Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling
Abstract
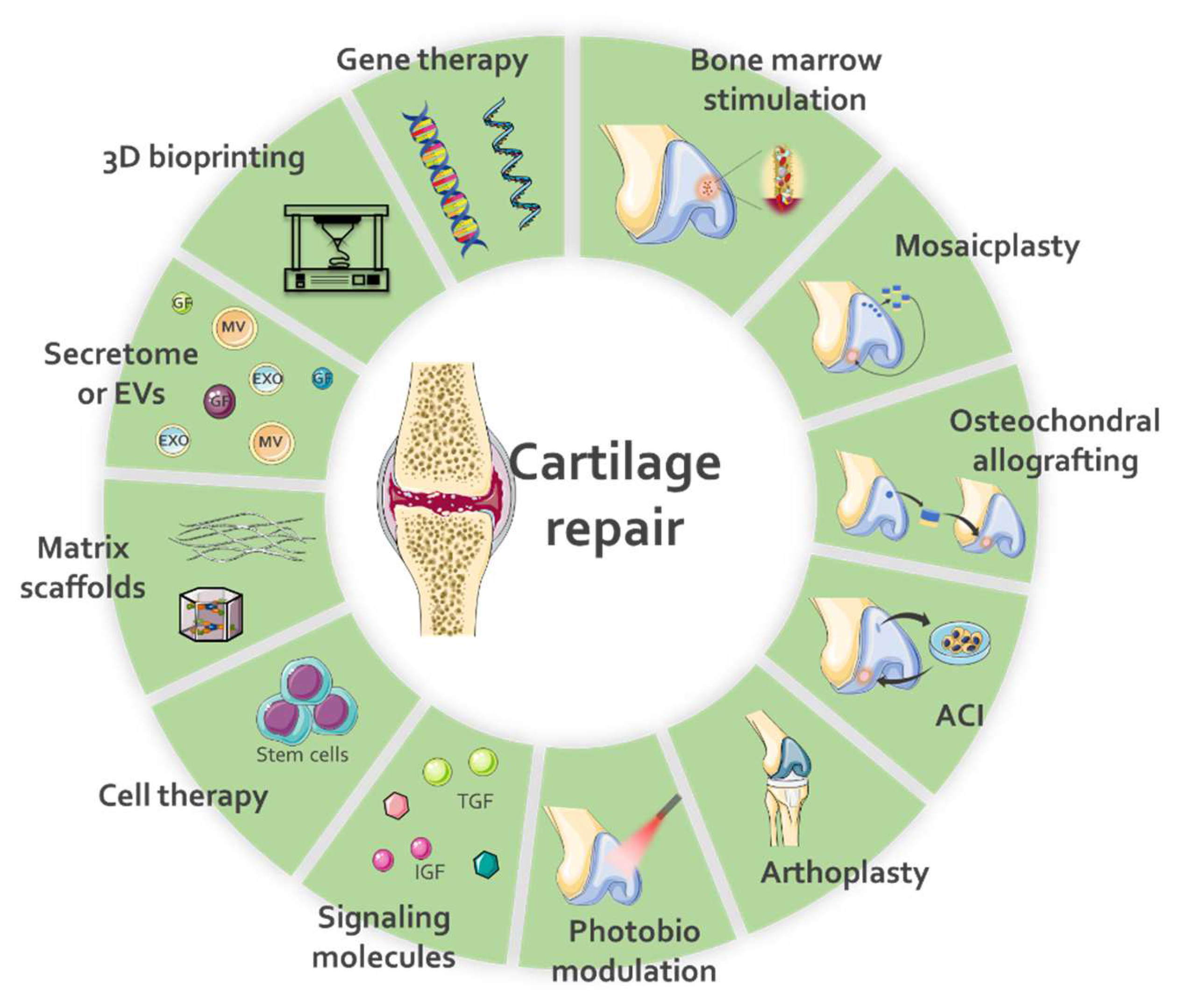
1. Introduction
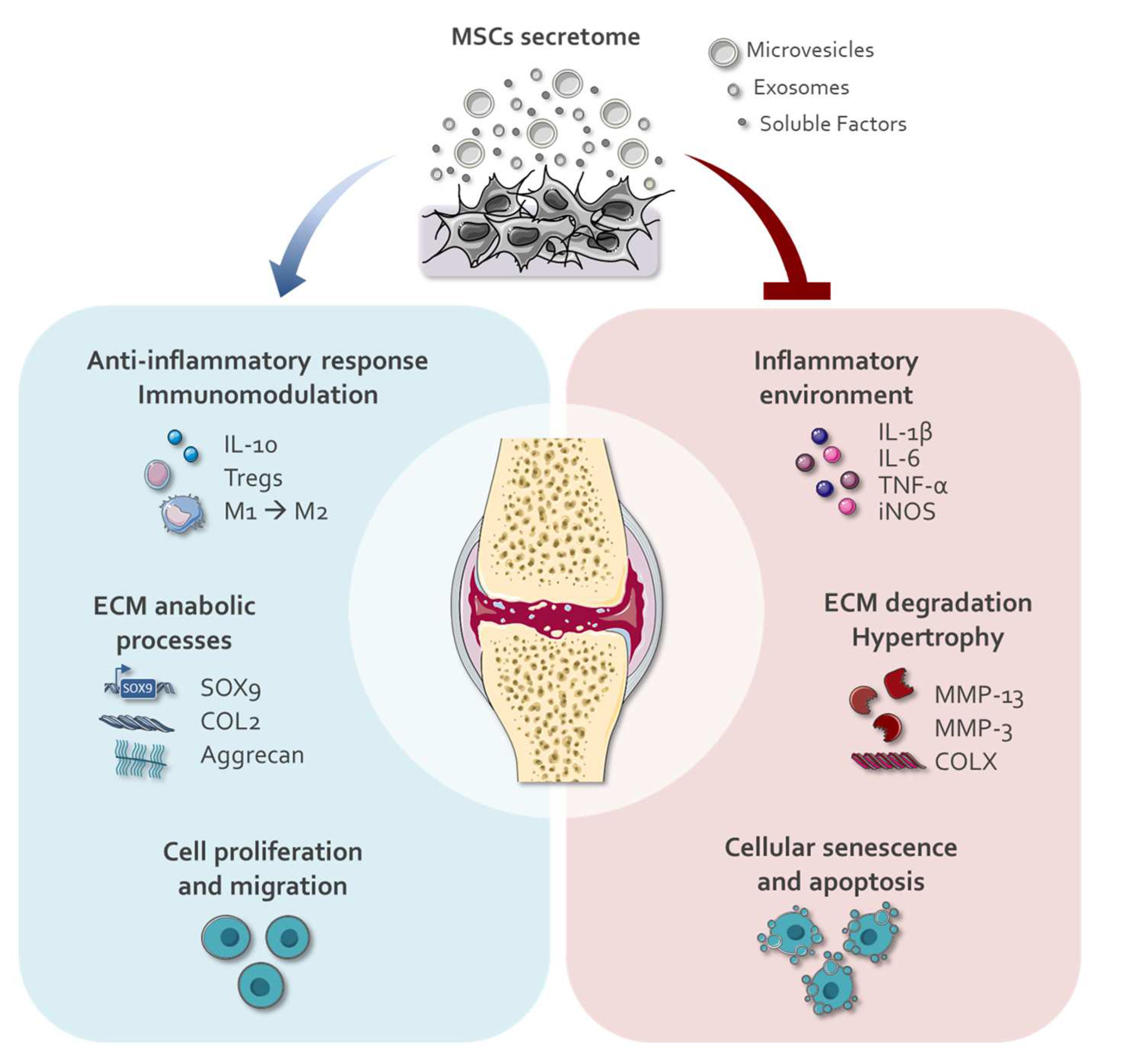
2. Application of Secretomes for Cartilage Repair
2.1. Modulation of Secretomes
3. Hydrogel and Cartilage Regeneration
| Hydrogel Composition | GF/EXO/EV | Delivery System * | Release Time | Ref. |
|---|---|---|---|---|
| Thiolated chitosan + carboxymethyl cellulose | TGF-β1 | Scaffold | 21 days | [125] |
| Sulfated carboxymethyl cellulose + carboxymethyl cellulose + gelatin | TGF-β1 | Scaffold | 30 days | [126] |
| Alginate-poly(acrylamide) | TGF-β3 | Poly(lactide-co-glycolide) nanoparticle | 60 days | [127] |
| Oligo (poly(ethylene glycol) fumarate) | TGF-β1 | Gelatin microparticles | 28 days | [128] |
| Thiolated gelatin + poly(ethylene glycol) diacrylate | IGF-1 | Poly(ethylene adipate)/ heparin coacervates | 21 days | [129] |
| Silk fibroin hydrogel | TGF-β1 and BMP-2 | Chitosan nanoparticles (TGF-β1); Scaffold (BMP-2) | Up to 15 days (both) | [130] |
| Silk fibroin hydrogel | MGF and TGF-β3 | Scaffold | 28 days (both) | [131] |
| Aldehyde-functionalized chondroitin sulfate (OCS) + gelatin methacryloyl (GM) | EXO from BM-MSCs | Scaffold | 14 days | [132] |
| dECM + gelatin methacrylate | EXO from BM-MSCs | Scaffold | 14 days | [133] |
| dECM | EXO from ASCs | Scaffold | 28 days | [134] |
| O-nitrobenzyl alcohol moieties modified hyaluronic acids (HA-NB) + gelatin | EXO from hiPSCs-MSC | Scaffold | 14 days | [135] |
| Poloxamer-407 and 188 mixture | PRP-EXO | Scaffold | 1 month | [136] |
| Poly(D,L-lactide)-b-poly(ethylene glycol)-b-poly(D,L-lactide) (PDLLA-PEG-PDLLA, PLEL) | Small EVs (circRNA3503) from Synovium MSCs | Scaffold | up to 35 days | [137] |
| Gelatin methacrylate (Gelma) + nanoclay | Small EV from hUMSC | Scaffold | 31 days | [138] |
3.1. Growth-Factor-Functionalized Hydrogels for Cartilage Repair
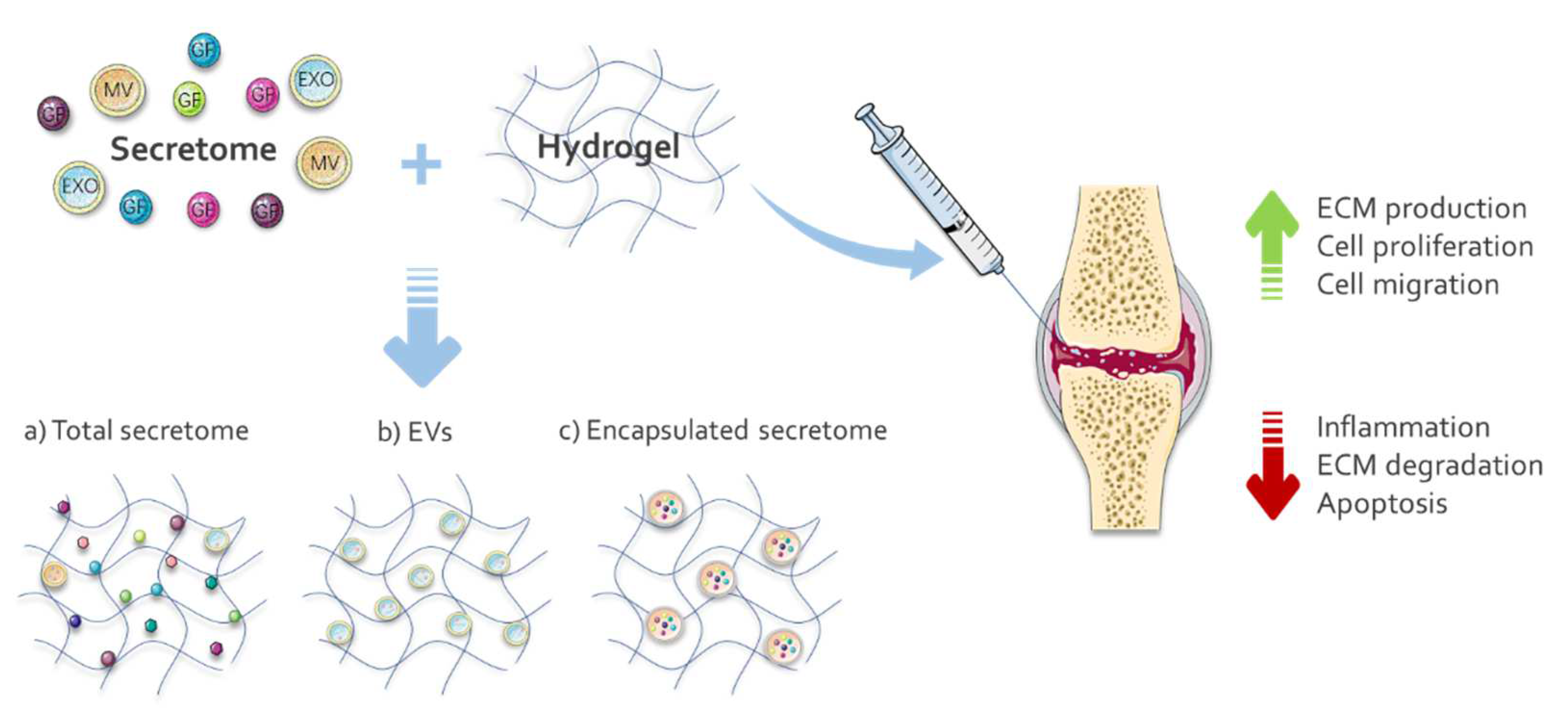
3.2. Secretome-Functionalized Hydrogels for Cartilage Repair
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage Diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 1328. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bironaite, D.; Bernotas, P.; Sobolev, A.; Bernotiene, E. Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation in Vitro. Int. J. Mol. Sci. 2021, 22, 9690. [Google Scholar] [CrossRef] [PubMed]
- Pap, T.; Korb-Pap, A. Cartilage Damage in Osteoarthritis and Rheumatoid Arthritis—Two Unequal Siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.P.A.; Smit, T.H. Osteoarthritis and Intervertebral Disc Degeneration: Quite Different, Quite Similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef]
- Chilelli, B.J.; Cole, B.J.; Farr, J.; Lattermann, C.; Gomoll, A.H. The Four Most Common Types of Knee Cartilage Damage Encountered in Practice: How and Why Orthopaedic Surgeons Manage Them. Instr. Course Lect. 2017, 66, 507–530. [Google Scholar]
- Song, H.J.; Seo, H.J.; Kim, D. Effectiveness of High-Intensity Laser Therapy in the Management of Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Back Musculoskelet. Rehabil. 2020, 33, 875–884. [Google Scholar] [CrossRef]
- Rayegani, S.M.; Raeissadat, S.A.; Heidari, S.; Moradi-Joo, M. Safety and Effectiveness of Low-Level Laser Therapy in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. J. Lasers Med. Sci. 2017, 8, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Deng, H.; Cheng, K.; Liu, H.; Lin, L.; Qu, X.; Liu, S.; Shen, X. Laser Photobiomodulation for Cartilage Defect in Animal Models of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2020, 35, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Akaltun, M.S.; Altindag, O.; Turan, N.; Gursoy, S.; Gur, A. Efficacy of High Intensity Laser Therapy in Knee Osteoarthritis: A Double-Blind Controlled Randomized Study. Clin. Rheumatol. 2021, 40, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.P.D.O.; Dos Santos, F.F.; Costa, T.E.D.; Lacerda, A.C.R.; Dos Santos, J.M.; Costa, K.B.; Santos, A.P.; Gaiad, T.P.; Pinfildi, C.E.; Rocha-Vieira, E.; et al. Photobiomodulation Therapy (Light-Emitting Diode 630 Nm) Favored the Oxidative Stress and the Preservation of Articular Cartilage in an Induced Knee Osteoarthritis Model. Photobiomodul. Photomed. Laser Surg. 2021, 39, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kheshie, A.R.; Alayat, M.S.M.; Ali, M.M.E. High-Intensity versus Low-Level Laser Therapy in the Treatment of Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Lasers Med. Sci. 2014, 29, 1371–1376. [Google Scholar] [CrossRef]
- da Rosa, A.S.; dos Santos, A.F.; da Silva, M.M.; Facco, G.G.; Perreira, D.M.; Alves, A.C.A.; Leal, E.C.P., Jr.; de Carvalho, P.D.T.C. Effects of Low-Level Laser Therapy at Wavelengths of 660 and 808 Nm in Experimental Model of Osteoarthritis. Photochem. Photobiol. 2012, 88, 161–166. [Google Scholar] [CrossRef]
- Alves, A.C.A.; Vieira, R.D.P.; Leal, E.C.P., Jr.; dos Santos, S.A.; Ligeiro, A.P.; Albertini, R.; Silva, J.A., Jr.; De Carvalho, P.D.T.C. Effect of Low-Level Laser Therapy on the Expression of Inflammatory Mediators and on Neutrophils and Macrophages in Acute Joint Inflammation. Arthritis Res. Ther. 2013, 15, R116. [Google Scholar] [CrossRef]
- Felizatti, A.L.; do Bomfim, F.R.C.; Bovo, J.L.; de Aro, A.A.; do Amaral, M.E.C.; Esquisatto, M.A.M. Effects of Low-Level Laser Therapy on the Organization of Articular Cartilage in an Experimental Microcrystalline Arthritis Model. Lasers Med. Sci. 2019, 34, 1401–1412. [Google Scholar] [CrossRef]
- Vassão, P.G.; de Souza, A.C.F.; da Silveira, C.R.M.; Garcia, L.A.; Tucci, H.T.; Renno, A.C.M. Effects of Photobiomodulation and a Physical Exercise Program on the Expression of Inflammatory and Cartilage Degradation Biomarkers and Functional Capacity in Women with Knee Osteoarthritis: A Randomized Blinded Study. Adv. Rheumatol. 2021, 61, 62. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An Educational Review of Cartilage Repair: Precepts & Practice—Myths & Misconceptions—Progress & Prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Chahla, J.; LaPrade, R.F.; Pascual-Garrido, C. Biologic Options for Articular Cartilage Wear (Platelet-Rich Plasma, Stem Cells, Bone Marrow Aspirate Concentrate). Clin. Sports Med. 2017, 36, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and Tissue Engineering Strategies for Articular Cartilage and Meniscus Repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Hulme, C.H.; Perry, J.; McCarthy, H.S.; Wright, K.T.; Snow, M.; Mennan, C.; Roberts, S. Cell Therapy for Cartilage Repair. Emerg. Top. Life Sci. 2021, 5, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced Hydrogels for the Repair of Cartilage Defects and Regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Jiang, S.; Tian, G.; Li, X.; Yang, Z.; Wang, F.; Tian, Z.; Huang, B.; Wei, F.; Zha, K.; Sun, Z.; et al. Research Progress on Stem Cell Therapies for Articular Cartilage Regeneration. Stem Cells Int. 2021, 2021, 8882505. [Google Scholar] [CrossRef]
- Xiang, X.N.; Zhu, S.Y.; He, H.C.; Yu, X.; Xu, Y.; He, C.Q. Mesenchymal Stromal Cell-Based Therapy for Cartilage Regeneration in Knee Osteoarthritis. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory Effects of Mesenchymal Stem Cell–Derived Exosomes on Experimental Type-1 Autoimmune Diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef]
- Xia, X.; Chiu, P.W.Y.; Lam, P.K.; Chin, W.C.; Ng, E.K.W.; Lau, J.Y.W. Secretome from Hypoxia-Conditioned Adipose-Derived Mesenchymal Stem Cells Promotes the Healing of Gastric Mucosal Injury in a Rodent Model. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Gomes, F.A.; Rode, M.P.; da Silva, M.M.; Veleirinho, M.B.R.; Maraschin, M.; Hayashi, L.; Calloni, G.W.; Stimamiglio, M.A. The Skin Regeneration Potential of a Pro-Angiogenic Secretome from Human Skin-Derived Multipotent Stromal Cells. J. Tissue Eng. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The Mesenchymal Stem Cell Secretome: A New Paradigm towards Cell-Free Therapeutic Mode in Regenerative Medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Mesenchymal Stem Cell Secretome: Cell-Free Therapeutic Strategy in Regenerative Medicine. Indones. Biomed. J. 2019, 11, 113–124. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Hafezi, M.; Khorasani, S.N.; Zare, M.; Neisiany, R.E.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Ren, Y.; Qiang, L.; Liu, Y.; Wang, J.; Dai, K. 3D Printed Hydrogel for Articular Cartilage Regeneration. Compos. Part B Eng. 2022, 237, 109863. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef]
- Cuccia, N.L.; Pothineni, S.; Wu, B.; Harper, J.M.; Burton, J.C. Pore-Size Dependence and Slow Relaxation of Hydrogel Friction on Smooth Surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 11247–11256. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent Advances in Hydrogels for Cartilage Tissue Engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of Mesenchymal Stem Cells and Bioactive Molecules in Hydrogels for Osteoarthritis Treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ye, J.; Yuan, F.-Z.; Zhang, J.-Y.; Chen, Y.-R.; Fan, B.-S.; Jiang, D.; Jiang, W.-B.; Wang, X.; Yu, J.-K. Advances of Stem Cell-Laden Hydrogels With Biomimetic Microenvironment for Osteochondral Repair. Front. Bioeng. Biotechnol. 2020, 8, 247. [Google Scholar] [CrossRef]
- Kuroda, K.; Kabata, T.; Hayashi, K.; Maeda, T.; Kajino, Y.; Iwai, S.; Fujita, K.; Hasegawa, K.; Inoue, D.; Sugimoto, N.; et al. The Paracrine Effect of Adipose-Derived Stem Cells Inhibits Osteoarthritis Progression. BMC Musculoskelet. Disord. 2015, 16, 236. [Google Scholar] [CrossRef]
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-Derived Mesenchymal Stem Cells Exert Antiinflammatory Effects on Chondrocytes and Synoviocytes from Osteoarthritis Patients through Prostaglandin E2. Arthritis Rheum. 2013, 65, 1271–1281. [Google Scholar] [CrossRef]
- Platas, J.; Guillén, M.I.; Del Caz, M.D.P.; Gomar, F.; Mirabet, V.; Alcaraz, M.J. Conditioned Media from Adipose-Tissue-Derived Mesenchymal Stem Cells Downregulate Degradative Mediators Induced by Interleukin-1β in Osteoarthritic Chondrocytes. Mediat. Inflamm. 2013, 2013, 357014. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Gomarasca, M.; Stanco, D.; Casati, S.; Brini, A.T. Adipose-Derived Stromal Cell Secretome Reduces TNFα-Induced Hypertrophy and Catabolic Markers in Primary Human Articular Chondrocytes. Stem Cell Res. 2019, 38, 101463. [Google Scholar] [CrossRef]
- Platas, J.; Guillén, M.I.; del Caz, M.D.P.; Gomar, F.; Castejón, M.A.; Mirabet, V.; Alcaraz, M.J. Paracrine Effects of Human Adipose-Derived Mesenchymal Stem Cells in Inflammatory Stress-Induced Senescence Features of Osteoarthritic Chondrocytes. Aging 2016, 8, 1703–1717. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, Y.W.; Tan, K.P.; Shen, Y.S.; Wang, Y.H.; Chang, C.H. Can Mesenchymal Stem Cells and Their Conditioned Medium Assist Inflammatory Chondrocytes Recovery? PLoS ONE 2018, 13, e0205563. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Kasim, N.H.A.; Fakurazi, S. Protective Effects of Stem Cells from Human Exfoliated Deciduous Teeth Derived Conditioned Medium on Osteoarthritic Chondrocytes. PLoS ONE 2020, 15, e0238449. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, K.; Pang, Y.; Xiang, F.; Gao, J.; Zhang, C.; Du, D. Anti-Hypertrophic Effect of Synovium-Derived Stromal Cells on Costal Chondrocytes Promotes Cartilage Repairs. J. Orthop. Transl. 2021, 32, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Hsu, C.C.; Hsu, S.L.; Chou, W.Y.; Wu, Y.N.; Kuo, C.E.A.; Hsu, T.C.; Shiu, L.Y.; Jhan, S.W. Adipose-Derived Mesenchymal Stem Cells-Conditioned Medium Modulates the Expression of Inflammation Induced Bone Morphogenetic Protein-2, -5 and -6 as Well as Compared with Shockwave Therapy on Rat Knee Osteoarthritis. Biomedicines 2021, 9, 1399. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Gu, X.; Hao, Y.; Liu, X.; Lin, J.; Chen, J.; Chen, S. Conditioned Medium of Mesenchymal Stem Cells Delays Osteoarthritis Progression in a Rat Model by Protecting Subchondral Bone, Maintaining Matrix Homeostasis, and Enhancing Autophagy. J. Tissue Eng. Regen. Med. 2019, 13, 1618–1628. [Google Scholar] [CrossRef]
- Kay, A.G.; Long, G.; Tyler, G.; Stefan, A.; Broadfoot, S.J.; Piccinini, A.M.; Middleton, J.; Kehoe, O. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci. Rep. 2017, 7, 18019. [Google Scholar] [CrossRef]
- Ogasawara, N.; Kano, F.; Hashimoto, N.; Mori, H.; Liu, Y.; Xia, L.; Sakamaki, T.; Hibi, H.; Iwamoto, T.; Tanaka, E.; et al. Factors Secreted from Dental Pulp Stem Cells Show Multifaceted Benefits for Treating Experimental Temporomandibular Joint Osteoarthritis. Osteoarthr. Cartil. 2020, 28, 831–841. [Google Scholar] [CrossRef]
- Giannasi, C.; Niada, S.; Della Morte, E.; Casati, S.; Orioli, M.; Gualerzi, A.; Brini, A.T. Towards Secretome Standardization: Identifying Key Ingredients of MSC-Derived Therapeutic Cocktail. Stem Cells Int. 2021, 2021, 3086122. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef]
- Youssef, A.; Aboalola, D.; Han, V.K.M. The Roles of Insulin-like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017, 2017, 9453108. [Google Scholar] [CrossRef]
- Zhen, G.; Cao, X. Targeting TGFβ Signaling in Subchondral Bone and Articular Cartilage Homeostasis. Trends Pharmacol. Sci. 2014, 35, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Ringe, J.; Häupl, T.; Notter, M.; Manz, R.; Burmester, G.R.; Sittinger, M.; Kaps, C. BMP2 Initiates Chondrogenic Lineage Development of Adult Human Mesenchymal Stem Cells in High-Density Culture. Differentiation 2003, 71, 567–577. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, D.H.; Ha, J.; Jin, H.J.; Kwon, S.J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; Kim, G.; et al. Thrombospondin-2 Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Promotes Chondrogenic Differentiation. Stem Cells 2013, 31, 2136–2148. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal Stem Cells Inhibit Both Endogenous and Exogenous MMPs via Secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose Mesenchymal Stem Cells Protect Chondrocytes from Degeneration Associated with Osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D. Secretion of Immunoregulatory Cytokines by Mesenchymal Stem Cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Maumus, M.; Manferdini, C.; Toupet, K.; Chuchana, P.; Casteilla, L.; Gachet, M.; Jorgensen, C.; Lisignoli, G.; Noël, D. Thrombospondin-1 Partly Mediates the Cartilage Protective Effect of Adipose-Derived Mesenchymal Stem Cells in Osteoarthritis. Front. Immunol. 2017, 8, 1638. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ Signaling in Cartilage Development and Maintenance. Birth Defects Res. C Embryo Today 2014, 102, 37–51. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.S.; Choi, N.Y.; Kim, Y.I.; Koh, Y.G. Proteomic Analysis Reveals Commonly Secreted Proteins of Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Synovial Membrane to Show Potential for Cartilage Regeneration in Knee Osteoarthritis. Stem Cells Int. 2021, 2021, 6694299. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Marbán, E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu. Rev. Physiol. 2016, 78, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Vian, M.; Guillén, M.I.; Del Caz, M.D.P.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of Peroxiredoxin 6 in the Chondroprotective Effects of Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Orthop. Transl. 2021, 30, 61–69. [Google Scholar] [CrossRef]
- Qi, H.; Liu, D.P.; Xiao, D.W.; Tian, D.C.; Su, Y.W.; Jin, S.F. Exosomes Derived from Mesenchymal Stem Cells Inhibit Mitochondrial Dysfunction-Induced Apoptosis of Chondrocytes via P38, ERK, and Akt Pathways. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 203–210. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Protect Cartilage Damage and Relieve Knee Osteoarthritis Pain in a Rat Model of Osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Ruiz, M.; Toupet, K.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noël, D. TGFBI Secreted by Mesenchymal Stromal Cells Ameliorates Osteoarthritis and Is Detected in Extracellular Vesicles. Biomaterials 2020, 226, 119544. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory Network of MiRNA on Its Target: Coordination between Transcriptional and Post-Transcriptional Regulation of Gene Expression. Cell. Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The Function of MicroRNAs in Cartilage and Osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 40–47. [Google Scholar] [PubMed]
- Sfikakis, P.P.; Vlachogiannis, N.I.; Christopoulos, P.F. Cadherin-11 as a Therapeutic Target in Chronic, Inflammatory Rheumatic Diseases. Clin. Immunol. 2017, 176, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, L.; Fang, X.; Zang, M. Exosome-Encapsulated MicroRNA-127-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Osteoarthritis Through Regulating CDH11-Mediated Wnt/β-Catenin Pathway. J. Pain Res. 2021, 14, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Song, G.; Guo, J.B.; Su, X.; Chen, Y.M.; Yang, Z.; Chen, P.J.; Wang, X.Q. Interactions Among LncRNA/CircRNA, MiRNA, and MRNA in Musculoskeletal Degenerative Diseases. Front. Cell Dev. Biol. 2021, 9, 753931. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Xu, Y.; Long, D.; Sun, H.; Li, H.; Xin, R.; Zhang, Z.; Li, Z.; Yang, Z.; Kang, Y. Exosome-Transported CircRNA_0001236 Enhances Chondrogenesis and Suppress Cartilage Degradation via the MiR-3677-3p/Sox9 Axis. Stem Cell Res. Ther. 2021, 12, 389. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.-J. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl. Med. 2016, 5, 816–825. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-Elicited Mesenchymal Stem Cell-Derived Exosomes Facilitates Cardiac Repair through MiR-125b-Mediated Prevention of Cell Death in Myocardial Infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Wong, S.W.; Lenzini, S.; Cooper, M.H.; Mooney, D.J.; Shin, J.W. Soft Extracellular Matrix Enhances Inflammatory Activation of Mesenchymal Stromal Cells to Induce Monocyte Production and Trafficking. Sci. Adv. 2020, 6, eaaw0158. [Google Scholar] [CrossRef]
- Wan, S.; Fu, X.; Ji, Y.; Li, M.; Shi, X.; Wang, Y. FAK- and YAP/TAZ Dependent Mechanotransduction Pathways Are Required for Enhanced Immunomodulatory Properties of Adipose-Derived Mesenchymal Stem Cells Induced by Aligned Fibrous Scaffolds. Biomaterials 2018, 171, 107–117. [Google Scholar] [CrossRef]
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous Scaffolds Potentiate the Paracrine Function of Mesenchymal Stem Cells: A New Dimension in Cell-Material Interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef]
- Gorin, C.; Rochefort, G.Y.; Bascetin, R.; Ying, H.; Lesieur, J.; Sadoine, J.; Beckouche, N.; Berndt, S.; Novais, A.; Lesage, M.; et al. Priming Dental Pulp Stem Cells With Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion. Stem Cells Transl. Med. 2016, 5, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Sivanathan, K.N.; Rojas-Canales, D.M.; Hope, C.M.; Krishnan, R.; Carroll, R.P.; Gronthos, S.; Grey, S.T.; Coates, P.T. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells 2015, 33, 2850–2863. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids Are Self-Activated to Produce Prostaglandin E2 That Directs Stimulated Macrophages into an Anti-Inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.J.; Miller, J.; Brown, H.; Allan, S.M.; Pinteaux, E. Changes in the Secretome of Tri-Dimensional Spheroid-Cultured Human Mesenchymal Stem Cells in Vitro by Interleukin-1 Priming. Stem Cell Res. Ther. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L. Adipose Tissue Oxygenation: Effects on Metabolic Function. Adipocyte 2014, 3, 75–80. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Silver, I.A. Measurement of PH and Ionic Composition of Pericellular Sites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1975, 271, 261–272. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Qu, Z.; Hao, J.; Zhang, W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes 2022, 12, 225. [Google Scholar] [CrossRef]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A Review of the Effects of Insulin-like Growth Factor and Platelet Derived Growth Factor on in Vivo Cartilage Healing and Repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Marlina, M.; Rahmadian, R.; Armenia, A.; Aviani, J.K.; Sholihah, I.A.; Kusuma, H.S.W.; Azizah, A.M.; Elida, N.; Widowati, W. Conditioned Medium of IGF1-Induced Synovial Membrane Mesenchymal Stem Cells Increases Chondrogenic and Chondroprotective Markers in Chondrocyte Inflammation. Biosci. Rep. 2021, 41, BSR20202038. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B. TGF-Β1-Modified MSC-Derived Exosomal MiR-135b Attenuates Cartilage Injury via Promoting M2 Synovial Macrophage Polarization by Targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef]
- van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; van Osch, G.J.V.M. Mesenchymal Stem Cells Secrete Factors That Inhibit Inflammatory Processes in Short-Term Osteoarthritic Synovium and Cartilage Explant Culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B.; et al. A Low Dose Cell Therapy System for Treating Osteoarthritis: In Vivo Study and in Vitro Mechanistic Investigations. Bioact. Mater. 2021, 7, 478–490. [Google Scholar] [CrossRef]
- Sahu, N.; Agarwal, P.; Grandi, F.; Bruschi, M.; Goodman, S.; Amanatullah, D.; Bhutani, N. Encapsulated Mesenchymal Stromal Cell Microbeads Promote Endogenous Regeneration of Osteoarthritic Cartilage Ex Vivo. Adv. Healthc. Mater. 2021, 10, e2002118. [Google Scholar] [CrossRef]
- Kadir, N.D.; Yang, Z.; Hassan, A.; Denslin, V.; Lee, E.H. Electrospun Fibers Enhanced the Paracrine Signaling of Mesenchymal Stem Cells for Cartilage Regeneration. Stem Cell Res. Ther. 2021, 12, 100. [Google Scholar] [CrossRef]
- Calloni, G.W.; Stimamiglio, M.A. Tuning Mesenchymal Stem Cell Secretome Therapeutic Potential through Mechanotransduction. Biocell 2022, 46, 1375–1381. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes Derived from MiR-126-3p-Overexpressing Synovial Fibroblasts Suppress Chondrocyte Inflammation and Cartilage Degradation in a Rat Model of Osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Xiong, J.; Zhu, W.; Liu, Q.; Wang, D.; Liu, W.; Li, Z.; Wang, D. E2 Regulates MMP-13 via Targeting MiR-140 in IL-1β-Induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res. Ther. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. The Role of Chondroitin Sulfate in Regulating Hypertrophy during MSC Chondrogenesis in a Cartilage Mimetic Hydrogel under Dynamic Loading. Biomaterials 2019, 190, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, S.; Eglin, D.; Pötter, N.; Ravari, M.K.; Stoddart, M.J.; Samadikuchaksaraei, A.; Alini, M.; Eslaminejad, M.B.; Safa, M. Inhibition of Hypertrophy and Improving Chondrocyte Differentiation by MMP-13 Inhibitor Small Molecule Encapsulated in Alginate-Chondroitin Sulfate-Platelet Lysate Hydrogel. Stem Cell Res. Ther. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.-A.; Ge, Z. Modified Hyaluronic Acid Hydrogels with Chemical Groups That Facilitate Adhesion to Host Tissues Enhance Cartilage Regeneration. Bioact. Mater. 2021, 6, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L.; et al. Ultrafast, Tough, and Adhesive Hydrogel Based on Hybrid Photocrosslinking for Articular Cartilage Repair in Water-Filled Arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Skaalure, S.C.; Bryant, S.J. Gel Structure Has an Impact on Pericellular and Extracellular Matrix Deposition, Which Subsequently Alters Metabolic Activities in Chondrocyte-Laden PEG Hydrogels. Acta Biomater. 2011, 7, 492–504. [Google Scholar] [CrossRef]
- Gupta, A.; Lee, J.; Ghosh, T.; Nguyen, V.Q.; Dey, A.; Yoon, B.; Um, W.; Park, J.H. Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis. Pharmaceutics 2022, 14, 540. [Google Scholar] [CrossRef]
- Bodenberger, N.; Kubiczek, D.; Abrosimova, I.; Scharm, A.; Kipper, F.; Walther, P.; Rosenau, F. Evaluation of Methods for Pore Generation and Their Influence on Physio-Chemical Properties of a Protein Based Hydrogel. Biotechnol. Rep. 2016, 12, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Haung, S.M.; Lin, Y.T.; Liu, S.M.; Chen, J.C.; Chen, W.C. In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid– Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Calore, A.; Wilbers, A.; Harings, J.; Moroni, L. Additive Manufacturing of an Elastic Poly(Ester)Urethane for Cartilage Tissue Engineering. Acta Biomater. 2020, 102, 192–204. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-Based Controlled Delivery Systems for Articular Cartilage Repair. Biomed. Res. Int. 2016, 2016, 1215263. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, S.; Yan, Y.; You, X.; Ye, H. Enhanced Efficacy of Transforming Growth Factor-Β1 Loaded an Injectable Cross-Linked Thiolated Chitosan and Carboxymethyl Cellulose-Based Hydrogels for Cartilage Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2021, 32, 2402–2422. [Google Scholar] [CrossRef]
- Arora, A.; Mahajan, A.; Katti, D.S. TGF-Β1 Presenting Enzymatically Cross-Linked Injectable Hydrogels for Improved Chondrogenesis. Colloids Surfaces B Biointerfaces 2017, 159, 838–848. [Google Scholar] [CrossRef]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.W.; Gunes, K.; Eren-Ozcan, G.G.; et al. An Alginate-Poly(Acrylamide) Hydrogel with TGF-Β3 Loaded Nanoparticles for Cartilage Repair: Biodegradability, Biocompatibility and Protein Adsorption. Int. J. Biol. Macromol. 2021, 172, 381–393. [Google Scholar] [CrossRef]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable Biodegradable Hydrogel Composites for Rabbit Marrow Mesenchymal Stem Cell and Growth Factor Delivery for Cartilage Tissue Engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, S.; Jung, Y.C.; Wang, Y.; Kang, B.J.; Kim, K. Dual Delivery of Stem Cells and Insulin-like Growth Factor-1 in Coacervate-Embedded Composite Hydrogels for Enhanced Cartilage Regeneration in Osteochondral Defects. J. Control. Release 2020, 327, 284–295. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk Fibroin Hydrogel Scaffolds Incorporated with Chitosan Nanoparticles Repair Articular Cartilage Defects by Regulating TGF-Β1 and BMP-2. Arthritis Res. Ther. 2021, 23, 50. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, L.; Xu, Y.; Li, H.; Xu, W.; Wu, S.; Wang, Y.; Tang, Z.; Lv, Y.; Yang, L. Mechano Growth Factor (MGF) and Transforming Growth Factor (TGF)-Β3 Functionalized Silk Scaffolds Enhance Articular Hyaline Cartilage Regeneration in Rabbit Model. Biomaterials 2015, 52, 463–475. [Google Scholar] [CrossRef]
- Guan, P.; Liu, C.; Xie, D.; Mao, S.; Ji, Y.; Lin, Y.; Chen, Z.; Wang, Q.; Fan, L.; Sun, Y. Exosome-Loaded Extracellular Matrix-Mimic Hydrogel with Anti-Inflammatory Property Facilitates/Promotes Growth Plate Injury Repair. Bioact. Mater. 2022, 10, 145–158. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-Stereolithography 3D Printing of a Radially Oriented Extracellular Matrix/Mesenchymal Stem Cell Exosome Bioink for Osteochondral Defect Regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, Z.; Mao, Q.; Wang, C.; Zhou, Y.; Zhou, X.; Ying, L.; Xu, H.; Hu, S.; Zhang, N. Injectable Exosome-Functionalized Extracellular Matrix Hydrogel for Metabolism Balance and Pyroptosis Regulation in Intervertebral Disc Degeneration. J. Nanobiotechnology 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of Stem Cell-Derived Exosomes with in Situ Hydrogel Glue as a Promising Tissue Patch for Articular Cartilage Regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, J.; Qian, D.; Gao, P.; Qin, T.; Jiang, T.; Yi, J.; Xu, T.; Huang, Y.; et al. Exosomes Derived from Platelet-Rich Plasma Administration in Site Mediate Cartilage Protection in Subtalar Osteoarthritis. J. Nanobiotechnol. 2022, 20, 1–22. [Google Scholar] [CrossRef]
- Tao, S.C.; Huang, J.Y.; Gao, Y.; Li, Z.X.; Wei, Z.Y.; Dawes, H.; Guo, S.C. Small Extracellular Vesicles in Combination with Sleep-Related CircRNA3503: A Targeted Therapeutic Agent with Injectable Thermosensitive Hydrogel to Prevent Osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef]
- Hu, H.; Dong, L.; Bu, Z.; Shen, Y.; Luo, J.; Zhang, H.; Zhao, S.; Lv, F.; Liu, Z. MiR-23a-3p-Abundant Small Extracellular Vesicles Released from Gelma/Nanoclay Hydrogel for Cartilage Regeneration. J. Extracell. Vesicles 2020, 9, 1778883. [Google Scholar] [CrossRef]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune Modulation to Improve Tissue Engineering Outcomes for Cartilage Repair in the Osteoarthritic Joint. Tissue Eng.-Part B Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells 2020, 9, 419. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of Decellularized Extracellular Matrix in Bone and Cartilage Tissue Engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular Cartilage: From Formation to Tissue Engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Differential Role of Transforming Growth Factor-Beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef]
- Davies, L.C.; Blain, E.J.; Gilbert, S.J.; Caterson, B.; Duance, V.C. The Potential of IGF-1 and TGFβ1 for Promoting “Adult” Articular Cartilage Repair: An in Vitro Study. Tissue Eng.-Part A. 2008, 14, 1251–1261. [Google Scholar] [CrossRef]
- Mullen, L.M.; Best, S.M.; Ghose, S.; Wardale, J.; Rushton, N.; Cameron, R.E. Bioactive IGF-1 Release from Collagen–GAG Scaffold to Enhance Cartilage Repair in Vitro. J. Mater. Sci. Mater. Med. 2015, 26, 2. [Google Scholar] [CrossRef]
- Sohier, J.; Moroni, L.; van Blitterswijk, C.; de Groot, K.; Bezemer, J.M. Critical Factors in the Design of Growth Factor Releasing Scaffolds for Cartilage Tissue Engineering. Expert Opin. Drug Deliv. 2008, 5, 543–566. [Google Scholar] [CrossRef]
- Ma, Q.; Liao, J.; Cai, X. Different Sources of Stem Cells and Their Application in Cartilage Tissue Engineering. Curr. Stem Cell Res. Ther. 2018, 13, 568–575. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Nejadnik, M.R. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435-17. [Google Scholar] [CrossRef]
- Zhang, S.; Uludağ, H. Nanoparticulate Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef]
- Aguilar, L.M.C.; Silva, S.M.; Moulton, S.E. Growth Factor Delivery: Defining the next Generation Platforms for Tissue Engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Freeman, I.; Kedem, A.; Cohen, S. The Effect of Sulfation of Alginate Hydrogels on the Specific Binding and Controlled Release of Heparin-Binding Proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. Dual Delivery of TGF-Β3 and Ghrelin in Microsphere/ Hydrogel Systems for Cartilage Regeneration. Molecules 2021, 26, 5732. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. In Vitro Release of Transforming Growth Factor-Β1 from Gelatin Microparticles Encapsulated in Biodegradable, Injectable Oligo(Poly(Ethylene Glycol) Fumarate) Hydrogels. J. Control. Release 2003, 91, 299–313. [Google Scholar] [CrossRef]
- Park, H.; Temenoff, J.S.; Holland, T.A.; Tabata, Y.; Mikos, A.G. Delivery of TGF-Β1 and Chondrocytes via Injectable, Biodegradable Hydrogels for Cartilage Tissue Engineering Applications. Biomaterials 2005, 26, 7095–7103. [Google Scholar] [CrossRef]
- Matta, C.; Mobasheri, A. Regulation of Chondrogenesis by Protein Kinase C: Emerging New Roles in Calcium Signalling. Cell. Signal. 2014, 26, 979–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.C.; Bang, E.; Rejon, C.A.; Libasci, V.; Pertchenko, P.; Hébert, T.E.; Bernard, D.J. Bone Morphogenetic Protein 2 Stimulates Noncanonical Smad2/3 Signaling via the Bmp Type 1a Receptor in Gonadotrope-like Cells: Implications for FSH Synthesis. Endocrinology 2014, 155, 1970–1981. [Google Scholar] [CrossRef]
- Lee, P.T.; Li, W.-J. Chondrogenesis of Embryonic Stem Cell-Derived Mesenchymal Stem Cells Induced by TGFβ1 and BMP7 Through Increased TGFβ Receptor Expression and Endogenous TGFβ1 Production. J. Cell. Biochem. 2017, 118, 172–181. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Cremers, A.; Surtel, D.A.M.; Coolsen, M.M.E.; van Rhijn, L.W.; Welting, T.J.M. Hypertrophic Differentiation during Chondrogenic Differentiation of Progenitor Cells Is Stimulated by BMP-2 but Suppressed by BMP-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef]
- Pogue, R.; Lyons, K. BMP Signaling in the Cartilage Growth Plate. Curr. Top. Dev. Biol. 2006, 76, 1–48. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bilousova, G.; Payne, K.; Bryant, S.J. Dynamic Mechanical Loading and Growth Factors Influence Chondrogenesis of Induced Pluripotent Mesenchymal Progenitor Cells in a Cartilage-Mimetic Hydrogel. Biomater. Sci. 2019, 7, 5388–5403. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yi, Q.; Feng, J.; Yang, L.; Tang, L. Mechano Growth Factor E Peptide Regulates Migration and Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Mol. Endocrinol. 2013, 52, 111–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, e1900411. [Google Scholar] [CrossRef]
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26, e923328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired Highly Adhesive Hydrogel with Exosomes for Endogenous Cell Recruitment and Cartilage Defect Regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barisón, M.J.; Nogoceke, R.; Josino, R.; Horinouchi, C.D.d.S.; Marcon, B.H.; Correa, A.; Stimamiglio, M.A.; Robert, A.W. Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. Int. J. Mol. Sci. 2022, 23, 6010. https://doi.org/10.3390/ijms23116010
Barisón MJ, Nogoceke R, Josino R, Horinouchi CDdS, Marcon BH, Correa A, Stimamiglio MA, Robert AW. Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. International Journal of Molecular Sciences. 2022; 23(11):6010. https://doi.org/10.3390/ijms23116010
Chicago/Turabian StyleBarisón, María Julia, Rodrigo Nogoceke, Raphaella Josino, Cintia Delai da Silva Horinouchi, Bruna Hilzendeger Marcon, Alejandro Correa, Marco Augusto Stimamiglio, and Anny Waloski Robert. 2022. "Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling" International Journal of Molecular Sciences 23, no. 11: 6010. https://doi.org/10.3390/ijms23116010
APA StyleBarisón, M. J., Nogoceke, R., Josino, R., Horinouchi, C. D. d. S., Marcon, B. H., Correa, A., Stimamiglio, M. A., & Robert, A. W. (2022). Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. International Journal of Molecular Sciences, 23(11), 6010. https://doi.org/10.3390/ijms23116010






