Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
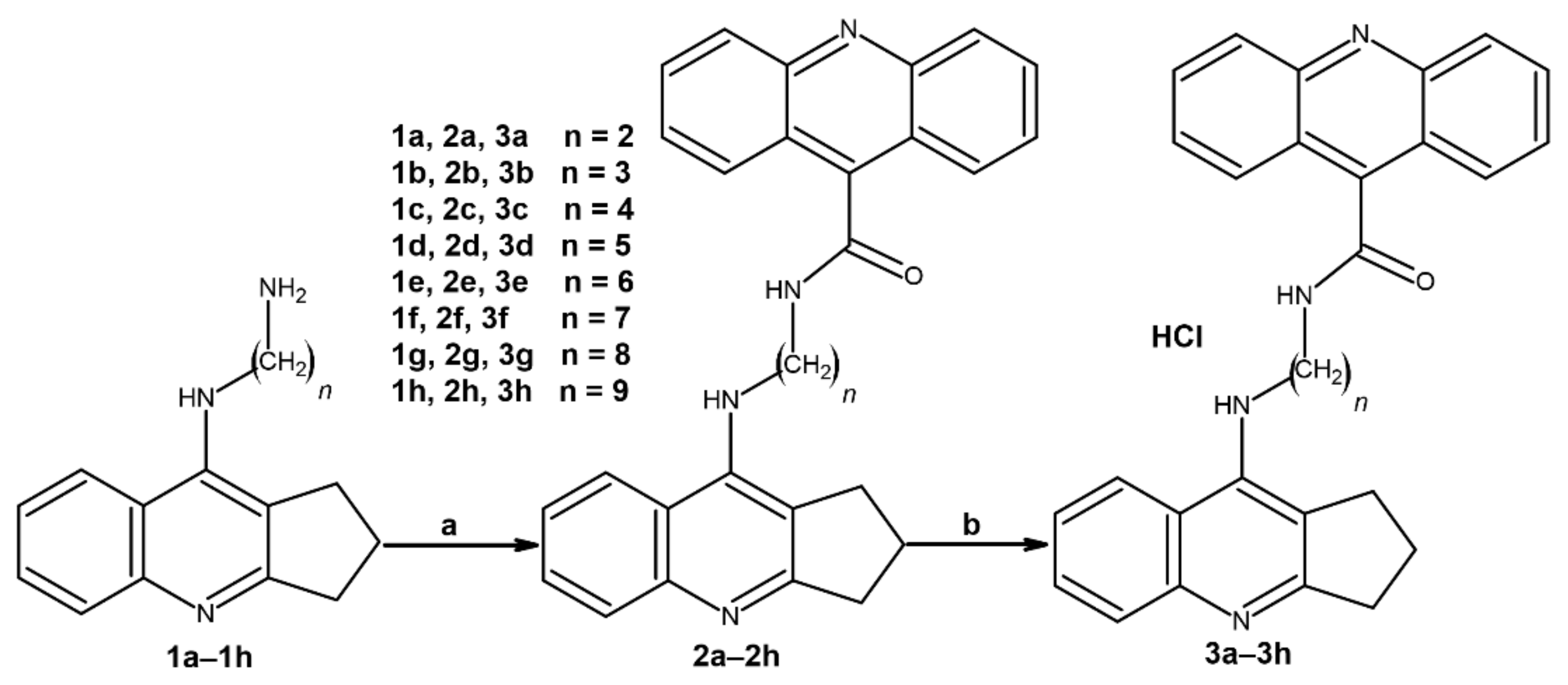
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Inhibition Studies AChE and BuChE
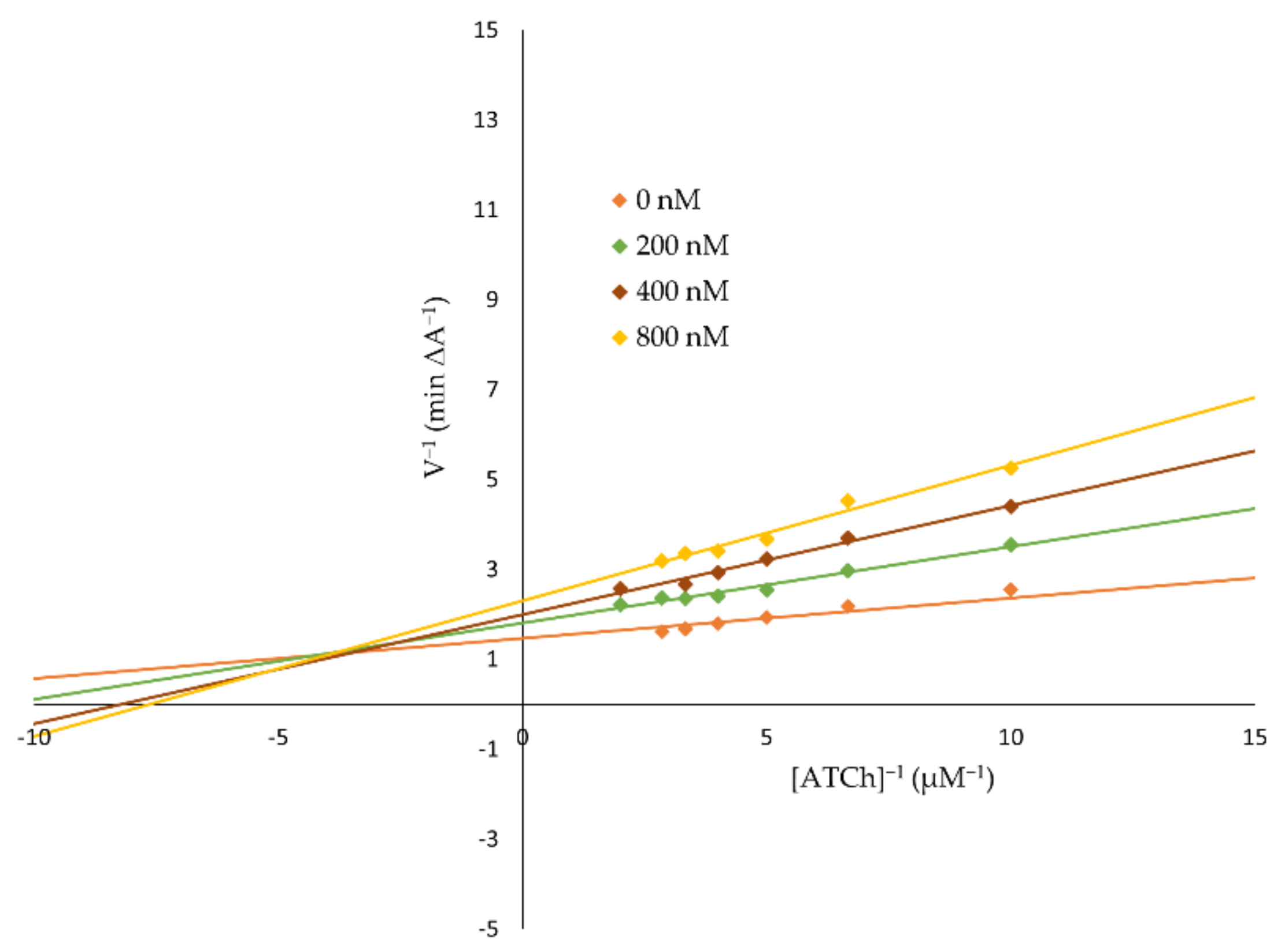
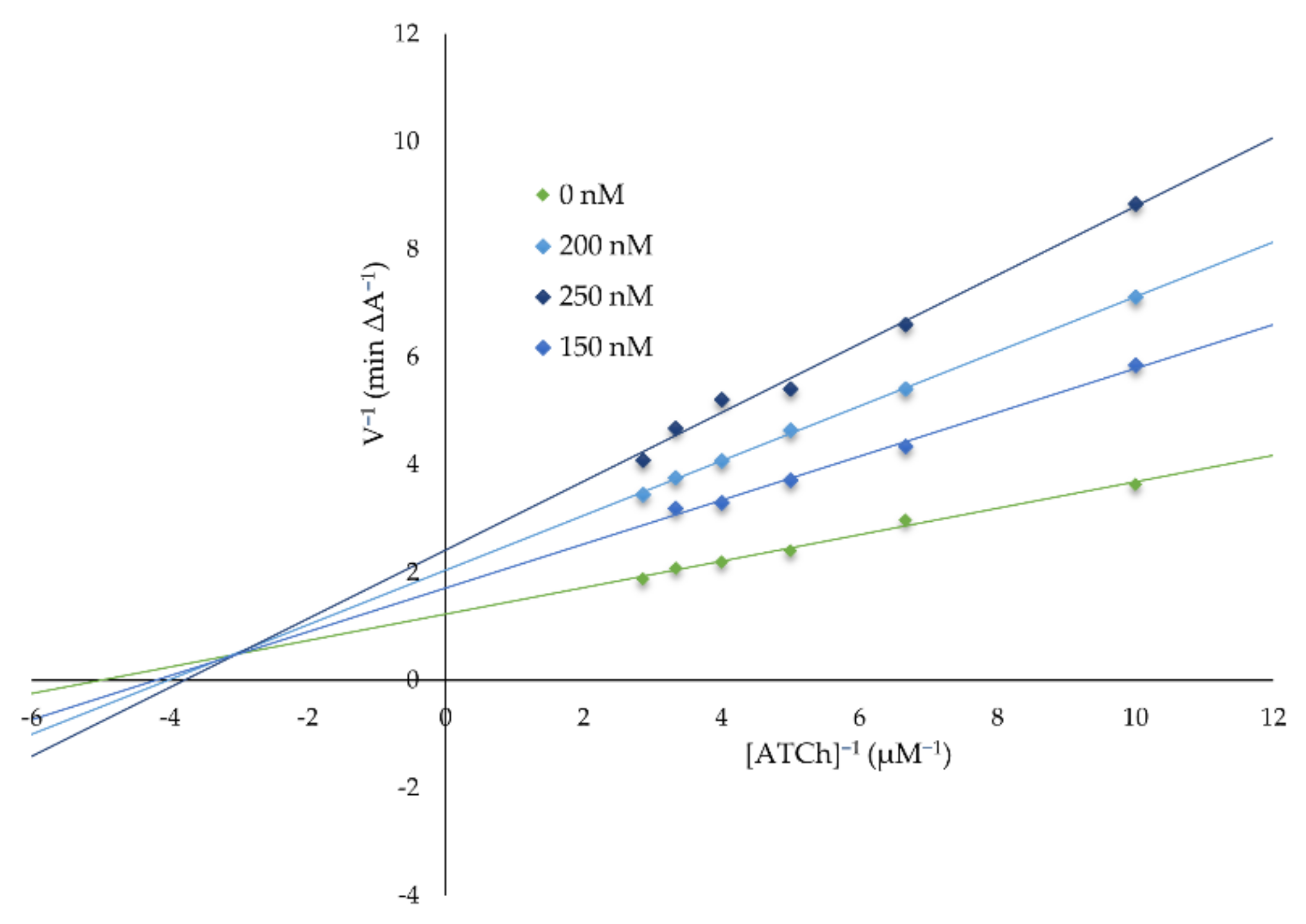
2.2.2. Kinetic Characterization of AChE and BuChE Inhibition
2.2.3. Antioxidant Activity Evaluation
2.2.4. In Vitro Hepatotoxicity Assay
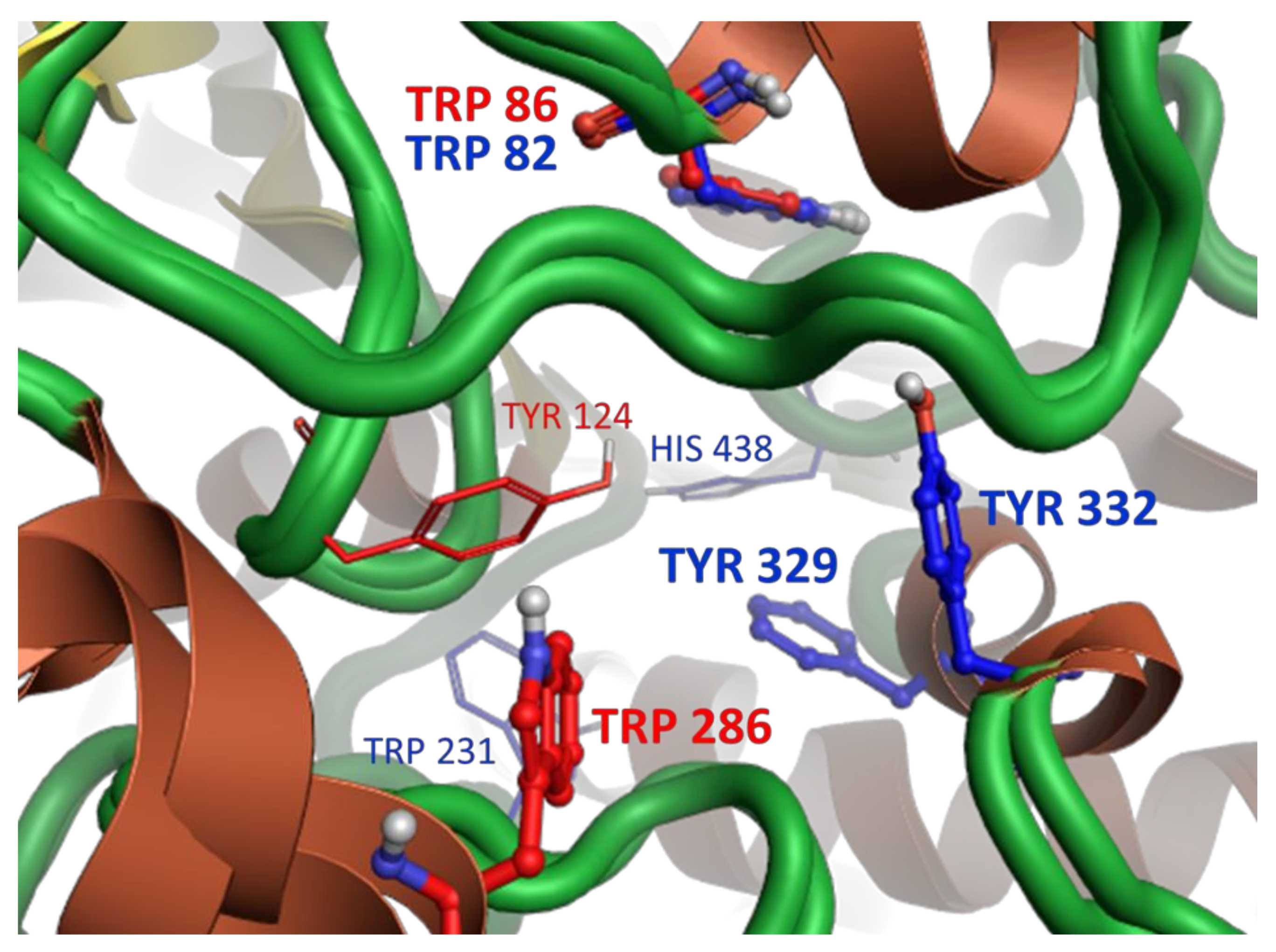
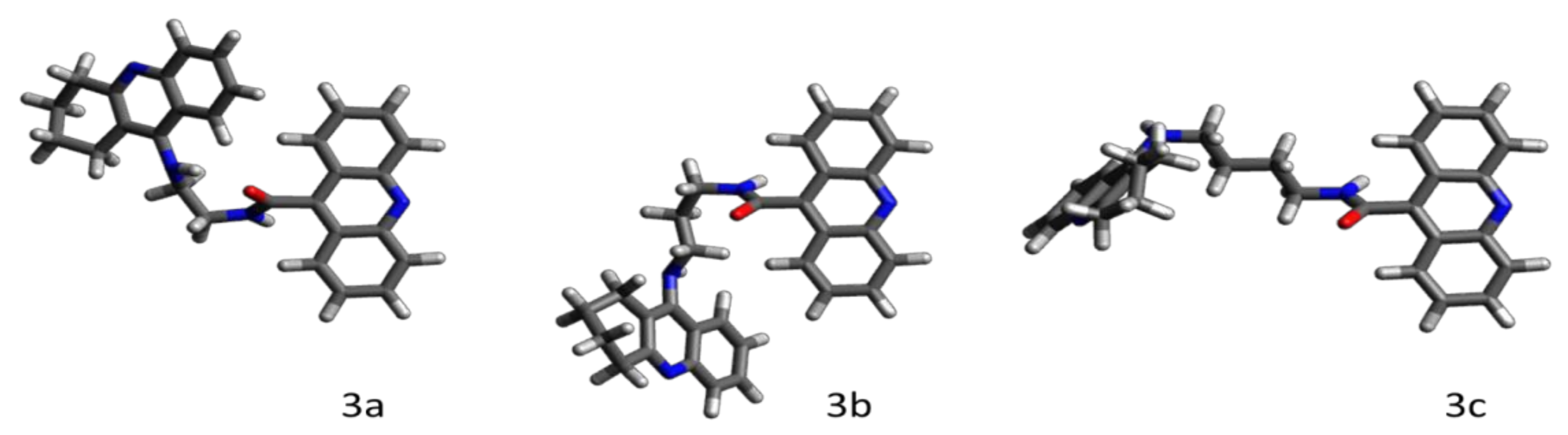
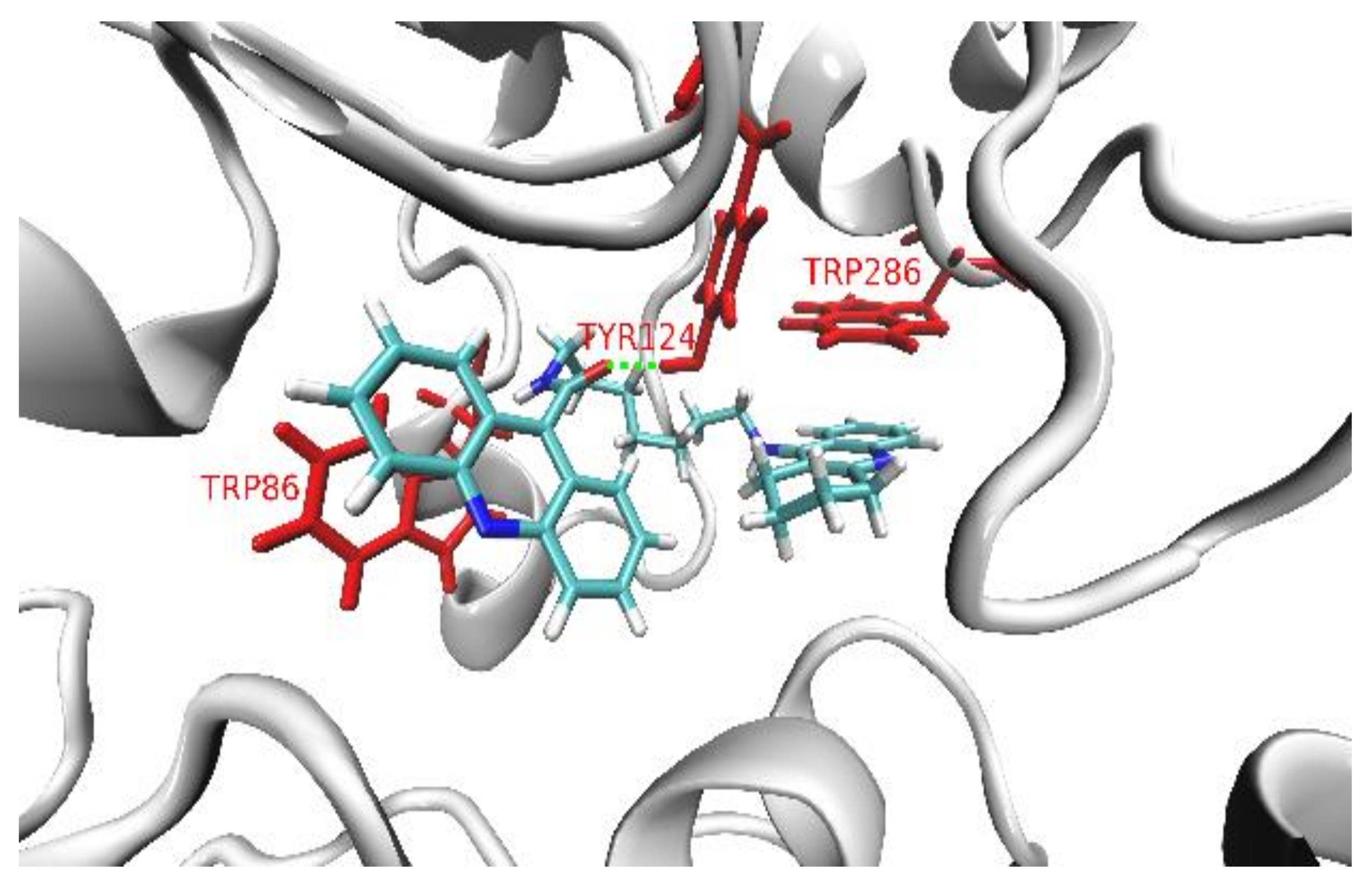
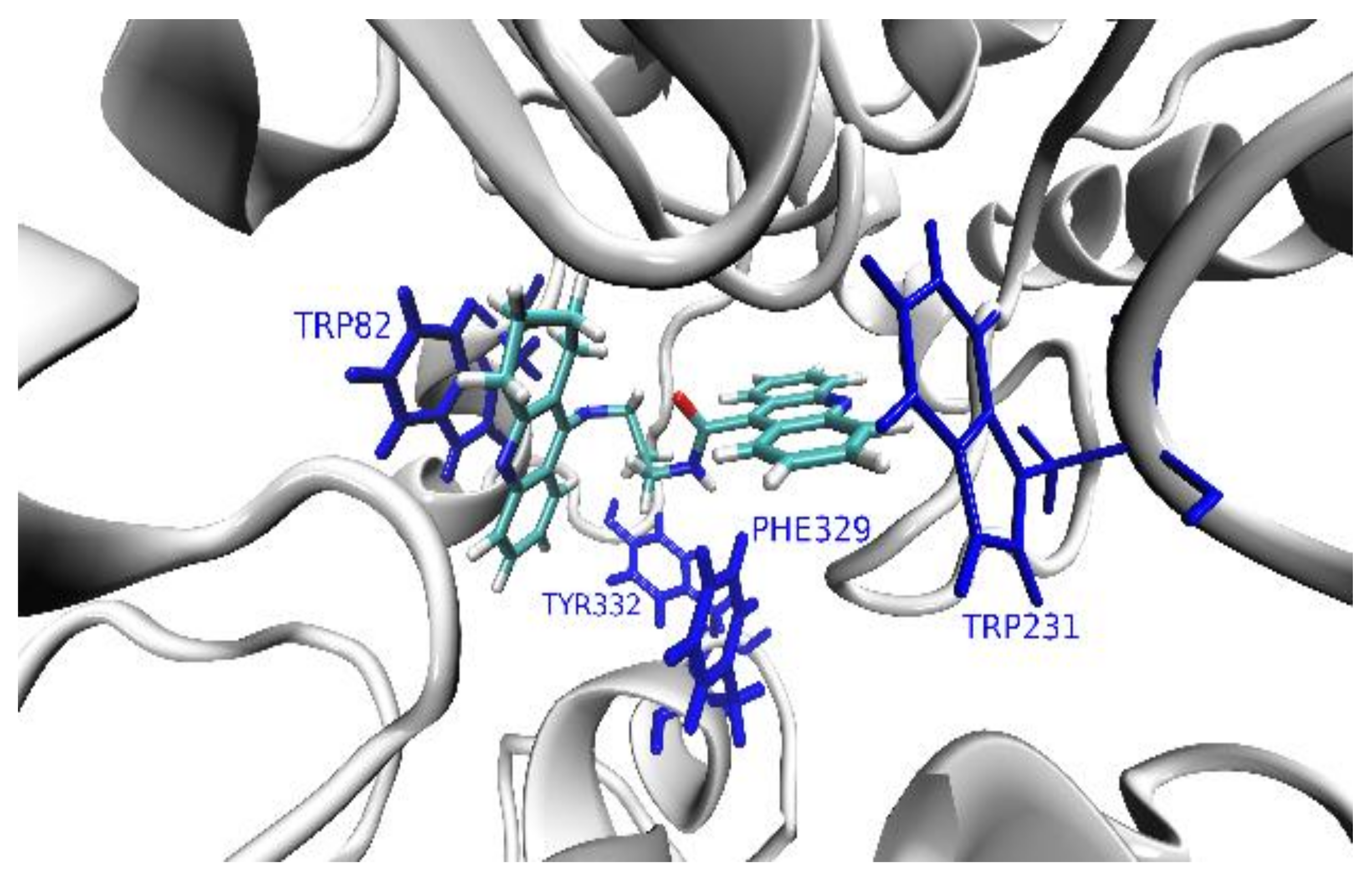
2.2.5. Molecular Modeling
2.2.6. ADMET Analysis
3. Discussion
- the amino acid context and, in turn, the tridimensional structures of the entrance and the interior of the binding pocket of an enzyme,
- the reciprocal conformational of the acridine and cyclopentaquinoline moieties depending on the (odd or even) number of the methylene segments in the aliphatic linker chain,
- the presence of extra- and intramolecular hydrogen bonds, which additionally stabilize both the ligand’s conformation and binding pose inside the pocket,
- the length and flexibility of the linker chain, with longer conformational rearrangements of aliphatic chains promoting more favorable positions of ligands inside the pocket.
4. Materials and Methods
4.1. Synthesis
4.1.1. General Procedure of Compound 2a–2h Synthesis
N-[2-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)ethyl]acridine-9-carboxamide (2a)
N-[3-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)propyl]acridine-9-carboxamide (2b)
N-[4-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)butyl]acridine-9-carboxamide (2c)
N-[5-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)pentyl]acridine-9-carboxamide (2d)
N-[6-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)hexyl]acridine-9-carboxamide (2e)
N-[7-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)heptyl]acridine-9-carboxamide (2f)
N-[8-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)octyl]acridine-9-carboxamide (2g)
N-[9-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)nonyl]acridine-9-carboxamide (2h)
4.1.2. General Procedure of Compound 3a–3h Synthesis
N-[2-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)ethyl]acridine-9-carboxamide hydrochloride (3a)
N-[3-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)propyl]acridine-9-carboxamide hydrochloride (3b)
N-[4-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)butyl]acridine-9-carboxamide hydrochloride (3c)
N-[5-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)pentyl]acridine-9-carboxamide hydrochloride (3d)
N-[6-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)hexyl]acridine-9-carboxamide hydrochloride (3e)
N-[7-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)heptyl]acridine-9-carboxamide hydrochloride (3f)
N-[8-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)octyl]acridine-9-carboxamide hydrochloride (3g)
N-[9-({1H,2H,3H-cyclopenta[b]quinolin-9-yl}amino)nonyl]acridine-9-carboxamide hydrochloride (3h)
4.2. Biological Evaluation
4.2.1. In Vitro Inhibition Studies on AChE and BuChE
4.2.2. Kinetic Characterization of AChE and BuChE Inhibition
4.2.3. Antioxidant Activity Evaluation
ORAC-FL Assay
4.2.4. Hepatotoxicity Assay
4.2.5. Molecular Modeling
4.2.6. ADMET Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Growdon, J.H. Is Alzheimer’s Disease Risk Modifiable? J. Alzheimers Dis. 2019, 67, 795–819. [Google Scholar] [CrossRef]
- Kozlov, S.; Afonin, A.; Evsyukov, I.; Bondarenko, A. Alzheimer’s disease: As it was in the beginning. Rev. Neurosci. 2017, 28, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Genetics of Alzheimer’s Disease. Dement. Neurocogn. Disord. 2018, 17, 131–136. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Whitwell, J.L. Alzheimer’s disease neuroimaging. Curr. Opin. Neurol. 2018, 31, 396–404. [Google Scholar] [CrossRef]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s Disease Diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Reddy, P.H. Therapeutics of Neurotransmitters in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1049–1069. [Google Scholar] [CrossRef] [Green Version]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Sagal, J.P.; Quintanilla, R.A.; Godoy, J.A.; Arrazola, M.S.; Inestrosa, N.C. Amyloid-beta-Acetylcholinesterase complexes potentiate neurodegenerative changes induced by the A beta peptide. Implications for the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Maramai, S.; Benchekroun, M.; Gabr, M.T.; Yahiaoui, S. Multitarget Therapeutic Strategies for Alzheimer’s Disease: Review on Emerging Target Combinations. BioMed Res. Int. 2020, 2020, 27. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s Disease, Oligomers, and Inflammation. J. Alzheimers Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef] [Green Version]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W.J. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Yang, P.; Sun, F. Aducanumab: The first targeted Alzheimer’s therapy. Drug Discov. Ther. 2021, 15, 166–168. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis. 2021, 8, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 13. [Google Scholar] [CrossRef] [PubMed]
- Chufarova, N.; Czarnecka, K.; Skibinski, R.; Cuchra, M.; Majsterek, I.; Szymanski, P. New tacrine-acridine hybrids as promising multifunctional drugs for potential treatment of Alzheimer’s disease. Arch. Pharm. 2018, 351, 11. [Google Scholar] [CrossRef]
- Szymanski, P.; Zurek, E.; Mikiciuk-Olasik, E. New tacrine-hydrazinonicotinamide hybrids as acetylcholinesterase inhibitors of potential interest for the early diagnostics of Alzheimer’s disease. Pharmazie 2006, 61, 269–273. [Google Scholar] [CrossRef]
- Szymanski, P.; Markowicz, M.; Mikiciuk-Olasik, E. Synthesis and biological activity of derivatives of tetrahydroacridine as acetylcholinesterase inhibitors. Bioorg. Chem. 2011, 39, 138–142. [Google Scholar] [CrossRef]
- Joullie, M.M.; Lassen, K.M. Evolution of amide bond formation. Arkivoc 2010, 8, 189–250. [Google Scholar] [CrossRef] [Green Version]
- Dunetz, J.R.; Magano, J.; Weisenburger, G.A. Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Org. Process Res. Dev. 2016, 20, 140–177. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Czarnecka, K.; Girek, M.; Maciejewska, K.; Skibinski, R.; Jonczyk, J.; Bajda, M.; Kabzinski, J.; Solowiej, P.; Majsterek, I.; Szymanski, P. New cyclopentaquinoline hybrids with multifunctional capacities for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017, 33, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 23, 3–25, reprinted in Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Czarnecka, K.; Girek, M.; Wojtowicz, P.; Krecisz, P.; Skibinski, R.; Jonczyk, J.; Latka, K.; Bajda, M.; Walczak, A.; Galita, G.; et al. New Tetrahydroacridine Hybrids with Dichlorobenzoic Acid Moiety Demonstrating Multifunctional Potential for the Treatment of Alzheimer’s Disease. J. Mol. Sci. 2020, 21, 3765. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14 (Suppl. 1), S77–S91. [Google Scholar] [CrossRef]
- Czarnecka, K.; Girek, M.; Krecisz, P.; Skibinski, R.; Latka, K.; Jonczyk, J.; Bajda, M.; Kabzinski, J.; Majsterek, I.; Szymczyk, P.; et al. Discovery of New Cyclopentaquinoline Analogues as Multifunctional Agents for the Treatment of Alzheimer’s Disease. J. Mol. Sci. 2019, 20, 498. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.H.; Wang, J.S.; Dong, G.; Guo, P.P.; Wei, D.D.; Xie, S.S.; Yang, M.H.; Kong, L.Y. The acute hepatotoxicity of tacrine explained by H-1 NMR based metabolomic profiling. Toxicol. Res. 2015, 4, 1465–1478. [Google Scholar] [CrossRef]
- Przybylowska, M.; Kowalski, S.; Dzierzbicka, K.; Inkielewicz-Stepniak, I. Therapeutic Potential of Multifunctional Tacrine Analogues. Curr. Neuropharmacol. 2019, 17, 472–490. [Google Scholar] [CrossRef]
- Hirono, H.; Watanabe, K.; Hasegawa, K.; Hiroyasu, K.; Shibasaki, K.; Ohkoshi, S. Anti-Dementia Drugs and Hepatotoxicity-Report of Two Cases. Int. J. Gerontol. 2018, 12, 261–263. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Mitic, M.; Lazarevic-Pasti, T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, K.; Czarnecka, K.; Szymanski, P. A review of the mechanisms underlying selected comorbidities in Alzheimer’s disease. Pharmacol. Rep. 2021, 17, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. Methods Mol. Med. 1999, 28, 25–30. [Google Scholar] [CrossRef]
- Mao, F.; Li, J.H.; Wei, H.; Huang, L.; Li, X.S. Tacrine-propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2015, 30, 995–1001. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zenger, K.; Lupp, A.; Kling, B.; Heilmann, J.; Fleck, C.; Kraus, B.; Decker, M. Tacrine-Silibinin Codrug Shows Neuro- and Hepato protective Effects in Vitro and Pro-Cognitive and Hepatoprotective Effects in Vivo. J. Med. Chem. 2012, 55, 5231–5242. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bauer, M.R.; Mackey, M.D. Electrostatic Complementarity as a Fast and Effective Tool to Optimize Binding and Selectivity of Protein-Ligand Complexes. J. Med. Chem. 2019, 62, 3036–3050. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Liu, Q.F.; Yin, W.C. Crystal Structure of Recombinant Human Acetylcholinesterase in Complex with Compound 2. Protein Data Bank 2021, 1844–1855. [Google Scholar] [CrossRef]
- Rossi, M.; Freschi, M.; Nascente, L.D.; Salerno, A.; Teixeira, S.D.V.; Nachon, F.; Chantegreil, F.; Soukup, O.; Prchal, L.; Malaguti, M.; et al. Sustainable Drug Discovery of Multi-Target-Directed Ligands for Alzheimer’s Disease. J. Med. Chem. 2021, 64, 4972–4990. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.B.; Lou, C.F.; Sun, L.X.; Li, J.; Cai, Y.C.; Wang, Z.; Li, W.H.; Liu, G.X.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
| Compound | AChE IC50 ± SD [nM] a | BuChE IC50 ± SD [nM] b | Selectivity for AChE c | Selectivity for BuChE d | ORAC (TE) e |
|---|---|---|---|---|---|
| 3a | 8619.37 ± 606.33 | 539.92 ± 66.90 | 0.063 | 15.96 | n.d. |
| 3b | 272.33 ± 35.11 | 103.73 ± 7.63 | 0.38 | 2.62 | 0.4614 ± 0.056 |
| 3c | 272.79 ± 22.11 | 200.20 ± 18.07 | 0.73 | 1.36 | n.d. |
| 3d | 4057.16 ± 535.79 | 173.41 ± 18.89 | 0.043 | 23.39 | n.d. |
| 3e | 394.69 ± 36.07 | 194.88 ± 11.02 | 0.49 | 2.02 | n.d. |
| 3f | 113.34 ± 7.14 | 203.52 ± 16.02 | 1.79 | 0.56 | 0.3254 ± 0.014 |
| 3g | 700.98 ± 67.08 | 177.63 ± 17.03 | 0.25 | 3.95 | n.d. |
| 3h | 669.42 ± 83.39 | 226.97 ± 27.05 | 0.34 | 2.95 | n.d. |
| Tacrine | 226.97 ± 27.05 | 7.2 ± 0.51 | 0.032 | 31.52 | 0.0132 ± 0.009 |
| Bistacrine | 405.10 ± 28.62 | 226 ± 30.40 | 0.56 | 1.79 | n.d. |
| Compound | Molecular Weight [g/mol] | LogP | pKa (acid) | pKa (base) | TPSA [Å2] | Molar Refractivity [m3/mol] | H-bond Acceptors | H-bond Donors |
|---|---|---|---|---|---|---|---|---|
| 3a | 432.52 | 4.70 | 13.19 | 8.71 | 66.91 | 133.80 | 3 | 2 |
| 3b | 446.54 | 5.03 | 13.52 | 8.90 | 66.91 | 138.61 | 3 | 2 |
| 3c | 460.57 | 5.31 | 13.65 | 8.97 | 66.91 | 143.42 | 3 | 2 |
| 3d | 474.60 | 5.47 | 13.71 | 9.00 | 66.91 | 148.23 | 3 | 2 |
| 3e | 488.62 | 5.93 | 13.73 | 9.01 | 66.91 | 153.03 | 3 | 2 |
| 3f | 502.65 | 6.17 | 13.73 | 9.02 | 66.91 | 157.84 | 3 | 2 |
| 3g | 516.68 | 6.60 | 13.74 | 9.02 | 66.91 | 162.65 | 3 | 2 |
| 3h | 530.70 | 6.96 | 13.74 | 9.02 | 66.91 | 167.45 | 3 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska, K.; Czarnecka, K.; Kręcisz, P.; Niedziałek, D.; Wieczorek, G.; Skibiński, R.; Szymański, P. Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 5876. https://doi.org/10.3390/ijms23115876
Maciejewska K, Czarnecka K, Kręcisz P, Niedziałek D, Wieczorek G, Skibiński R, Szymański P. Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(11):5876. https://doi.org/10.3390/ijms23115876
Chicago/Turabian StyleMaciejewska, Karolina, Kamila Czarnecka, Paweł Kręcisz, Dorota Niedziałek, Grzegorz Wieczorek, Robert Skibiński, and Paweł Szymański. 2022. "Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 11: 5876. https://doi.org/10.3390/ijms23115876
APA StyleMaciejewska, K., Czarnecka, K., Kręcisz, P., Niedziałek, D., Wieczorek, G., Skibiński, R., & Szymański, P. (2022). Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease. International Journal of Molecular Sciences, 23(11), 5876. https://doi.org/10.3390/ijms23115876







