Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption
Abstract
:1. Introduction
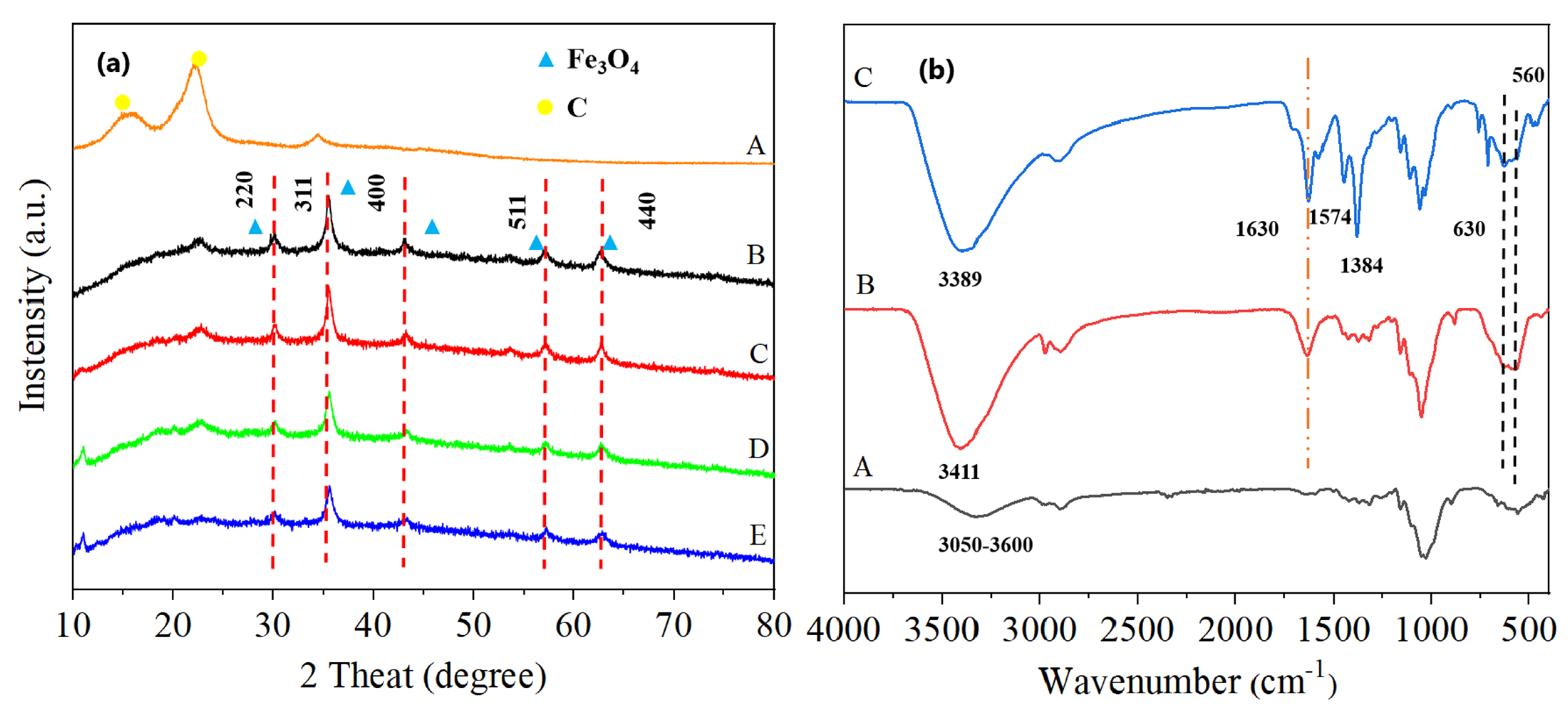
2. Results and Discussion
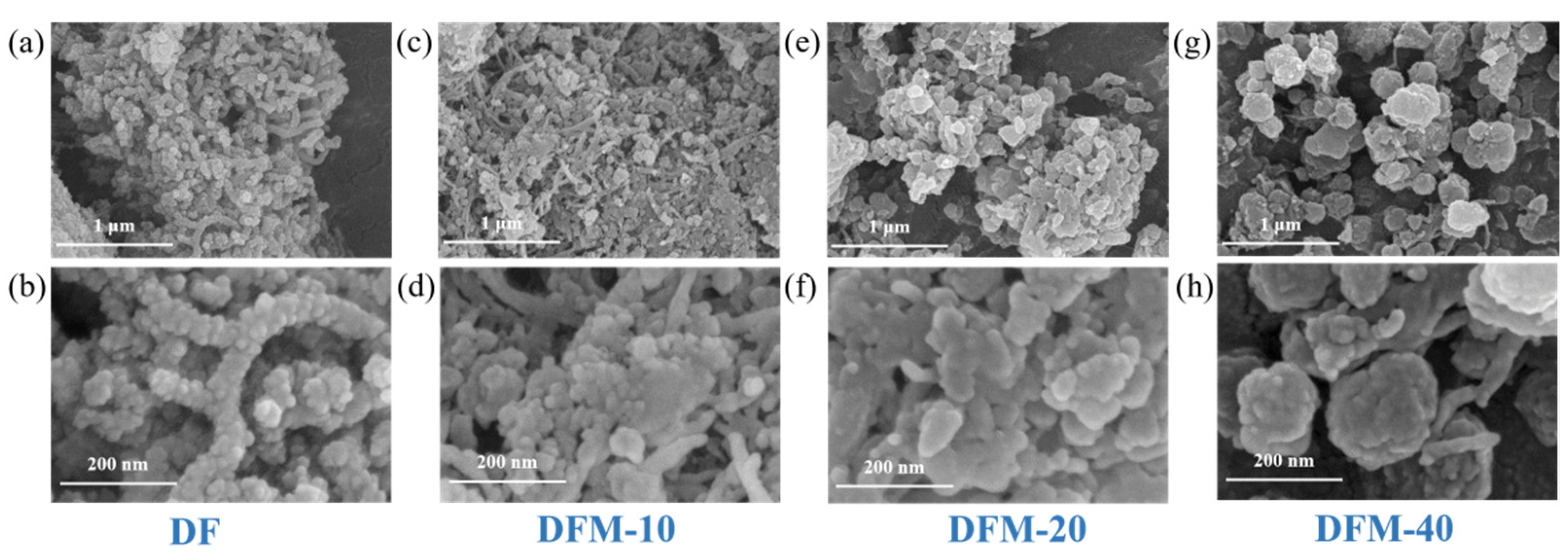
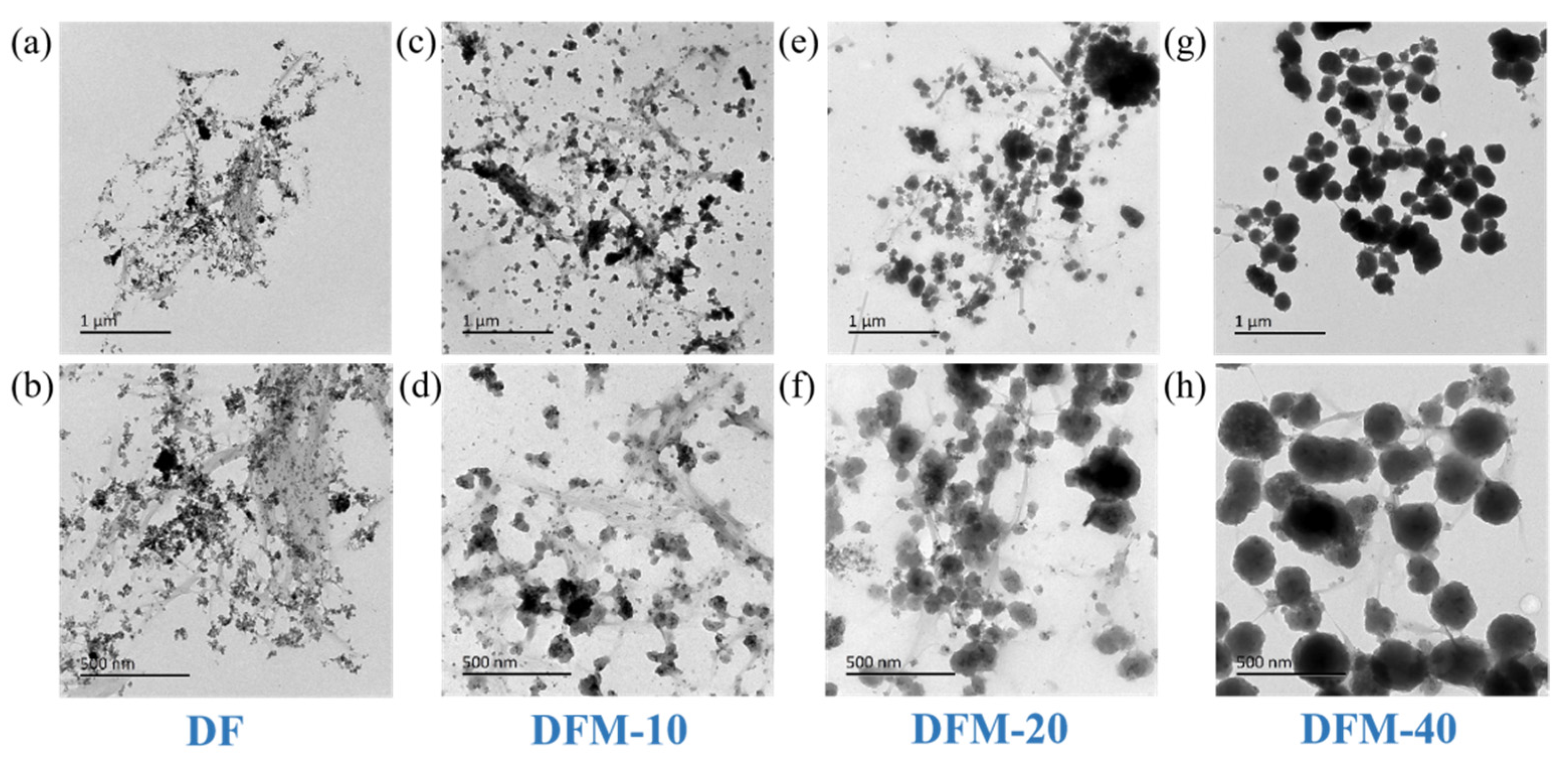
2.1. SEM and TEM Analysis
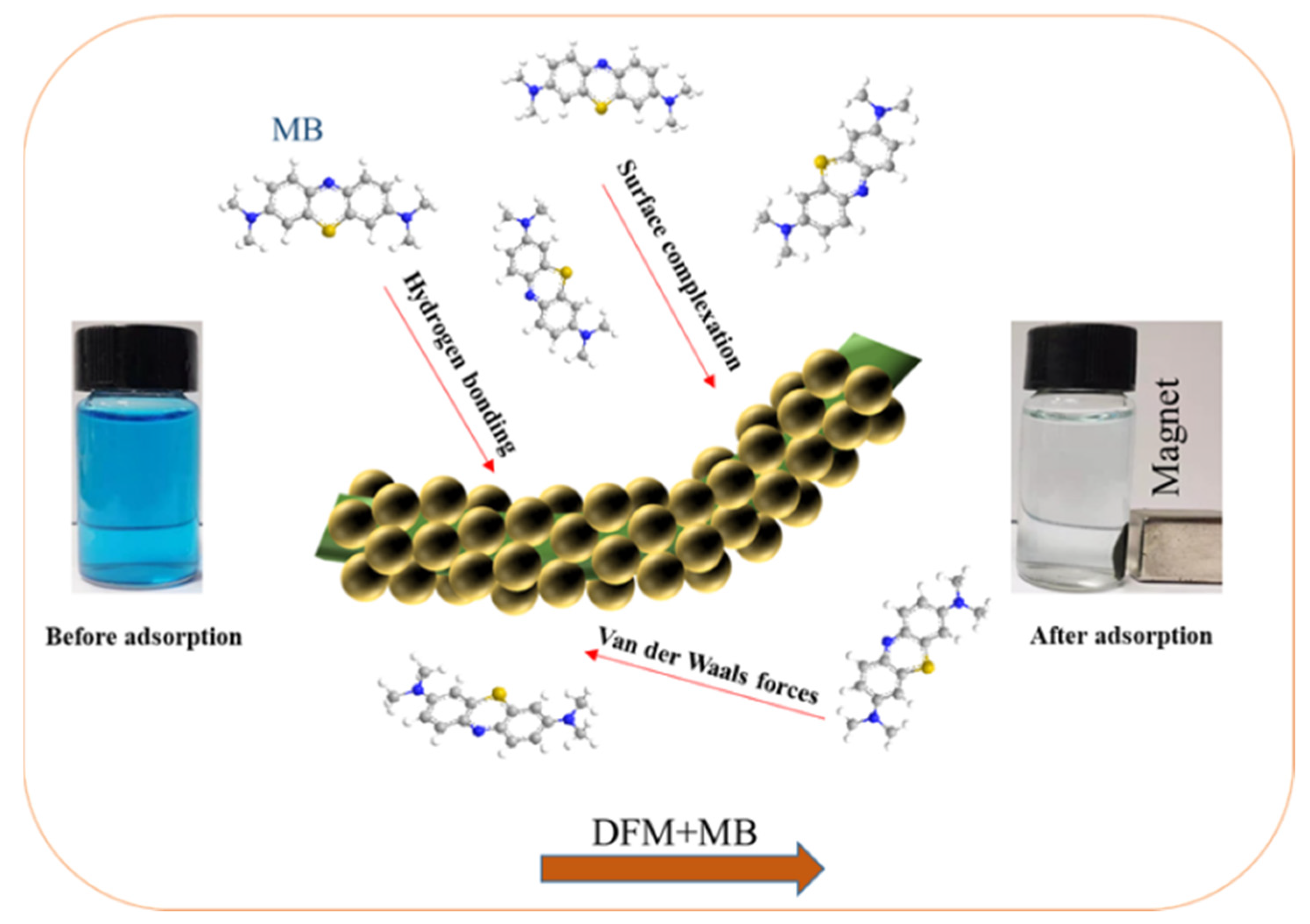
2.2. Adsorption Experiments
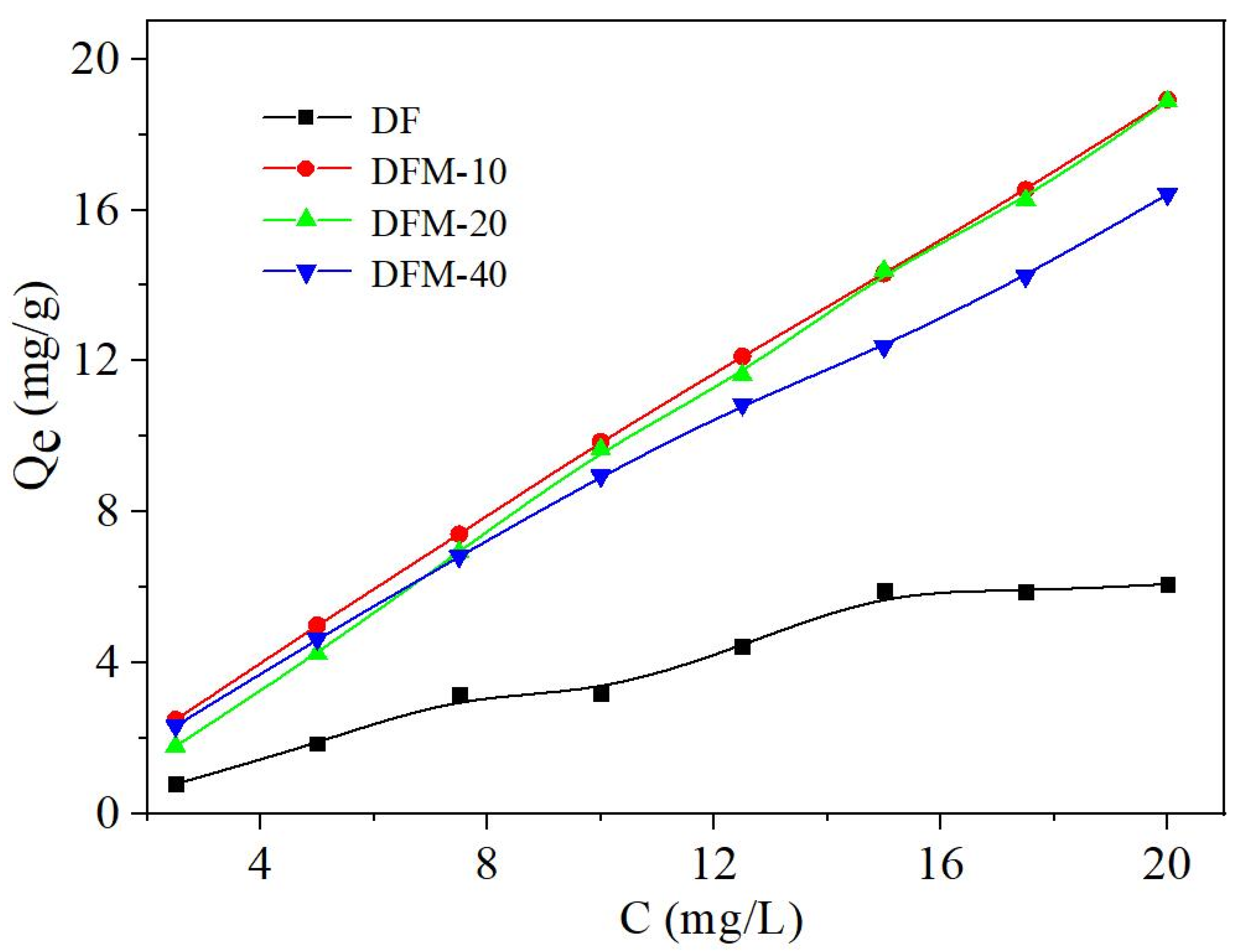
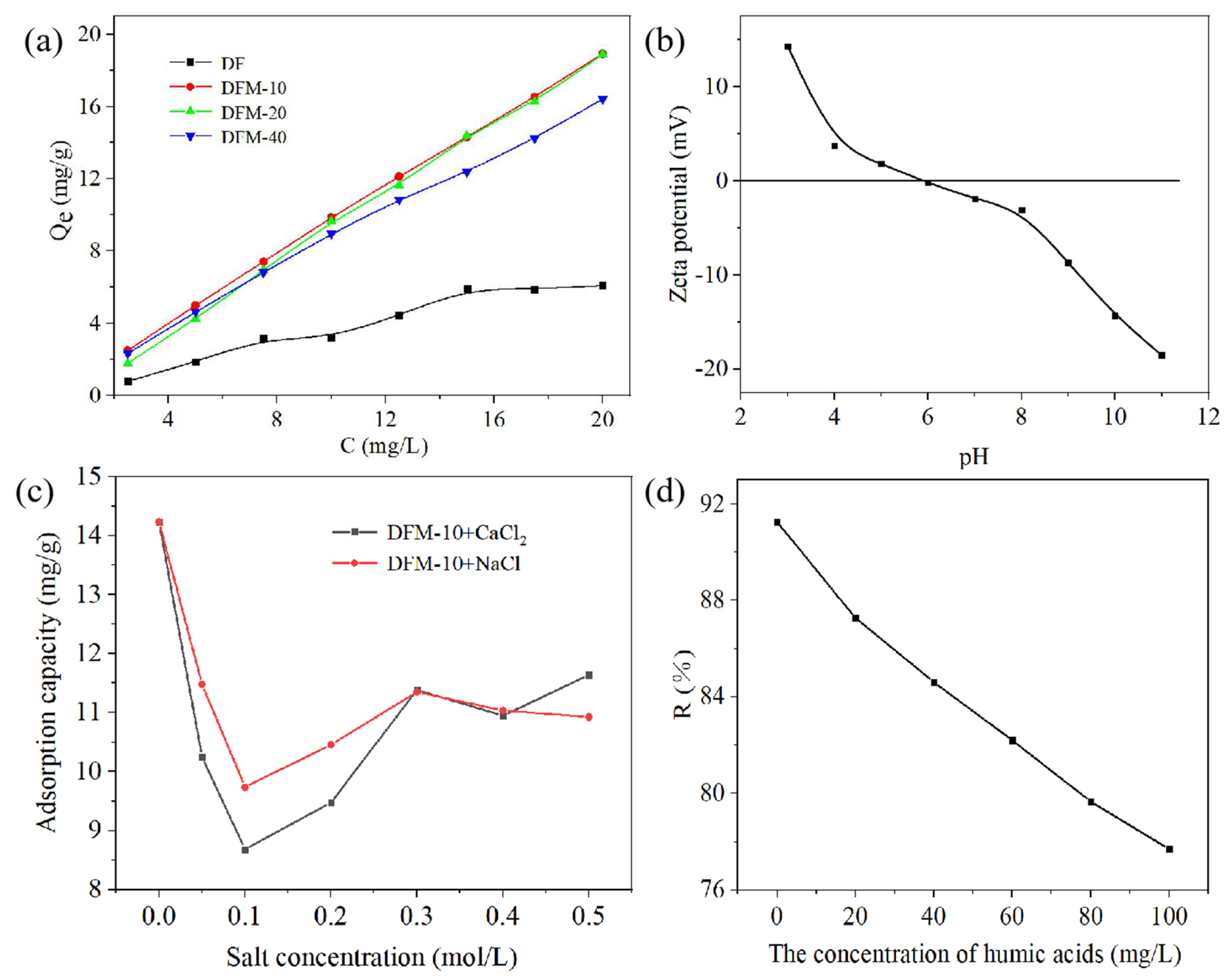
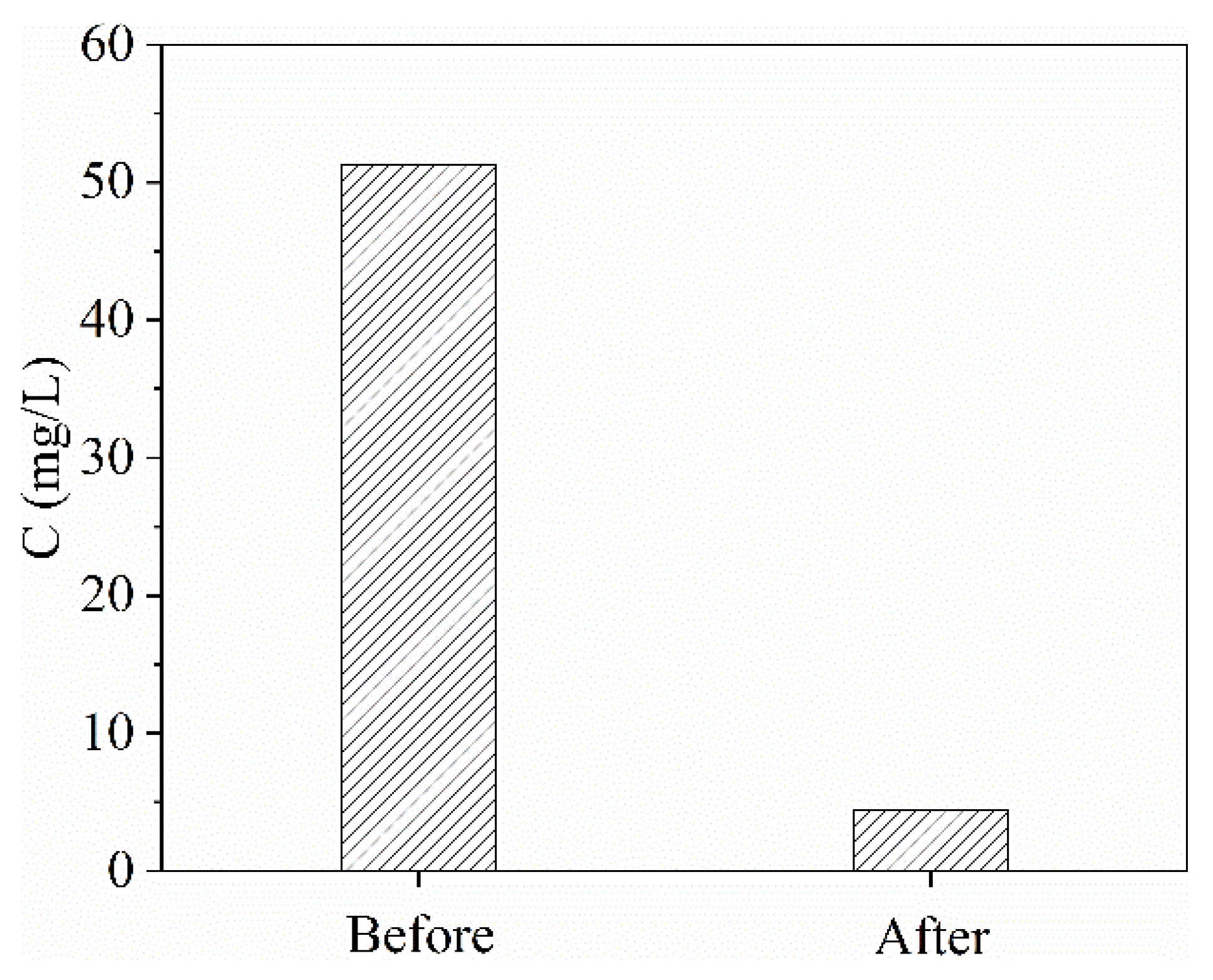
2.2.1. Effect of Different Concentrations
2.2.2. Effect of pH and Ionic Strength
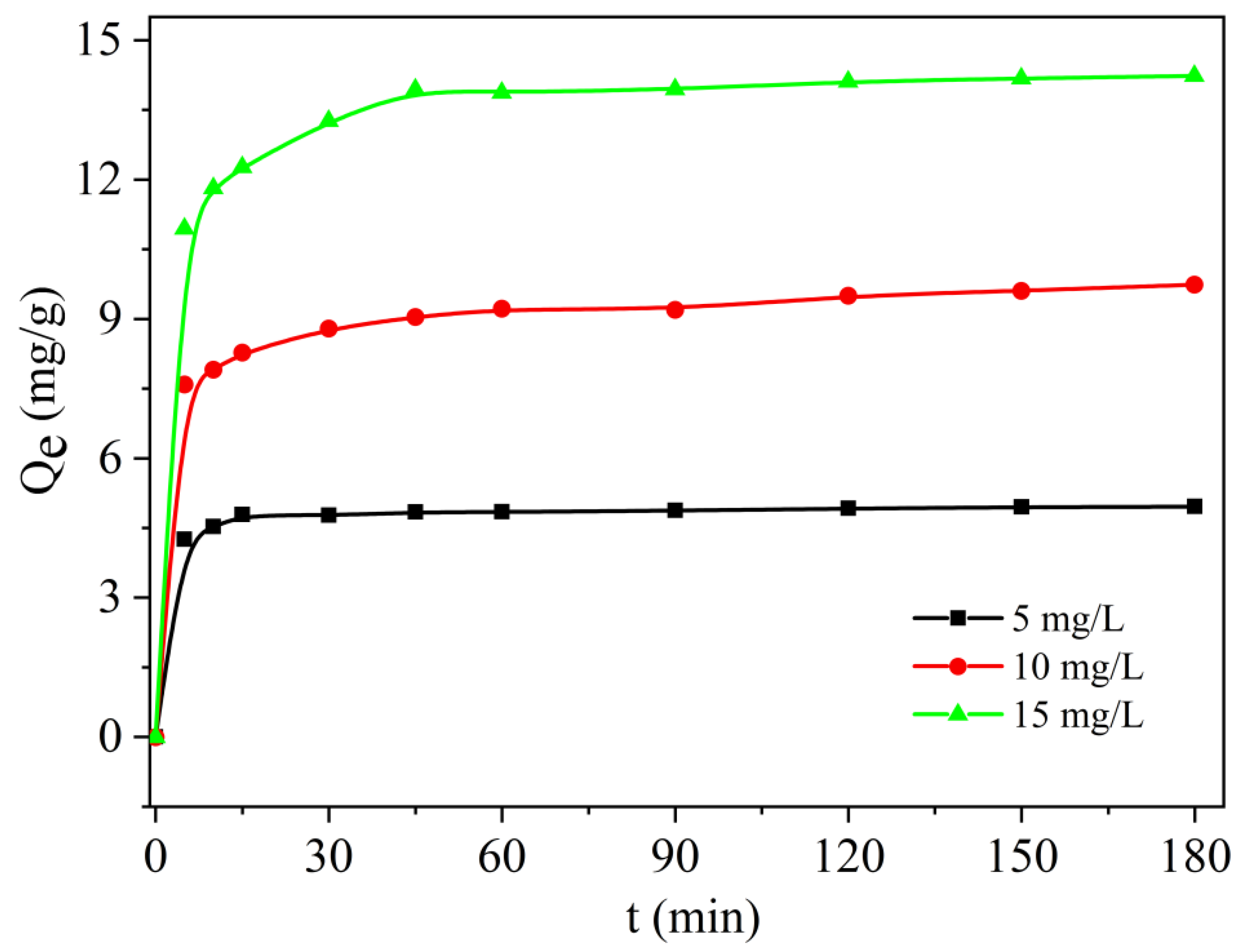
2.2.3. Effect of Time and Initial Concentration of MB
2.3. Adsorption Isotherms
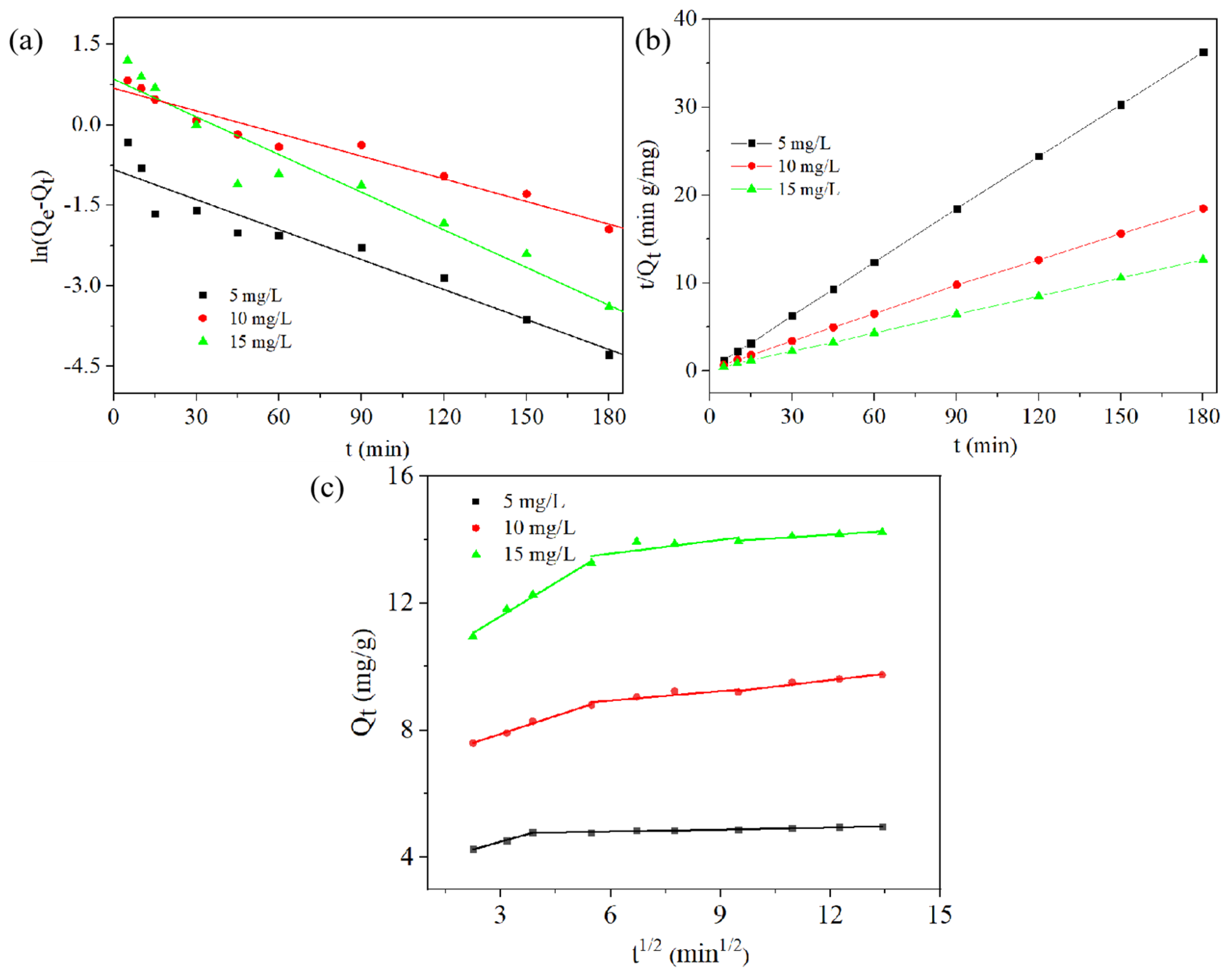
2.4. Adsorption Kinetics
2.5. Thermodynamic Study
2.6. Total Organic Carbon (TOC) Analysis
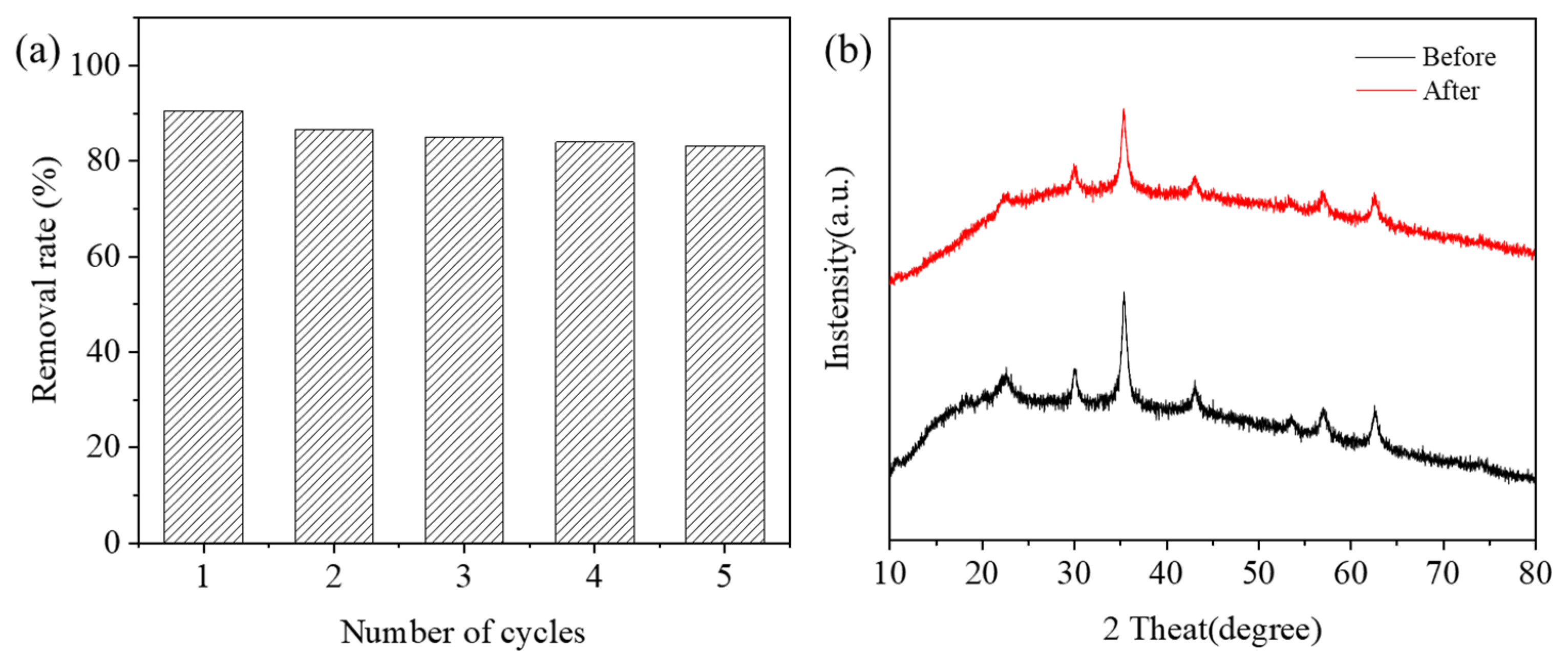
2.7. Recycling Ability
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Adsorbent
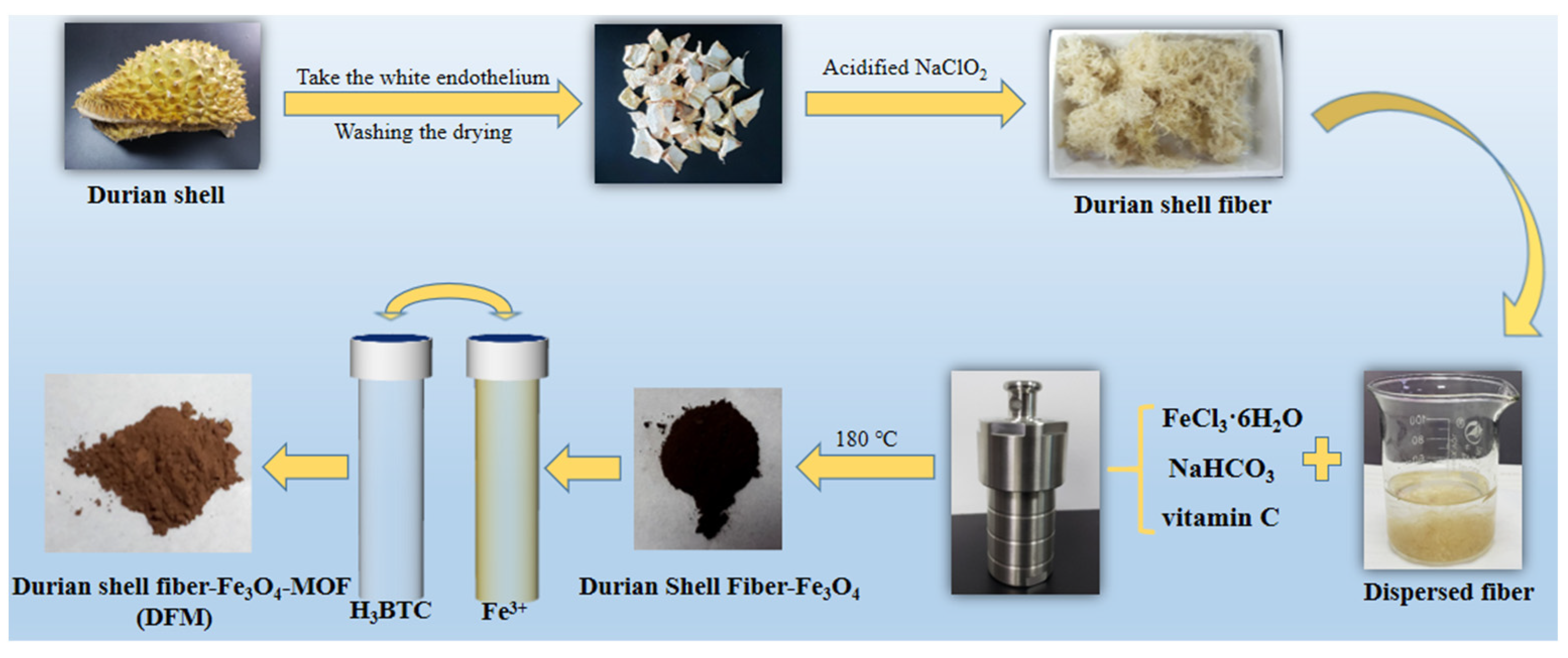
3.2.1. Extraction of Durian Shell Fiber
3.2.2. Preparation of Durian Shell Fiber–Fe3O4 (DF)
3.2.3. Preparation of Durian Shell Fiber–Fe3O4 MOF (DFM)
3.3. Adsorption Properties
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Reynel, H.E.; Mendoza, D.I.; Bonilla, A. Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves. J. Mol. Liq. 2016, 219, 425–434. [Google Scholar] [CrossRef]
- Atrous, M.; Sellaoui, L.; Bouzid, M.; Lima, E.C.; Thue, P.S.; Bonilla-Petriciolet, A.; Ben Lamine, A. Adsorption of dyes acid red 1 and acid green 25 on grafted clay: Modeling and statistical physics interpretation. J. Mol. Liq. 2019, 294, 111610. [Google Scholar] [CrossRef]
- Rashid, J.; Tehreem, F.; Rehman, A.; Kumar, R. Synthesis using natural functionalization of activated carbon from pumpkin peels for decolourization of aqueous methylene blue. Sci. Total Environ. 2019, 671, 369–376. [Google Scholar] [CrossRef]
- Wu, G.; Xing, W. Fabrication of ternary visible-light-driven semiconductor photocatalyst and its effective photocatalytic performance. Mater. Technol. 2018, 34, 292–300. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Wang, J.; Cai, Z.; Li, H.; Zhang, T.; Lu, R.; Li, P.; Han, J.; Xing, W. Synthesis of silver-based composite photocatalysis material and its visible-light-driven photocatalytic degradation of dye pollutants. Fresenius Environ Bull. 2021, 30, 9696–9706. [Google Scholar]
- Huang, X.; Wei, D.; Zhang, X.; Fan, D.; Sun, X.; Du, B.; Wei, Q. Synthesis of amino-functionalized magnetic aerobic granular sludge-biochar for Pb(II) removal: Adsorption performance and mechanism studies. Sci. Total Environ. 2019, 685, 681–689. [Google Scholar] [CrossRef]
- Wu, G.; Ma, F.; Xiong, R.; Xu, Z.; Li, P.; Han, J.; Xing, W. Fabrication of semiconductor photocatalyst and its high photo-catalytic performance under visble light irradiation. Fresenius Env. Bull. 2021, 30, 10482–10491. [Google Scholar]
- AliYounis, S.; Maitlo, H.A.; Lee, J.; Kim, K.-H. Nanotechnology-based sorption and membrane technologies for the treatment of petroleum-based pollutants in natural ecosystems and wastewater streams. Adv. Colloid Interface Sci. 2019, 275, 102071. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Wang, J.; Zhang, Y.; Yu, C.; Bian, H.; Hegazy, M.; Han, J.; Xing, W. Facile fabrication of Bi2WO6/biochar composites with enhanced charge carrier separation for photodecomposition of dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 634, 127945. [Google Scholar] [CrossRef]
- Wu, G.; Xing, W.; Han, J.; Li, P. Fabrication of silver-based visible-light-driven photocatalyst and degradation of organic pollutions in wastewater. Fresenius Env. Bull. 2020, 29, 445–453. [Google Scholar]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biore-finery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, Y.; Cheng, K.; Zou, J.; Wu, G. Fabrication of novel carbon species into porous g-C3N4 nanosheet frameworks with enhanced photocatalytic performance. New J. Chem. 2021, 45, 10589–10593. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Abdulhameed, A.S. Hybrid Crosslinked Chitosan-Epichlorohydrin/TiO2 Nanocomposite for Reactive Red 120 Dye Adsorption: Kinetic, Isotherm, Thermodynamic, and Mechanism Study. J. Polym. Environ. 2020, 28, 624–637. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Hasanzadehab, M.; Simchi, A.; Far, H.S. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2019, 81, 405–414. [Google Scholar] [CrossRef]
- Thines, K.; Abdullah, E.C.; Mubarak, N.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.-E.; Zuo, L.; Lu, H.; Huang, Y.; Liu, T. Biomass-Derived Nitrogen-Doped Carbon Nanofiber Network: A Facile Template for Decoration of Ultrathin Nickel-Cobalt Layered Double Hydroxide Nanosheets as High-Performance Asymmetric Supercapacitor Electrode. Small 2016, 12, 3235–3244. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Q.; Wang, J.; Xia, S.; Ma, L.; Lu, R.; Zhang, Y.; Huang, Y.; Wu, G. High Selectivity and Reusability of Bi-omass-Based Adsorbent for Chloramphenicol Removal. Nanomaterials 2021, 11, 2950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Son, E.-B.; Poo, K.-M.; Chang, J.-S.; Chae, K.-J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Luo, M.-T.; Zhao, C.; Huang, C.; Chen, X.-F.; Huang, Q.-L.; Qi, G.-X.; Tian, L.-L.; Xiong, L.; Li, H.-L.; Chen, X.-D. Efficient Using Durian Shell Hydrolysate as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Indian J. Microbiol. 2017, 57, 393–399. [Google Scholar] [CrossRef]
- Yazidi, A.; Atrous, M.; Soetaredjo, F.E.; Sellaoui, L.; Ismadji, S.; Erto, A.; Bonilla-Petriciolet, A.; Dotto, G.L.; Ben Lamine, A. Adsorption of amoxicillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: Experimental study and modeling analysis. Chem. Eng. J. 2019, 379, 122320. [Google Scholar] [CrossRef]
- Laysandra, L.; Santosa, F.H.; Austen, V.; Soetaredjo, F.E.; Foe, K.; Putro, J.N.; Ju, Y.H.; Ismadji, S. Rarasapo-nin-bentonite-activated biochar from durian shells composite for removal of crystal violet and Cr(VI) from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 30680–30695. [Google Scholar] [CrossRef]
- Kurniawan, A.; Sisnandy, V.O.A.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of durian shell waste as high capacity biosorbent for Cr(VI) removal from synthetic wastewater. Ecol. Eng. 2011, 37, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhao, Q.; Ren, S.; Lu, Q.; Guo, X.; Chen, Z. Fabrication of core-shell Fe3O4@MIL-100(Fe) magnetic microspheres for the removal of Cr(VI) in aqueous solution. J. Solid State Chem. 2016, 244, 25–30. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Xue, X.; Liu, W.; Kong, Y.; Cheng, R.; Yuan, D. Facile synthesis of Fe3O4@MOF-100(Fe) magnetic microspheres for the adsorption of diclofenac sodium in aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 31705–31717. [Google Scholar] [CrossRef]
- Luu, Q.S.; Do, U.T.; Kim, D.; Kim, J.; Jo, D.; Nguyen, Q.T.; Lee, Y. Enhancing adsorption efficiencies of organic molecules through covalently bonded structures of magnetic carbon nanoparticles. J. Ind. Eng. Chem. 2021, 105, 74–82. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Wang, J.; Xia, S.; Wu, H.; Zong, J.; Han, J.; Xing, W. Facile fabrication of rape straw biomass fi-ber/β-CD/Fe3O4 as adsorbent for effective removal of ibuprofen. Ind. Crops. Prod. 2021, 173, 114150. [Google Scholar] [CrossRef]
- Sun, Y.; Ni, P.Y.; Zhu, M.Y.; Yao, Y.L.; Fu, S.W. Preparation of Fe3O4@C submicron rods for adsorption of methylene blue and fast separation from water. Micro. Nano Lett. 2019, 14, 962–966. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Yu, F. Facile preparation of Fe3O4@MOF core-shell microspheres for lipase immobilization. J. Taiwan Inst. Chem. Eng. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Su, M.; Fang, Y.; Li, B.; Yin, W.; Gu, J.; Liang, H.; Li, P.; Wu, J. Enhanced hexavalent chromium removal by activated carbon modified with micro-sized goethite using a facile impregnation method. Sci. Total Environ. 2018, 647, 47–56. [Google Scholar] [CrossRef]
- Sun, X.; Gao, G.; Yan, D.; Feng, C. Synthesis and electrochemical properties of Fe3O4@MOF core-shell microspheres as an anode for lithium ion battery application. Appl. Surf. Sci. 2017, 405, 52–59. [Google Scholar] [CrossRef]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interf. Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal-Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef]
- Xia, L.J.; Zhou, S.J.; Zhang, C.H.; Fu, Z.A.; Wang, A.M.; Zhang, Q.; Wang, Y.L.; Liu, X.; Wang, X.G.; Xu, W.L. En-vironment-friendly Juncus effusus-based adsorbent with a three-dimensional network structure for highly efficient removal of dyes from wastewater. J. Clean Prod. 2020, 259, 120812. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agri-cultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 2021, 126, 293–304. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef]
- Eren, E.; Cubuk, O.; Ciftci, H.; Eren, B.; Caglar, B. Adsorption of basic dye from aqueous solutions by modified sepiolite: Equilibrium, kinetics and thermodynamics study. Desalination 2010, 252, 88–96. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, J.; Li, X.; Song, C.; Meng, H.; Zhang, X. Adsorption behavior of methylene blue on Fe3O4-embedded hybrid magnetic metal–organic framework. Desalination Water Treat. 2016, 57, 25216–25225. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Hu, X.; Tan, X.; Liu, S.; Wang, H.; Gu, Y.; Luo, L. Design and Preparation of Chitosan-Crosslinked Bismuth Ferrite/Biochar Coupled Magnetic Material for Methylene Blue Removal. Int. J. Environ. Res. Public Health. 2020, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, Y.; Zhang, Y.; Wang, G.; Li, S.; Han, R.; Wei, W. Reed biochar supported hydroxyapatite nanocomposite: Characterization and reactivity for methylene blue removal from aqueous media. J. Mol. Liq. 2018, 263, 53–63. [Google Scholar] [CrossRef]
- Aslam Malana, M.; Parveen, S.; Beenish Qureshi, R. Adsorptive removal of organic dyes from aqueous solutions using acrylic acid-acrylonitrile-N-isopropylacrylamide polymeric gels as adsorbents: Linear and non linear isotherms. Desalination Water Treat. 2016, 57, 22543–22550. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, D.; Han, C.; Huang, J. Comprehensive Utilization of Phosphogypsum: Adsorption of Methylene Blue and its Application in Bricks. Surf. Rev. Lett. 2021, 28, 2150075. [Google Scholar] [CrossRef]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M. Adsorption of methylene blue dye from aqueous solutions using eichhornia crassipes. Bull. Environ. Contam. Toxicol. 2013, 91, 362–366. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, W.; Xu, W.; Liu, Y. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Chem. Eng. J. 2018, 339, 468–478. [Google Scholar] [CrossRef]
- Selvaraju, G.; Abu Bakar, N.K. Production of a new industrially viable green-activated carbon from Artocarpus integer fruit processing waste and evaluation of its chemical, morphological and adsorption properties. J. Clean. Prod. 2016, 141, 989–999. [Google Scholar] [CrossRef]

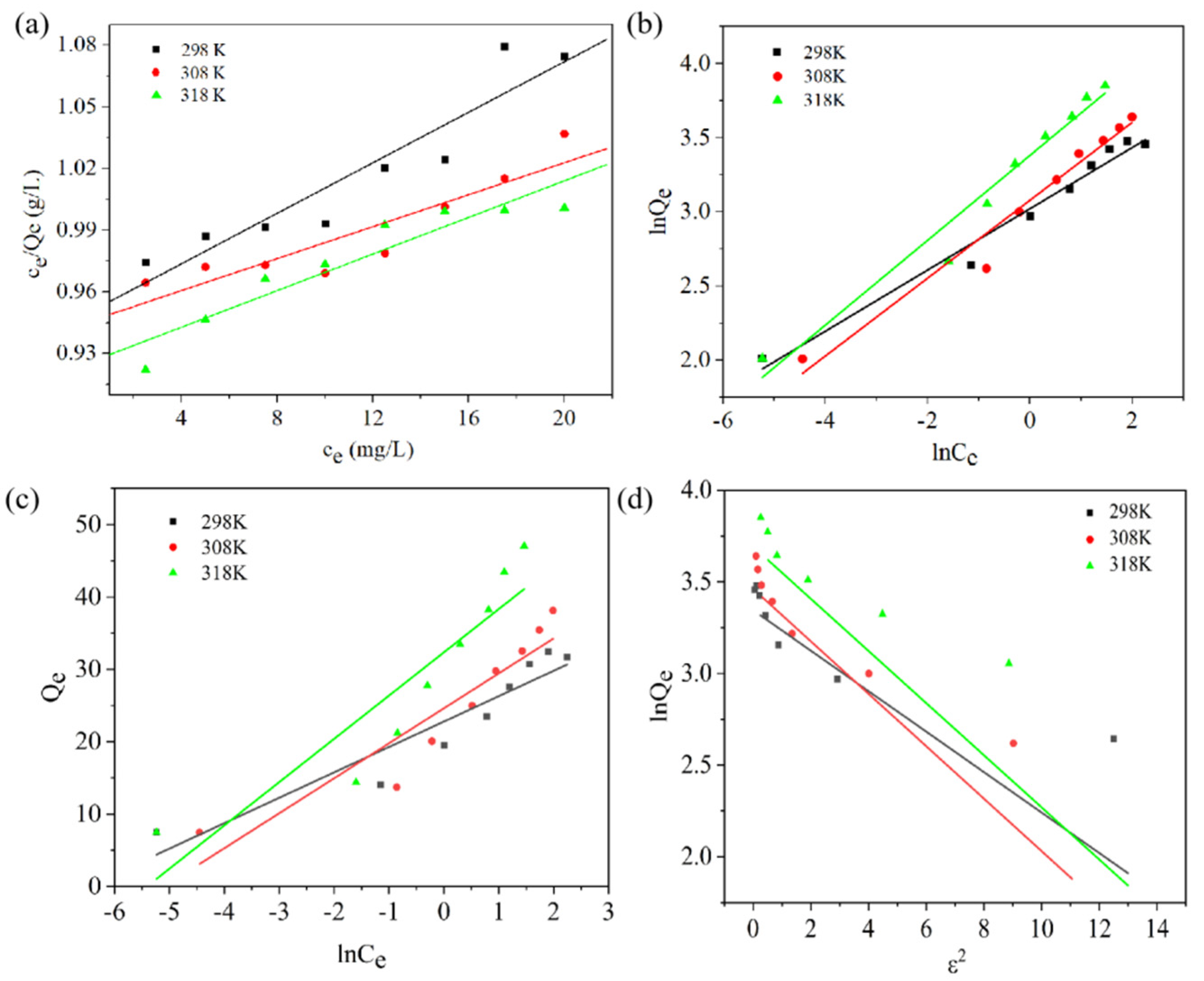

| Model | Parameter | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| Langmuir | Ql (mg/g) | 34.8100 | 42.2500 | 53.3100 |
| KL (L/mg) | 1.8427 | 1.3402 | 1.9642 | |
| R2 | 0.9931 | 0.9948 | 0.9791 | |
| Freundich | KF (mg1−1/n L1/n /g) | 20.5600 | 22.6900 | 30.6800 |
| 1/n | 0.2065 | 0.2624 | 0.2862 | |
| R2 | 0.9766 | 0.9609 | 0.9570 | |
| Temkin | bT (J.g/mol.mg) | 0.7053 | 0.5124 | 0.4142 |
| KT (L/mg) | 1.0065 | 1.0051 | 1.0054 | |
| R2 | 0.8910 | 0.8735 | 0.8447 | |
| Dubinin–Radushkevich | Ql (mg/g) | 28.3731 | 31.8753 | 40.1395 |
| KDR (molJ) | 0.1105 | 0.1432 | 0.1423 | |
| R2 | 0.8802 | 0.8567 | 0.8811 |
| Model | Parameter | 5 (mg/L) | 10 (mg/L) | 15 (mg/L) |
|---|---|---|---|---|
| Pseudo-first-order | Qe (mg/g) | 1.5100 | 4.0900 | 6.8100 |
| K1 (/min) | 0.0225 | 0.0301 | 0.0438 | |
| R2 | 0.6817 | 0.8041 | 0.9523 | |
| Pseudo-second-order | Qe (mg/g) | 15.0900 | 27.6000 | 37.5100 |
| K2 (g/(mg min)) | 0.0581 | 0.0171 | 0.0101 | |
| R2 | 0.9998 | 0.9998 | 0.9998 | |
| Kp1 (mg/g/min1/2) | 0.3237 | 0.3767 | 0.7017 | |
| C1 (mg/g) | 3.5209 | 6.7550 | 9.4854 | |
| R2 | 0. 9961 | 0. 9928 | 0.9884 | |
| Intraparticle diffusion | Kp2 (mg/g/min1/2) | 0.0184 | 0.0993 | 0.1144 |
| C2 (mg/g) | 4.7022 | 8.3343 | 12.6905 | |
| R2 | 0. 8202 | 0.7468 | 0.5608 | |
| Kp3 (mg/g/min1/2) | 0.0230 | 0.1345 | 0.0730 | |
| C3 (mg/g) | 4.6616 | 7.9615 | 13.2741 | |
| R2 | 0. 9665 | 0.9591 | 0. 9548 |
| ΔH | ΔS | ΔG (kJ/mol) | R2 | ||
|---|---|---|---|---|---|
| (KJ/mol) | (J/(mol·K)) | 298 K | 308 K | 318 K | |
| 4.6747 | 16.8786 | −3.0115 | −4.2291 | −5.8263 | 0.9851 |
| Simples | DF | DFM-10 |
|---|---|---|
| Mean pore radius (nm) | 10.53 | 5.45 |
| Total pore volume (cm3 g−1) | 0.19 | 0.24 |
| BET surface area (m2 g−1) | 60.55 | 226.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption. Int. J. Mol. Sci. 2022, 23, 5900. https://doi.org/10.3390/ijms23115900
Cai Z, Liu Q, Li H, Wang J, Tai G, Wang F, Han J, Zhu Y, Wu G. Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption. International Journal of Molecular Sciences. 2022; 23(11):5900. https://doi.org/10.3390/ijms23115900
Chicago/Turabian StyleCai, Zhangzhen, Qi Liu, Haoxin Li, Jingyi Wang, Guoyu Tai, Fan Wang, Jiangang Han, Yongli Zhu, and Guangyu Wu. 2022. "Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption" International Journal of Molecular Sciences 23, no. 11: 5900. https://doi.org/10.3390/ijms23115900
APA StyleCai, Z., Liu, Q., Li, H., Wang, J., Tai, G., Wang, F., Han, J., Zhu, Y., & Wu, G. (2022). Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption. International Journal of Molecular Sciences, 23(11), 5900. https://doi.org/10.3390/ijms23115900








