Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin
Abstract
:1. Introduction
2. Results
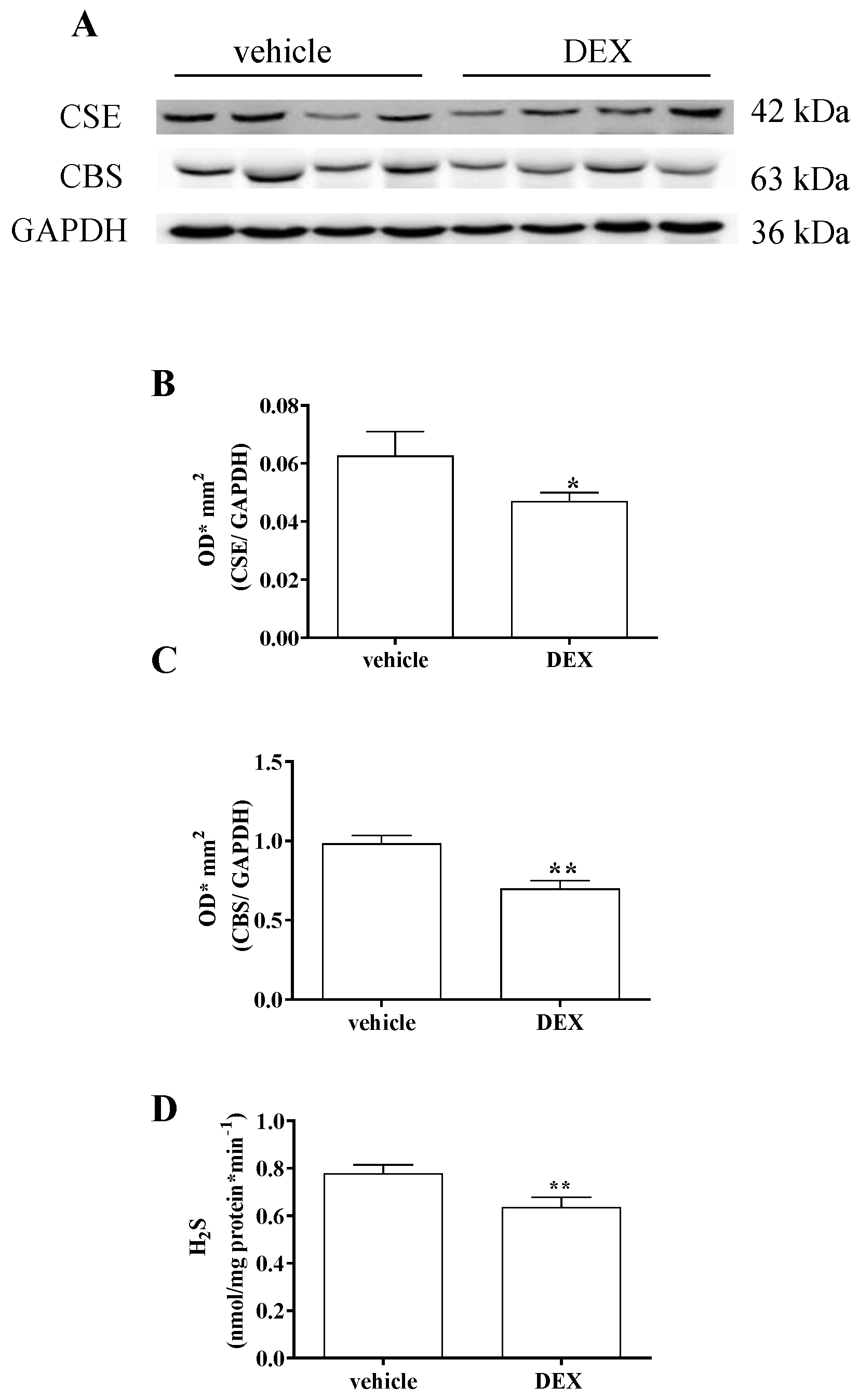
2.1. Impairment of H2S Signalling in DEX-Induced In Vitro Model of Sarcopenia in C2C12 Myotubes
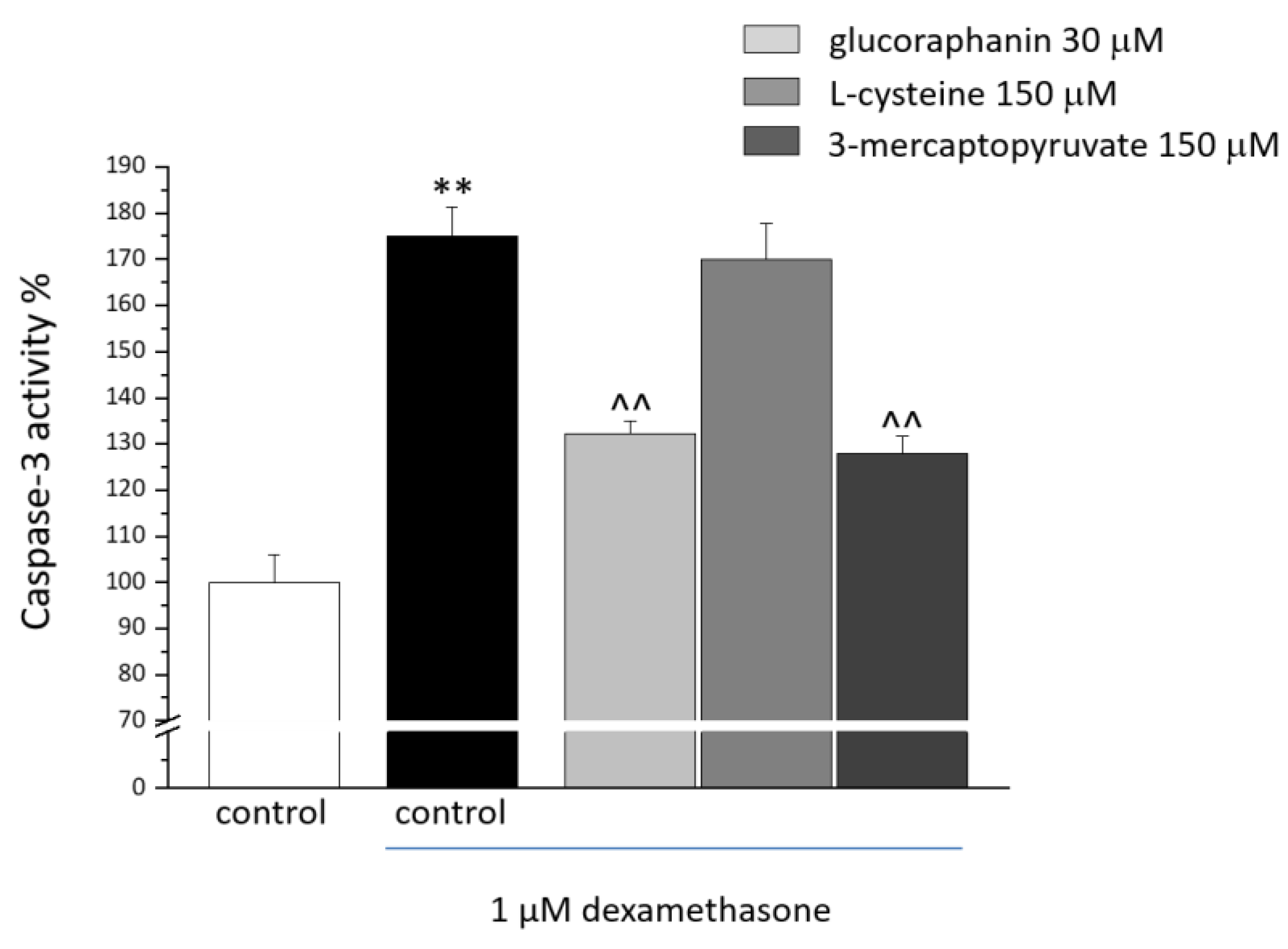
2.2. C2C12 Myotube Viability and Caspase-3 Activity
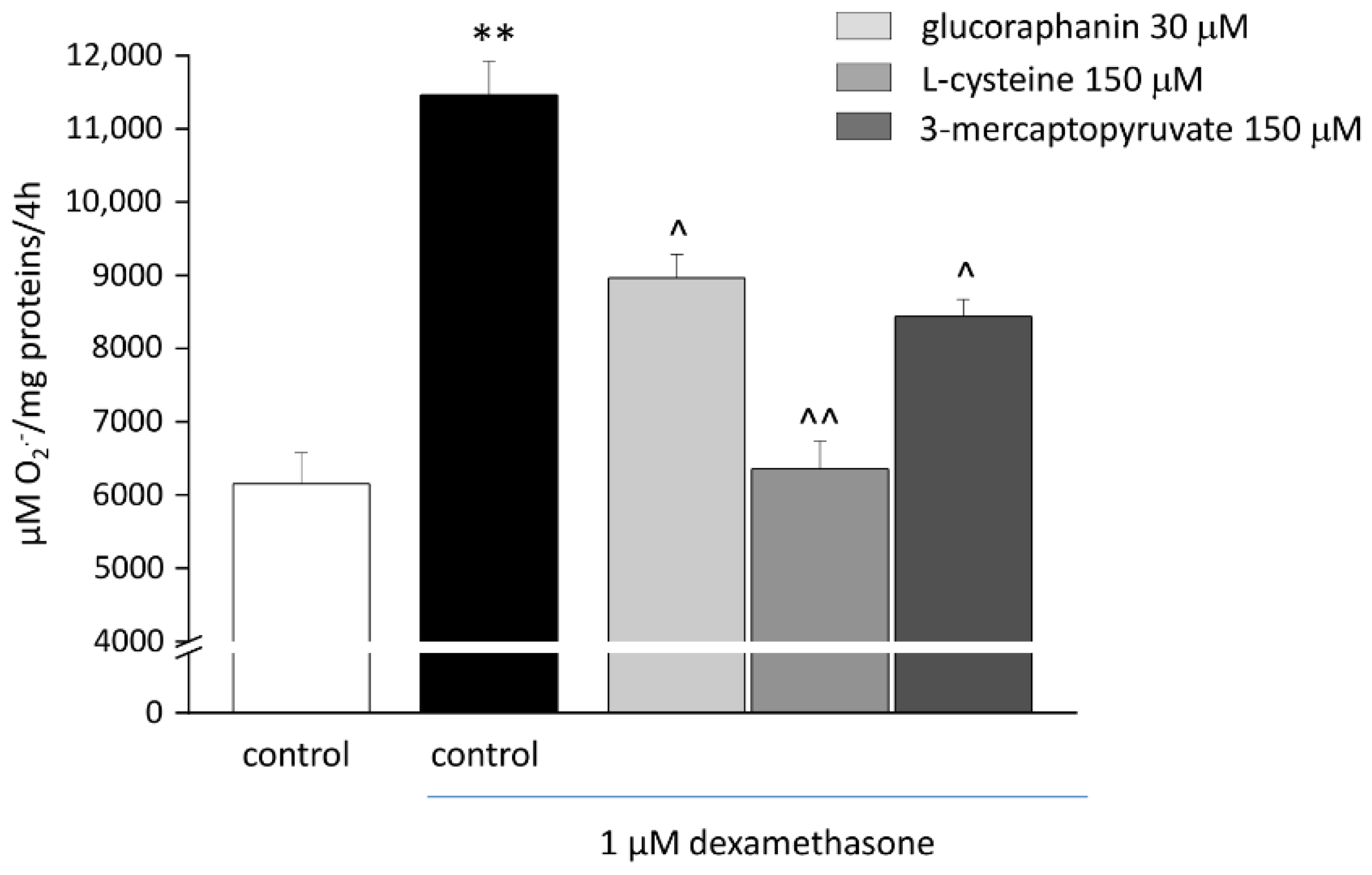
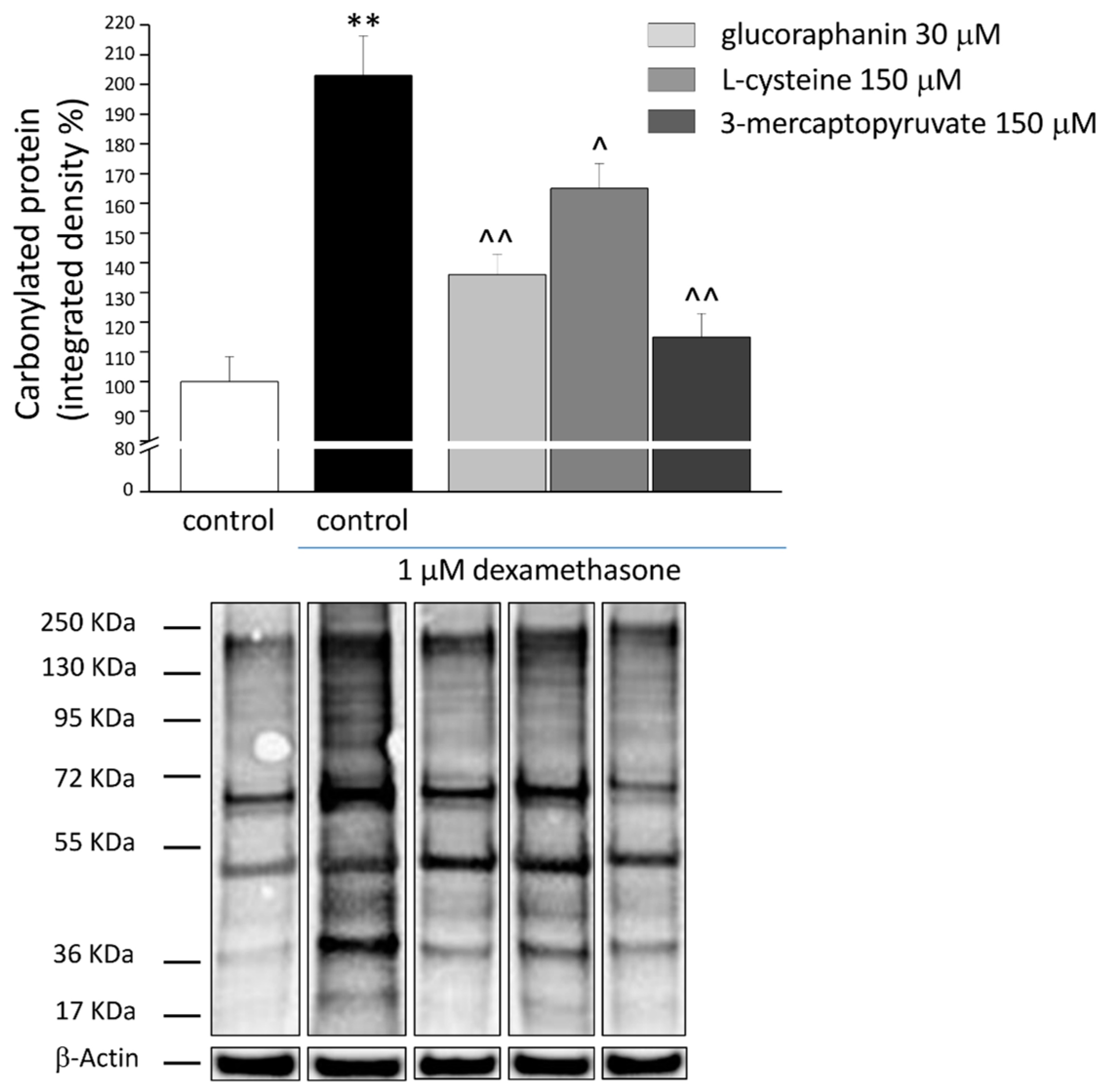
2.3. Protective Effects from Oxidative Stress Induced by DEX
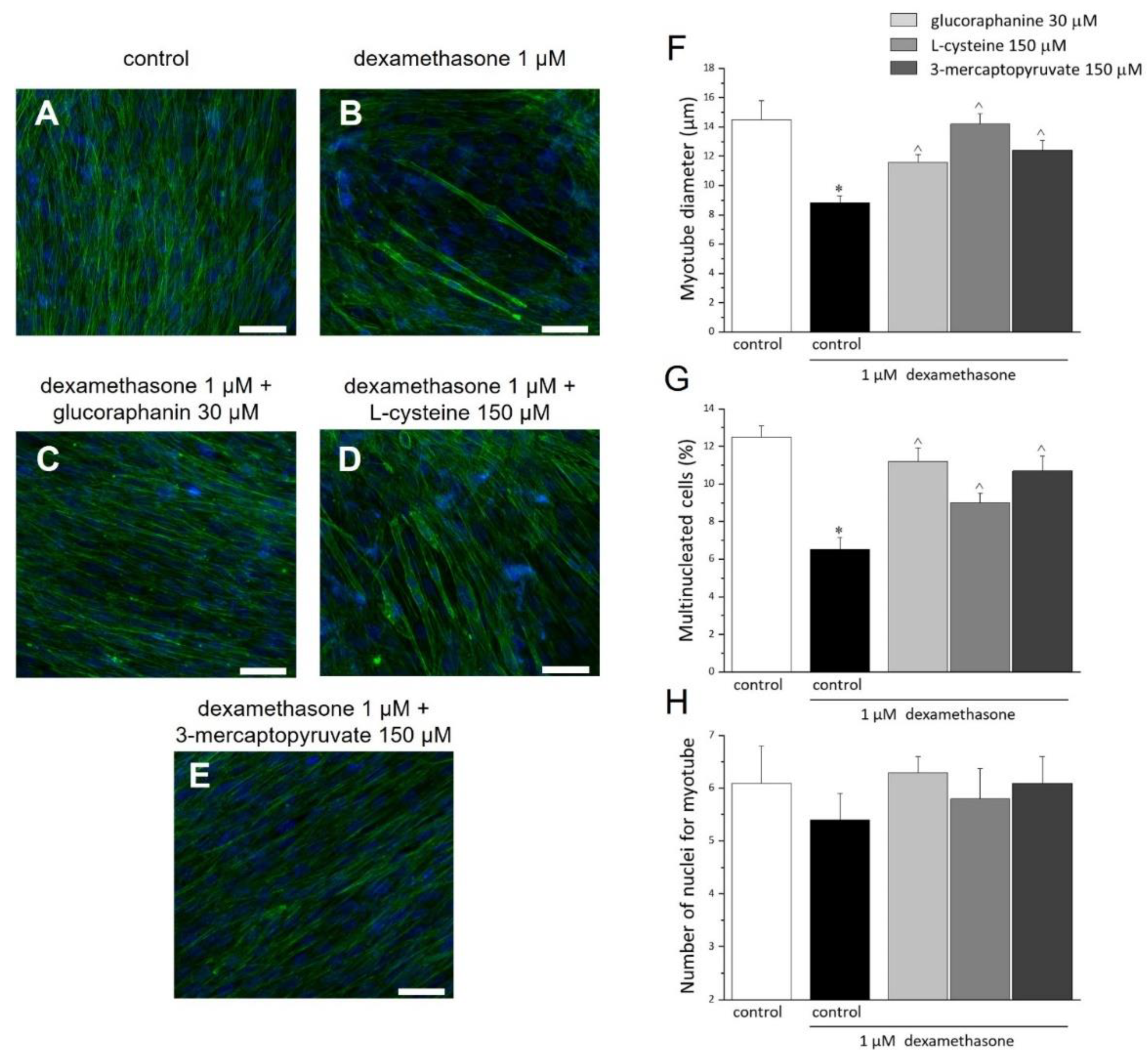
2.4. Morphology and Morphometrics of C2C12 Myotubes
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Pharmacological Treatments
4.3. Cell Viability Assay (MTT Test)
4.4. Western Blot Analysis
4.5. H2S Determination
4.6. Caspase-3 Activity
4.7. Catalase Activity
4.8. Superoxide Dismutase (SOD)-Inhibitable Superoxide Anion (O2−) Production Evaluated by Cytochrome c Assay
4.9. Carbonylated Protein Evaluation
4.10. Morphologic Evaluations
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delmonico, M.J.; Harris, T.B.; Lee, J.-S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. For the Health, Aging and Body Composition Study Alternative Definitions of Sarcopenia, Lower Extremity Performance, and Functional Impairment with Aging in Older Men and Women: Sarcopenia Indices, Performance, and Aging. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J. Sarcopenia, the Last Organ Insufficiency. Eur. Geriatr. Med. 2016, 7, 195–196. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; van Kan, G.A.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its Assessment, Etiology, Pathogenesis, Consequences and Future Perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, Incidence, and Clinical Impact of Sarcopenia: Facts, Numbers, and Epidemiology-Update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef]
- Garatachea, N.; Lucía, A. Genes and the Ageing Muscle: A Review on Genetic Association Studies. Age 2013, 35, 207–233. [Google Scholar] [CrossRef]
- Pratt, J.; Boreham, C.; Ennis, S.; Ryan, A.W.; De Vito, G. Genetic Associations with Aging Muscle: A Systematic Review. Cells 2019, 9, 12. [Google Scholar] [CrossRef]
- Waters, D.L.; Baumgartner, R.N. Sarcopenia and Obesity. Clin. Geriatr. Med. 2011, 27, 401–421. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: Effects on Body Composition and Function. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M1012–M1017. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and Age-Related Endocrine Function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Guerriero, F.; Morazzoni, P.; Riva, A.; Guido, D.; Perna, S. A Systematic Review on the Effects of Botanicals on Skeletal Muscle Health in Order to Prevent Sarcopenia. Evid.-Based Complement. Altern. Med. 2016, 2016, 5970367. [Google Scholar] [CrossRef]
- Veeranki, S.; Tyagi, S.C. Role of Hydrogen Sulfide in Skeletal Muscle Biology and Metabolism. Nitric Oxide 2015, 46, 66–71. [Google Scholar] [CrossRef]
- Vellecco, V.; Armogida, C.; Bucci, M. Hydrogen Sulfide Pathway and Skeletal Muscle: An Introductory Review: Hydrogen Sulfide and Skeletal Muscle. Br. J. Pharmacol. 2018, 175, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Synthesis, Metabolism, and Signaling Mechanisms of Hydrogen Sulfide: An Overview. In Vascular Effects of Hydrogen Sulfide; Bełtowski, J., Ed.; Springer: New York, NY, USA, 2019; Volume 2007, pp. 1–8. ISBN 9781493995271. [Google Scholar]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Módis, K.; Panopoulos, P.; Coletta, C.; Papapetropoulos, A.; Szabo, C. Hydrogen Sulfide-Mediated Stimulation of Mitochondrial Electron Transport Involves Inhibition of the Mitochondrial Phosphodiesterase 2A, Elevation of cAMP and Activation of Protein Kinase A. Biochem. Pharmacol. 2013, 86, 1311–1319. [Google Scholar] [CrossRef]
- Vellecco, V.; Mancini, A.; Ianaro, A.; Calderone, V.; Attanasio, C.; Cantalupo, A.; Andria, B.; Savoia, G.; Panza, E.; Di Martino, A.; et al. Cystathionine β-Synthase-Derived Hydrogen Sulfide Is Involved in Human Malignant Hyperthermia. Clin. Sci. 2016, 130, 35–44. [Google Scholar] [CrossRef]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine γ-Lyase-Deficient Mice Require Dietary Cysteine to Protect against Acute Lethal Myopathy and Oxidative Injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; Behera, J.; Theilen, N.T.; George, A.K.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Hydrogen Sulfide Alleviates Hyperhomocysteinemia-Mediated Skeletal Muscle Atrophy via Mitigation of Oxidative and Endoplasmic Reticulum Stress Injury. Am. J. Physiol. Physiol. 2018, 315, C609–C622. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Sinha-Hikim, A.P.; Parveen, M.; Shen, R.; Goswami, R.; Tran, P.; Crum, A.; Norris, K.C. Long-Term Supplementation with a Cystine-Based Antioxidant Delays Loss of Muscle Mass in Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Willmann, R.; Possekel, S.; Dubach-Powell, J.; Meier, T.; Ruegg, M.A. Mammalian Animal Models for Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2009, 19, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.; O’Malley, D. What Has the Mdx Mouse Model of Duchenne Muscular Dystrophy Contributed to Our Understanding of This Disease? J. Muscle Res. Cell Motil. 2015, 36, 155–167. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal Models of Duchenne Muscular Dystrophy: From Basic Mechanisms to Gene Therapy. Dis. Model. Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef]
- Terrill, J.R.; Boyatzis, A.; Grounds, M.D.; Arthur, P.G. Treatment with the Cysteine Precursor L-2-Oxothiazolidine-4-Carboxylate (OTC) Implicates Taurine Deficiency in Severity of Dystropathology in Mdx Mice. Int. J. Biochem. Cell Biol. 2013, 45, 2097–2108. [Google Scholar] [CrossRef]
- Panza, E.; Vellecco, V.; Iannotti, F.A.; Paris, D.; Manzo, O.L.; Smimmo, M.; Mitilini, N.; Boscaino, A.; de Dominicis, G.; Bucci, M.; et al. Duchenne’s Muscular Dystrophy Involves a Defective Transsulfuration Pathway Activity. Redox Biol. 2021, 45, 102040. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R. V Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Mitidieri, E.; Tramontano, T.; Gurgone, D.; Citi, V.; Calderone, V.; Brancaleone, V.; Katsouda, A.; Nagahara, N.; Papapetropoulos, A.; Cirino, G.; et al. Mercaptopyruvate Acts as Endogenous Vasodilator Independently of 3-Mercaptopyruvate Sulfurtransferase Activity. Nitric Oxide 2018, 75, 53–59. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; Parisio, C.; Toti, A.; Tenci, B.; Zanardelli, M.; Branca, J.; Pacini, A.; Ghelardini, C. Effects of the Combination of β-Hydroxy-β-Methyl Butyrate and R(+) Lipoic Acid in a Cellular Model of Sarcopenia. Molecules 2020, 25, 2117. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-Related Inflammation and Insulin Resistance: A Review of Their Intricate Interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef]
- Aleman-Mateo, H.; Lopez Teros, M.T.; Ramirez, C.F.A.; Astiazaran-Garcia, H. Association between Insulin Resistance and Low Relative Appendicular Skeletal Muscle Mass: Evidence from a Cohort Study in Community-Dwelling Older Men and Women Participants. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and Risk of Physical Disability in Adults: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Graham, J.E.; Al Snih, S.; Raji, M.; Samper-Ternent, R.; Ostir, G.V.; Markides, K.S. Mexican Americans and Frailty: Findings From the Hispanic Established Populations Epidemiologic Studies of the Elderly. Am. J. Public Health 2009, 99, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Andrew, M.K.; Fallah, N.; Rockwood, K. Comparison of the Prognostic Importance of Diagnosed Diabetes, Co-Morbidity and Frailty in Older People: Diabetes and Frailty. Diabet. Med. 2010, 27, 603–606. [Google Scholar] [CrossRef]
- Saum, K.-U.; Dieffenbach, A.K.; Müller, H.; Holleczek, B.; Hauer, K.; Brenner, H. Frailty Prevalence and 10-Year Survival in Community-Dwelling Older Adults: Results from the ESTHER Cohort Study. Eur. J. Epidemiol. 2014, 29, 171–179. [Google Scholar] [CrossRef]
- Lee, J.S.W.; Auyeung, T.-W.; Leung, J.; Kwok, T.; Leung, P.-C.; Woo, J. Physical Frailty in Older Adults Is Associated with Metabolic and Atherosclerotic Risk Factors and Cognitive Impairment Independent of Muscle Mass. J. Nutr. Health Aging 2011, 15, 857–862. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J.C. Patients with Type 2 Diabetes Show a Greater Decline in Muscle Mass, Muscle Strength, and Functional Capacity with Aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Mone, P.; Pansini, A.; Frullone, S.; de Donato, A.; Buonincontri, V.; De Blasiis, P.; Marro, A.; Morgante, M.; De Luca, A.; Santulli, G. Physical Decline and Cognitive Impairment in Frail Hypertensive Elders during COVID-19. Eur. J. Intern. Med. 2022, 99, 89–92. [Google Scholar] [CrossRef]
- Camafort, M.; Kario, K. Hypertension, Heart Failure, and Frailty in Older People: A Common but Unclear Situation. J. Clin. Hypertens. 2020, 22, 1763–1768. [Google Scholar] [CrossRef]
- Roshanravan, B.; Gamboa, J.; Wilund, K. Exercise and CKD: Skeletal Muscle Dysfunction and Practical Application of Exercise to Prevent and Treat Physical Impairments in CKD. Am. J. Kidney Dis. 2017, 69, 837–852. [Google Scholar] [CrossRef]
- Kaasik, P.; Umnova, M.; Pehme, A.; Alev, K.; Aru, M.; Selart, A.; Seene, T. Ageing and Dexamethasone Associated Sarcopenia: Peculiarities of Regeneration. J. Steroid Biochem. Mol. Biol. 2007, 105, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Aru, M.; Alev, K.; Pehme, A.; Purge, P.; Õnnik, L.; Ellam, A.; Kaasik, P.; Seene, T. Changes in Body Composition of Old Rats at Different Time Points After Dexamethasone Administration. Curr. Aging Sci. 2019, 11, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Attaix, D.; Ventadour, S.; Codran, A.; Béchet, D.; Taillandier, D.; Combaret, L. The Ubiquitin–Proteasome System and Skeletal Muscle Wasting. Essays Biochem. 2005, 41, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Auclair, D.; Garrel, D.R.; Chaouki Zerouala, A.; Ferland, L.H. Activation of the Ubiquitin Pathway in Rat Skeletal Muscle by Catabolic Doses of Glucocorticoids. Am. J. Physiol. Physiol. 1997, 272, C1007–C1016. [Google Scholar] [CrossRef]
- Santidrian, S.; Moreyra, M.; Munro, H.N.; Young, V.R. Effect of Corticosterone and Its Route of Administration on Muscle Protein Breakdown, Measured in Vivo by Urinary Excretion of Nτ-Methylhistidine in Rats: Response to Different Levels of Dietary Protein and Energy. Metabolism 1981, 30, 798–804. [Google Scholar] [CrossRef]
- Thompson, M.G.; Thom, A.; Partridge, K.; Garden, K.; Campbell, G.P.; Calder, G.; Palmer, R.M. Stimulation of Myofibrillar Protein Degradation and Expression of mRNA Encoding the Ubiquitin-Proteasome System in C2C12 Myotubes by Dexamethasone: Effect of the Proteasome Inhibitor MG-132. J. Cell. Physiol. 1999, 181, 455–461. [Google Scholar] [CrossRef]
- Menconi, M.; Gonnella, P.; Petkova, V.; Lecker, S.; Hasselgren, P.-O. Dexamethasone and Corticosterone Induce Similar, but Not Identical, Muscle Wasting Responses in Cultured L6 and C2C12 Myotubes. J. Cell. Biochem. 2008, 105, 353–364. [Google Scholar] [CrossRef]
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; Jackson, T.A. COVID-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345. [Google Scholar] [CrossRef]
- Szabo, C. Roles of Hydrogen Sulfide in the Pathogenesis of Diabetes Mellitus and Its Complications. Antioxid. Redox Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef]
- di Villa Bianca, R.D.E.; Coletta, C.; Mitidieri, E.; De Dominicis, G.; Rossi, A.; Sautebin, L.; Cirino, G.; Bucci, M.; Sorrentino, R. Hydrogen Sulphide Induces Mouse Paw Oedema through Activation of Phospholipase A2: H2S Induced Oedema Formation in Mice. Br. J. Pharmacol. 2010, 161, 1835–1842. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ferraz, J.G.P.; Muscara, M.N. Hydrogen Sulfide: An Endogenous Mediator of Resolution of Inflammation and Injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Gaddam, R.R. Hydrogen Sulfide in Inflammation: A Novel Mediator and Therapeutic Target. Antioxid. Redox Signal. 2021, 34, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Donnarumma, E.; Tramontano, T.; Brancaleone, V.; Cirino, G.; Bucci, M.; Sorrentino, R. Hydrogen Sulfide Is Involved in Dexamethasone-Induced Hypertension in Rat. Nitric Oxide 2015, 46, 80–86. [Google Scholar] [CrossRef]
- Ma, J.; Shi, C.; Liu, Z.; Han, B.; Guo, L.; Zhu, L.; Ye, T. Hydrogen Sulfide Is a Novel Regulator Implicated in Glucocorticoids-Inhibited Bone Formation. Aging 2019, 11, 7537–7552. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Moore, P.K. Dexamethasone Inhibits Lipopolysaccharide-induced Hydrogen Sulphide Biosynthesis in Intact Cells and in an Animal Model of Endotoxic Shock. J. Cell. Mol. Med. 2009, 13, 2684–2692. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-harbi, S.; Almasan, A. Caspase-3 Activation Is a Critical Determinant of Genotoxic Stress-Induced Apoptosis. In Apoptosis and Cancer; Mor, G., Alvero, A.B., Eds.; Springer: New York, NY, USA, 2015; Volume 1219, pp. 1–9. ISBN 9781493916603. [Google Scholar]
- Alway, S.E.; Siu, P.M. Nuclear Apoptosis Contributes to Sarcopenia. Exerc. Sport Sci. Rev. 2008, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Versteegden, E.E. Apoptosis in Muscle Atrophy: Relevance to Sarcopenia. Exp. Gerontol. 2005, 40, 473–481. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; De Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phyther. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome C. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–Initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–Dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Ow, Y.-L.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond Respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kalpage, H.A.; Bazylianska, V.; Recanati, M.A.; Fite, A.; Liu, J.; Wan, J.; Mantena, N.; Malek, M.H.; Podgorski, I.; Heath, E.I.; et al. Tissue-specific Regulation of Cytochrome c by Post-translational Modifications: Respiration, the Mitochondrial Membrane Potential, ROS, and Apoptosis. FASEB J. 2019, 33, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Kim, J.-K. Sulphide as an Inhibitor and Electron Donor for the Cytochrome c Oxidase System. Can. J. Biochem. 1982, 60, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide Inhibition of and Metabolism by Cytochrome c Oxidase. Biochem. Soc. Trans. 2013, 41, 1312–1316. [Google Scholar] [CrossRef]
- Zanardelli, M.; Micheli, L.; Nicolai, R.; Failli, P.; Ghelardini, C.; Di Cesare Mannelli, L. Different Apoptotic Pathways Activated by Oxaliplatin in Primary Astrocytes vs. Colo-Rectal Cancer Cells. Int. J. Mol. Sci. 2015, 16, 5386–5399. [Google Scholar] [CrossRef]
- Levine, R.L. Carbonyl Modified Proteins in Cellular Regulation, Aging, and Disease. Free Radic. Biol. Med. 2002, 32, 790–796. [Google Scholar] [CrossRef]
- Wedmann, R.; Bertlein, S.; Macinkovic, I.; Böltz, S.; Miljkovic, J.L.; Muñoz, L.E.; Herrmann, M.; Filipovic, M.R. Working with “H2S”: Facts and Apparent Artifacts. Nitric Oxide 2014, 41, 85–96. [Google Scholar] [CrossRef]
- Hansen, J.; Klass, M.; Harris, C.; Csete, M. A Reducing Redox Environment Promotes C2C12 Myogenesis: Implications for Regeneration in Aged Muscle. Cell Biol. Int. 2007, 31, 546–553. [Google Scholar] [CrossRef]
- Liu, X.; Heras, G.; Lauschke, V.M.; Mi, J.; Tian, G.; Gastaldello, S. High Glucose-Induced Oxidative Stress Accelerates Myogenesis by Altering SUMO Reactions. Exp. Cell Res. 2020, 395, 112234. [Google Scholar] [CrossRef]
- Steil, A.W.; Kailing, J.W.; Armstrong, C.J.; Walgenbach, D.G.; Klein, J.C. The Calmodulin Redox Sensor Controls Myogenesis. PLoS ONE 2020, 15, e0239047. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Villalobo, A. The Many Faces of Calmodulin in Cell Proliferation, Programmed Cell Death, Autophagy, and Cancer. Biochim. Biophys. Acta—Mol. Cell Res. 2014, 1843, 398–435. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J.; Han, Z.Q.; Li, Z.Y. Modulating Protein Activity and Cellular Function by Methionine Residue Oxidation. Amino Acids 2012, 43, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilisation in C2C12 mouse myotubes. Free Radic. Res. 2018, 52, 288–303. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione Synthesis Is Diminished in Patients with Uncontrolled Diabetes and Restored by Dietary Supplementation with Cysteine and Glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or Decrease Hydrogen Sulfide Exert Opposite Lipolysis, but Reduce Global Insulin Resistance in High Fatty Diet Induced Obese Mice. PLoS ONE 2013, 8, e73892. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic Improves Insulin Sensitivity and Associated Metabolic Syndromes in Fructose Fed Rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Vainio, H. Allium Vegetables and Organosulfur Compounds: Do They Help Prevent Cancer? Environ. Health Perspect. 2001, 109, 893–902. [Google Scholar] [CrossRef]
- Tocmo, R.; Liang, D.; Lin, Y.; Huang, D. Chemical and Biochemical Mechanisms Underlying the Cardioprotective Roles of Dietary Organopolysulfides. Front. Nutr. 2015, 2, 1. [Google Scholar] [CrossRef]
- Angelino, D.; Jeffery, E. Glucosinolate Hydrolysis and Bioavailability of Resulting Isothiocyanates: Focus on Glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- De Nicola, G.; Rollin, P.; Mazzon, E.; Iori, R. Novel Gram-Scale Production of Enantiopure R-Sulforaphane from Tuscan Black Kale Seeds. Molecules 2014, 19, 6975–6986. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Barone, M.; Amore, E.; Grigolo, B.; Filardo, G.; Iori, R.; Citi, V.; Calderone, V.; Grassi, F. Glucoraphanin Increases Intracellular Hydrogen Sulfide (H2S) Levels and Stimulates Osteogenic Differentiation in Human Mesenchymal Stromal Cell. Nutrients 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Fusco, F.; Russo, A.; Pagliara, V.; Tramontano, T.; Donnarumma, E.; Mirone, V.; Cirino, G.; Russo, G.; et al. Urothelium Muscarinic Activation Phosphorylates CBSSer227 via cGMP/PKG Pathway Causing Human Bladder Relaxation through H2S Production. Sci. Rep. 2016, 6, 31491. [Google Scholar] [CrossRef] [PubMed]
- Mitidieri, E.; Vanacore, D.; Turnaturi, C.; Sorrentino, R.; d’Emmanuele di Villa Bianca, R. Uterine Dysfunction in Diabetic Mice: The Role of Hydrogen Sulfide. Antioxidants 2020, 9, 917. [Google Scholar] [CrossRef]
- Disatnik, M.-H.; Chamberlain, J.S.; Rando, T.A. Dystrophin Mutations Predict Cellular Susceptibility to Oxidative Stress. Muscle Nerve 2000, 23, 784–792. [Google Scholar] [CrossRef]

| Treatment | Cell Viability |
|---|---|
| control | 100 ± 2.4 |
| dexamethasone 1 μM | 92.3 ± 0.6 |
| dexamethasone 1 μM + glucoraphanin 30 μM | 103.3 ± 2.3 |
| dexamethasone 1 μM + L-cysteine 150 μM | 99.8 ± 3.1 |
| dexamethasone 1 μM + 3-mercaptopyruvate 150 μM | 105.4 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, L.; Mitidieri, E.; Turnaturi, C.; Vanacore, D.; Ciampi, C.; Lucarini, E.; Cirino, G.; Ghelardini, C.; Sorrentino, R.; Di Cesare Mannelli, L.; et al. Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin. Int. J. Mol. Sci. 2022, 23, 5955. https://doi.org/10.3390/ijms23115955
Micheli L, Mitidieri E, Turnaturi C, Vanacore D, Ciampi C, Lucarini E, Cirino G, Ghelardini C, Sorrentino R, Di Cesare Mannelli L, et al. Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin. International Journal of Molecular Sciences. 2022; 23(11):5955. https://doi.org/10.3390/ijms23115955
Chicago/Turabian StyleMicheli, Laura, Emma Mitidieri, Carlotta Turnaturi, Domenico Vanacore, Clara Ciampi, Elena Lucarini, Giuseppe Cirino, Carla Ghelardini, Raffaella Sorrentino, Lorenzo Di Cesare Mannelli, and et al. 2022. "Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin" International Journal of Molecular Sciences 23, no. 11: 5955. https://doi.org/10.3390/ijms23115955
APA StyleMicheli, L., Mitidieri, E., Turnaturi, C., Vanacore, D., Ciampi, C., Lucarini, E., Cirino, G., Ghelardini, C., Sorrentino, R., Di Cesare Mannelli, L., & d’Emmanuele di Villa Bianca, R. (2022). Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin. International Journal of Molecular Sciences, 23(11), 5955. https://doi.org/10.3390/ijms23115955









