Multiwalled Carbon Nanotubes Induce Fibrosis and Telomere Length Alterations
Abstract
:1. Introduction
2. Results
2.1. Effects of MWCNT on Animal Growth and Histopathology
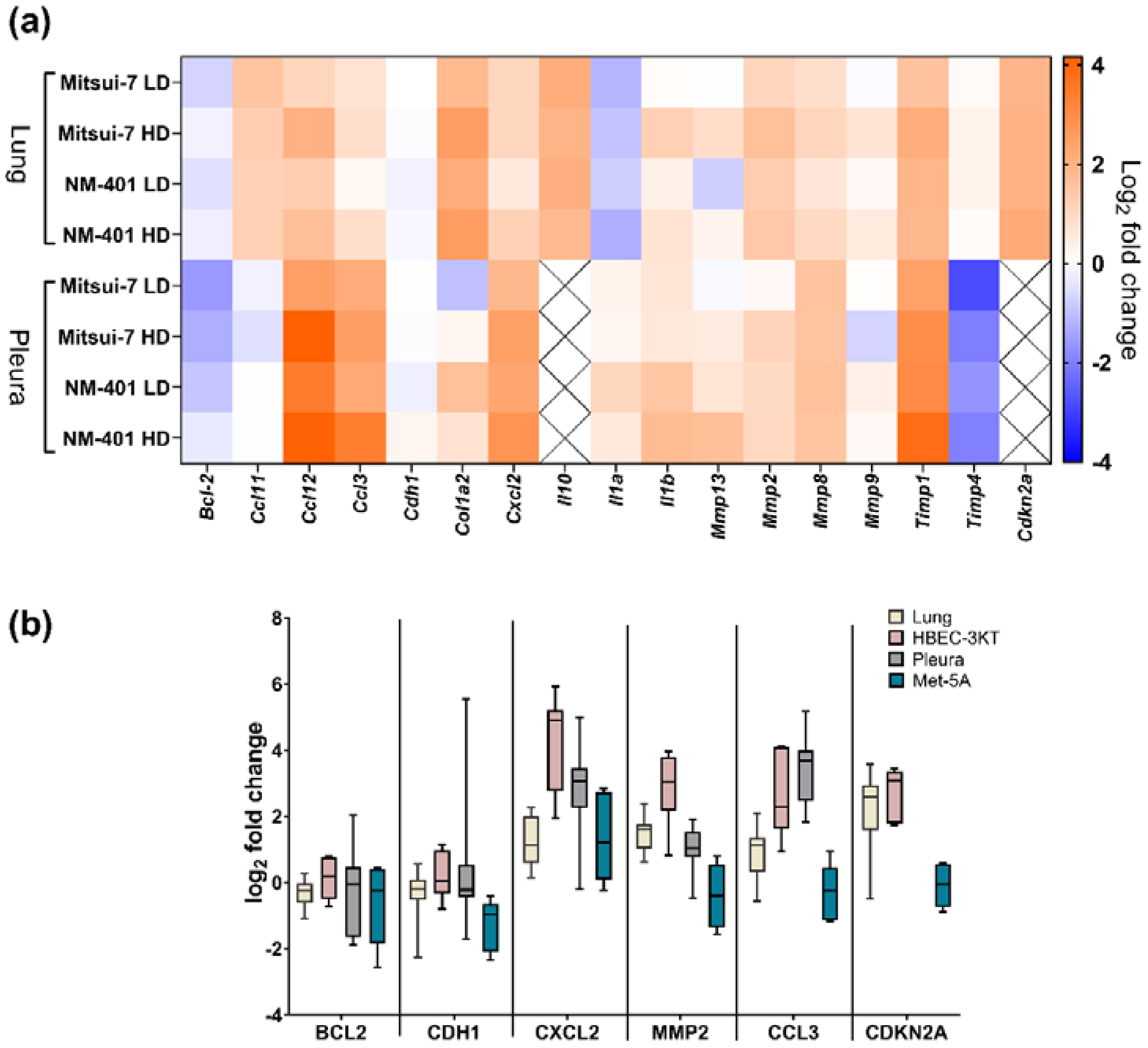
2.2. Effects of MWCNT on Genes Involved in Fibrosis and Inflammation
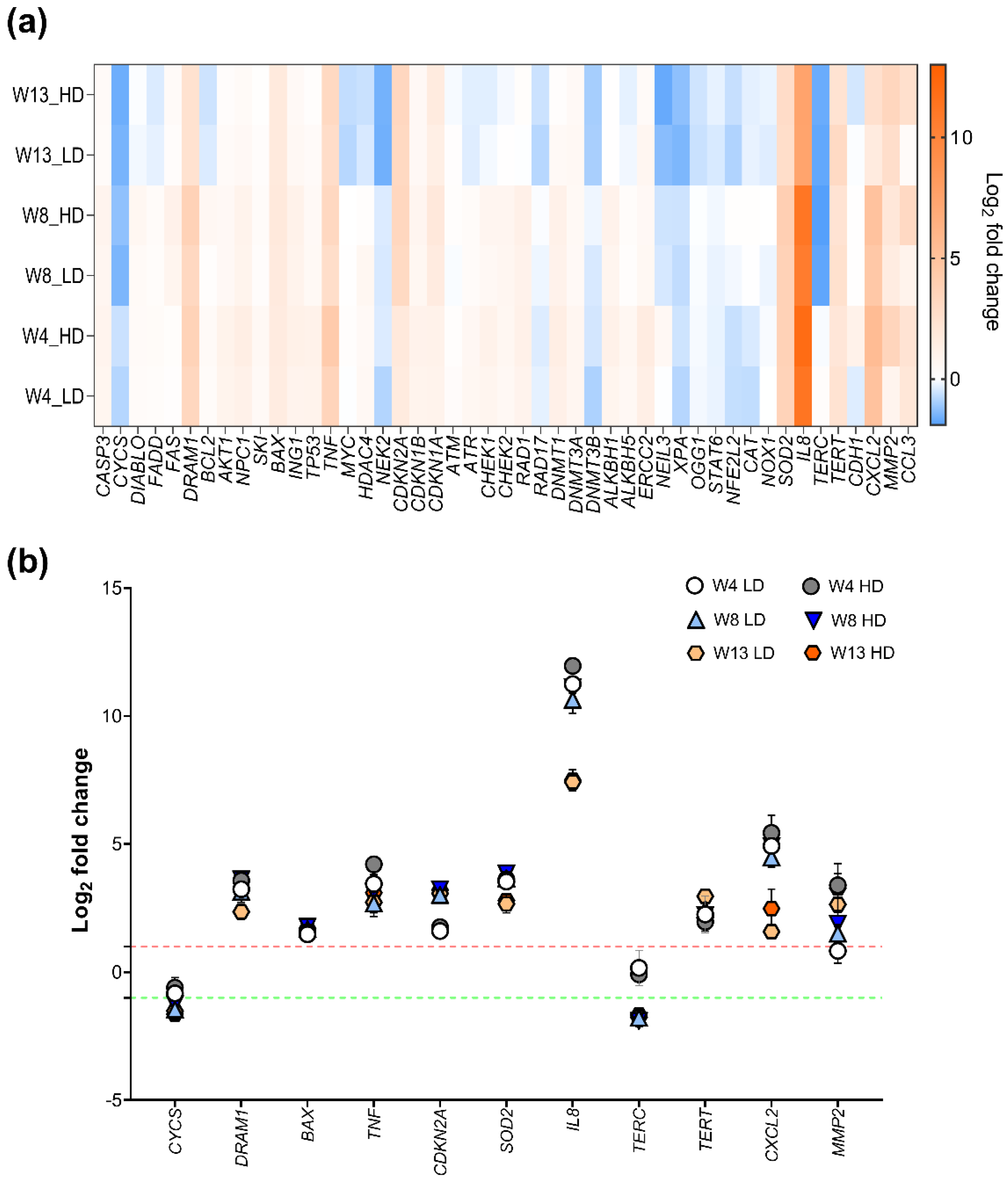
2.3. Effects of MWCNT-Exposure on Telomere Length
2.4. Effects of NM-401 on Pathway-Based Gene Expression Patterns in HBEC-3KT Cells
2.5. Effects of NM-401-Exposure on Cell Cycle and Apoptosis in Lung Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Nanomaterials
4.2. In Vivo Experiments
4.2.1. Intrapleural Injection of the MWCNT
4.2.2. Collection and Preparation of Biological Samples for Histological and Molecular Analysis
4.3. In Vitro Cell Culture Experiments
4.3.1. Assessment of In Vitro Deposited Dose
4.3.2. Assessment of Cell Cycle and Apoptosis
4.4. Gene Expression
4.5. Telomere Length Assessment
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, C.L.; Hankin, S.M.; Ross, B.; Aitken, R.J.; Jones, A.D.; Donaldson, K.; Stone, V.; Trantra, R. An Outline Scoping Study to Determine Whether High Aspect Ratio Nanoparticles (Harn) Should Raise the Same Concerns as Do Asbestos Fibres. In Report on Project CB0406; IOM: London, UK, 2008. [Google Scholar]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, Carbon Nanotubes and the Pleural Mesothelium: A Review of the Hypothesis Regarding the Role of Long Fibre Retention in the Parietal Pleura, Inflammation and Mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbarino, M.; Giordano, A. Assessment of the Carcinogenicity of Carbon Nanotubes in the Respiratory System. Cancers 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Nanomaterials and Some Fibres: Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 111. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Nanomaterials-And-Some-Fibres-2017 (accessed on 19 May 2017).
- Poulsen, S.S.; Jackson, P.; Kling, K.; Knudsen, K.B.; Skaug, V.; Kyjovska, Z.O.; Thomsen, B.L.; Clausen, P.A.; Atluri, R.; Berthing, T.; et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology 2016, 10, 1263–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargent, L.M.; Porter, D.W.; Staska, L.M.; Hubbs, A.F.; Lowry, D.T.; Battelli, L.; Siegrist, K.J.; Kashon, M.L.; Mercer, R.R.; Bauer, A.K.; et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Arnoldussen, Y.J.; Skaug, V.; Aleksandersen, M.; Ropstad, E.; Anmarkrud, K.H.; Einarsdottir, E.; Chin-Lin, F.; Bjørklund, C.G.; Kasem, M.; Eilertsen, E.; et al. Inflammation in the pleural cavity following injection of multi-walled carbon nanotubes is dependent on their characteristics and the presence of IL-1 genes. Nanotoxicology 2018, 12, 522–538. [Google Scholar] [CrossRef]
- Møller, P.; Vest Christophersen, D.; Jensen, D.M.; Kermanizadeh, A.; Roursgaard, M.; Jacobsen, N.R.; Hemmingsen, J.G.; Danielsen, P.H.; Cao, Y.; Jantzen, K.; et al. Role of oxidative stress in carbon nanotube-generated health effects. Arch. Toxicol. 2014, 88, 1939–1964. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Jaurand, M.C.; Møller, P.; Morimoto, Y.; Kobayashi, N.; Pinkerton, K.E.; Sargent, L.M.; Vermeulen, R.C.; Fubini, B.; Kane, A.B. Evaluating the Mechanistic Evidence and Key Data Gaps in Assessing the Potential Carcinogenicity of Carbon Nanotubes and Nanofibers in Humans. Crit. Rev. Toxicol. 2017, 47, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2020, 34, 24–46. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase Catalytic Subunit Homologs from Fission Yeast and Human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. Mol. Mech. Mutagen. 2012, 730, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Meier, H.C.S.; Antonini, J.M. Telomeres in Toxicology: Occupational Health. Pharmacol. Ther. 2021, 220, 107742. [Google Scholar] [CrossRef]
- Borghini, A.; Roursgaard, M.; Andreassi, M.G.; Kermanizadeh, A.; Møller, P. Repair activity of oxidatively damaged DNA and telomere length in human lung epithelial cells after exposure to multi-walled carbon nanotubes. Mutagenesis 2016, 32, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Wils, R.S.; Jacobsen, N.R.; Di Ianni, E.; Roursgaard, M.; Møller, P. Reactive Oxygen Species Production, Genotoxicity and Telomere Length in Fe1-Muta™Mouse Lung Epithelial Cells Exposed to Carbon Nanotubes. Nanotoxicology 2021, 15, 661–672. [Google Scholar] [CrossRef]
- Ghosh, M.; Janssen, L.; Martens, D.S.; Öner, D.; Vlaanderen, J.; Pronk, A.; Kuijpers, E.; Vermeulen, R.; Nawrot, T.S.; Godderis, L.; et al. Increased telomere length and mtDNA copy number induced by multi-walled carbon nanotube exposure in the workplace. J. Hazard. Mater. 2020, 394, 122569. [Google Scholar] [CrossRef]
- Murphy, F.A.; Poland, C.; Duffin, R.; Al-Jamal, K.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-Dependent Retention of Carbon Nanotubes in the Pleural Space of Mice Initiates Sustained Inflammation and Progressive Fibrosis on the Parietal Pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meeren, A.; Fleury, J.; Nebut, M.; Monchaux, G.; Janson, X.; Jaurand, M.C. Mesothelioma in rats following intrapleural injection of chrysotile and phosphorylated chrysotile (chrysophosphate). Int. J. Cancer 1992, 50, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J.; Dehner, L.P. Nodular mesothelial hyperplasia in hernia sacs.A benign reactive condition simulating a neoplastic process. Cancer 1975, 35, 165–175. [Google Scholar] [CrossRef]
- Di Ianni, E.; Erdem, J.S.; Møller, P.; Sahlgren, N.M.; Poulsen, S.S.; Knudsen, K.B.; Zienolddiny, S.; Saber, A.T.; Wallin, H.; Vogel, U.; et al. In Vitro-in Vivo Correlations of Pulmonary Inflammogenicity and Genotoxicity of Mwcnt. Part. Fibre Toxicol. 2021, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Jacobsen, N.R.; Aziz, S.A.; Wu, D.; Williams, A.; Yauk, C.; White, P.; Wallin, H.; Vogel, U.; Halappanavar, S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. Toxicol. Environ. Mutagen. 2017, 823, 28–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reamon-Buettner, S.M.; Hackbarth, A.; Leonhardt, A.; Braun, A.; Ziemann, C. Cellular senescence as a response to multiwalled carbon nanotube (MWCNT) exposure in human mesothelial cells. Mech. Ageing Dev. 2020, 193, 111412. [Google Scholar] [CrossRef]
- Lucas, J.; Wang, Q.; Muthumalage, T.; Rahman, I. Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells. Toxics 2021, 9, 144. [Google Scholar] [CrossRef]
- Verdun, R.E.; Karlseder, J. Replication and protection of telomeres. Nature 2007, 447, 924–931. [Google Scholar] [CrossRef]
- Turner, K.; Vasu, V.; Griffin, D. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Aida, S.; Aida, J.; Naoi, M.; Kato, M.; Tsuura, Y.; Natsume, I.; Takubo, K. Measurement of telomere length in cells from pleural effusion: Asbestos exposure causes telomere shortening in pleural mesothelial cells. Pathol. Int. 2018, 68, 503–508. [Google Scholar] [CrossRef]
- Alswady-Hoff, M.; Erdem, J.S.; Phuyal, S.; Knittelfelder, O.; Sharma, A.; Fonseca, D.D.M.; Skare, Ø.; Slupphaug, G.; Zienolddiny, S. Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5349. [Google Scholar] [CrossRef]
- Jensen, D.M.; Løhr, M.; Sheykhzade, M.; Lykkesfeldt, J.; Wils, R.S.; Loft, S.; Møller, P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis 2019, 34, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA Damage Foci at Dysfunctional Telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- Catalán, J.; Siivola, K.M.; Nymark, P.; Lindberg, H.; Suhonen, S.; Järventaus, H.; Koivisto, A.J.; Moreno, C.; Vanhala, E.; Wolff, H.; et al. In vitro and in vivo genotoxic effects of straight versus tangled multi-walled carbon nanotubes. Nanotoxicology 2016, 10, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Phuyal, S.; Kasem, M.; Knittelfelder, O.; Sharma, A.; Fonseca, D.D.M.; Vebraite, V.; Shaposhnikov, S.; Slupphaug, G.; Skaug, V.; Zienolddiny, S. Characterization of the proteome and lipidome profiles of human lung cells after low dose and chronic exposure to multiwalled carbon nanotubes. Nanotoxicology 2018, 12, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Al-Ajmi, N.; Saretzki, G.; Miles, C.; Spyridopoulos, I. Dietary restriction ameliorates haematopoietic ageing independent of telomerase, whilst lack of telomerase and short telomeres exacerbates the ageing phenotype. Exp. Gerontol. 2014, 58, 113–119. [Google Scholar] [CrossRef]
- Blasco, M.A.; Rizen, M.; Greider, C.; Hanahan, D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat. Genet. 1996, 12, 200–204. [Google Scholar] [CrossRef]
- Harrington, L.; Lansdorp, P.; Hande, M.; Robinson, M.; Siderovski, D.; Itie, A.; Erdmann, N.; Snow, B.; Yeung, D.; Wakeham, A.; et al. The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr. Biol. 2000, 10, 1459–1462. [Google Scholar] [CrossRef]
- Lee, J.; Sung, Y.H.; Cheong, C.; Choi, Y.S.; Jeon, H.K.; Sun, W.; Hahn, W.C.; Ishikawa, F.; Lee, H.-W. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene 2008, 27, 3754–3760. [Google Scholar] [CrossRef] [Green Version]
- Wright, W.E.; Pereira-Smith, O.M.; Shay, J.W. Reversible cellular senescence: Implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 1989, 9, 3088–3092. [Google Scholar] [CrossRef]
- Baker, D.J.; Perez-Terzic, C.; Jin, F.; Pitel, K.S.; Niederländer, N.J.; Jeganathan, K.; Yamada, S.; Reyes, S.; Rowe, L.; Hiddinga, H.J.; et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 2008, 10, 825–836. [Google Scholar] [CrossRef]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Mancera, P.A.; Young, A.R.J.; Narita, M. Inside and out: The activities of senescence in cancer. Nat. Rev. Cancer 2014, 14, 547–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, Lifestyle, Cancer, and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, V.; Swahari, V.; Schafer, J.; Kole, A.J.; Evans, A.; Huang, Y.; Cliffe, A.; Golitz, B.; Sciaky, N.; Pei, X.-H.; et al. The E3 ligase PARC mediates the degradation of cytosolic cytochrome c to promote survival in neurons and cancer cells. Sci. Signal. 2014, 7, ra67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnoldussen, Y.J.; Skogstad, A.; Skaug, V.; Kasem, M.; Haugen, A.; Benker, N.; Weinbruch, S.; Apte, R.N.; Zienolddiny, S. Involvement of IL-1 genes in the cellular responses to carbon nanotube exposure. Cytokine 2015, 73, 128–137. [Google Scholar] [CrossRef]
- Phuyal, S.; Kasem, M.; Rubio, L.; Karlsson, H.L.; Marcos, R.; Skaug, V.; Zienolddiny, S. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol. Vitro 2017, 44, 230–240. [Google Scholar] [CrossRef]
- Rasmussen, K.; Cotogno, G.; Gaillard, C. Multi-Walled Carbon Nanotubes, Nm-400, Nm-401, Nm-402, Nm-403: Characterisation and Physico-Chemical Properties. JRC Science for Policy Report. 2014. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC91205/mwcnt-online.pdf (accessed on 29 August 2014).
- Jensen, K.A. Deliverable 4.1: Summary Report on Primary Physicochemical Properties of Manufactured Nanomaterials Used in Nanogenotox. 2014. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable.1.pdf (accessed on 26 March 2013).
- Jensen, K.A. The Nanogenotox Dispersion Protocol for Nanoreg; National Research Centre for the Working Environment: Copenhagen, Denmark, 2014. [Google Scholar]
- NIOSH. Occupational Exposure to Carbon Nanotubes and Nanofibers. 2013. Available online: https://www.cdc.gov/niosh/docs/2013-145/pdfs/2013-145.pdf?id=10.26616/NIOSHPUB2013145 (accessed on April 2013).
- Devoy, J.; Nunge, H.; Bonfanti, E.; Seidel, C.; Gaté, L.; Cosnier, F. Quantitative measurement of carbon nanotubes in rat lung. Nanotoxicology 2020, 14, 1227–1240. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef] [Green Version]
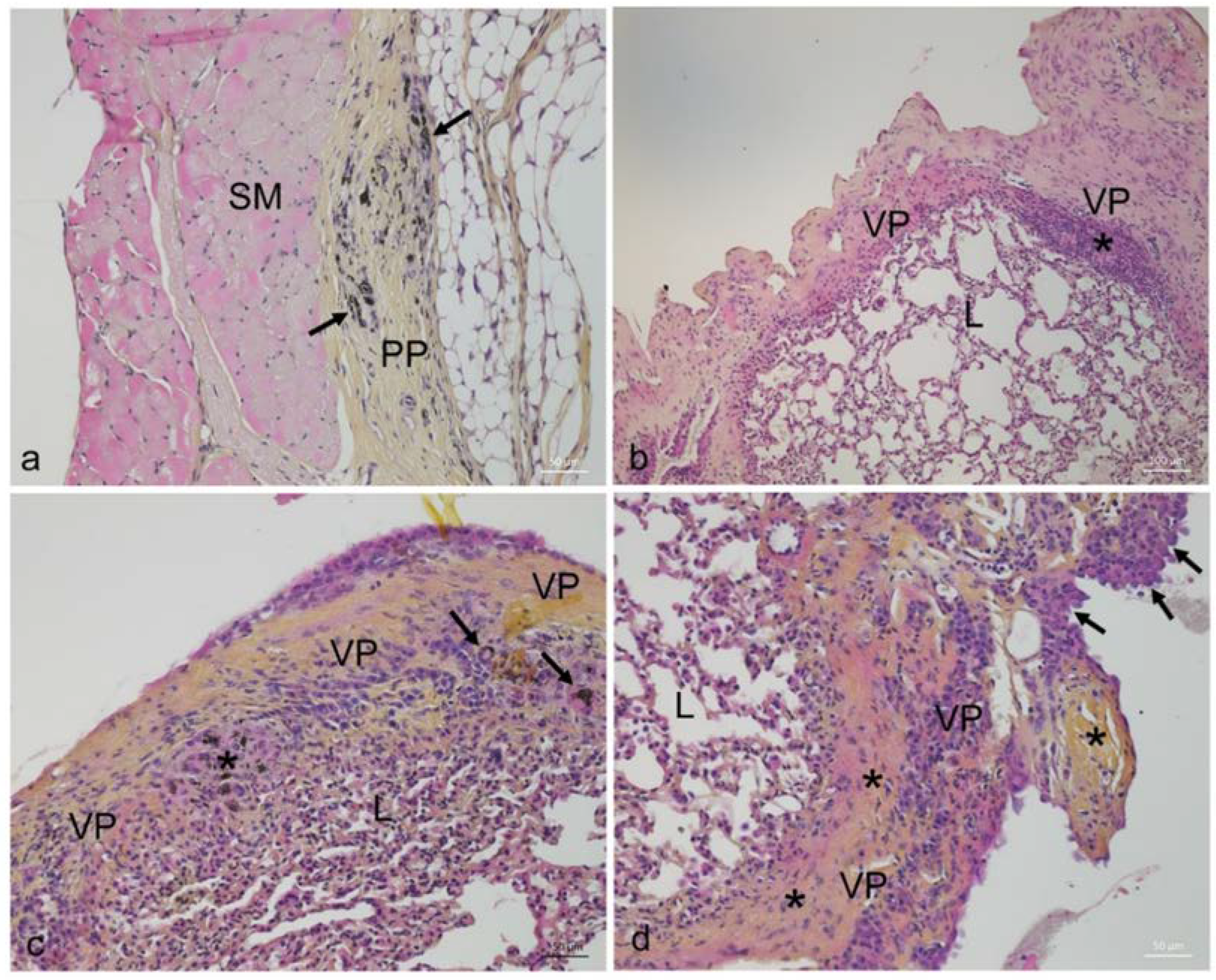


| Presence of Fibres | Infiltration of Leukocytes | Hyperplasia of Mesothelial Cells | Presence of Granulomas | ||
|---|---|---|---|---|---|
| Exposure | n | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Sham | 14 | 0 a | 0 a | 0 a | 0 a |
| Mitsui-7 LD | 15 | 1.57 b ± 0.15 | 2.47 b ± 0.11 | 2.03 b ± 0.12 | 0.00 a ± 0.00 |
| Mitsui-7 HD | 17 | 2.62 c ± 0.16 | 2.68 b ± 0.11 | 1.85 b ± 0.15 | 1.41 b ± 0.24 |
| NM-401 LD | 17 | 0.94 d ± 0.09 | 2.71 b ± 0.11 | 2.29 b ± 0.18 | 0.06 a ± 0.06 |
| NM-401 HD | 14 | 2.11 bc ± 0.13 | 2.82 b ± 0.10 | 1.89 b ± 0.22 | 1.15 b ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alswady-Hoff, M.; Erdem, J.S.; Aleksandersen, M.; Anmarkrud, K.H.; Skare, Ø.; Lin, F.-C.; Simensen, V.; Arnoldussen, Y.J.; Skaug, V.; Ropstad, E.; et al. Multiwalled Carbon Nanotubes Induce Fibrosis and Telomere Length Alterations. Int. J. Mol. Sci. 2022, 23, 6005. https://doi.org/10.3390/ijms23116005
Alswady-Hoff M, Erdem JS, Aleksandersen M, Anmarkrud KH, Skare Ø, Lin F-C, Simensen V, Arnoldussen YJ, Skaug V, Ropstad E, et al. Multiwalled Carbon Nanotubes Induce Fibrosis and Telomere Length Alterations. International Journal of Molecular Sciences. 2022; 23(11):6005. https://doi.org/10.3390/ijms23116005
Chicago/Turabian StyleAlswady-Hoff, Mayes, Johanna Samulin Erdem, Mona Aleksandersen, Kristine Haugen Anmarkrud, Øivind Skare, Fang-Chin Lin, Vincent Simensen, Yke Jildouw Arnoldussen, Vidar Skaug, Erik Ropstad, and et al. 2022. "Multiwalled Carbon Nanotubes Induce Fibrosis and Telomere Length Alterations" International Journal of Molecular Sciences 23, no. 11: 6005. https://doi.org/10.3390/ijms23116005
APA StyleAlswady-Hoff, M., Erdem, J. S., Aleksandersen, M., Anmarkrud, K. H., Skare, Ø., Lin, F.-C., Simensen, V., Arnoldussen, Y. J., Skaug, V., Ropstad, E., & Zienolddiny-Narui, S. (2022). Multiwalled Carbon Nanotubes Induce Fibrosis and Telomere Length Alterations. International Journal of Molecular Sciences, 23(11), 6005. https://doi.org/10.3390/ijms23116005








