Full-Length Transcriptome Sequencing Reveals the Impact of Cold Stress on Alternative Splicing in Quinoa
Abstract
1. Introduction
2. Results
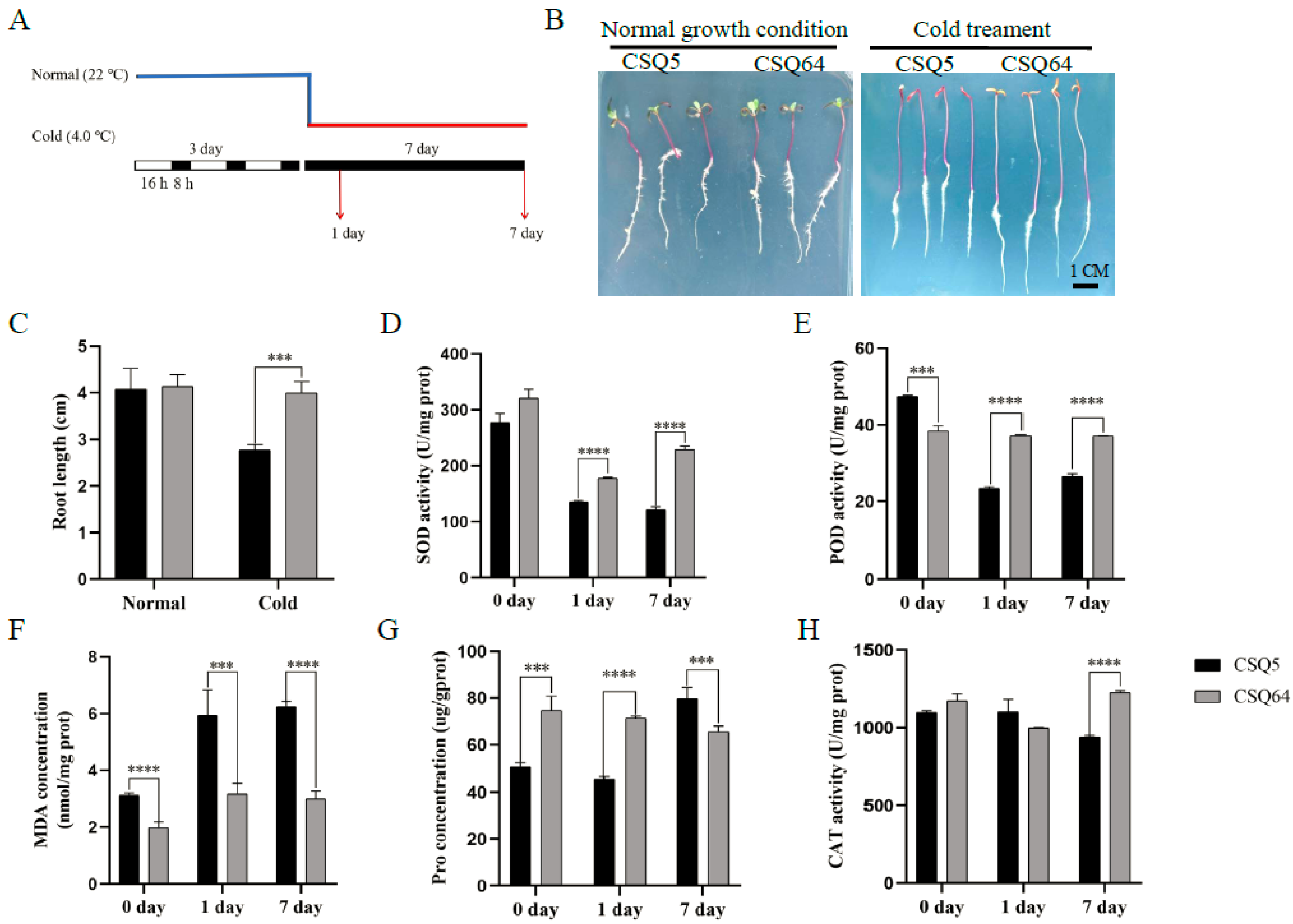
2.1. Comparing Cold Sensitivity in CSQ5 and CRQ64 Quinoa Varieties
2.2. ONT Sequencing Enables the Sequencing of Full-Length Transcripts
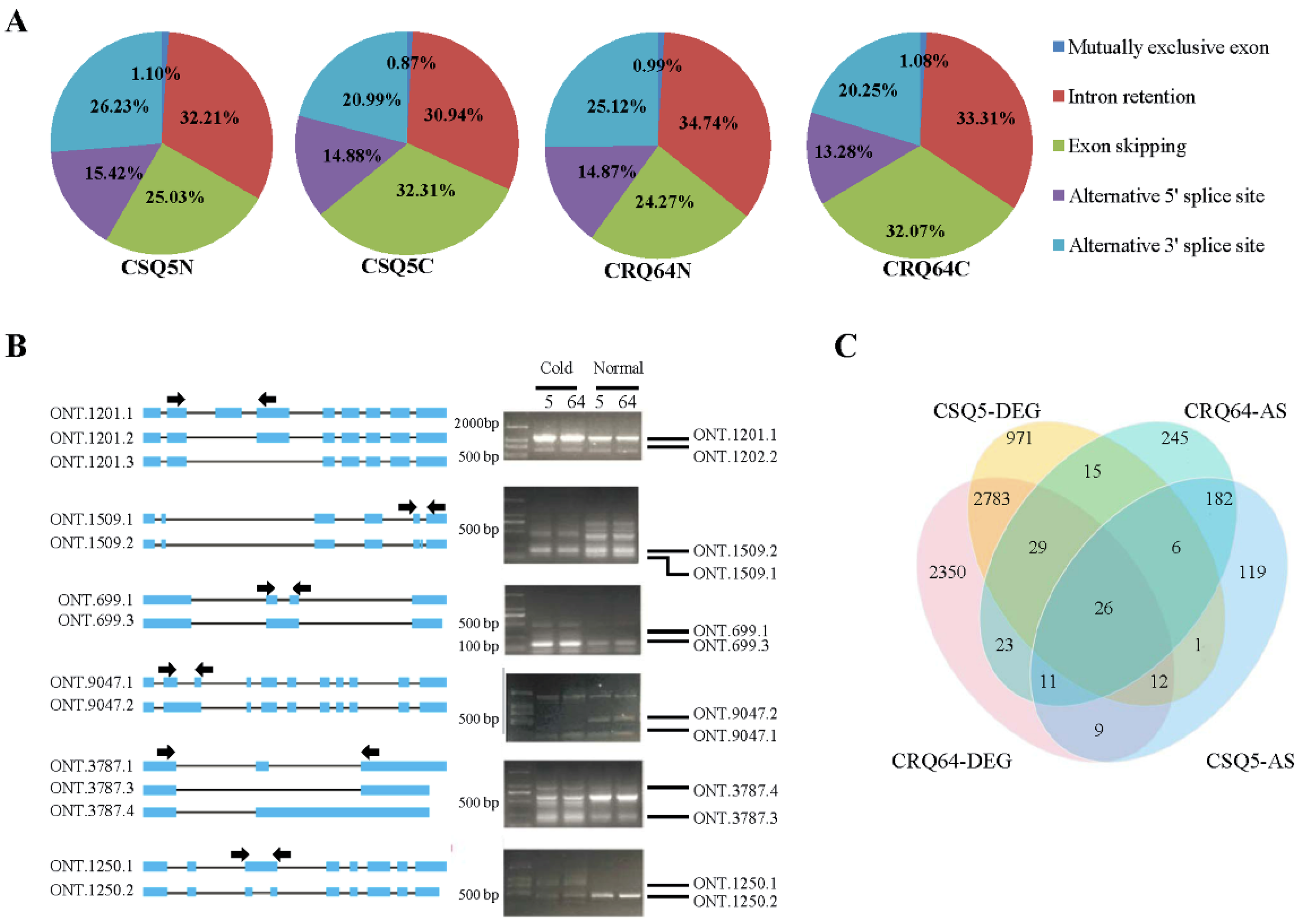
2.3. Novel Isoforms Identified by ONT Sequencing
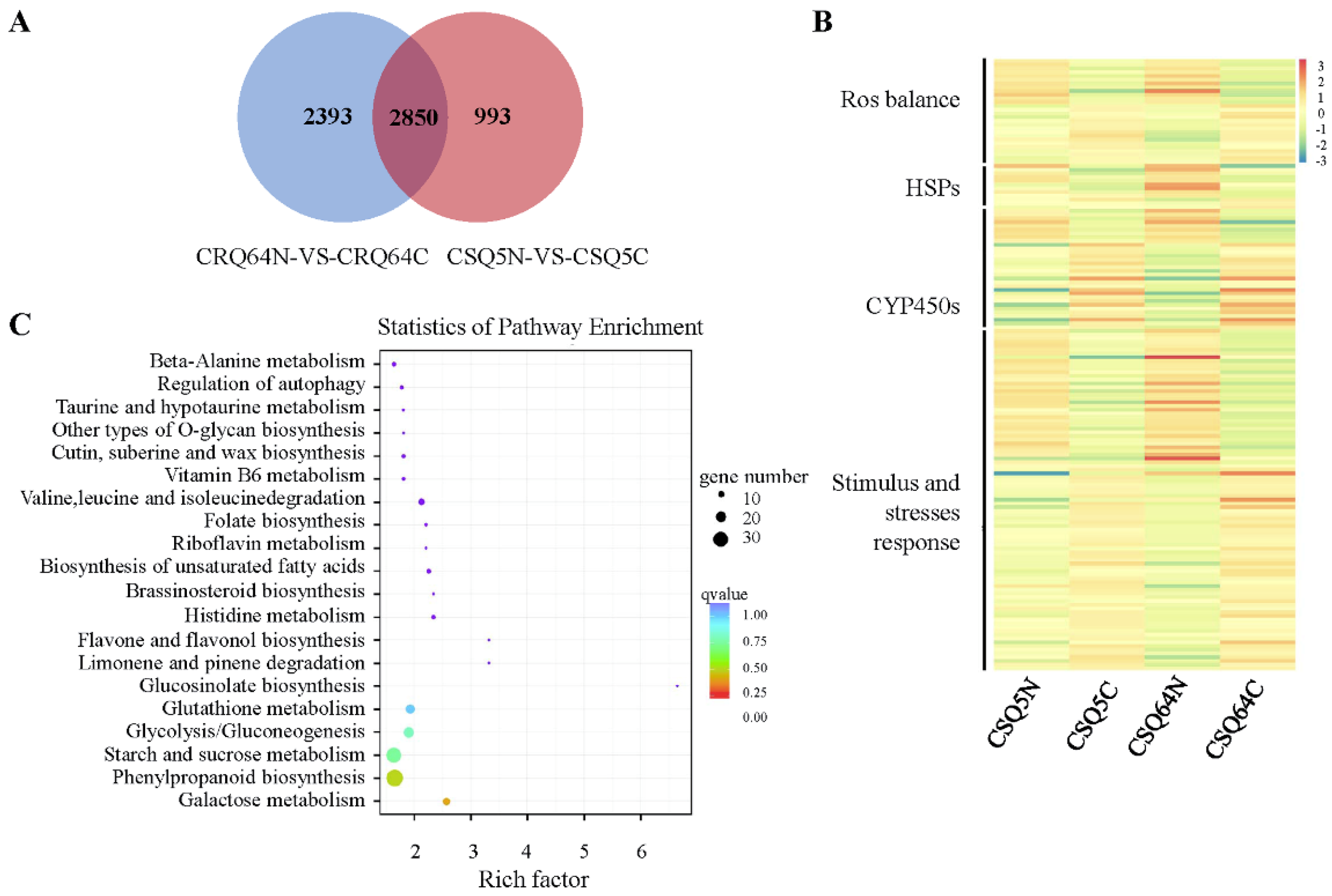
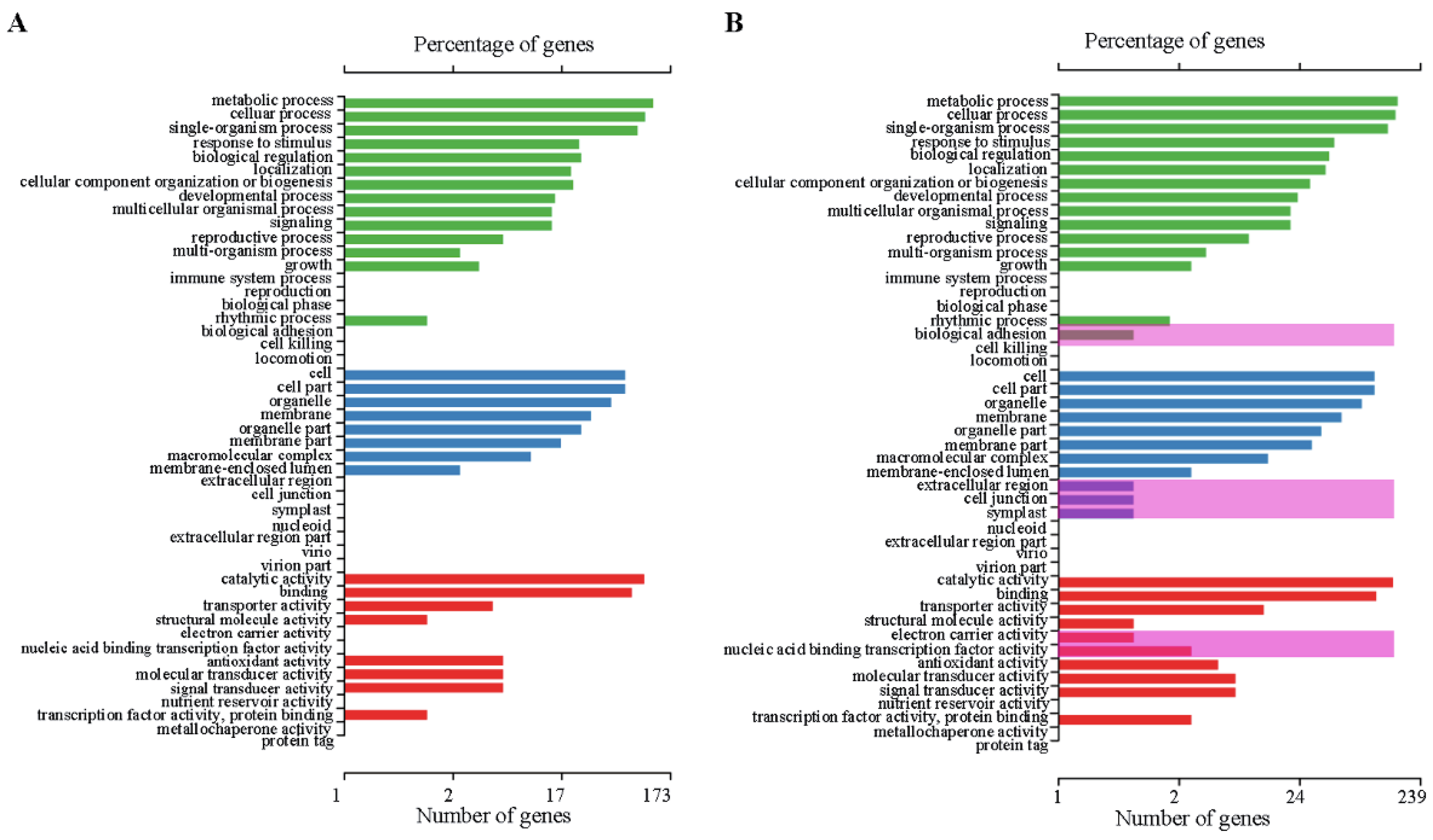
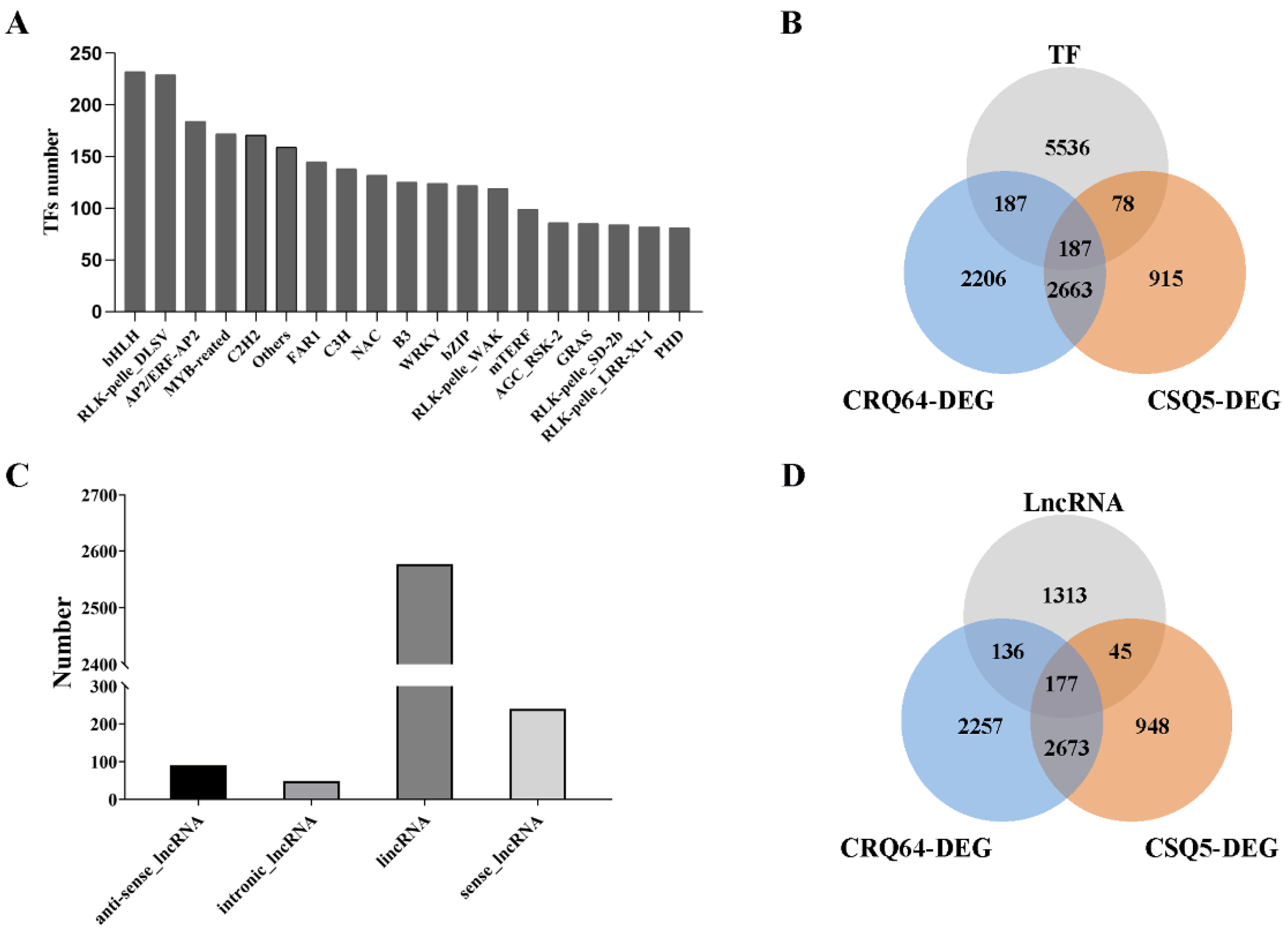
2.4. Differentially Expressed Genes (DEGs)
2.5. Analysis of Alternative Splicing Events and Corresponding Pathways in Response to Cold Stress
2.6. Genome-Wide Analysis of Cold-Stress-Associated Transcription Factors
2.7. Distinct Expression Patterns of LncRNAs under Cold Stress
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Cold Treatment
4.2. Physiological Assays
4.3. RT-PCR and qPCR
4.4. Oxford Nanopore Technologies Long Read Processing
4.5. Gene Functional Annotation and Functional Enrichment Analysis
4.6. Quantification of Gene/Transcript Expression Levels
4.7. Structure and Transcription Factors Analysis
4.8. lncRNA Analysis
- PfamScan: http://pfam.xfam.org/ (accessed on 6 October 2021)
- CPC: http://cpc.cbi.pku.edu.cn/ (accessed on 6 October 2021)
- CNCI: https://github.com/www-bioinfo-org/CNCI (accessed on 6 October 2021)
- CPAT: http://lilab.research.bcm.edu/ (accessed on 6 October 2021)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, F.; Bendevis, M.; Shabala, S.; Jacobsen, S.E. Sensitivity of two quinoa (Chenopodium quinoa Willd.) varieties to progressive drought stress. J. Agron. Crop Sci. 2014, 200, 12–23. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa-a model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.A.F.; Martínez-Mosqueira, E.A.C.A.; Canahua-Murillo, A.; Zurita-Silva, A. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Gehan, M.A.; Greenham, K.; Mockler, T.C.; Mcclung, C.R. Transcriptional networks-crops, clocks, and abiotic stress. Curr. Opin. Plant Biol. 2015, 24, 39–46. [Google Scholar] [CrossRef]
- Majláth, I.; Darko, E.; Palla, B.; Nagy, Z.; Szalai, G. Reduced light and moderate water deficiency sustain nitrogen assimilation and sucrose degradation at low temperature in durum wheat. J. Plant Physiol. 2016, 191, 149–158. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Shi, Y.; Huy, P.; Liu, Y.; Cao, S.; Zhang, Z.; Chu, C.; Schlppi, M.R. Glycosyltransferase OsUGT90A1 helps protect the plasma membrane during chilling stress in rice. J. Exp. Bot. 2020, 9, 2723–2739. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Z.; Luo, W.; Tang, Y.; Niu, Y.; Chong, K.; Xu, Y. OsGRF6 interacts with SLR1 to regulate OsGA2ox1 expression for coordinating chilling tolerance and growth in rice. J. Plant Physiol. 2021, 260, 153406. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Wan, J. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 15. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Amandin, R.; Alice, M.; Jean, C.H.; Millicent, A.; Geofrey, L. Effect of low temperature stress on field performance of highland sorghum (Sorghum bicolor (L.) Moench) at flowering stages. J. Plant Breed. Crop Sci. 2020, 12, 25–33. [Google Scholar] [CrossRef]
- Cui, G.; Chai, H.; Yin, H.; Yang, M.; Hu, G.; Guo, M.; Yi, R.; Zhang, P. Full-length transcriptome sequencing reveals the low-temperature-tolerance mechanism of Medicago falcata roots. BMC Plant Biol. 2019, 19, 575. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Li, B.; Nakayama, T.; Kawamura, Y.; Uemura, M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013, 4, 90. [Google Scholar] [CrossRef]
- Kanchupati, P.; Wang, Y.; Anower, M.R.; Boe, A.; Hu, T.; Wu, Y. The gene family in alfalfa: Expression analyses and identification of potential functional homologs of Arabidopsis CBF3. Crop Sci. 2017, 57, 2051–2063. [Google Scholar] [CrossRef]
- Artlip, T.S.; Wisniewski, M.E.; Bassett, C.L.; Norelli, J.L. CBF gene expression in peach leaf and bark tissues is gated by a circadian clock. Tree Physiol. 2013, 33, 866–877. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.K. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R. Arabidopsis CBF1 Overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Zoran, J.; Pillman, K.A.; Dhillon, T.; Skinner, J.S.; Stockinger, E.J. Hv-CBF2A overexpression in barley accelerates COR gene transcript accumulation and acquisition of freezing tolerance during cold acclimation. Plant Mol. Biol. 2014, 84, 67–82. [Google Scholar]
- Chinnusamy, V. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lang, Z.; Zhu, J.K. Cold responsive gene transcription becomes more complex. Trends Plant Sci. 2015, 20, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef]
- Cui, J.; Shen, N.; Lu, Z.; Xu, G.; Jin, B. Analysis and comprehensive comparison of PacBio and nanopore-based RNA sequencing of the Arabidopsis transcriptome. Plant Methods 2020, 16, 85. [Google Scholar] [CrossRef]
- Ali, N.; Chen, H.; Zhang, C.; Khan, S.A.; Zhuang, W. Ectopic expression of AhGLK1b (GOLDEN2-like transcription factor) in Arabidopsis confers dual resistance to fungal and Bacterial Pathogens. Genes 2020, 11, 343. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, B.; Dong, H.; He, G.; Sun, J. BIC 1 acts as a transcriptional coactivator to promote brassinosteroid signaling and plant growth. EMBO J. 2020, 40, e104615. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yan, Y.; Wang, W.; Gebretsadik, K.; Qi, X.; Xu, Q.; Chen, X. Comparative proteomic analysis of cucumber powdery mildew resistance between a single-segment substitution line and its recurrent parent. Hortic. Res. 2019, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Davletova, S.; Kchlauch, K.; Coutu, J.; Mittler, R. The Zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Soitamo, A.J.; Piippo, M.; Allahverdiyeva, Y.; Battchikova, N.; Aro, E.M. Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Dahal, P.; Kunusoth, K.; McCallum, C.M.; Bradford, K.J. Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. Plant Cell 2013, 25, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, I.W.W. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperons in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Seo, P.J.; Kim, M.J.; Ryu, J.Y.; Jeong, E.Y.; Park, C.M. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat. Commun. 2011, 2, 303. [Google Scholar] [CrossRef]
- Kamata, T.; Uemura, M. Solute accumulation in wheat seedlings during cold acclimation: Contribution to increased freezing tolerance. Cryoletters 2004, 25, 311–322. [Google Scholar]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2021, 171, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Rekarte-Cowie, I.; Ebshish, O.S.; Mohamed, K.S.; Pearce, R.S. Sucrose helps regulate cold acclimation of Arabidopsis thaliana. J. Exp. Bot. 2008, 59, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Prateek, T.; Abbu, Z.; Challa, G.S.; Anuj, K.; Vinay, K.; Jyoti, U.; Rohit, J.; Manoj, B. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol. Biol. 2018, 97, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Ivan, C.; Cécile, S.; Gwenola, G.; Abdelhak, E.A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar]
- Chiou, D.; Bush, D. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 1998, 95, 4784–4788. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.-J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radical Res. 2019, 31, 273–300. [Google Scholar] [CrossRef]
- Chen, J.H.; Jiang, H.W.; Hsieh, E.J.; Chen, H.Y.; Chien, C.T.; Hsieh, H.L.; Lin, T.P. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef]
- Szalai, G.; Kells, T.; Galiba, G.; Kocsy, G. Glutathione as an Antioxidant and Regulatory Molecule in Plants Under Abiotic Stress Conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Thomashow, F.M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar]
- Candida, V.; Franca, L.; Marcella, B.; Enrico, M.; Milena, M.; Michela, O.; Monica, M.; Elena, B.; Immacolata, C. Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 2010, 37, 115–127. [Google Scholar]
- Vogel, J.T.; Zarka, D.G.; Buskirk, H.V.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2010, 41, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Dove, S.K.; Cooke, F.T.; Douglas, M.R.; Sayers, L.G.; Michell, R.H. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 1997, 390, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Clarke, S.M.; Mur, W.L.A.J. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004, 135, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Wei, J.; Ping, D.; Ru, H. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef]
- SchmöCkel, S.M.; Lightfoot, D.J.; Rozaimi, R.; Mark, T.; Jarvis, D.E. Identification of putative transmembrane proteins involved in salinity tolerance in chenopodium quinoa by integrating physiological data, RNAseq, and SNP analyses. Front. Plant Sci. 2017, 8, 1023. [Google Scholar] [CrossRef]
- Chou, W.C.; Lin, S.S.; Yeh, S.D.; Li, S.L.; Chen, T.C. Characterization of the genome of a phylogenetically distinct tospovirus and its interactions with the local lesion-induced host Chenopodium quinoa by whole-transcriptome analyses. PLoS ONE 2017, 12, e0182425. [Google Scholar] [CrossRef]
- Qi, W.; Bai, X.; Wu, X.; Xiang, D.; Wan, Y.; Luo, Y.; Shi, X.; Li, Q.; Zhao, J.; Qin, P.; et al. Transcriptome profiling identifies transcription factors and key homologs involved in seed dormancy and germination regulation of Chenopodium quinoa. Plant Physiol. Biochem. 2020, 151, 443–456. [Google Scholar]
- Filichkin, S.; Priest, H.D.; Megraw, M.; Mockler, T.C. Alternative splicing in plants: Directing traffic at the crossroads of adaptation and environmental stress. Curr. Opin. Plant Biol. 2015, 24, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Martín, G.; Márquez, Y.; Mantica, F.; Duque, P.; Irimia, M. Alternative splicing landscapes in Arabidopsis thaliana across tissues and stress conditions highlight major functional differences with animals. Genome Biol. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Guo, Q.H.; Xu, W.B.; Liu, P.; Yan, K. Rapid Regulation of Alternative Splicing in Response to Environmental Stresses. Front. Plant Sci. 2022, 13, 832177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zink, D.; Korn, B.; Vingron, M.; Haas, S. Genome wide identification and classification of alternative splicing based on EST data. Bioinformatics 2004, 20, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brendel, V. Genomewide comparative analysis of alternative splicing in plants. Proc. Natl. Acad. Sci. USA 2006, 103, 7175–7180. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Leborgne-Castel, N.; Luu, D.T. Regulation of endocytosis by external stimuli in plant cells. Plant Biosyst. 2009, 143, 630–635. [Google Scholar] [CrossRef]
- Poór, P.; Czékus, Z.; Tari, I.; Rdg, A. The multifaceted roles of plant hormone salicylic acid in endoplasmic reticulum stress and unfolded protein response. Int. J. Mol. Sci. 2019, 20, 5842. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.Q.; Yang, Y.; Chen, Q.; Xiao, J.X. Comparative transcriptome analysis reveals positive effects of arbuscular mycorrhizal fungi inoculation on photosynthesis and high-pH tolerance in blueberry seedlings. Trees 2019, 34, 433–444. [Google Scholar] [CrossRef]
- Cheng, C.; Yun, K.Y.; Ressom, H.W.; Mohanty, B.; Bajic, V.B.; Jia, Y.; Song, J.Y.; Reyes, B. An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 2007, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Mélida, H.; López, G.; Dabos, P.; Molina, A. Arabidopsis response regulator 6 (ARR6) modulates plant cell-wall composition and disease resistance. Mol. Plant Microbe Interact. 2020, 33, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Zwack, P.; Clercq, I.; Howton, T.; Hallmark, H.; Hurny, A.; Keshishian, E. Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress. Plant Physiol. 2016, 172, 1249–1258. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for rna-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Tao, C.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, S.E.; Hamilton, M.; Jacobi, J.L.; Ngam, P.; Devitt, N.; Schilkey, F.; Ben-Hur, A.; Reddy, A.S. Survey of the sorghum transcriptome using single-molecule long reads. Nat. Commun. 2016, 7, 11706. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhao, Y.; Gan, Y.; Li, H.; Luo, S.; Liu, X.; Li, Y.; Shao, Q.; Zhang, H.; Zhao, Y.; et al. Full-Length Transcriptome Sequencing Reveals the Impact of Cold Stress on Alternative Splicing in Quinoa. Int. J. Mol. Sci. 2022, 23, 5724. https://doi.org/10.3390/ijms23105724
Zheng L, Zhao Y, Gan Y, Li H, Luo S, Liu X, Li Y, Shao Q, Zhang H, Zhao Y, et al. Full-Length Transcriptome Sequencing Reveals the Impact of Cold Stress on Alternative Splicing in Quinoa. International Journal of Molecular Sciences. 2022; 23(10):5724. https://doi.org/10.3390/ijms23105724
Chicago/Turabian StyleZheng, Ling, Yiwu Zhao, Yifeng Gan, Hao Li, Shiqi Luo, Xiang Liu, Yuanyuan Li, Qun Shao, Hui Zhang, Yanxiu Zhao, and et al. 2022. "Full-Length Transcriptome Sequencing Reveals the Impact of Cold Stress on Alternative Splicing in Quinoa" International Journal of Molecular Sciences 23, no. 10: 5724. https://doi.org/10.3390/ijms23105724
APA StyleZheng, L., Zhao, Y., Gan, Y., Li, H., Luo, S., Liu, X., Li, Y., Shao, Q., Zhang, H., Zhao, Y., & Ma, C. (2022). Full-Length Transcriptome Sequencing Reveals the Impact of Cold Stress on Alternative Splicing in Quinoa. International Journal of Molecular Sciences, 23(10), 5724. https://doi.org/10.3390/ijms23105724





