Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
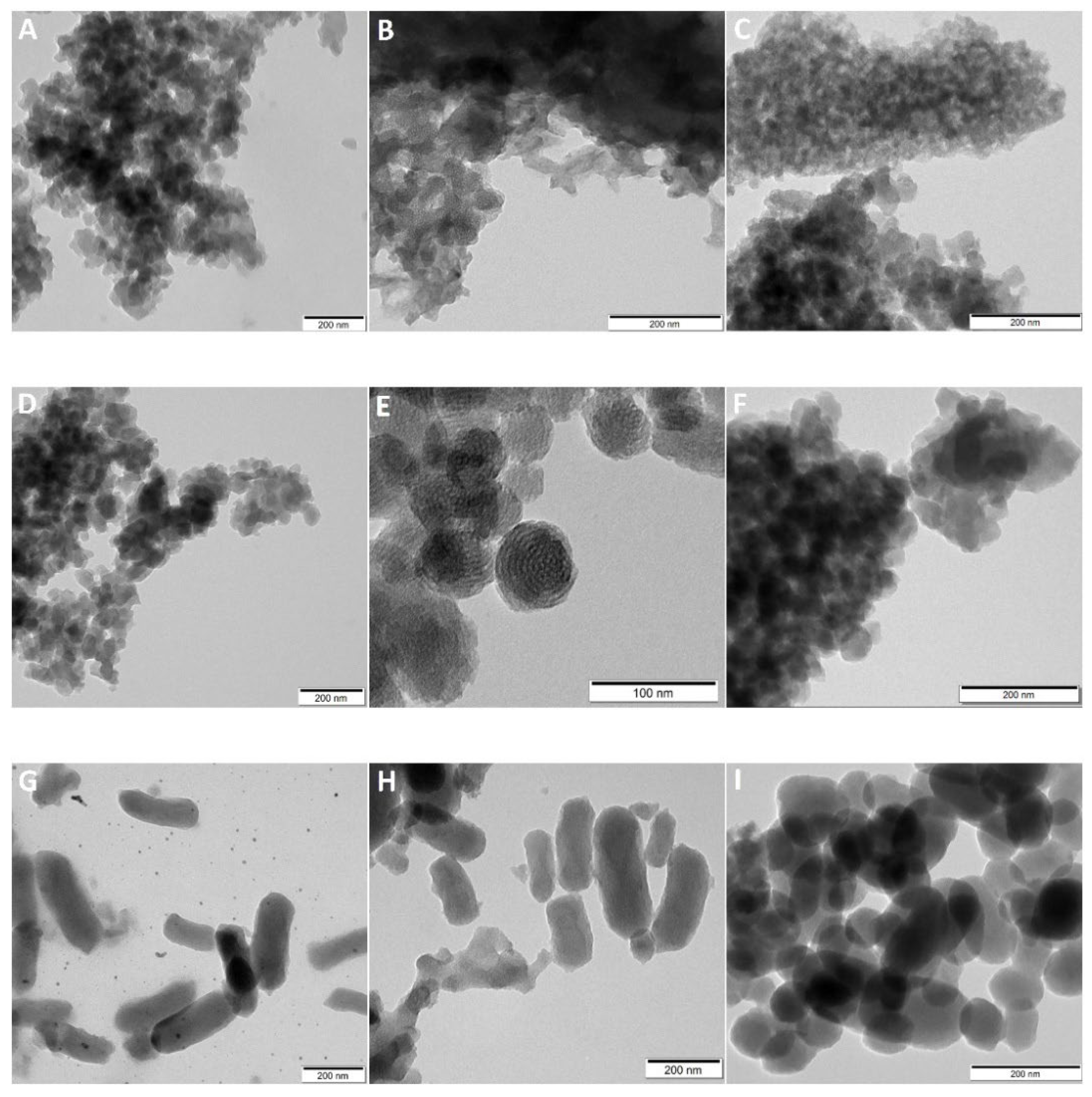
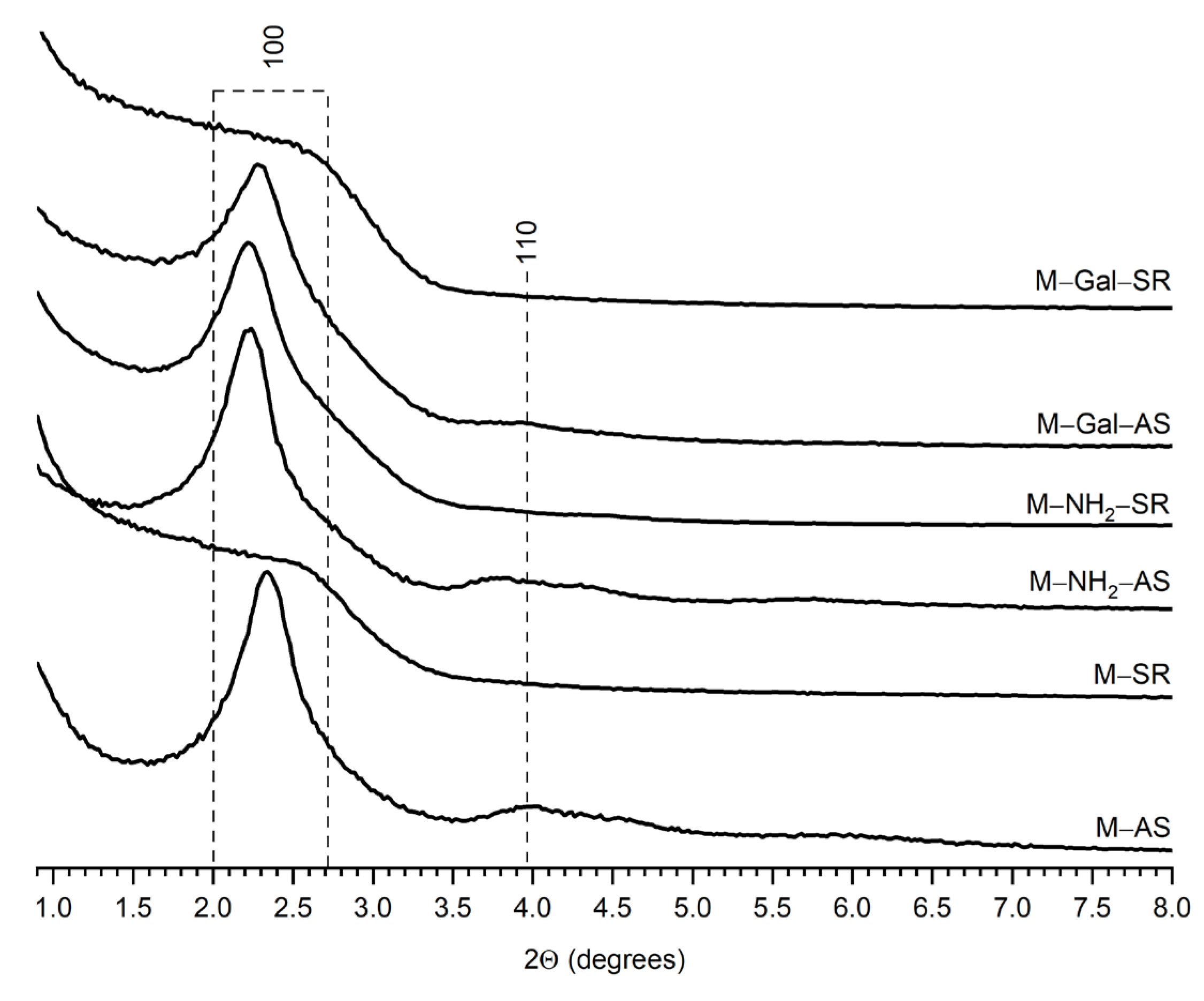
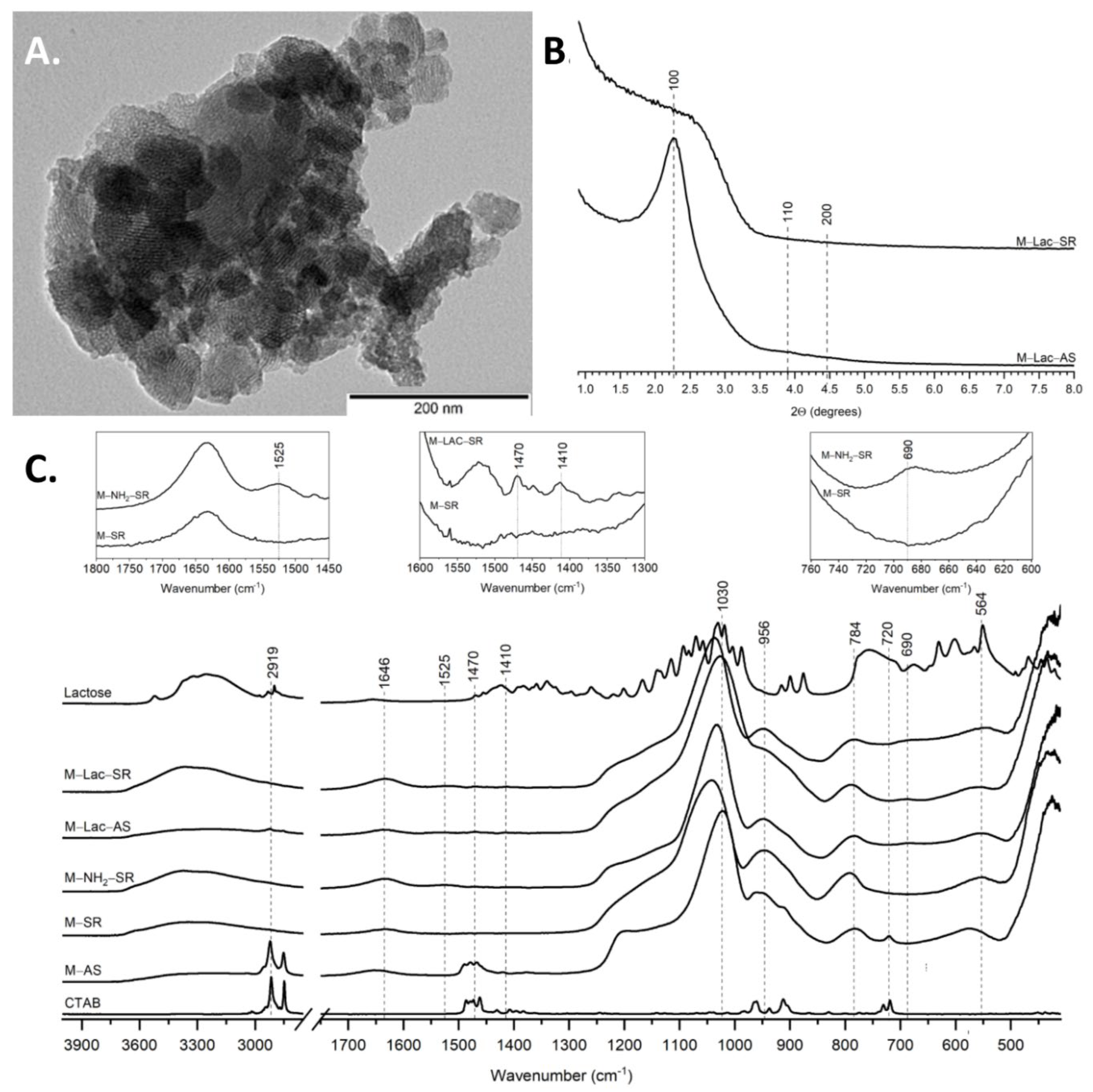
2.1. Particle Size, Morphology and Pore Architecture
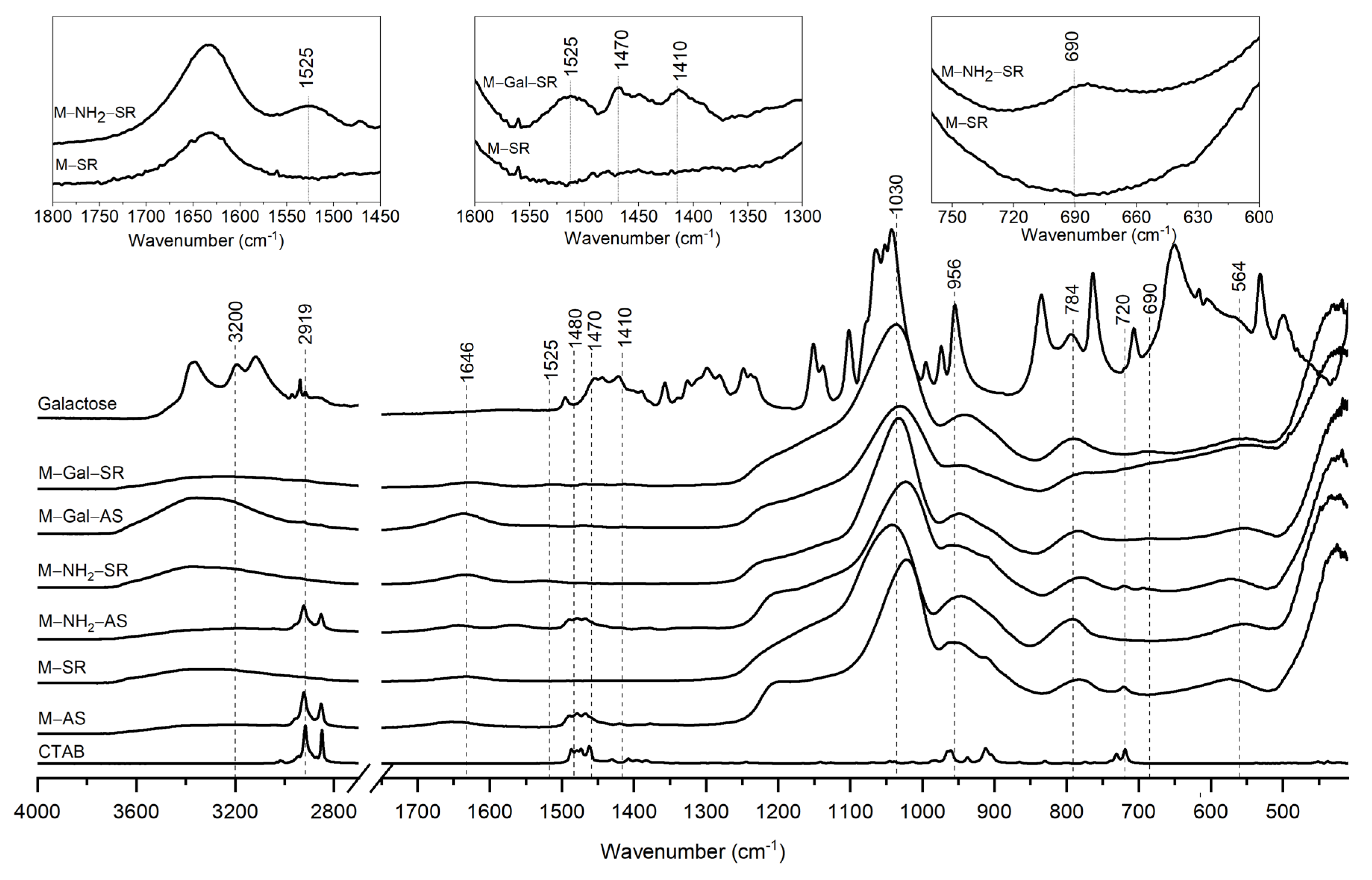
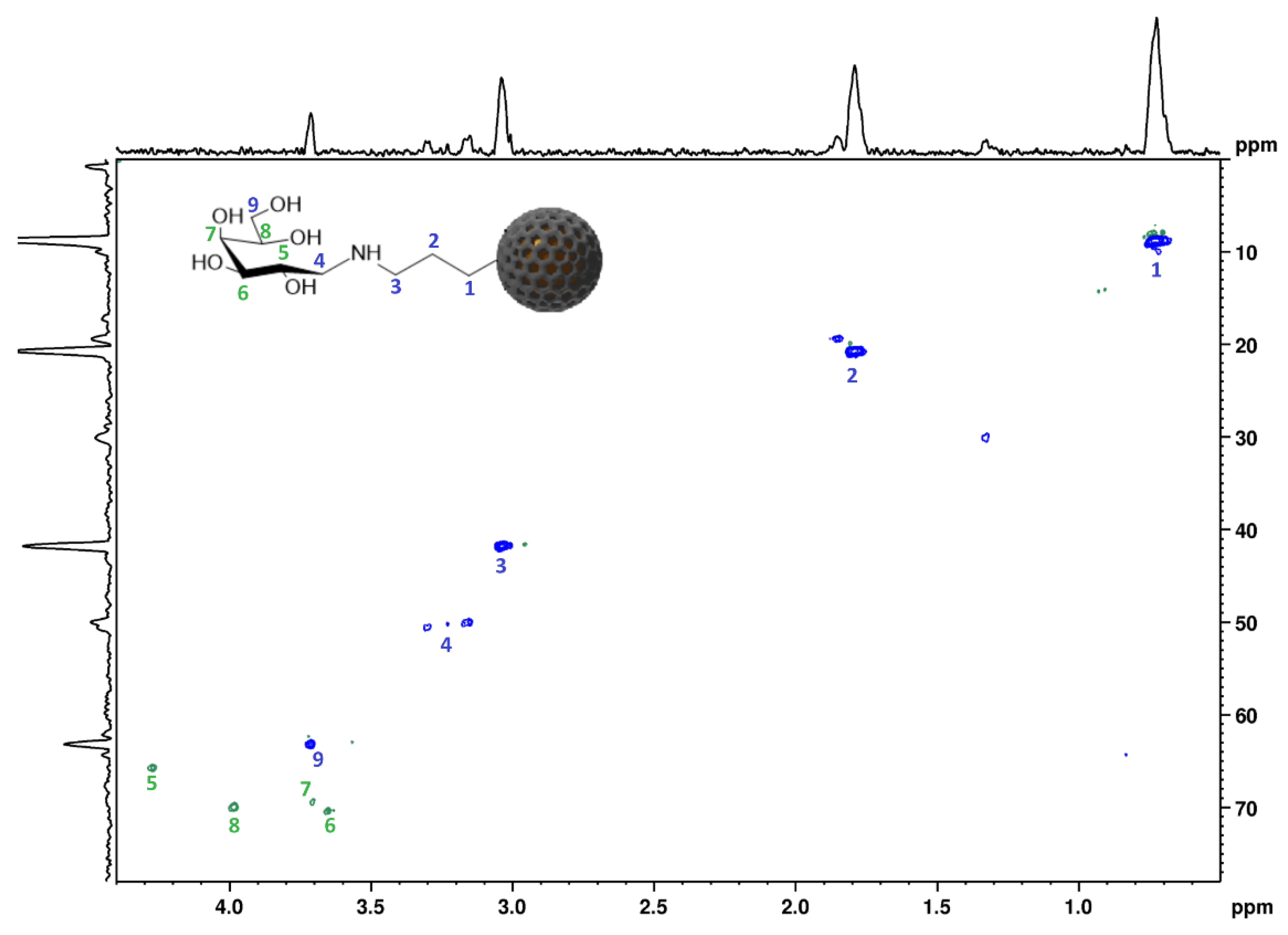
2.2. Surface Functionalisation with GAL and LAC
3. Materials and Methods
3.1. MSN Synthesis
3.2. MSN Functionalisation
3.3. Particle Size and Shape
3.4. Low Angle Powder X-ray Diffraction
3.5. N2 Adsorption
3.6. Fourier Transformed Infrared Spectroscopy (FTIR)
3.7. Nuclear Magnetic Resonance (NMR) Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862. [Google Scholar] [CrossRef] [PubMed]
- Giret, S.; Man, M.W.C.; Carcel, C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chem. A Eur. J. 2015, 21, 13850–13865. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol-Gel Process and Applications in Controlled Release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Shahabi, S.; Döscher, S.; Bollhorst, T.; Treccani, L.; Maas, M.; Dringen, R.; Rezwan, K. Enhancing Cellular Uptake and Doxorubicin Delivery of Mesoporous Silica Nanoparticles via Surface Functionalization: Effects of Serum. ACS Appl. Mater. Interfaces 2015, 7, 26880–26891. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Bharti, C.; Gulati, N.; Nagaich, U.; Pal, A. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles: Synthesis and Applications; Annual Review of Nanoresearch; World Scientific Publishing Co. Pte Ltd.: Singapore, 2009; Volume 3, pp. 191–231. [Google Scholar]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous Silica Nanoparticles: Synthesis, Classification, Drug Loading, Pharmacokinetics, Biocompatibility, and Application in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous Silica Nanoparticles in Medicine-Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Slowing, B.I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Mackowiak, S.A.; Schmidt, A.; Weiss, V.; Argyo, C.; Von Schirnding, C.; Bein, T.; Bräuchle, C. Targeted Drug Delivery in Cancer Cells with Red-Light Photoactivated Mesoporous Silica Nanoparticles. Nano Lett. 2013, 13, 2576–2583. [Google Scholar] [CrossRef]
- Mukherjee, M.B.; Mullick, R.; Reddy, B.U.; Das, S.; Raichur, A.M. Galactose Functionalized Mesoporous Silica Nanoparticles as Delivery Vehicle in the Treatment of Hepatitis C Infection. ACS Appl. Bio Mater. 2020, 3, 7598–7610. [Google Scholar] [CrossRef]
- Caldarelli, S.; Meden, A.; Tuel, A. Solid-State Nuclear Magnetic Resonance Study of the Microporous Aluminophosphate AlPO4-41. J. Phys. Chem. B 1999, 103, 5477–5487. [Google Scholar] [CrossRef]
- Davidowski, S.K.; Holland, G.P. Solid-State NMR Characterization of Mixed Phosphonic Acid Ligand Binding and Organization on Silica Nanoparticles. Langmuir 2016, 32, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Rapp, J.L.; Wiench, J.W.; Pruski, M. Characterization of nanostructured organic-inorganic hybrid materials using advanced solid-state NMR spectroscopy. In Proceedings of the Materials Research Society Symposium Proceedings, Ames, IA, USA, 5 January 2009; Volume 1184, pp. 175–183. [Google Scholar]
- Kobayashi, T.; Singappuli-Arachchige, D.; Wang, Z.; Slowing Ab, I.I.; Pruski, M. Spatial Distribution of Organic Functional Groups Supported on Mesoporous Silica Nanoparticles: A Study by Conventional and DNP-Enhanced 29 Si Solid-State NMR. Phys. Chem. Chem. Phys. 2017, 19, 1781. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Monin, G.; Duong, N.T.; Lopez, I.Z.; Bardet, M.; Mareau, V.; Gonon, L.; De Paëpe, G. Untangling the Condensation Network of Organosiloxanes on Nanoparticles Using 2D 29Si-29Si Solid-State NMR Enhanced by Dynamic Nuclear Polarization. J. Am. Chem. Soc. 2014, 136, 13781–13788. [Google Scholar] [CrossRef]
- Dai, F.R.; Sambasivam, U.; Hammerstrom, A.J.; Wang, Z. Synthetic Supercontainers Exhibit Distinct Solution versus Solid State Guest-Binding Behavior. J. Am. Chem. Soc. 2014, 136, 7480–7491. [Google Scholar] [CrossRef]
- Tataurova, Y.; Sealy, M.J.; Larsen, R.G.; Larsen, S.C. Surface-Selective Solution NMR Studies of Functionalized Zeolite Nanoparticles. J. Phys. Chem. Lett. 2012, 3, 425–429. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, L.; Zhang, R.; Wang, X.; Guo, C.; Sun, P.; Xue, G. Investigation on the Mechanism of the Synthesis of Gold (I) Thiolate Complexes by NMR. J. Phys. Chem. C 2014, 118, 10434–10440. [Google Scholar] [CrossRef]
- Hens, Z.; Moreels, I.; Martins, J.C. In Situ 1H NMR Study on the Trioctylphosphine Oxide Capping of Colloidal InP Nanocrystals. ChemPhysChem 2005, 6, 2578–2584. [Google Scholar] [CrossRef]
- Alam, T.M.; Jenkins, J.E. HR-MAS NMR Spectroscopy in Material Science; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Moestue, S.; Sitter, B.; Bathen, T.F.; Tessem, M.-B.; Susann Gribbestad, I. HR MAS MR Spectroscopy in Metabolic Characterization of Cancer. Curr. Top. Med. Chem. 2010, 11, 2–26. [Google Scholar] [CrossRef]
- Banfi, D.; Patiny, L. www.nmrdb.org: Resurrecting and Processing NMR Spectra On-Line. Chim. Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDB—Constructing a Free Chemical Information System with Open-Source Components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and Accurate Algorithm for the Simulation of NMR Spectra of Large Spin Systems. J. Magn. Reson. 2011, 209, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Hocine, O.; Brevet, D.; Maynadier, M.; Raehm, L.; Richeter, S.; Charasson, V.; Loock, B.; Morère, A.; Maillard, P.; et al. Cancer Therapy Improvement with Mesoporous Silica Nanoparticles Combining Targeting, Drug Delivery and PDT. Int. J. Pharm. 2012, 423, 509–515. [Google Scholar] [CrossRef]
- Oh, H.; Jo, H.-Y.; Park, J.; Kim, D.-E.; Cho, J.-Y.; Kim, P.-H.; Kim, K.-S. Galactosylated Liposomes for Targeted Co-Delivery of Doxorubicin/Vimentin SiRNA to Hepatocellular Carcinoma. Nanomaterials 2016, 6, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska, K.; Gołkowska, A.M.; Nowak, M.; Kozakiewicz-Latała, M.; Pudło, W.; Żak, A.; Karolewicz, B.; Khimyak, Y.Z.; Nartowski, K.P. Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy. Int. J. Mol. Sci. 2022, 23, 5906. https://doi.org/10.3390/ijms23115906
Krajewska K, Gołkowska AM, Nowak M, Kozakiewicz-Latała M, Pudło W, Żak A, Karolewicz B, Khimyak YZ, Nartowski KP. Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy. International Journal of Molecular Sciences. 2022; 23(11):5906. https://doi.org/10.3390/ijms23115906
Chicago/Turabian StyleKrajewska, Karolina, Anna M. Gołkowska, Maciej Nowak, Marta Kozakiewicz-Latała, Wojciech Pudło, Andrzej Żak, Bożena Karolewicz, Yaroslav Z. Khimyak, and Karol P. Nartowski. 2022. "Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy" International Journal of Molecular Sciences 23, no. 11: 5906. https://doi.org/10.3390/ijms23115906
APA StyleKrajewska, K., Gołkowska, A. M., Nowak, M., Kozakiewicz-Latała, M., Pudło, W., Żak, A., Karolewicz, B., Khimyak, Y. Z., & Nartowski, K. P. (2022). Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy. International Journal of Molecular Sciences, 23(11), 5906. https://doi.org/10.3390/ijms23115906








