.V.; Tiwari, A.K.; Rao, J.M. Antioxidants and a new germacrane sesquiterpene from Carissa spinarum. Nat. Prod. Res. 2005, 19, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S. The synthesis of lignans and neolignans. Chem. Soc. Rev. 1982, 11, 75–125. [Google Scholar] [CrossRef]
- Fang, X.; Hu, X. Advances in the synthesis of lignan natural products. Molecules 2018, 23, 3385. [Google Scholar] [CrossRef] [Green Version]
- Sih, C.J.; Ravikumar, P.R.; Huang, F.C.; Buckner, C.; Whitlock, H. Isolation and Synthesis of PinoresinolDiglucoside, a Major Antihypertensive Principle of Tu-Chung (Eucommia ulmoides, Oliver). J. Am. Chem. Soc. 1976, 98, 5412–5413. [Google Scholar] [CrossRef]
- Dickey, E.E. Liriodendrin, a New Lignan Diglucoside from the Inner Bark of Yellow Poplar (Liriodendron tulipifera L.). J. Org. Chem. 1958, 23, 179–184. [Google Scholar] [CrossRef]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the Preparation of Bioactive Lignans by Oxidative Coupling Reaction. II. Oxidative Coupling Reaction of Methyl (E)-3-(4,5-Dihydroxy-2-methoxyphenyl)propenoate and Lipid Peroxidation Inhibitory Effects of the Produced Lignans. Chem. Pharm. Bull. 1994, 42, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Lemière, G.; Gao, M.; De Groot, A.; Dommisse, R.; Lepoivre, J.; Pieters, L.; Buss, V. 3′,4-Di-O-methylcedrusin: Synthesis, resolution and absolute configuration. J. Chem. Soc. Perkin Trans. 1995, 13, 1775–1779. [Google Scholar] [CrossRef]
- Daquino, C.; Rescifina, A.; Spatafora, C.; Tringali, C. Biomimetic synthesis of natural and “unnatural” lignans by oxidative coupling of caffeic esters. Eur. J. Org. Chem. 2009, 2009, 6289–6300. [Google Scholar] [CrossRef]
- Kao, T.T.; Lin, C.C.; Shia, K.S. The Total Synthesis of Retrojusticidin B, Justicidin E, and Helioxanthin. J. Org. Chem. 2015, 80, 6708–6714. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A.; Albrecht, M.; Wray, V.; Gumbinger, H.G.; John Winterhoff, M.H.; Kemper, F.H. Structures of compounds with antigonadotropic activity obtained by in vitro oxidation of caffeic acid. Planta Med. 1990, 56, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Li, Y.; Yu, W.; Hou, Z.J. An efficient method for the synthesis of lignans. Tetrahedron 2006, 62, 6107–6112. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsumoto, K.; Ueda, M.; Miyake, Y.; Fukuyama, Y. Biomimetic syntheses of neurotrophic americanola and isoamericanola by horseradish peroxidase (HRP) catalyzed oxidative coupling. Heterocycles 2002, 56, 245–256. [Google Scholar] [CrossRef]
- Bruschi, M.; Orlandi, M.; Rindone, B.; Rummakko, P.; Zoia, L. Asymmetric biomimetic oxidations of phenols using oxazolidines as chiral auxiliaries: The enantioselective synthesis of (+)- and (-)-dehydrodiconiferyl alcohol. J. Phys. Org. Chem. 2006, 19, 592–596. [Google Scholar] [CrossRef]
- Singleton, V.L.; Cilliers, J.J.L. Characterization of the Products of Nonenzymic Autoxidative Phenolic Reactions in a Caffeic Acid Model System. J. Agric. Food Chem. 1991, 39, 1298–1303. [Google Scholar] [CrossRef]
- Agata, I.; Hatano, T.; Nishibe, S.; Okuda, T. A tetrameric derivative of caffeic acid from Rabdosia japonica. Phytochemistry 1989, 28, 2447–2450. [Google Scholar] [CrossRef]
- Bogucki, D.E.; Charlton, J.L. A non-enzymatic synthesis of (S)-(−)-rosmarinic acid and a study of a biomimetic route to (+)-rabdosiin. Can. J. Chem. 1997, 75, 1783–1794. [Google Scholar] [CrossRef]
- Belozerova, O.A.; Deigin, V.I.; Khrushchev, A.Y.; Dubinnyi, M.A.; Kublitski, V.S. The total synthesis of sevanol, a novel lignan isolated from the thyme plant (Thymus armeniacus). Tetrahedron 2018, 74, 1449–1453. [Google Scholar] [CrossRef]
- Datta, P.K.; Yau, C.; Hooper, T.S.; Yvon, B.L.; Charlton, J.L. Acid-catalyzed cyclization of 2,3-dibenzylidenesuccinates: Synthesis of lignans (±)-cagayanin and (±)-galbulin. J. Org. Chem. 2001, 66, 8606–8611. [Google Scholar] [CrossRef]
- Ishikawa, R.; Yoshida, N.; Akao, Y.; Kawabe, Y.; Inai, M.; Asakawa, T.; Hamashima, Y.; Kan, T. Total syntheses of (+)-sesamin and (+)-sesaminol. Chem. Lett. 2014, 43, 1572–1574. [Google Scholar] [CrossRef]
- Tanaka, M.; Mukaiyama, C.; Mitsuhashi, H.; Maruno, M.; Wakamatsu, T. Synthesis of Optically Pure Gomisi Lignans: The Total Synthesis of (+)-Schizandrin, (+)-Gomisin A, and (+)-Isoschizandrin in Naturally Occurring Forms. J. Org. Chem. 1995, 60, 4339–4352. [Google Scholar] [CrossRef]
- Tanaka, M.; Ohshima, T.; Mitsuhashi, H.; Maruno, M.; Wakamatsu, T. Total syntheses of the lignans isolated from Schisandra Chinensis. Tetrahedron 1995, 51, 11693–11702. [Google Scholar] [CrossRef]
- Fischer, J.; Reynolds, A.J.; Sharp, L.A.; Sherburn, M.S. Radical carboxyarylation approach to lignans. Total synthesis of (-)-arctigenin, (-)-matairesinol, and related natural products. Org. Lett. 2004, 6, 1345–1348. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Y.; Wang, W.; Ding, Y.; He, R. Synthesis and bioactivity of erythro-nordihydroguaiaretic acid, threo-(-)-saururenin and their analogues. J. Serb. Chem. Soc. 2010, 75, 1325–1335. [Google Scholar] [CrossRef]
- Holmes, T.L.; Stevenson, R. Arylnaphthalene Lignans. Synthesis of Justicidin E, Taiwanin C, Dehydrodimethylconidendrin, and Dehydrodimethylretrodendrin. J. Org. Chem. 1971, 36, 3450–3453. [Google Scholar] [CrossRef]
- Maclean, I.; Stevenson, R. Synthesis of (±)-otobain. J. Chem. Soc. C Org. 1966, 0, 1717–1719. [Google Scholar] [CrossRef]
- Ting, C.P.; Tschanen, E.; Jang, E.; Maimone, T.J. Total synthesis of podophyllotoxin and select analog designs via C–H activation. Tetrahedron 2019, 75, 3299–3308. [Google Scholar] [CrossRef]
- Gardner, K.D.; Reed, W.P.; Evan, A.P.; Zedalis, J.; Hylarides, M.D.; Leon, A.A. Endotoxin provocation of experimental renal cystic disease. Kidney Int. 1987, 32, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yu, B.; Fang, F.; Cao, H.; Ma, T.; Yang, H. Modulation of chloride channel functions by the plant lignan compounds kobusin and eudesmin. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Osmakov, D.I.; Andreev, Y.A.; Kozlov, S.A. Acid-sensing ion channels and their modulators. Biochemistry 2014, 79, 1528–1545. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Khasanov, T.A.; Andreev, Y.A.; Lyukmanova, E.N.; Kozlov, S.A. Animal, Herb, and Microbial Toxins for Structural and Pharmacological Study of Acid-Sensing Ion Channels. Front. Pharmacol. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Koshelev, S.G.; Lyukmanova, E.N.; Shulepko, M.A.; Andreev, Y.A.; Illes, P.; Kozlov, S.A. Multiple Modulation of Acid-Sensing Ion Channel 1a by the Alkaloid Daurisoline. Biomolecules 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmakov, D.I.; Koshelev, S.G.; Palikov, V.A.; Palikova, Y.A.; Shaykhutdinova, E.R.; Dyachenko, I.A.; Andreev, Y.A.; Kozlov, S.A. Alkaloid Lindoldhamine Inhibits Acid-Sensing Ion Channel 1a and Reveals Anti-Inflammatory Properties. Toxins 2019, 11, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Dubinnyi, M.A.; Kublitski, V.S.; Efremov, R.G.; Sobolevsky, A.I.; Kozlov, S.A. Proton-independent activation of acid-sensing ion channel 3 by an alkaloid, lindoldhamine, from Laurus nobilis. Br. J. Pharmacol. 2018, 175, 924–937. [Google Scholar] [CrossRef] [Green Version]
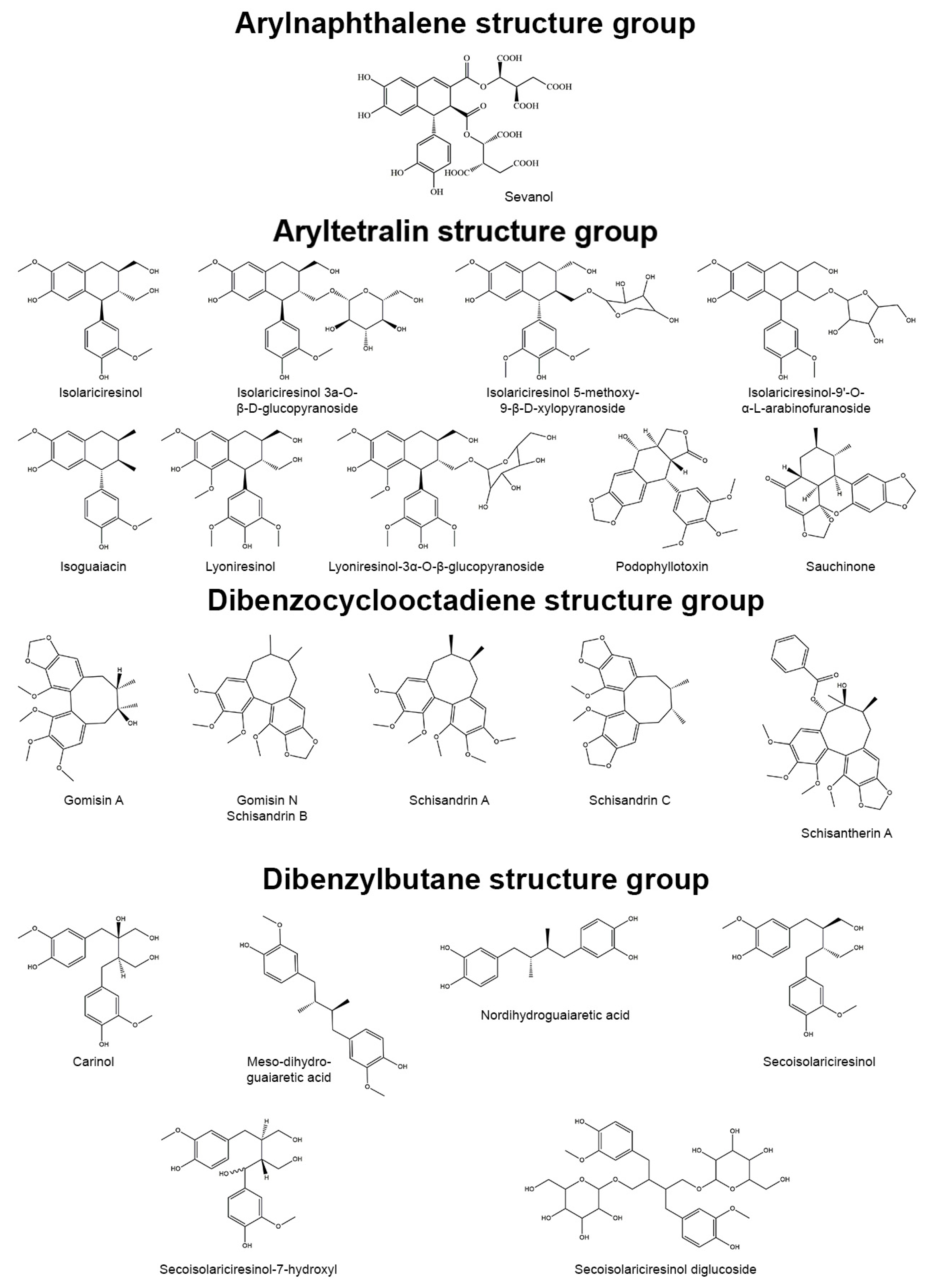
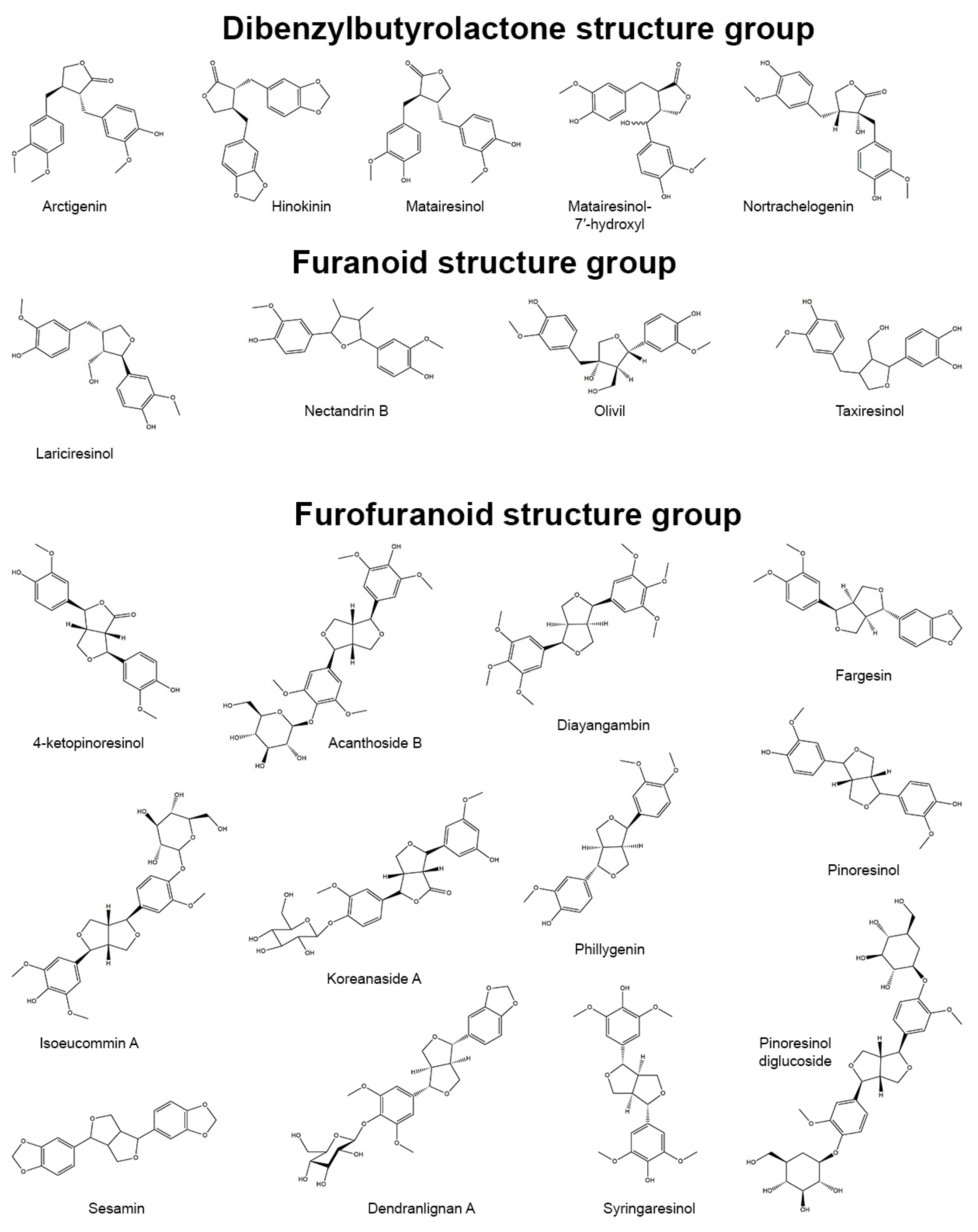
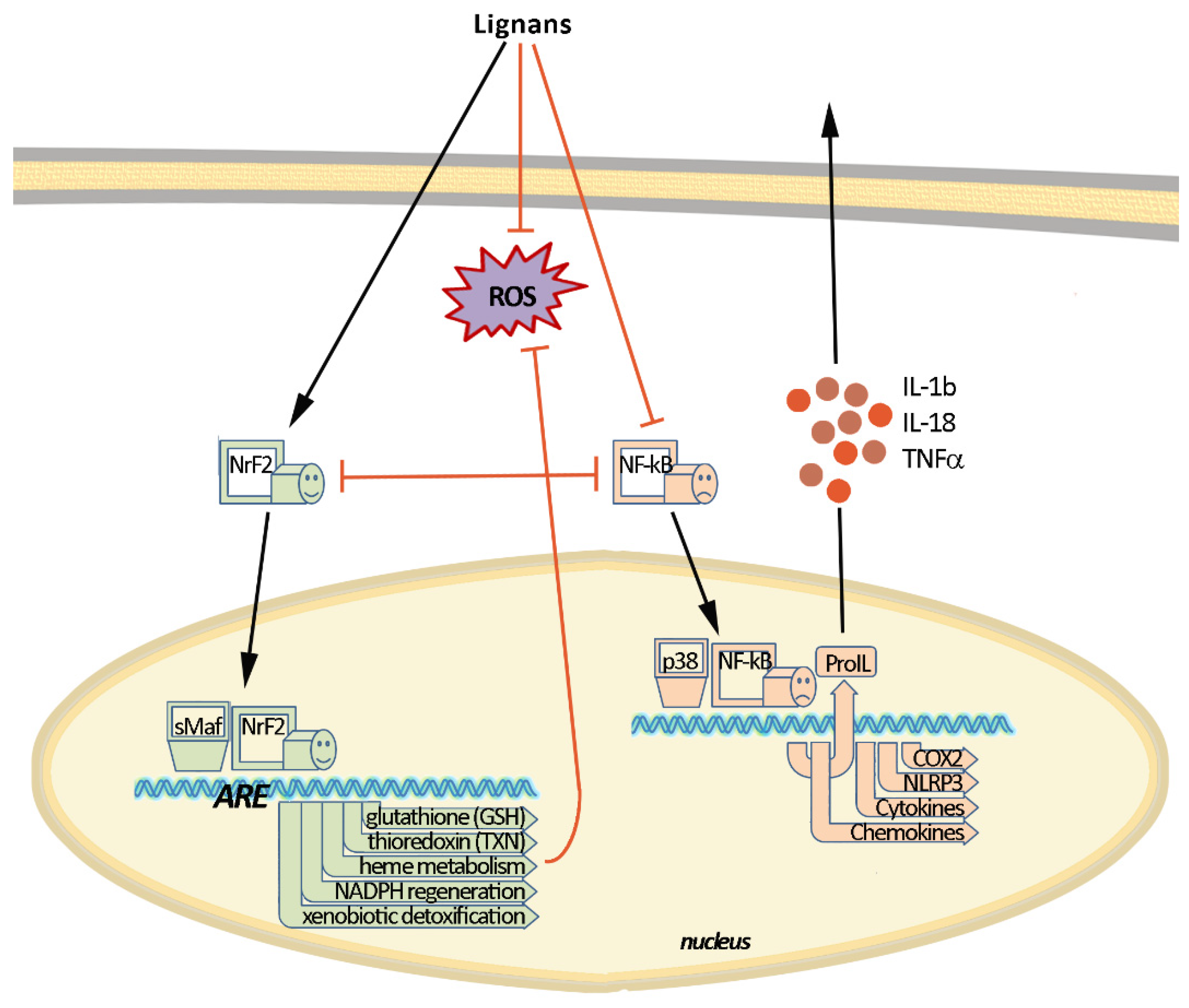
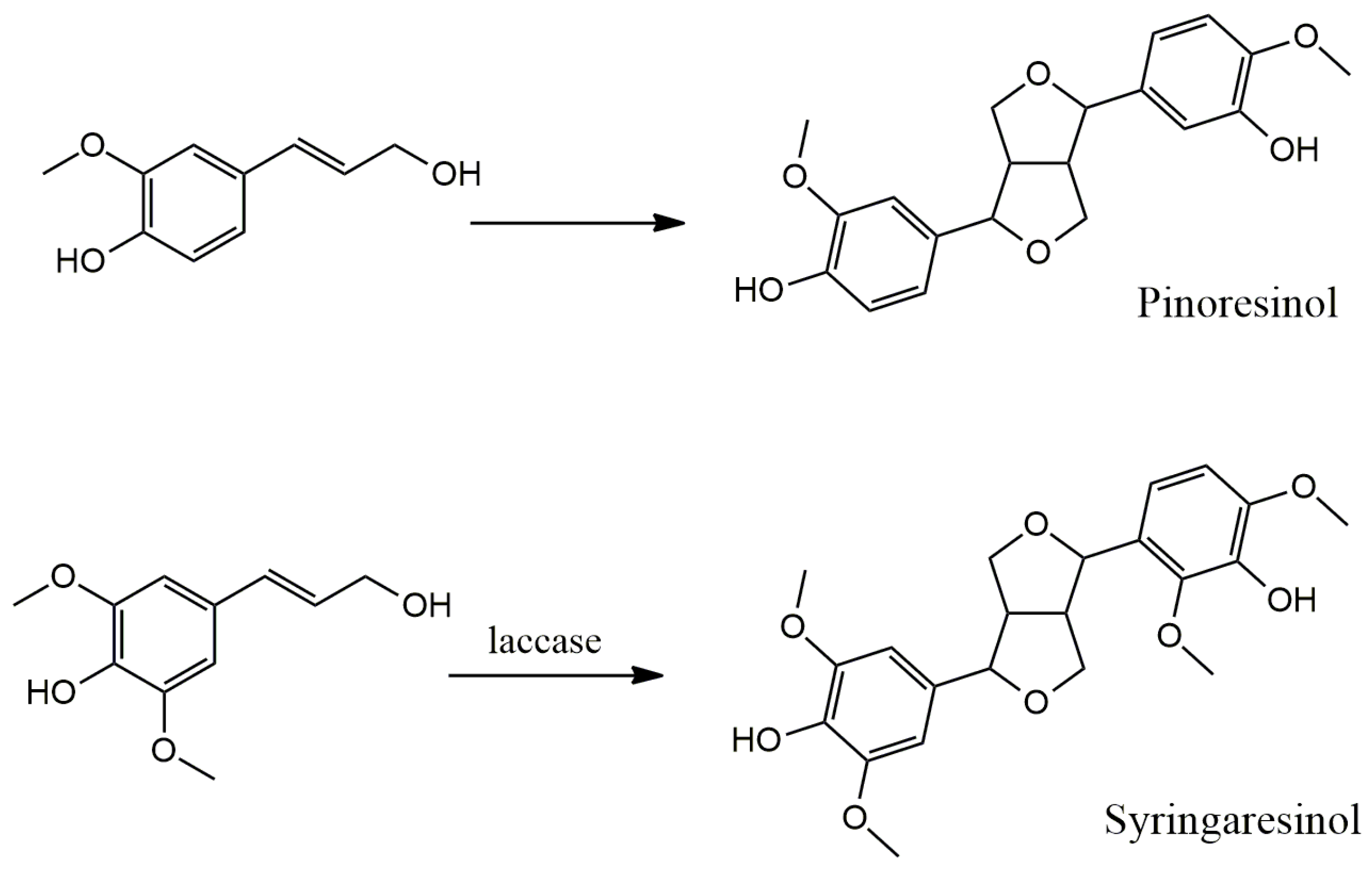
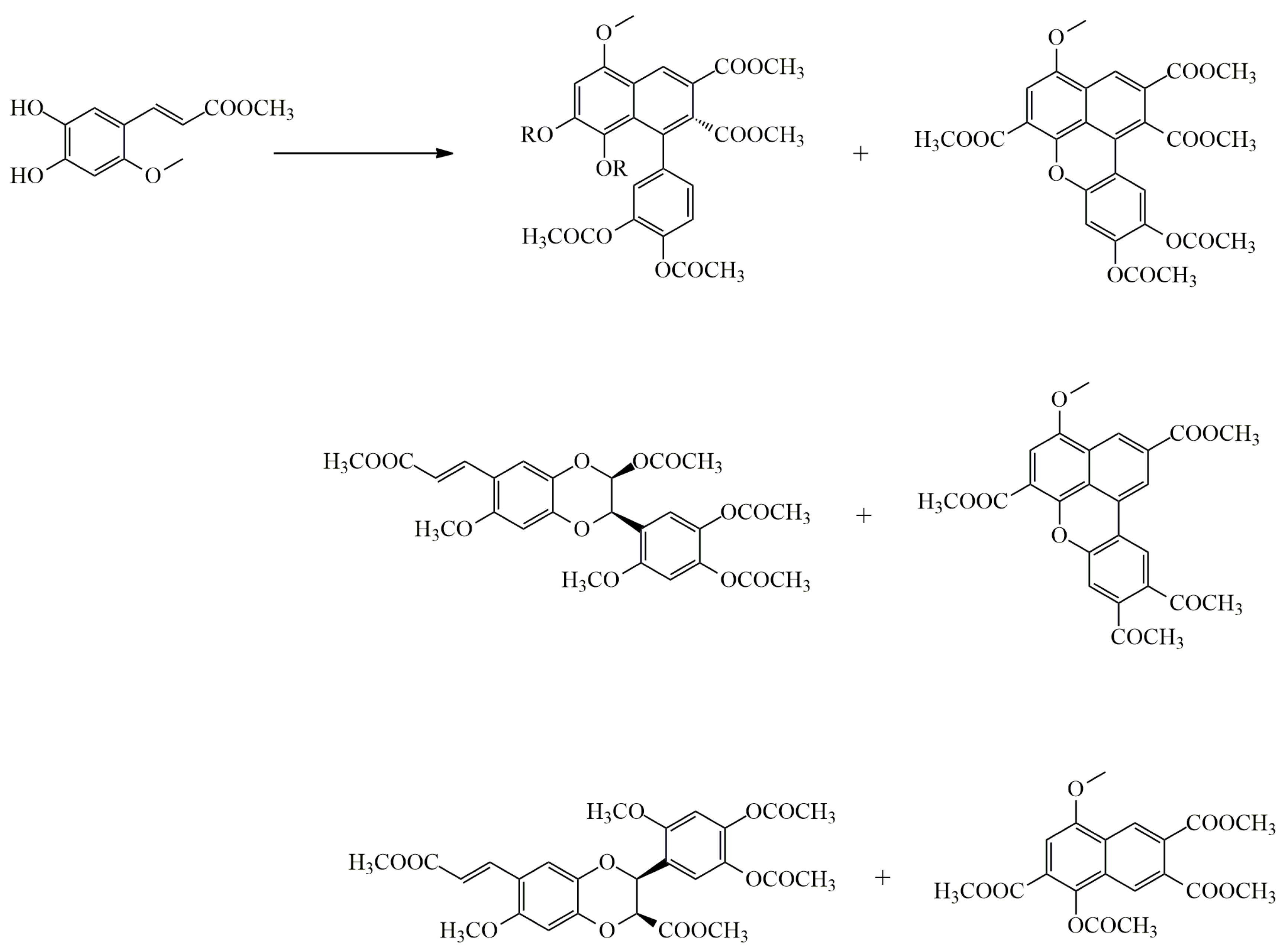
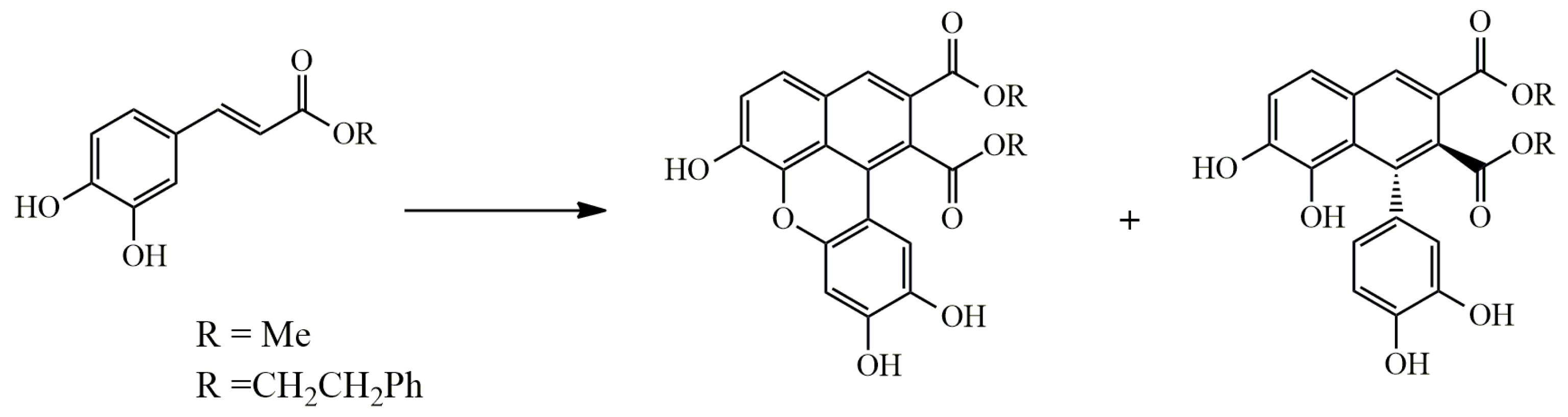
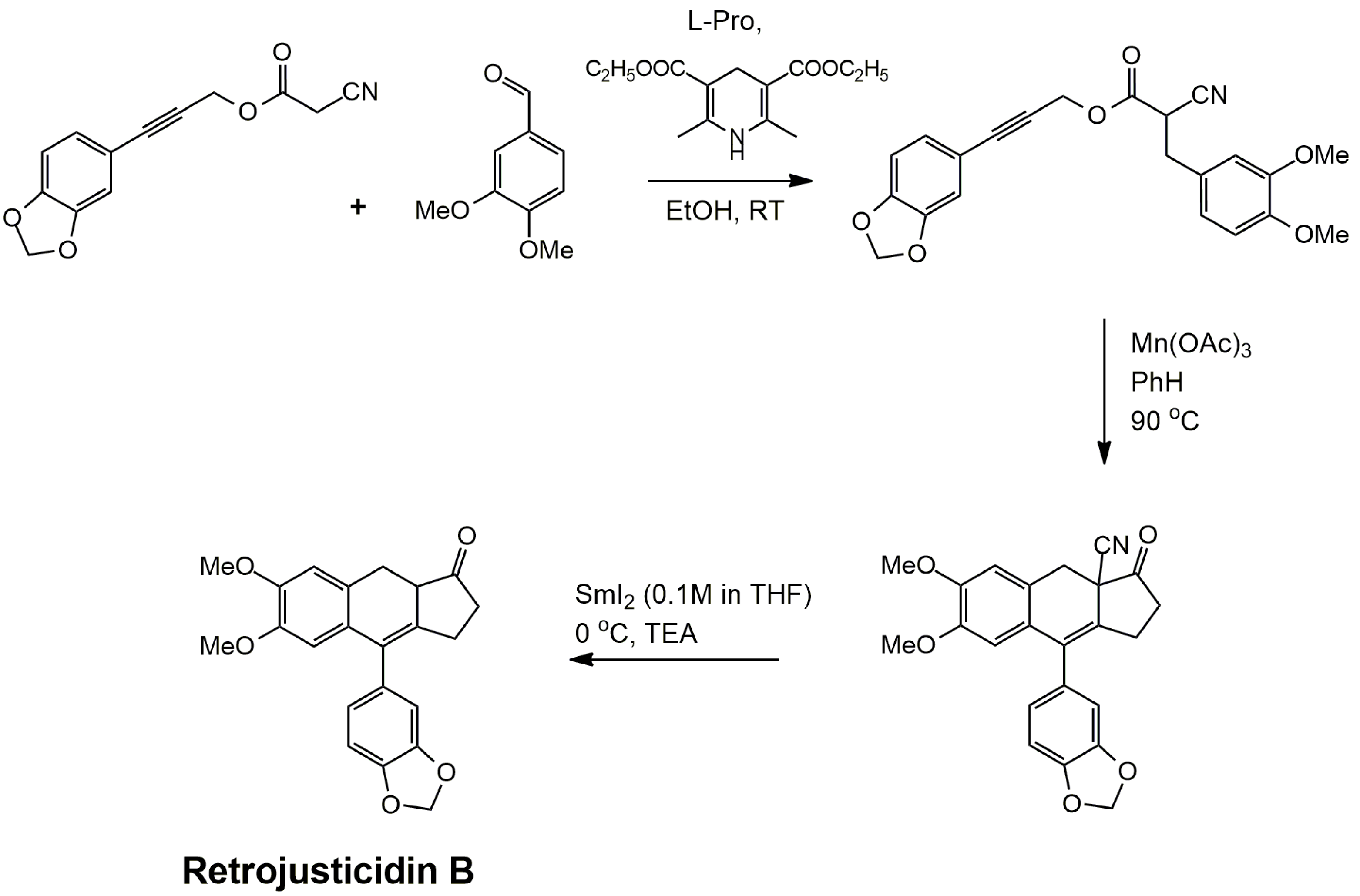
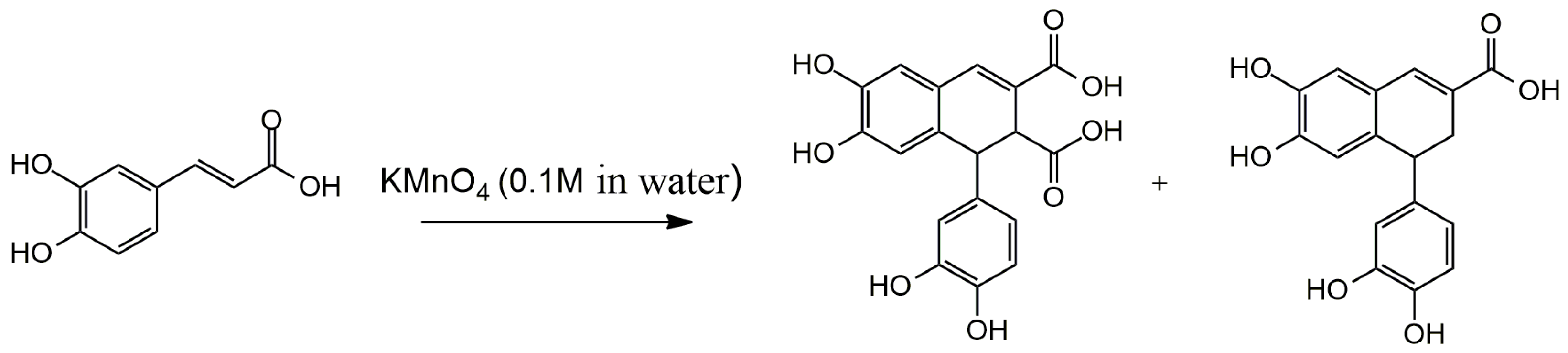
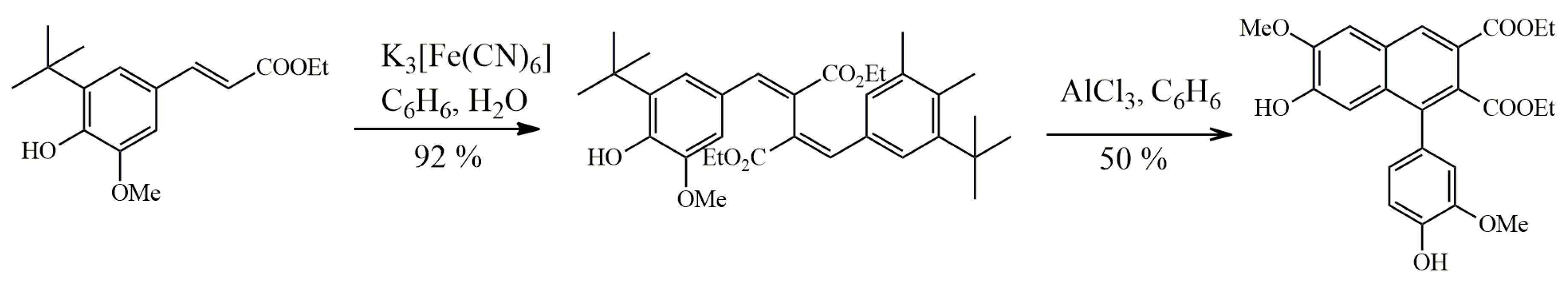
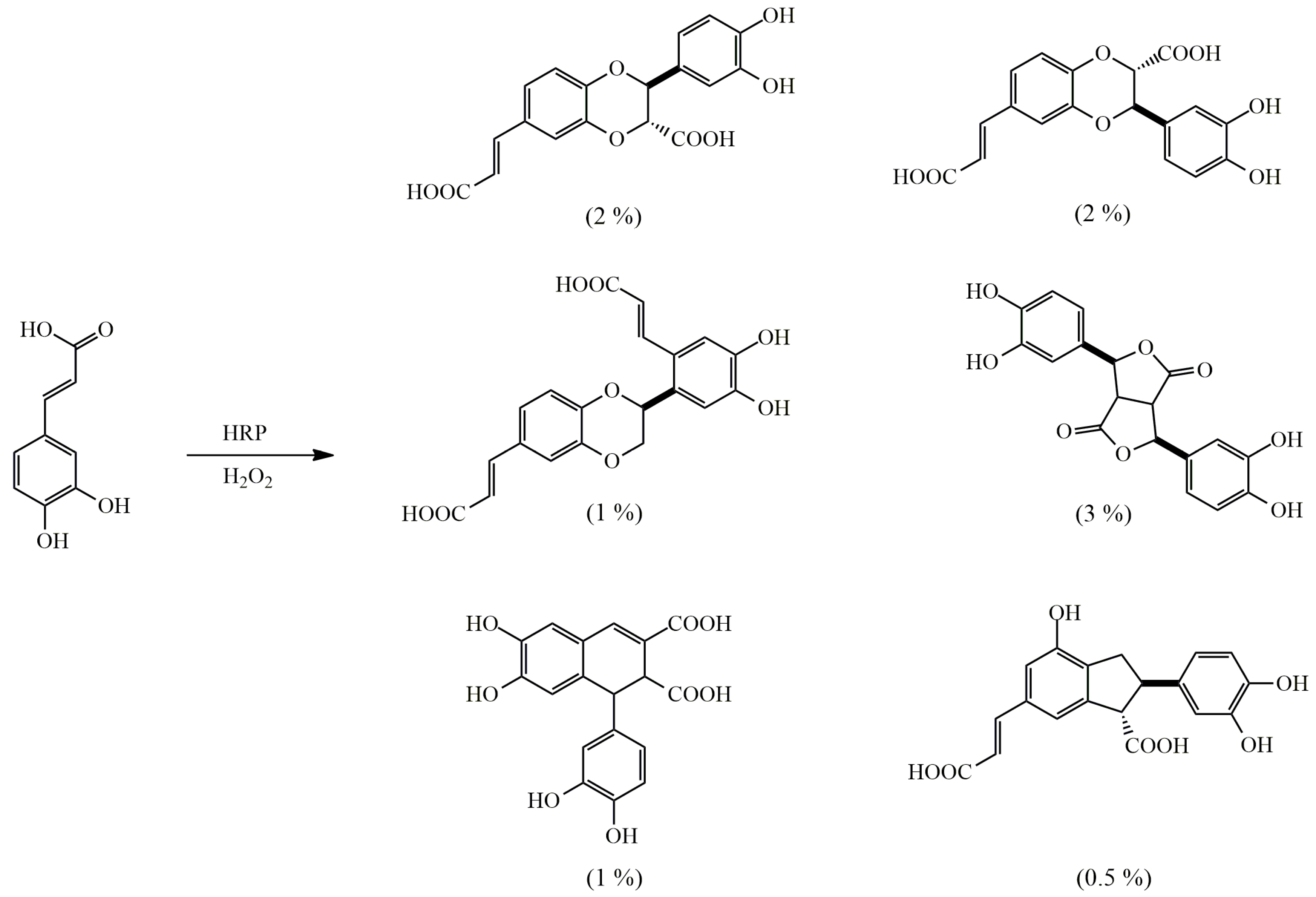
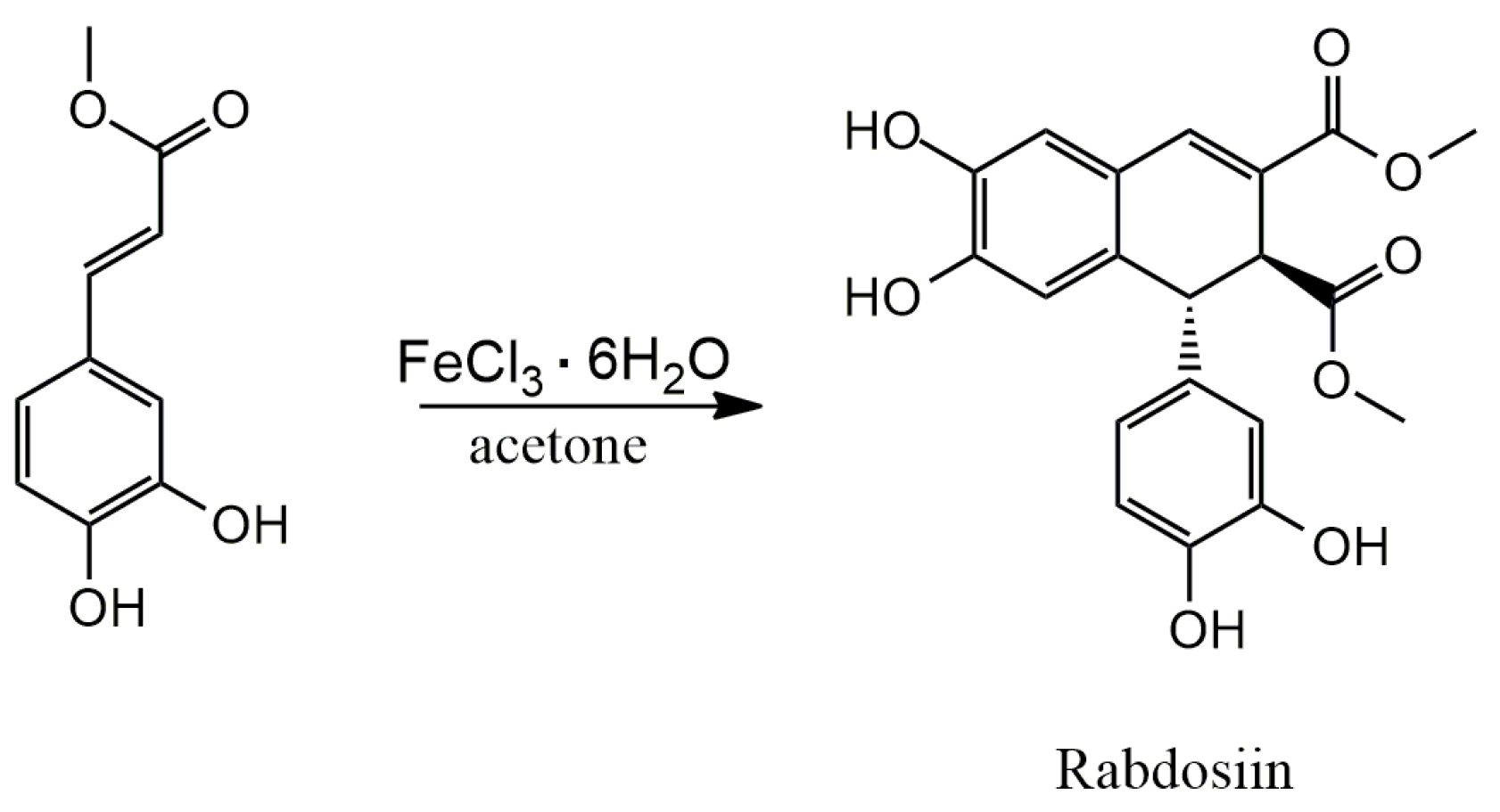
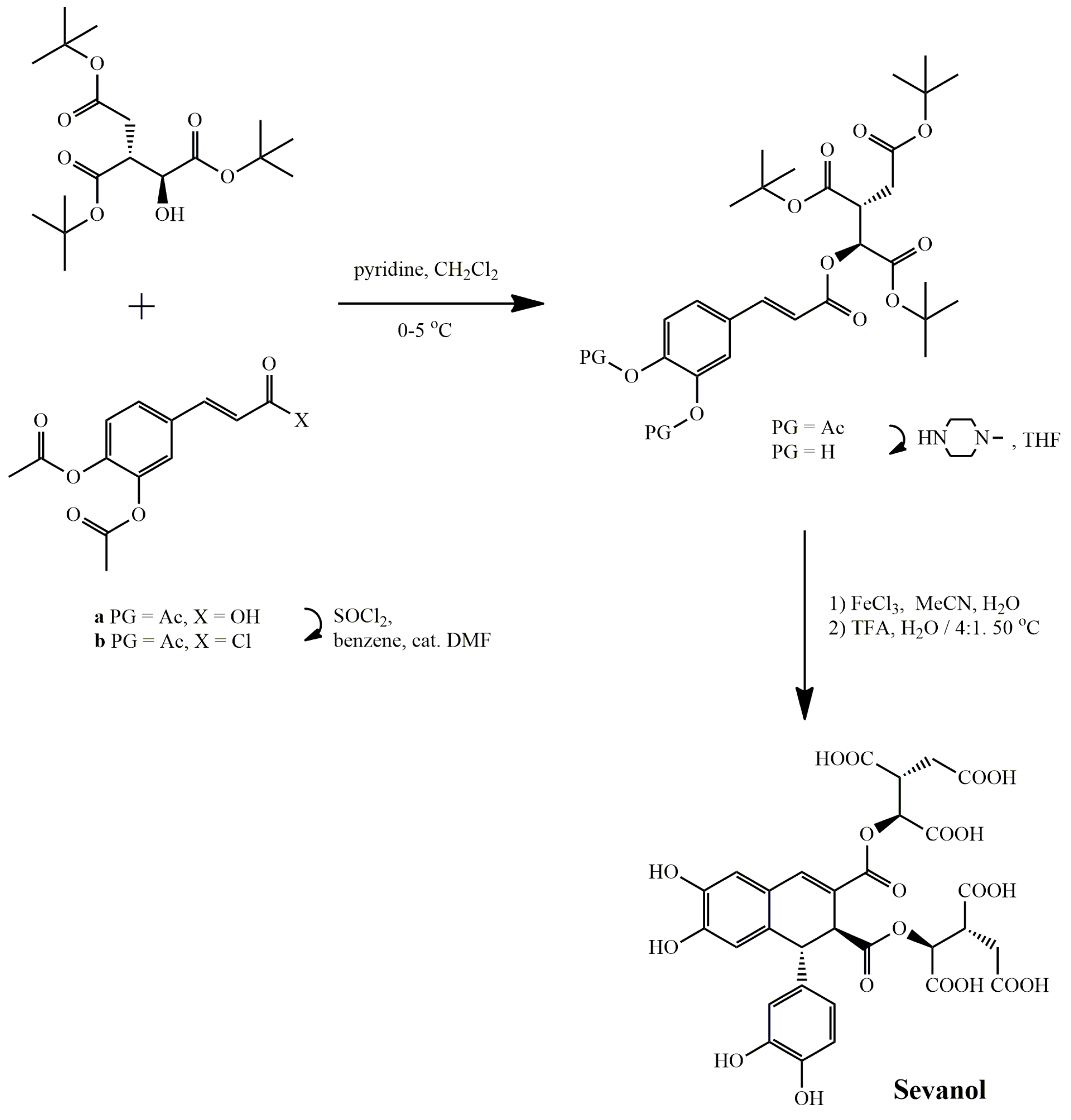

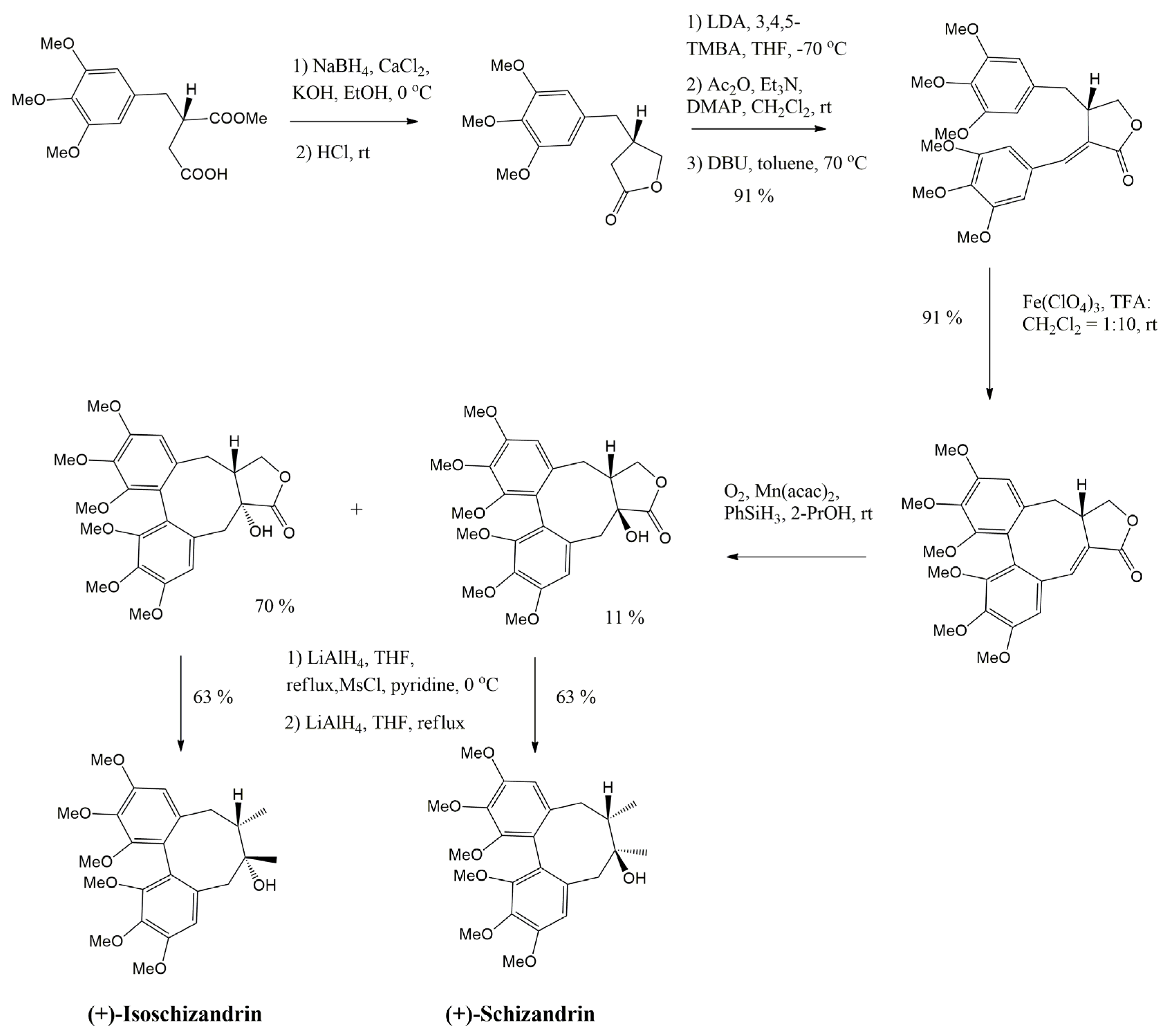
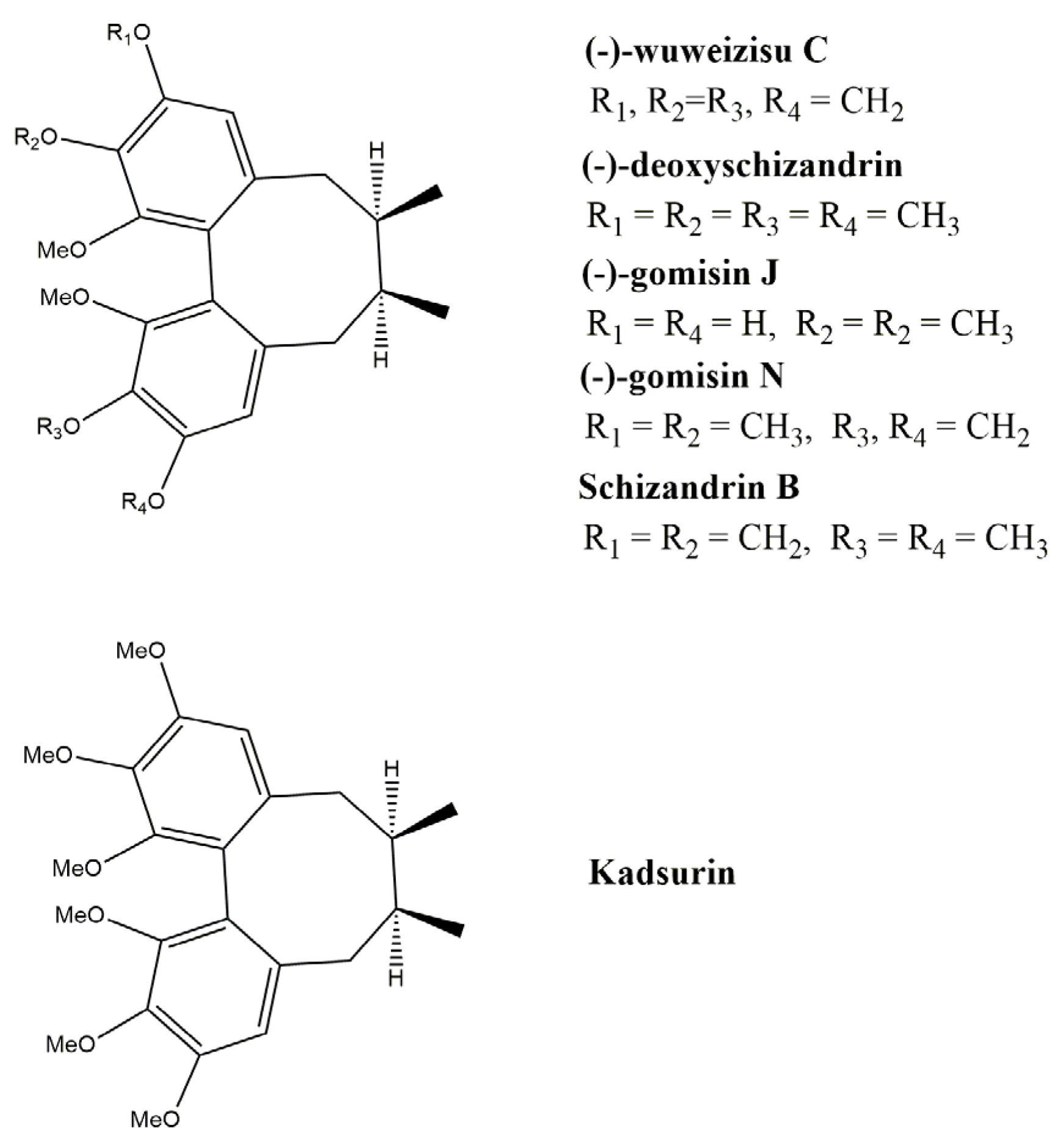
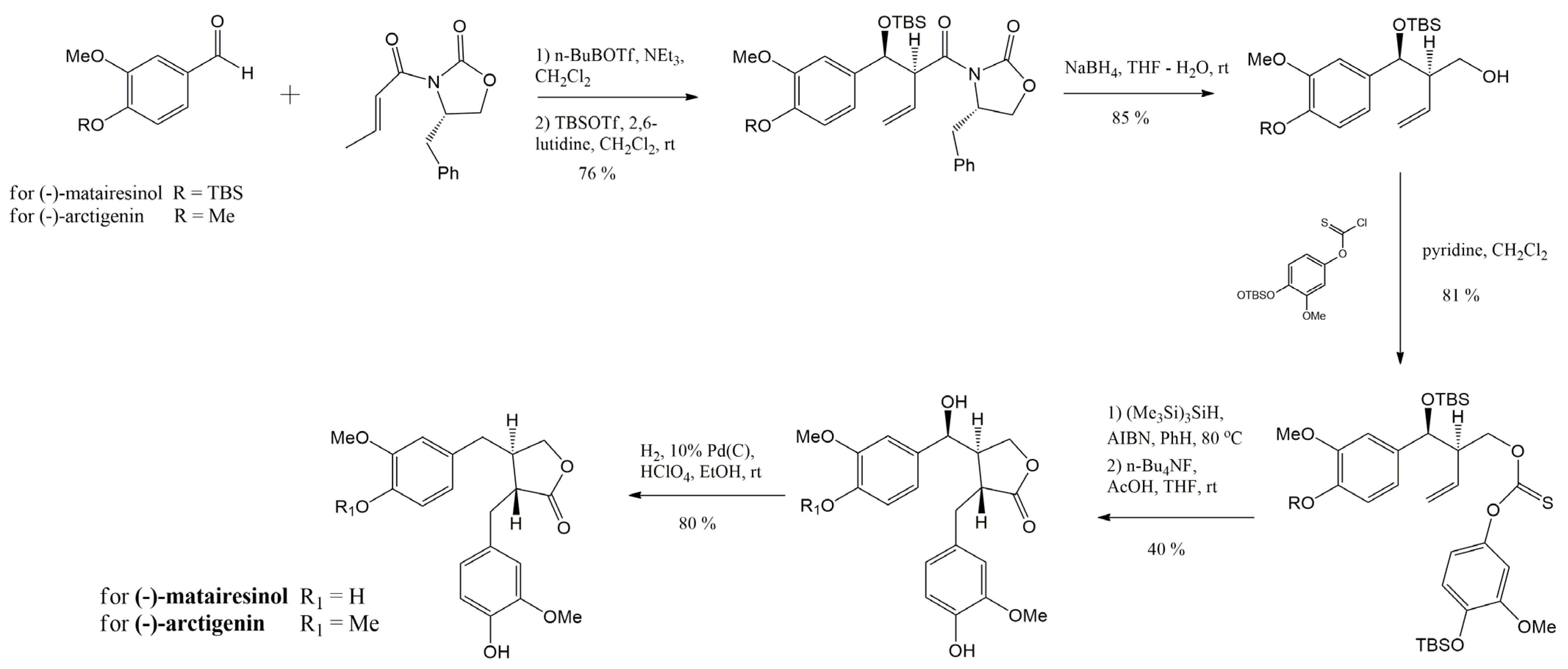
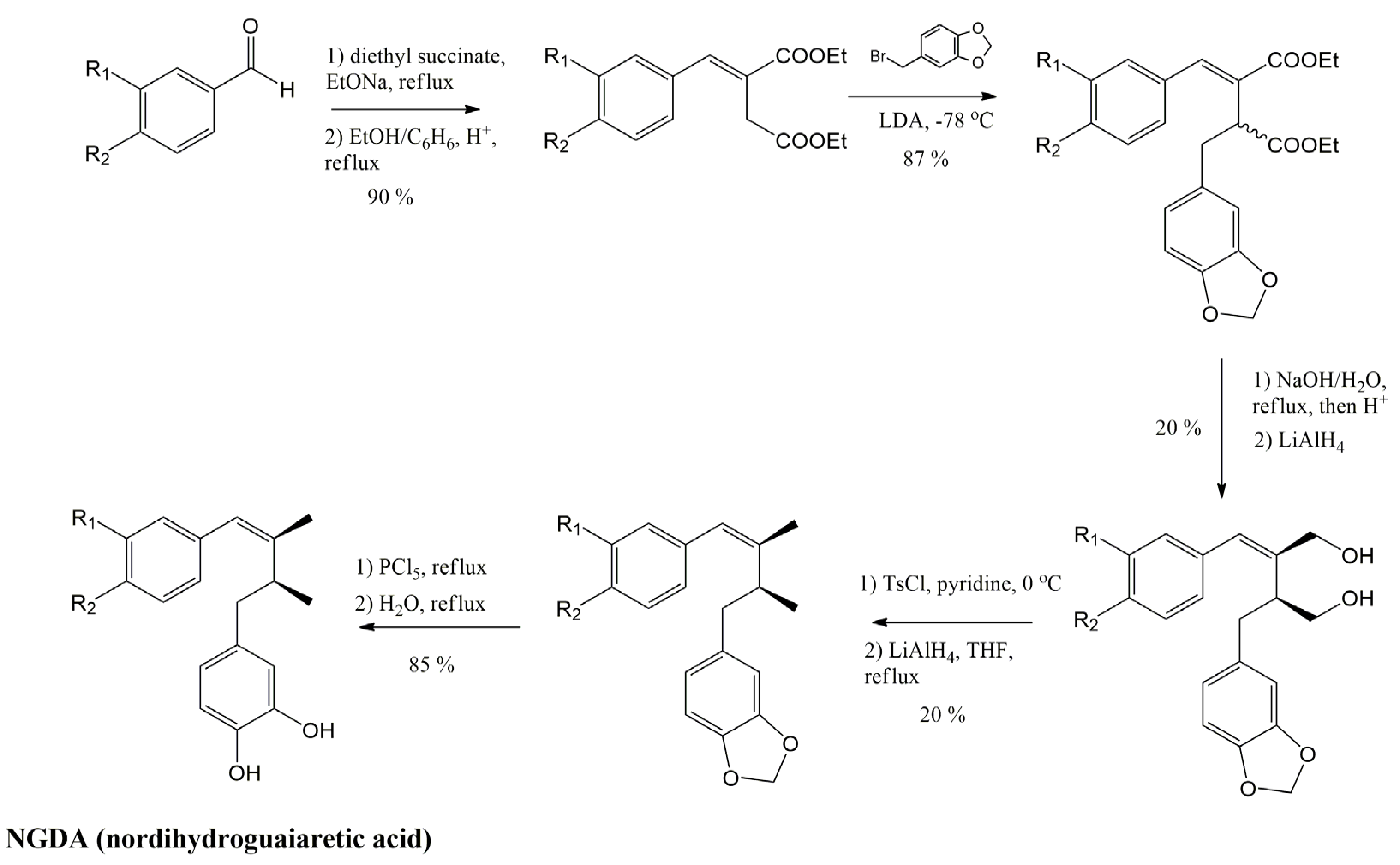
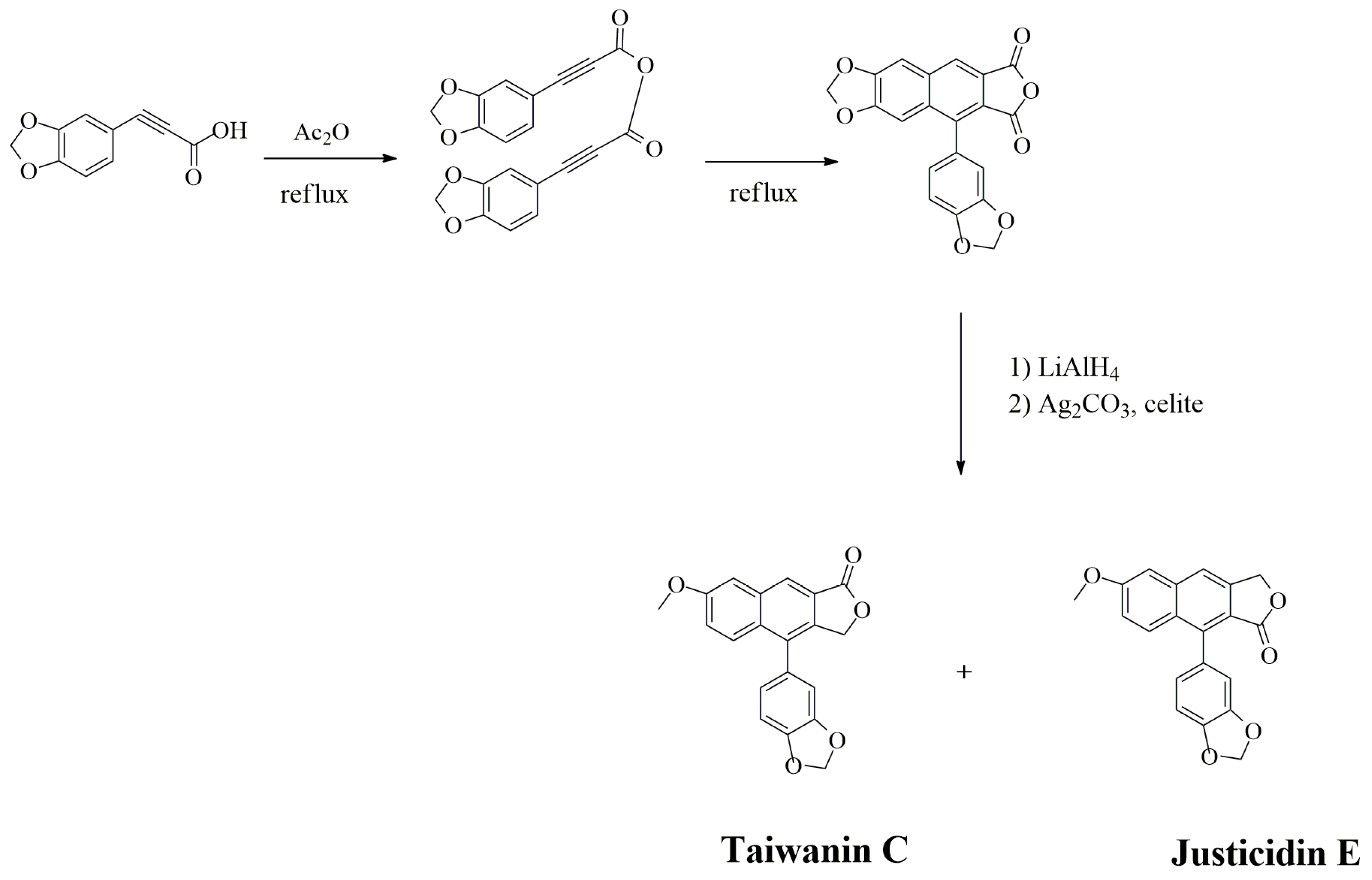
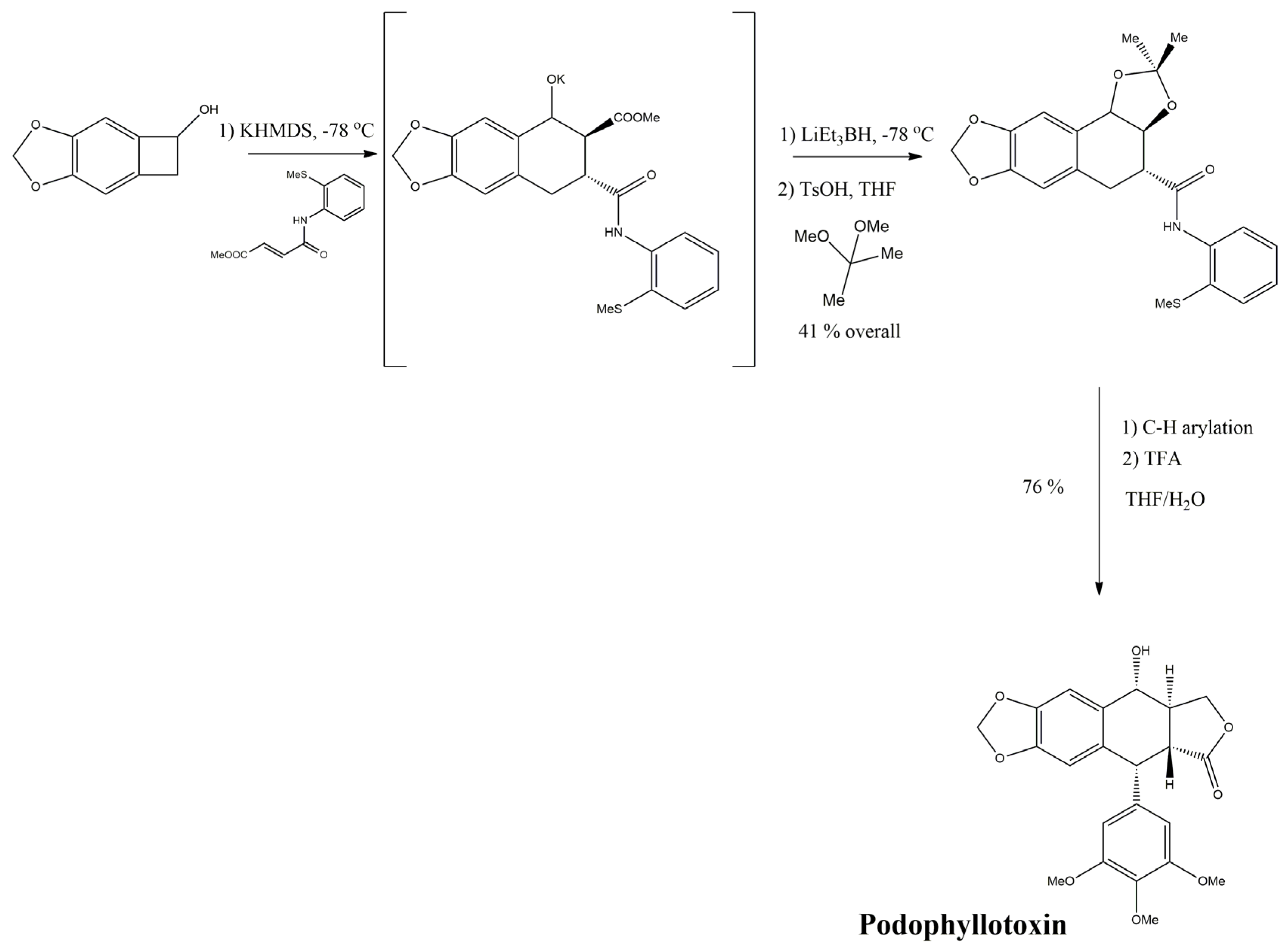
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. https://doi.org/10.3390/ijms23116031
Osmakov DI, Kalinovskii AP, Belozerova OA, Andreev YA, Kozlov SA. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. International Journal of Molecular Sciences. 2022; 23(11):6031. https://doi.org/10.3390/ijms23116031
Chicago/Turabian StyleOsmakov, Dmitry I., Aleksandr P. Kalinovskii, Olga A. Belozerova, Yaroslav A. Andreev, and Sergey A. Kozlov. 2022. "Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities" International Journal of Molecular Sciences 23, no. 11: 6031. https://doi.org/10.3390/ijms23116031
APA StyleOsmakov, D. I., Kalinovskii, A. P., Belozerova, O. A., Andreev, Y. A., & Kozlov, S. A. (2022). Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. International Journal of Molecular Sciences, 23(11), 6031. https://doi.org/10.3390/ijms23116031






