Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of HA-Dop
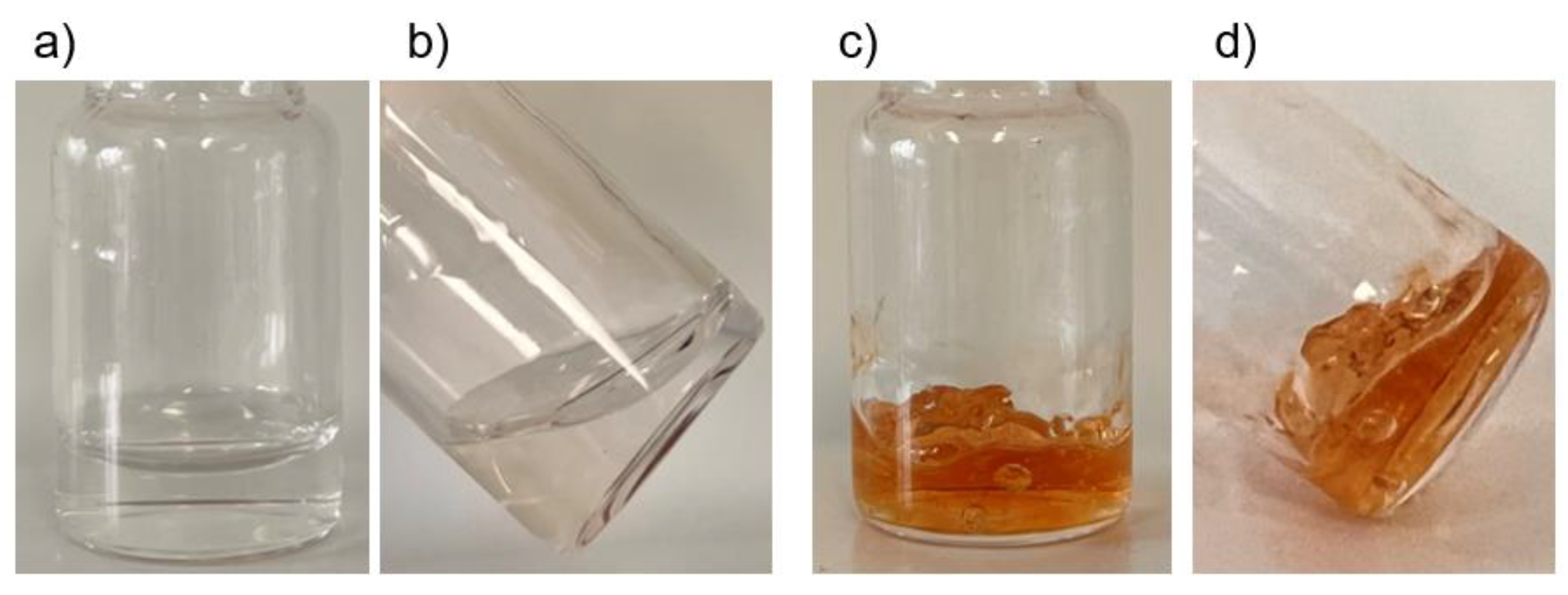
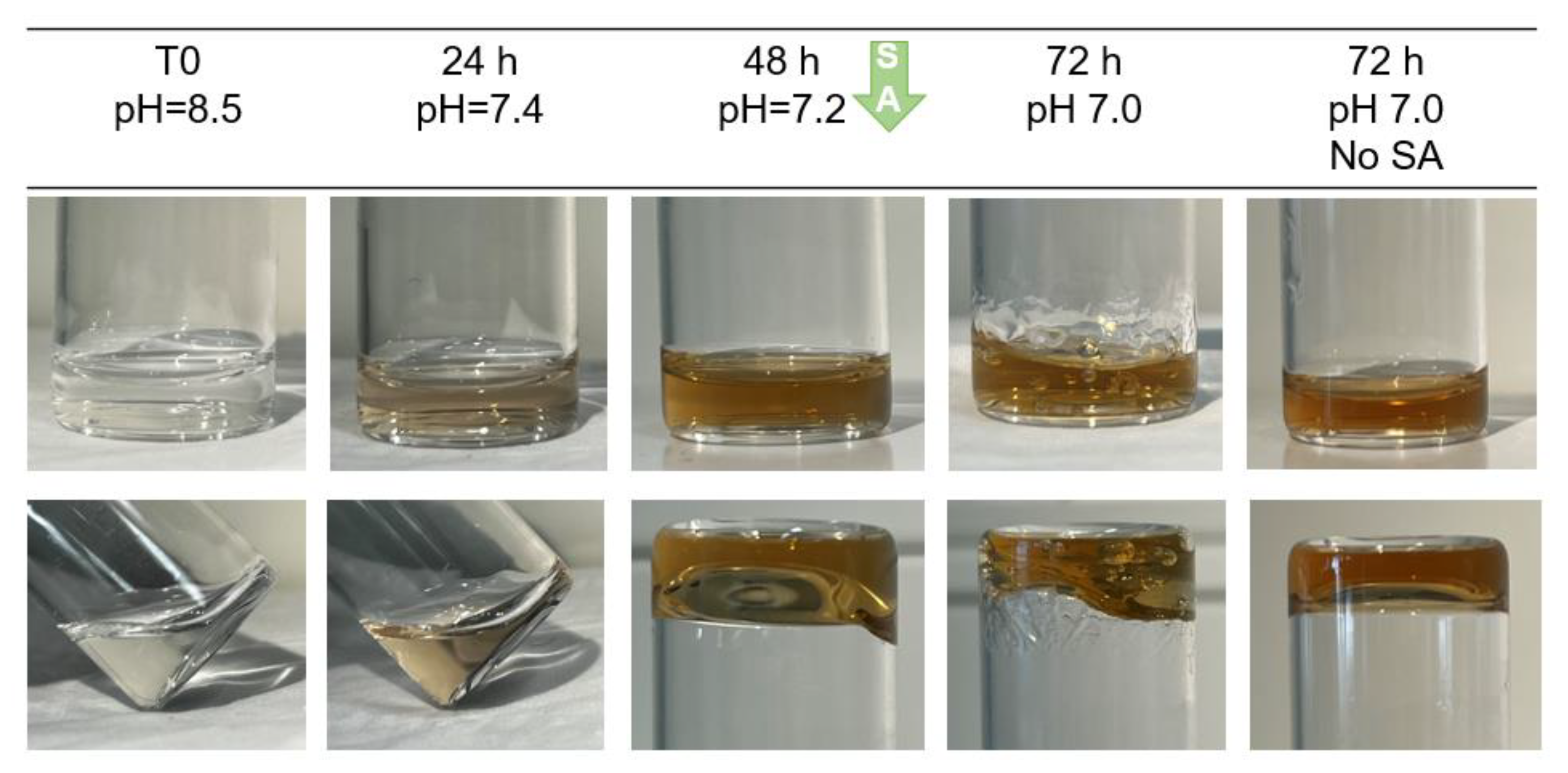
2.2. Crosslinking of HA-Dop with Either Sodium Periodate or Sodium Hydroxide
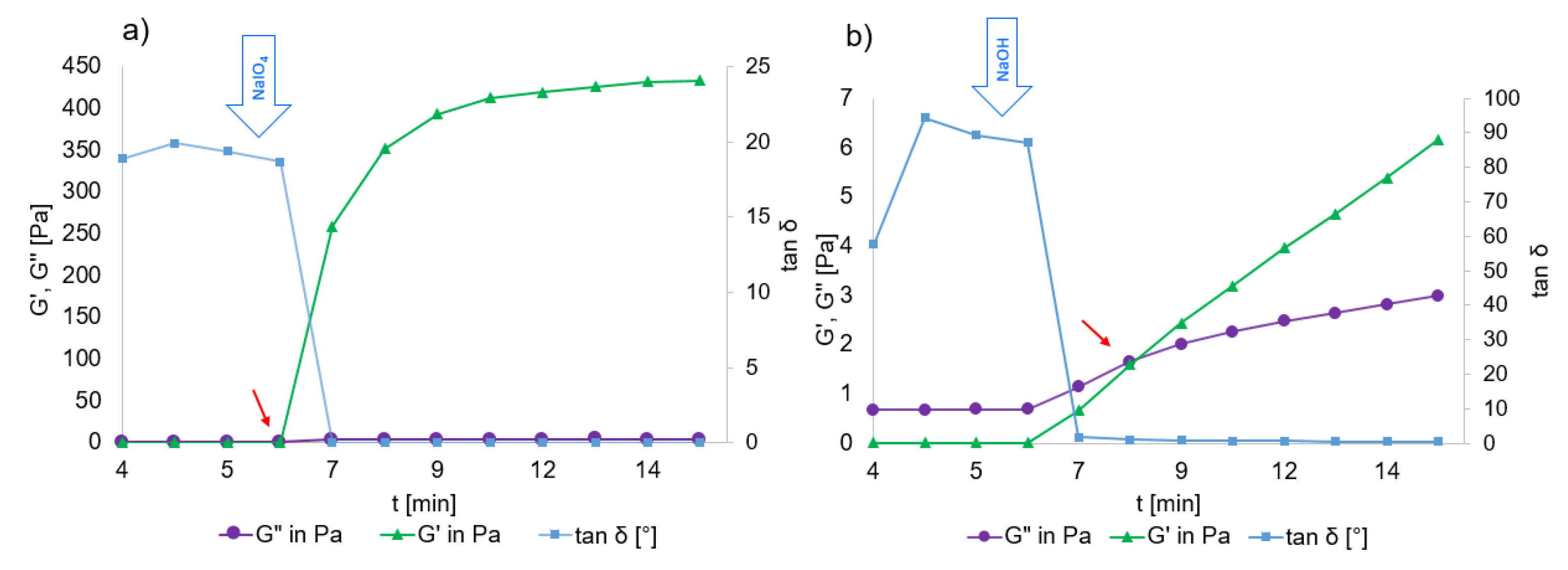
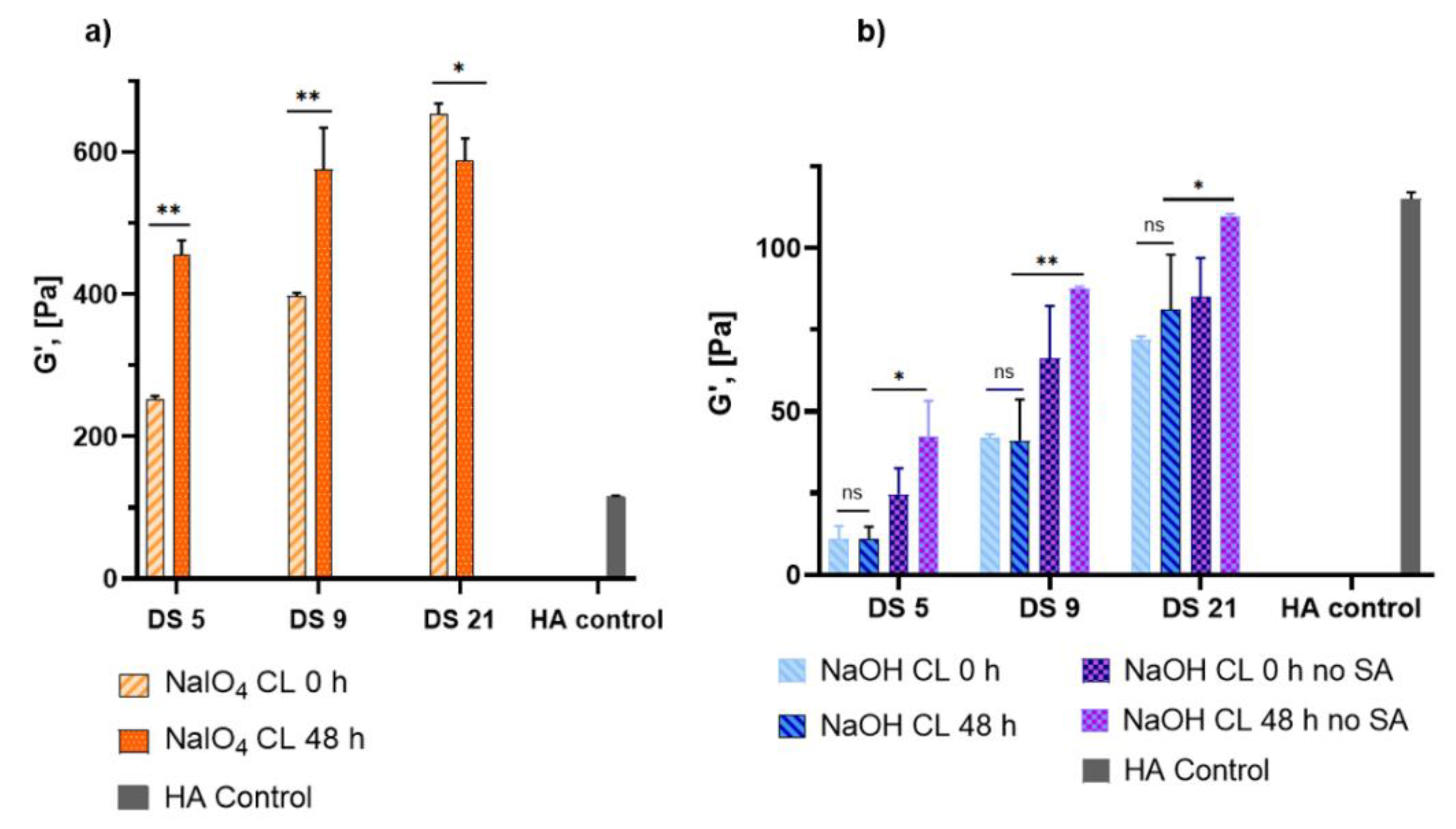
2.3. Rheological Properties of HA-Dop Crosslinked Gels
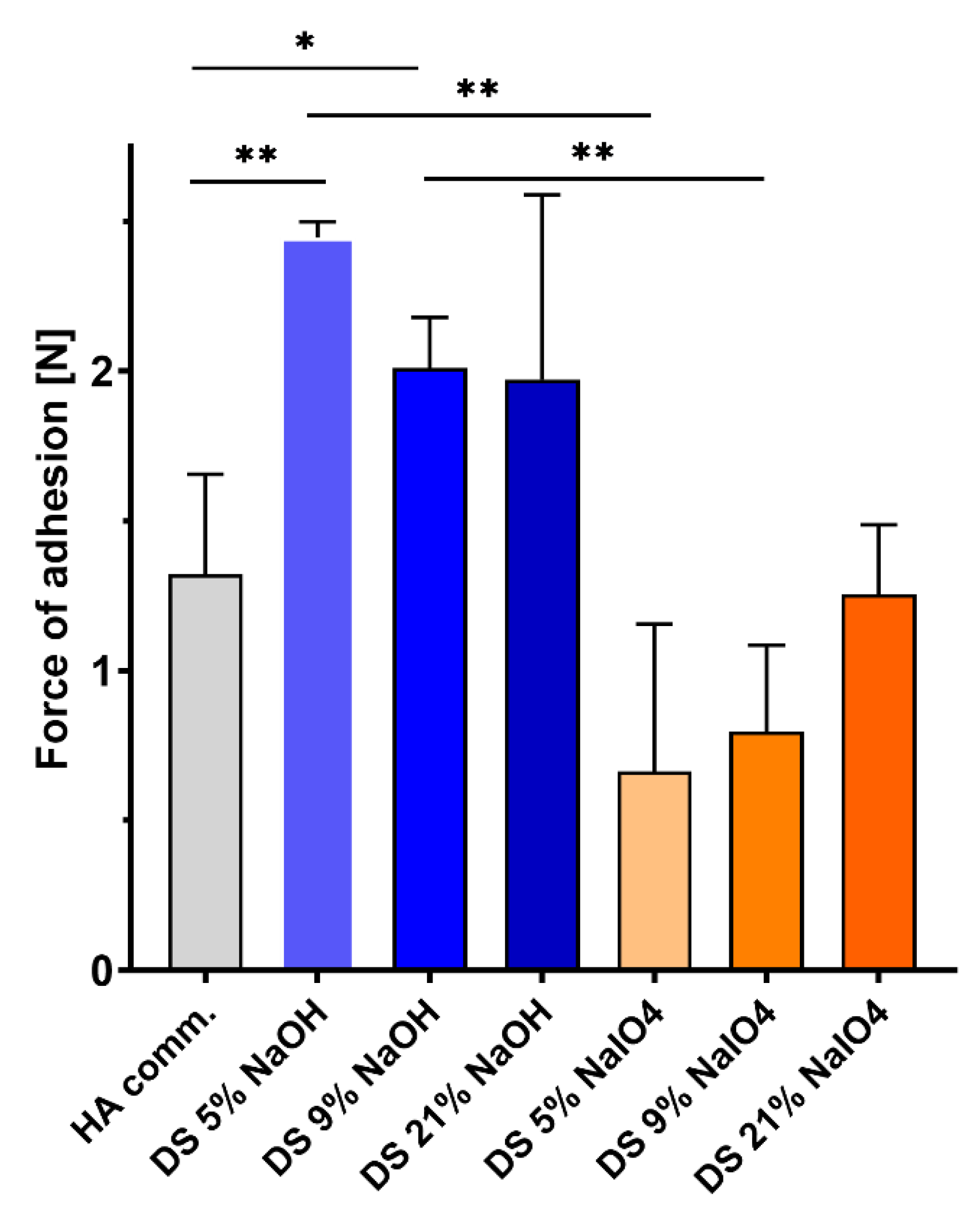
2.4. Ex Vivo Adhesion to Porcine Aorta Tissues
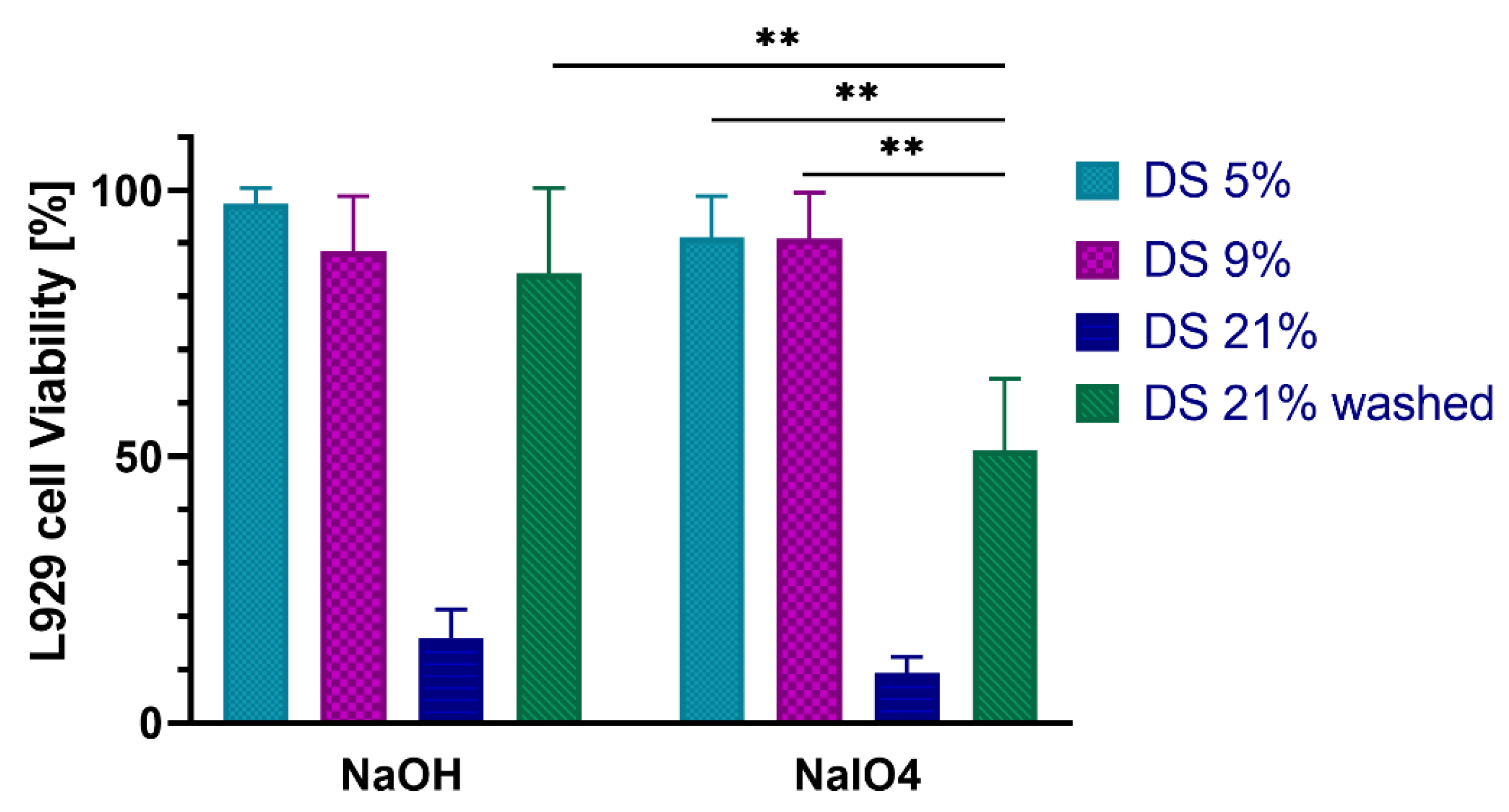
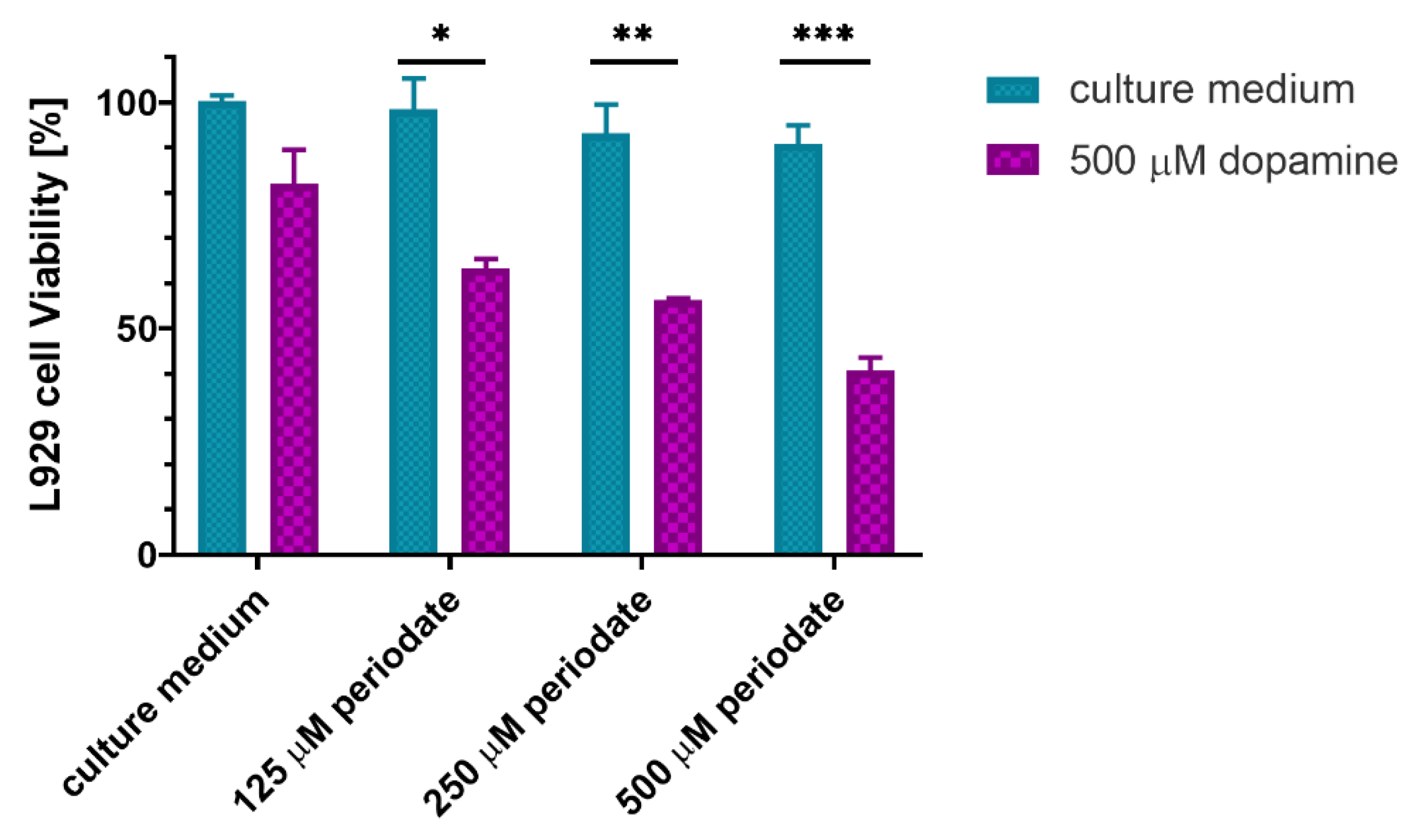
2.5. In Vitro Cytocompatibility with Fibroblast Cells
2.6. In Vitro Release of Atorvastatin from the HA-Dop NaOH-CL Gel
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. HA Molecular Weight Measurement
4.2.2. Synthesis of HA-Dop
4.2.3. DS Determination by NMR Spectroscopy
4.2.4. DS Determination by UV-Vis Spectroscopy
4.2.5. FTIR
4.2.6. HA-Dop Crosslinking with Sodium Periodate
4.2.7. HA-Dop Crosslinking with Sodium Hydroxide
4.2.8. Rheological Measurements
4.2.9. Adhesion to Porcine Aorta
4.2.10. Gel Extracts Preparation and Cytotoxicity Tests
4.2.11. Determination of Dopamine Concentration in the Gel Extract
4.2.12. Incorporation of ATV into the Gel, In Vitro Drug Release Study
4.2.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Gasper, W.J.; Rahman, A.S.; Conte, M.S. Vein graft failure. J. Vasc. Surg. 2015, 61, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylonaki, I.; Strano, F.; Deglise, S.; Allémann, E.; Alonso, F.; Corpataux, J.-M.; Dubuis, C.; Haefliger, J.-A.; Jordan, O.; Saucy, F.; et al. Perivascular sustained release of atorvastatin from a hydrogel-microparticle delivery system decreases intimal hyperplasia. J. Control. Release 2016, 232, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Mottola, G.; Chatterjee, A.; Lance, K.D.; Chen, M.; Siguenza, I.O.; Desai, T.A.; Conte, M.S. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J. Vasc. Surg. 2017, 65, 207–217.e3. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Shi, X.; Wang, B.; Xie, R.; Guo, L.-W.; Gong, S.; Kent, K.C. Unimolecular Micelle-Based Hybrid System for Perivascular Drug Delivery Produces Long-Term Efficacy for Neointima Attenuation in Rats. Biomacromolecules 2017, 18, 2205–2213. [Google Scholar] [CrossRef]
- Melnik, T.; Jordan, O.; Corpataux, J.-M.; Delie, F.; Saucy, F. Pharmacological prevention of intimal hyperplasia: A state-of-the-art review. Pharmacol. Ther. 2022, 235, 108157. [Google Scholar] [CrossRef]
- Chaudhary, M.A.; Guo, L.-W.; Shi, X.; Chen, G.; Gong, S.; Liu, B.; Kent, K.C. Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery. J. Control. Release 2016, 233, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Mylonaki, I.; Allémann, É.; Saucy, F.; Haefliger, J.-A.; Delie, F.; Jordan, O. Perivascular medical devices and drug delivery systems: Making the right choices. Biomaterials 2017, 128, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Rohrich, R.J.; Ghavami, A.; Crosby, M.A. The Role of Hyaluronic Acid Fillers (Restylane) in Facial Cosmetic Surgery: Review and Technical Considerations. Plast. Reconstr. Surg. 2007, 120, 41S–54S. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Park, H.-J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.-W. Catechol-Functionalized Hyaluronic Acid Hydrogels Enhance Angiogenesis and Osteogenesis of Human Adipose-Derived Stem Cells in Critical Tissue Defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic Acid Catechol: A Biopolymer Exhibiting a pH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, Z.; Xu, X.; Feng, Q.; Xu, J.; Bian, L. Efficient catechol functionalization of biopolymeric hydrogels for effective multiscale bioadhesion. Mater. Sci. Eng. C 2019, 103, 109835. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Sato, T.; Aoyagi, T.; Ebara, M.; Auzély-Velty, R. Catechol-modified hyaluronic acid: In situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym. Bull. 2017, 74, 4069–4085. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Behboodi-Sadabad, F.; Welle, A.; Li, J.; Heissler, S.; Zhang, H.; Plumeré, N.; Levkin, P.A. Bio-inspired strategy for controlled dopamine polymerization in basic solutions. Polym. Chem. 2017, 8, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Lih, E.; Choi, S.G.; Ahn, D.J.; Joung, Y.K.; Han, D.K. Optimal conjugation of catechol group onto hyaluronic acid in coronary stent substrate coating for the prevention of restenosis. J. Tissue Eng. 2016, 7, 2041731416683745. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ryu, J.H.; Lee, H. Effect of charge on in vivo adhesion stability of catechol-conjugated polysaccharides. J. Ind. Eng. Chem. 2019, 79, 425–430. [Google Scholar] [CrossRef]
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces 2019, 182, 110388. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lee, E.; Yeudall, W.A.; Yang, H. Dendrimer-triglycine-EGF nanoparticles for tumor imaging and targeted nucleic acid and drug delivery. Oral Oncol. 2010, 46, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, G.P.; Bernt, C.M.; Butler, A. Catechol oxidation: Considerations in the design of wet adhesive materials. Biomater. Sci. 2018, 6, 332–339. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Guo, Z.; Mi, S.; Sun, W. The multifaceted nature of catechol chemistry: Bioinspired pH-initiated hyaluronic acid hydrogels with tunable cohesive and adhesive properties. J. Mater. Chem. B 2018, 6, 6234–6244. [Google Scholar] [CrossRef]
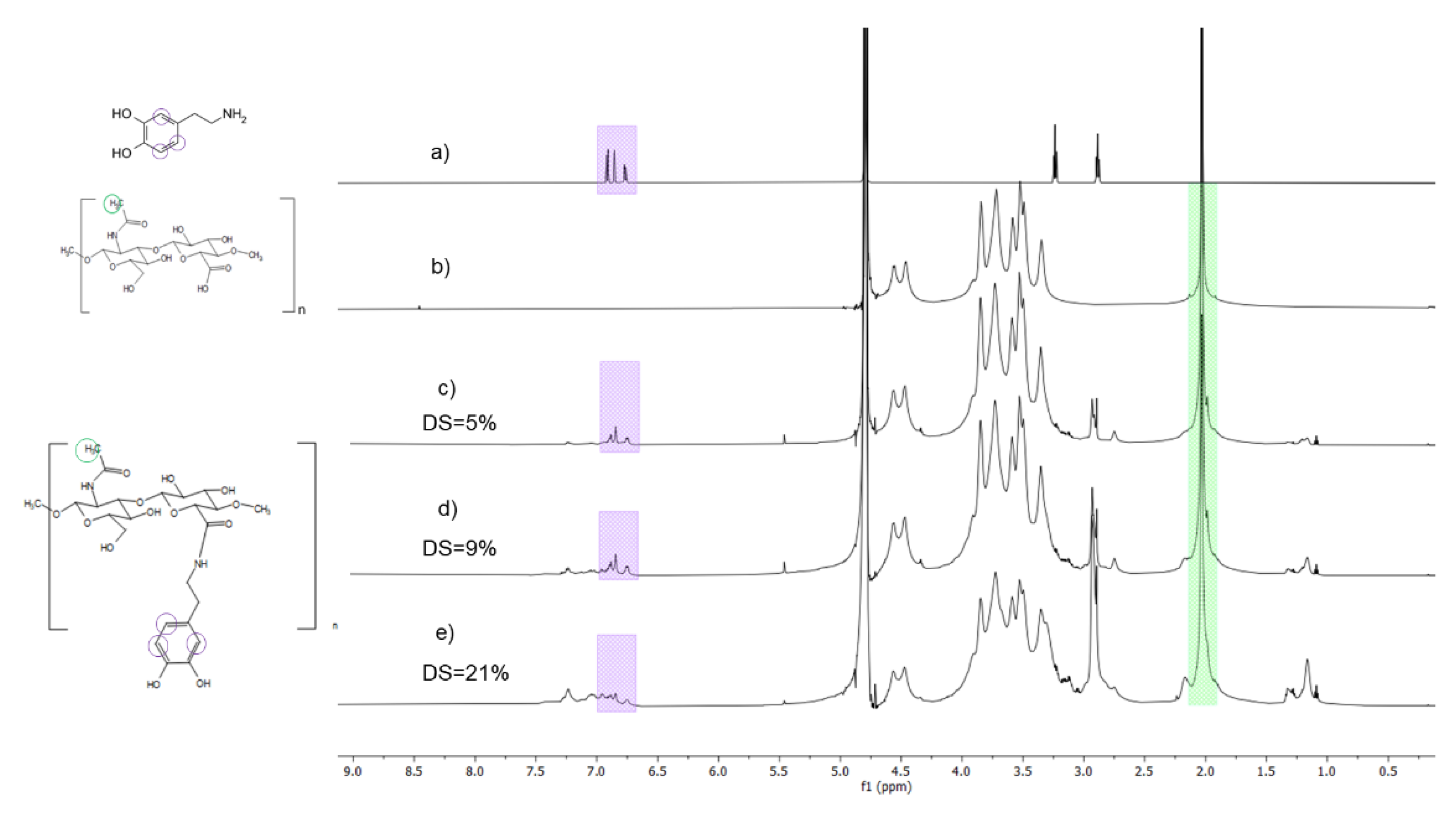

| Target DS | EDC | sNHS | Dopamine | HA-Dop DS (NMR) | HA-Dop DS (UV) |
|---|---|---|---|---|---|
| Low | 2.0 | 1.2 | 1.2 | 5.7 ± 0.6 | 5.4 ± 0.9 |
| Medium | 3.0 | 1.3 | 1.3 | 9 ± 1 | 9.4 ± 2.0 |
| High | 5.0 | 1.5 | 1.5 | 19 ± 2 | 18 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnik, T.; Ben Ameur, S.; Kanfar, N.; Vinet, L.; Delie, F.; Jordan, O. Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. Int. J. Mol. Sci. 2022, 23, 5706. https://doi.org/10.3390/ijms23105706
Melnik T, Ben Ameur S, Kanfar N, Vinet L, Delie F, Jordan O. Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. International Journal of Molecular Sciences. 2022; 23(10):5706. https://doi.org/10.3390/ijms23105706
Chicago/Turabian StyleMelnik, Tamara, Senda Ben Ameur, Nasreddine Kanfar, Laurent Vinet, Florence Delie, and Olivier Jordan. 2022. "Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking" International Journal of Molecular Sciences 23, no. 10: 5706. https://doi.org/10.3390/ijms23105706
APA StyleMelnik, T., Ben Ameur, S., Kanfar, N., Vinet, L., Delie, F., & Jordan, O. (2022). Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. International Journal of Molecular Sciences, 23(10), 5706. https://doi.org/10.3390/ijms23105706







