Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation
Abstract
:1. Introduction
2. Results
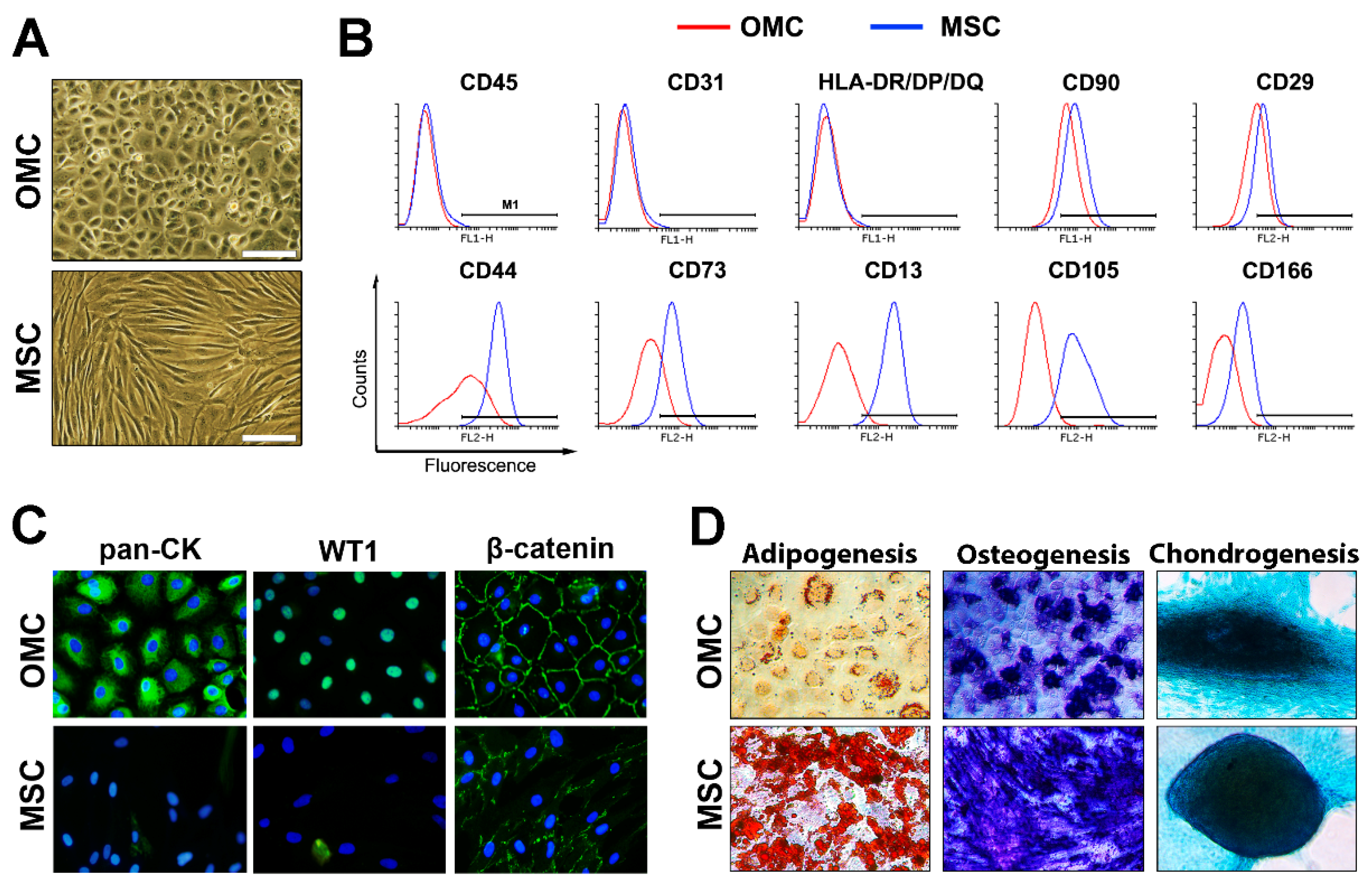
2.1. Omental-Derived Mesothelial Cells Exhibit a Typical Phenotype
2.2. Omental Mesothelial Cells Display a Mixed Pro- and Anti-Inflammatory Signature
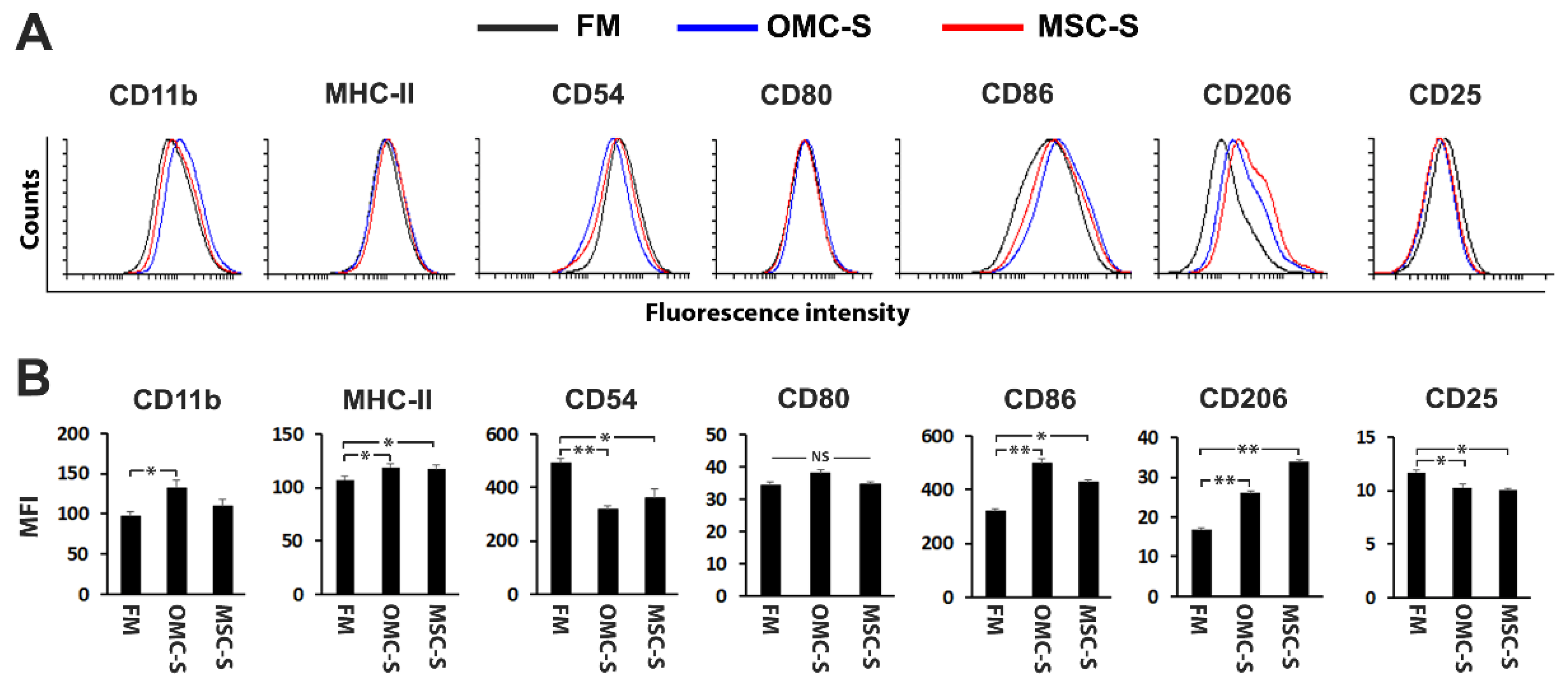
2.3. The Secretome of OMC Induces M2 Polarization and Inhibits Mouse Lymphocyte Proliferation and Agglutination
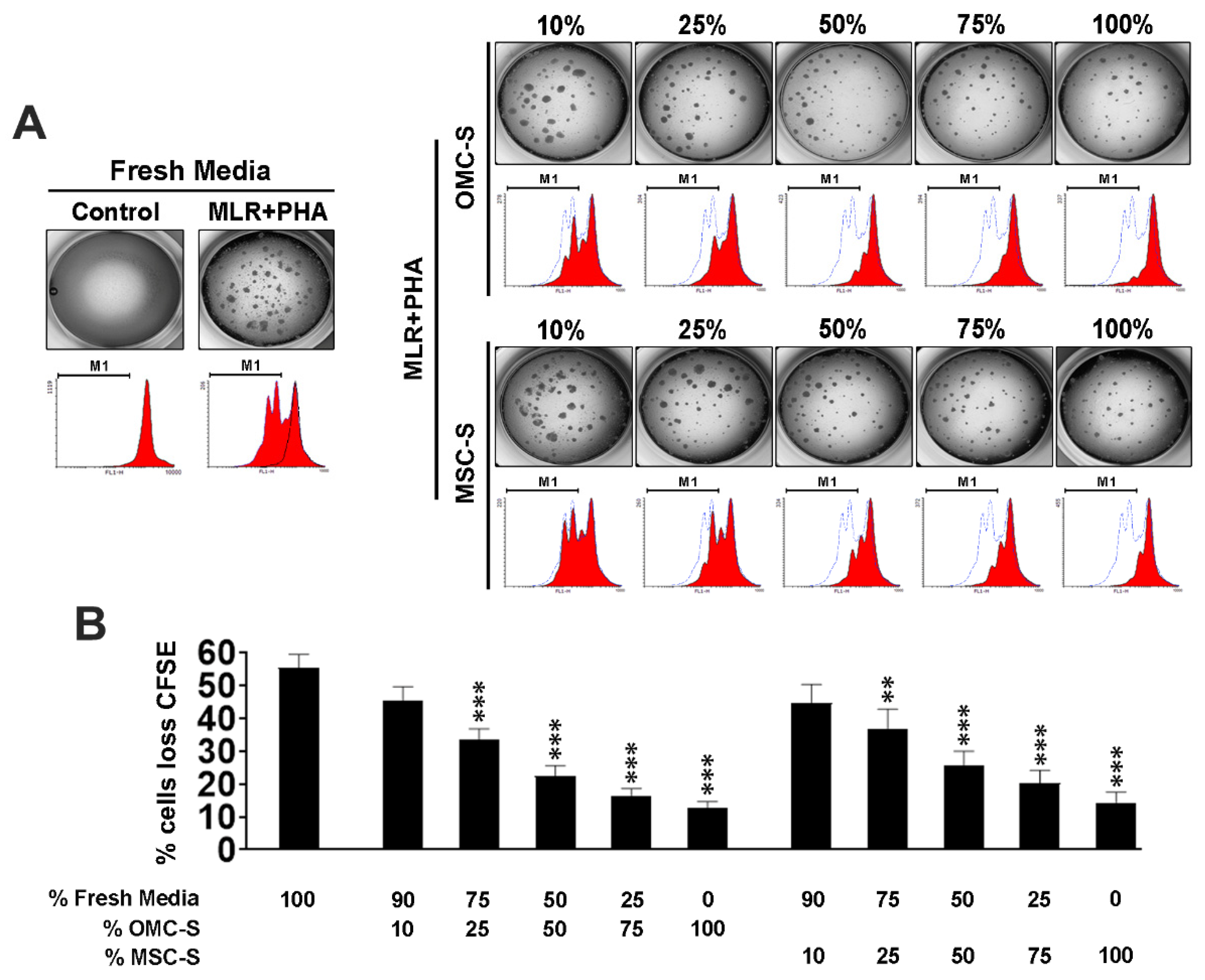
2.4. Omental Mesothelial Cell Secretome Inhibits the Activation of Mouse T and B Cells
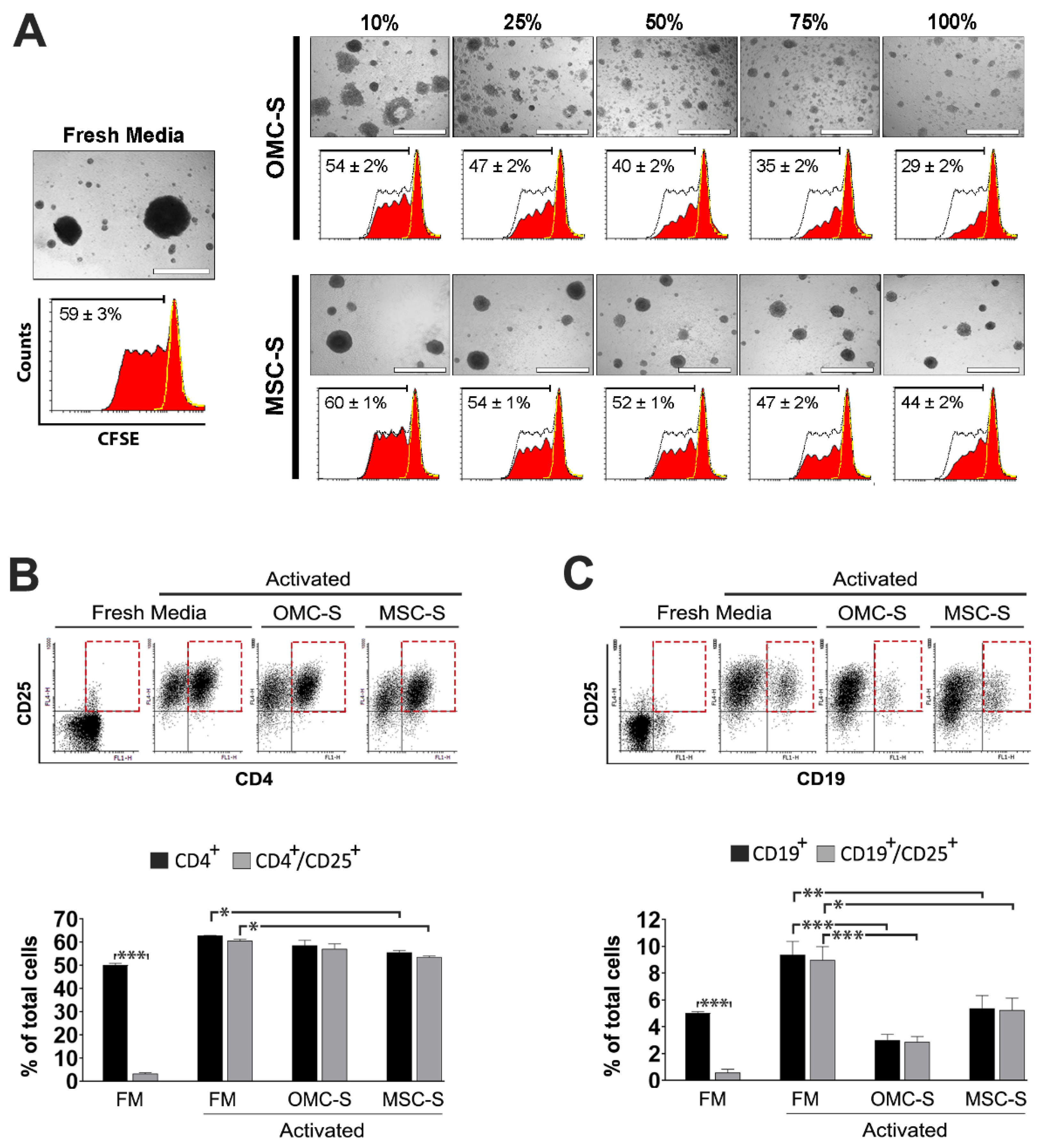
2.5. Activation and Proliferation of Human Lymphocytes and B Cells Are Blunted by OMC-S
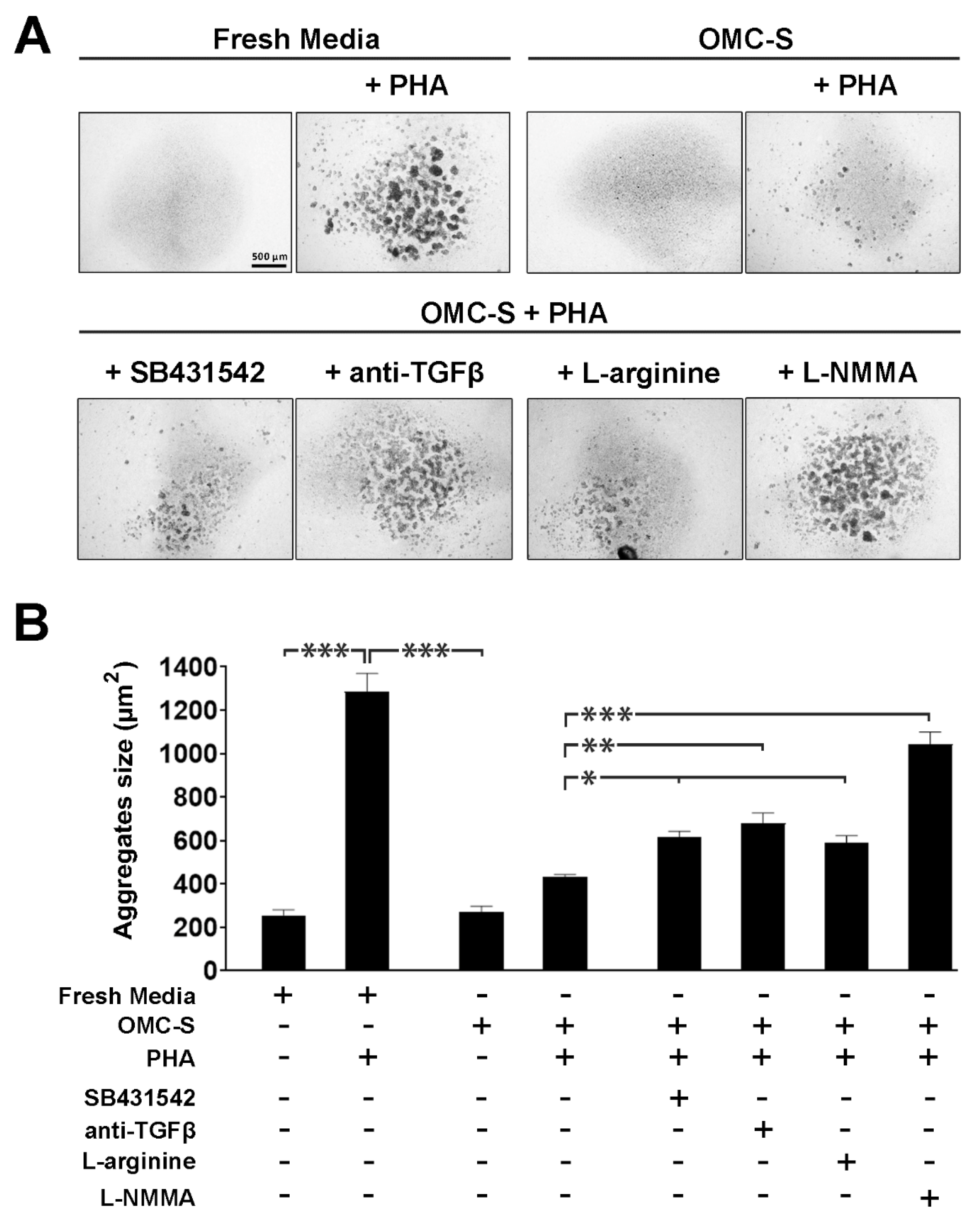
2.6. The Lymphosuppressive Activity of OMC-Derived Secretome Is Conveyed via TGFβ as Well as Nitric Oxide Synthase Activity
3. Discussion
4. Materials and Methods
4.1. Isolation of Human Omental Mesothelial Cells (OMC)
4.2. Culture of Human OMC
4.3. Culture of Human Mesenchymal Stem Cells (MSC)
4.4. Production of OMC- and MSC-Derived Secretomes
4.5. Immunofluorescence Characterization of OMC and MSC
4.6. Flow Cytometry
4.7. Mesodermal Multipotency Analysis of Human OMC and MSC
4.8. RNA Isolation and Quantitative PCR Analysis of OMC and MSC
4.9. Isolation of Mouse Lymphocytes
4.10. Cytokine Array
4.11. M1/M2 Macrophage Polarization Study
4.12. Human Peripheral Blood Mononuclear Cells Isolation
4.13. Quantification of Proliferation of Human Peripheral Blood Lymphocytes
4.14. Measurements of Activated T and B Cells in Human Peripheral Blood Lymphocytes Cultures
4.15. Culture Conditions of Mouse and Human Lymphocytes
4.16. Quantification of Lymphocytes Agglutination
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Wilkosz, S. Structure and function of mesothelial cells. Cancer Treat. Res. 2007, 134, 1–19. [Google Scholar]
- Mutsaers, S.E.; Prele, C.M.; Lansley, S.M.; Herrick, S.E. The origin of regenerating mesothelium: A historical perspective. Int. J. Artif. Organs 2007, 30, 484–494. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef]
- Kawanishi, K. Mesothelial cell transplantation: History, challenges and future directions. Pleura Peritoneum 2016, 1, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Colmont, C.S.; Raby, A.C.; Dioszeghy, V.; Lebouder, E.; Foster, T.L.; Jones, S.A.; Labeta, M.O.; Fielding, C.A.; Topley, N. Human peritoneal mesothelial cells respond to bacterial ligands through a specific subset of Toll-like receptors. Nephrol Dial. Transplant. 2011, 26, 4079–4090. [Google Scholar] [CrossRef] [Green Version]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural Heteropolysaccharide Adhesion to the Glycocalyx of Visceral Mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Katz, S.; Zsiros, V.; Kiss, A.L. Under inflammatory stimuli mesenteric mesothelial cells transdifferentiate into macrophages and produce pro-inflammatory cytokine IL-6. Inflamm. Res. 2019, 68, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Silva-Sanchez, A.; Randall, T.D.; Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2021, 109, 717–729. [Google Scholar] [CrossRef]
- Ivanov, S.; Gallerand, A.; Gros, M.; Stunault, M.I.; Merlin, J.; Vaillant, N.; Yvan-Charvet, L.; Guinamard, R.R. Mesothelial cell CSF1 sustains peritoneal macrophage proliferation. Eur. J. Immunol. 2019, 49, 2012–2018. [Google Scholar] [CrossRef]
- Riera, M.; McCulloch, P.; Pazmany, L.; Jagoe, T. Optimal method for isolation of human peritoneal mesothelial cells from clinical samples of omentum. J. Tissue Viability 2006, 16, 22–24. [Google Scholar] [CrossRef]
- Pronk, A.; Leguit, P.; Hoynck van Papendrecht, A.A.; Hagelen, E.; van Vroonhoven, T.J.; Verbrugh, H.A. A cobblestone cell isolated from the human omentum: The mesothelial cell; isolation, identification, and growth characteristics. Vitr. Cell. Dev. Biol. J. Tissue Cult. Assoc. 1993, 29A, 127–134. [Google Scholar] [CrossRef]
- Lachaud, C.C.; Lopez-Beas, J.; Soria, B.; Hmadcha, A. EGF-induced adipose tissue mesothelial cells undergo functional vascular smooth muscle differentiation. Cell Death Dis. 2014, 5, e1304. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, S.E.; Di Paolo, N. Future directions in mesothelial transplantation research. Int. J. Artif. Organs 2007, 30, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Lachaud, C.C.; Pezzolla, D.; Dominguez-Rodriguez, A.; Smani, T.; Soria, B.; Hmadcha, A. Functional vascular smooth muscle-like cells derived from adult mouse uterine mesothelial cells. PLoS ONE 2013, 8, e55181. [Google Scholar] [CrossRef] [Green Version]
- Lansley, S.M.; Searles, R.G.; Hoi, A.; Thomas, C.; Moneta, H.; Herrick, S.E.; Thompson, P.J.; Newman, M.; Sterrett, G.F.; Prele, C.M.; et al. Mesothelial cell differentiation into osteoblast- and adipocyte-like cells. J. Cell. Mol. Med. 2011, 15, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.; Knaan-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef]
- Elmadbouh, I.; Chen, Y.; Louedec, L.; Silberman, S.; Pouzet, B.; Meilhac, O.; Michel, J.B. Mesothelial cell transplantation in the infarct scar induces neovascularization and improves heart function. Cardiovasc. Res. 2005, 68, 307–317. [Google Scholar] [CrossRef]
- Elmadbouh, I.; Michel, J.B.; Chachques, J.C. Mesothelial cell transplantation in myocardial infarction. Int. J. Artif. Organs 2007, 30, 541–549. [Google Scholar] [CrossRef]
- Asano, T.; Takazawa, R.; Yamato, M.; Takagi, R.; Iimura, Y.; Masuda, H.; Kihara, K.; Okano, T. Transplantation of an autologous mesothelial cell sheet prepared from tunica vaginalis prevents post-operative adhesions in a canine model. Tissue Eng. 2006, 12, 2629–2637. [Google Scholar] [CrossRef]
- Bresson, L.; Leblanc, E.; Lemaire, A.S.; Okitsu, T.; Chai, F. Autologous peritoneal grafts permit rapid reperitonealization and prevent postoperative abdominal adhesions in an experimental rat study. Surgery 2017, 162, 863–870. [Google Scholar] [CrossRef]
- Kawanishi, K.; Nitta, K. Cell sheet-based tissue engineering for mesothelial cell injury. Contrib. Nephrol. 2015, 185, 66–75. [Google Scholar] [CrossRef]
- Kawanishi, K.; Nitta, K.; Yamato, M.; Okano, T. Therapeutic applications of mesothelial cell sheets. Ther. Apher. Dial. 2015, 19, 1–7. [Google Scholar] [CrossRef]
- Verhagen, H.J.; Heijnen-Snyder, G.J.; Pronk, A.; Vroom, T.M.; van Vroonhoven, T.J.; Eikelboom, B.C.; Sixma, J.J.; de Groot, P.G. Thrombomodulin activity on mesothelial cells: Perspectives for mesothelial cells as an alternative for endothelial cells for cell seeding on vascular grafts. Br. J. Haematol. 1996, 95, 542–549. [Google Scholar] [CrossRef]
- Kobori, L.; Nemeth, T.; Nemes, B.; Dallos, G.; Sotonyi, P., Jr.; Fehervari, I.; Patonai, A.; Slooff, M.J.; Jaray, J.; De Jong, K.P. Experimental vascular graft for liver transplantation. Acta Vet. Hung 2003, 51, 529–537. [Google Scholar] [CrossRef]
- Lachaud, C.C.; Soria, F.; Escacena, N.; Quesada-Hernandez, E.; Hmadcha, A.; Alio, J.; Soria, B. Mesothelial cells: A cellular surrogate for tissue engineering of corneal endothelium. Invest. Ophthalmol Vis. Sci 2014, 55, 5967–5978. [Google Scholar] [CrossRef] [Green Version]
- Lachaud, C.C.; Rodriguez-Campins, B.; Hmadcha, A.; Soria, B. Use of Mesothelial Cells and Biological Matrices for Tissue Engineering of Simple Epithelium Surrogates. Front. Bioeng. Biotechnol. 2015, 3, 117. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Kift-Morgan, A.; Moser, B.; Topley, N.; Eberl, M. Suppression of pro-inflammatory T-cell responses by human mesothelial cells. Nephrol. Dial. Transpl. 2013, 28, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, J.; Emoto, S.; Yamaguchi, H.; Ishigami, H.; Yamashita, H.; Seto, Y.; Matsuzaki, K.; Watanabe, T. CD90(+)CD45(−) intraperitoneal mesothelial-like cells inhibit T cell activation by production of arginase I. Cell Immunol. 2014, 288, 8–14. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Roszik, J.; Grimm, E.A.; Ekmekcioglu, S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front. Oncol. 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [Green Version]
- Herrick, S.E.; Mutsaers, S.E. The potential of mesothelial cells in tissue engineering and regenerative medicine applications. Int. J. Artif. Organs 2007, 30, 527–540. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Mercalli, A.; Calavita, I.; Dugnani, E.; Citro, A.; Cantarelli, E.; Nano, R.; Melzi, R.; Maffi, P.; Secchi, A.; Sordi, V.; et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology 2013, 140, 179–190. [Google Scholar] [CrossRef]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.H.; Paunel-Gorgulu, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Jostock, T.; Mullberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 35, e1488. [Google Scholar] [CrossRef]
- Yoshimoto, M. The ontogeny of murine B-1a cells. Int. J. Hematol. 2020, 111, 622–627. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espagnolle, N.; Balguerie, A.; Arnaud, E.; Sensebe, L.; Varin, A. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Rep. 2017, 8, 961–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef]
- Valenti, L.M.; Mathieu, J.; Chancerelle, Y.; De Sousa, M.; Levacher, M.; Dinh-Xuan, A.T.; Florentin, I. High levels of endogenous nitric oxide produced after burn injury in rats arrest activated T lymphocytes in the first G1 phase of the cell cycle and then induce their apoptosis. Exp. Cell Res. 2005, 306, 150–167. [Google Scholar] [CrossRef]
- Valenti, L.; Mathieu, J.; Chancerelle, Y.; Levacher, M.; Chanaud, B.; De Sousa, M.; Strzalko, S.; Dinh-Xuan, A.T.; Giroud, J.P.; Florentin, I. Nitric oxide inhibits spleen cell proliferative response after burn injury by inducing cytostasis, apoptosis, and necrosis of activated T lymphocytes: Role of the guanylate cyclase. Cell. Immunol. 2003, 221, 50–63. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Choi, K.S.; Song, E.K.; Yim, C.Y. Cytokines secreted by IL-2-activated lymphocytes induce endogenous nitric oxide synthesis and apoptosis in macrophages. J. Leukoc. Biol. 2008, 83, 1440–1450. [Google Scholar] [CrossRef]
- Lu, L.; Bonham, C.A.; Chambers, F.G.; Watkins, S.C.; Hoffman, R.A.; Simmons, R.L.; Thomson, A.W. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: Nitric oxide production is associated with dendritic cell apoptosis. J. Immunol. 1996, 157, 3577–3586. [Google Scholar]
- Connell, N.D.; Rheinwald, J.G. Regulation of the cytoskeleton in mesothelial cells: Reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell 1983, 34, 245–253. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, R.; Bao, Q.; Li, Y. Regulation of M1type and M2type macrophage polarization in RAW264.7 cells by Galectin9. Mol. Med. Rep. 2017, 16, 9111–9119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Xu, Y.; Lai, Y.; He, W.; Li, Y.; Wang, R.; Luo, X.; Chen, R.; Chen, T. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J. Cell. Biochem. 2018, 119, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Di Carluccio, A.R.; Tresoldi, E.; So, M.; Mannering, S.I. Quantification of Proliferating Human Antigen-specific CD4+ T Cells using Carboxyfluorescein Succinimidyl Ester. J. Vis. Exp. 2019, e59545. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, B.R.; Rubio-Contreras, D.; Gómez-Rosado, J.C.; Capitán-Morales, L.C.; Hmadcha, A.; Soria, B.; Lachaud, C.C. Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation. Int. J. Mol. Sci. 2022, 23, 5924. https://doi.org/10.3390/ijms23115924
Gauthier BR, Rubio-Contreras D, Gómez-Rosado JC, Capitán-Morales LC, Hmadcha A, Soria B, Lachaud CC. Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation. International Journal of Molecular Sciences. 2022; 23(11):5924. https://doi.org/10.3390/ijms23115924
Chicago/Turabian StyleGauthier, Benoit R., Diana Rubio-Contreras, Juan Carlos Gómez-Rosado, Luis Cristobal Capitán-Morales, Abdelkrim Hmadcha, Bernat Soria, and Christian Claude Lachaud. 2022. "Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation" International Journal of Molecular Sciences 23, no. 11: 5924. https://doi.org/10.3390/ijms23115924
APA StyleGauthier, B. R., Rubio-Contreras, D., Gómez-Rosado, J. C., Capitán-Morales, L. C., Hmadcha, A., Soria, B., & Lachaud, C. C. (2022). Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation. International Journal of Molecular Sciences, 23(11), 5924. https://doi.org/10.3390/ijms23115924








