The Cytoskeletal Protein Zyxin Inhibits Retinoic Acid Signaling by Destabilizing the Maternal mRNA of the RXRγ Nuclear Receptor
Abstract
:1. Introduction
2. Results
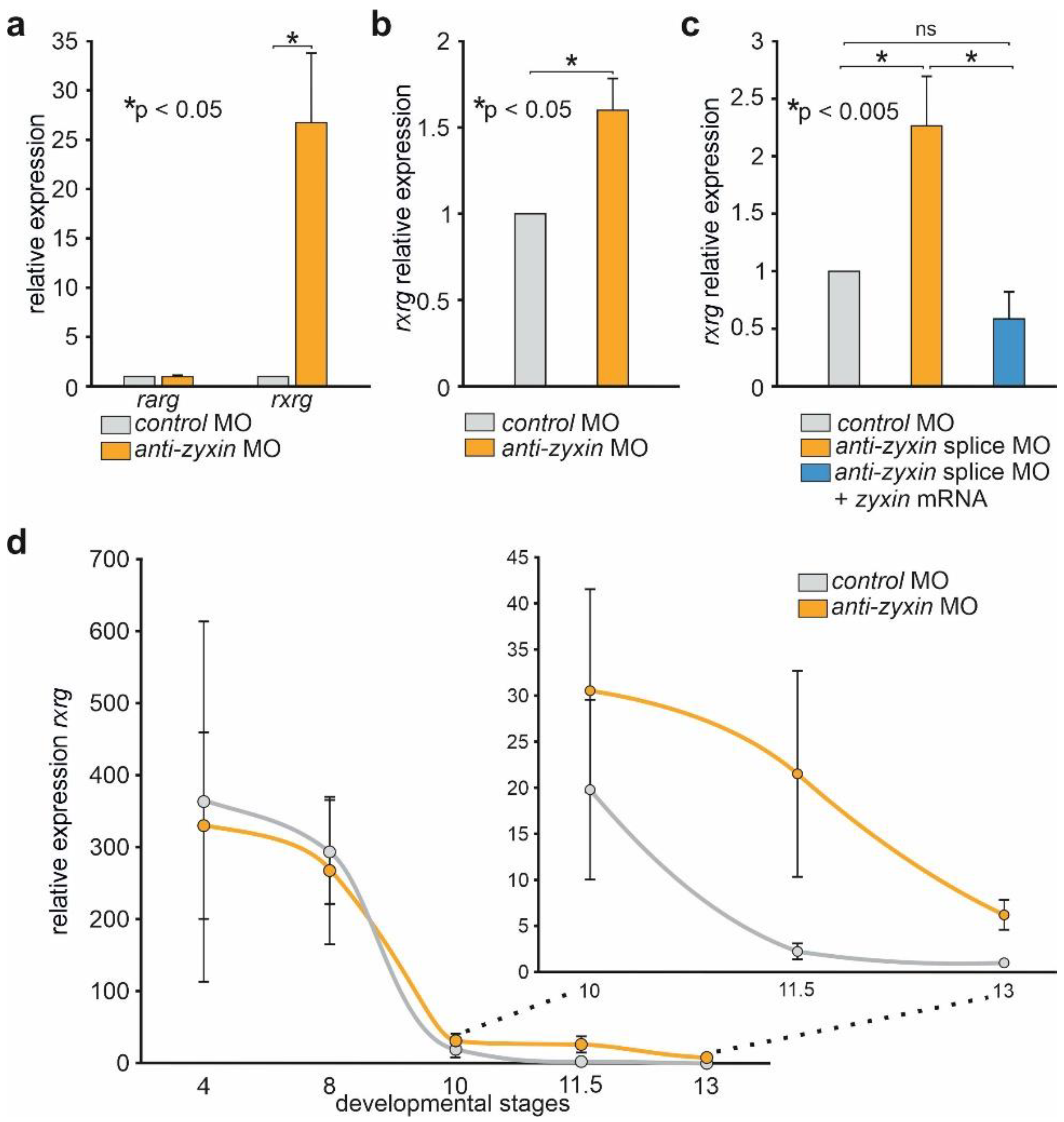
2.1. Downregulation of Zyxin Results in the Increase in rxrγ mRNA Level
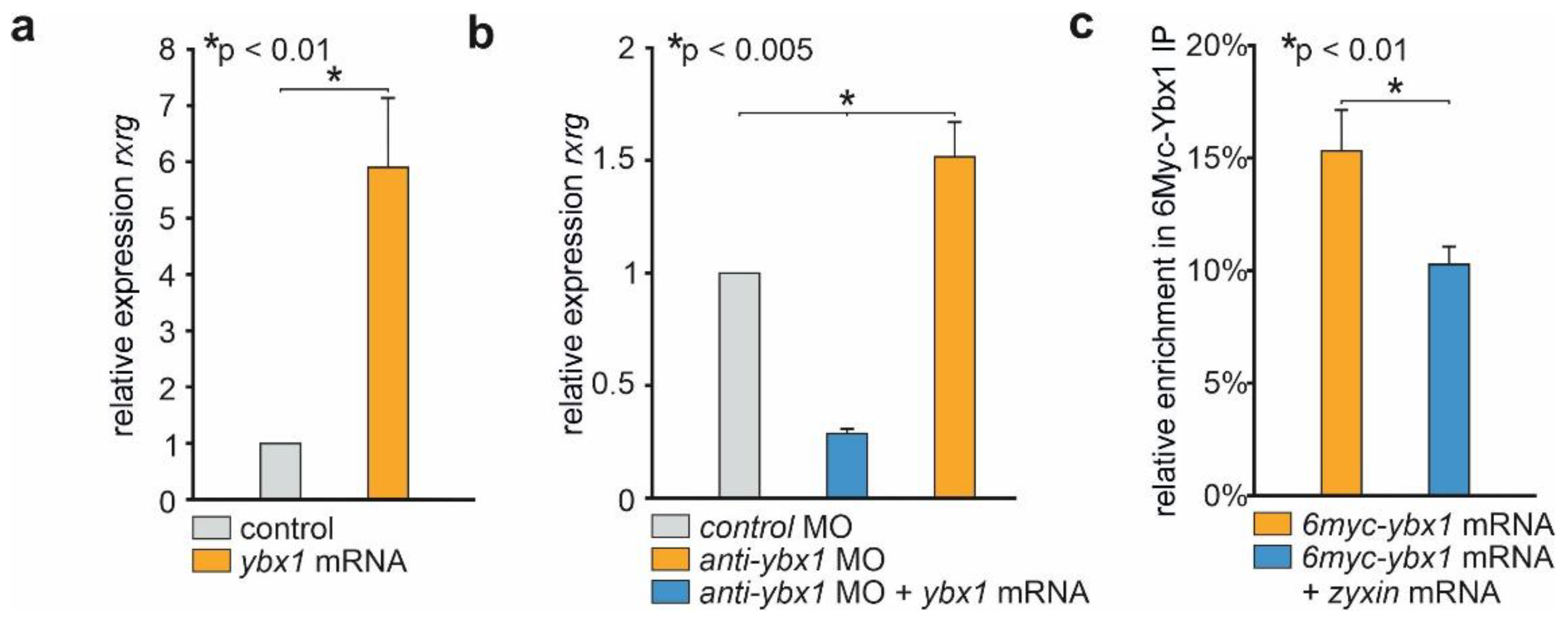
2.2. Zyxin Prevents Binding of Ybx1 to rxrγ mRNA
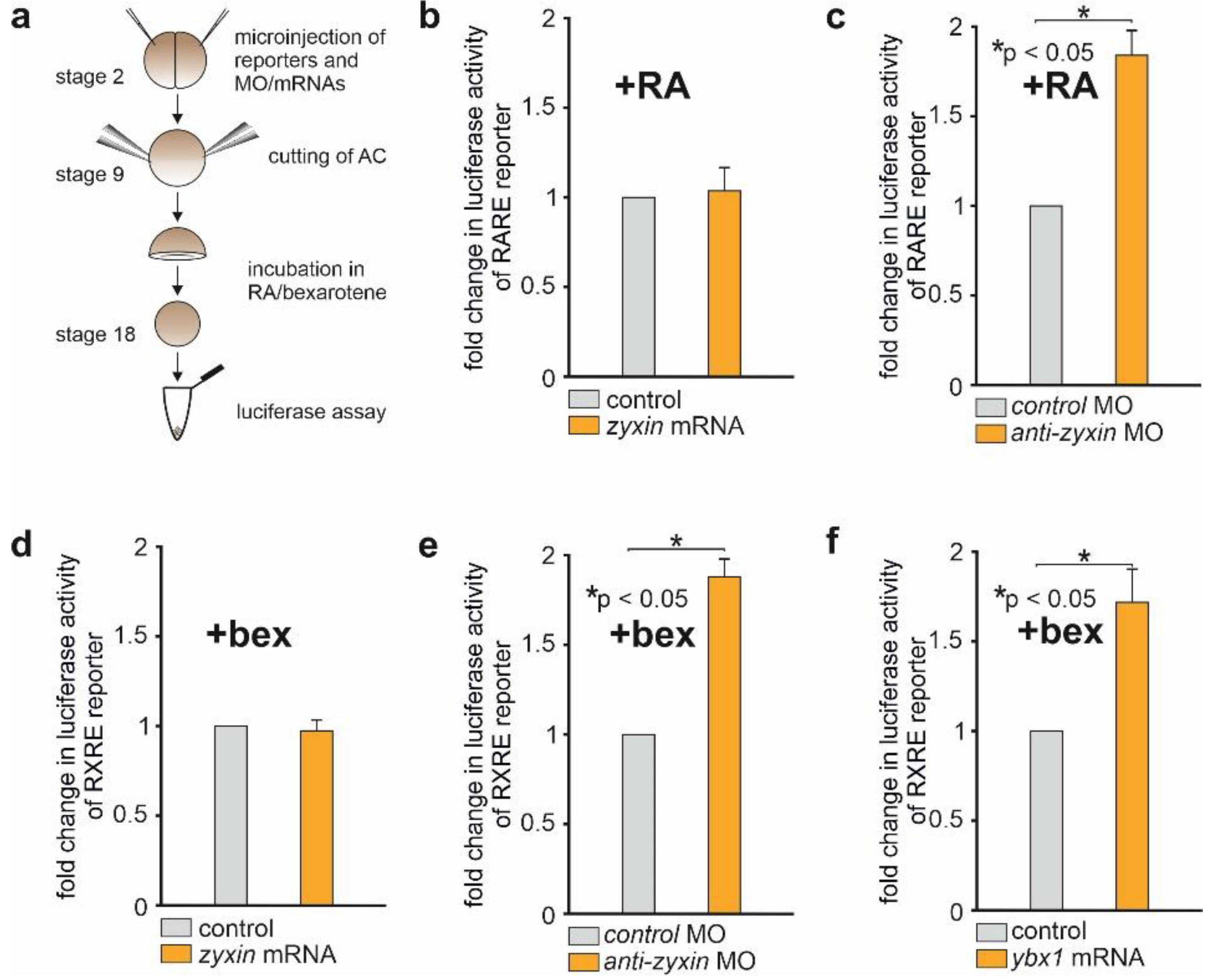
2.3. Downregulation of Zyxin Results in the Activation of Luciferase Reporters Containing the Cis-Regulatory Elements Essential for the RA-Dependent Signaling
2.4. The Distribution of Zyxin between the Cytoplasm and the Nucleus Is Not Changed upon Treatment of AC with RA or Bexarotene
3. Discussion
4. Materials and Methods
4.1. Experimental Model and Subject Details
Embryo Manipulations
4.2. Method Details
4.2.1. Embryonic Microinjections
4.2.2. mRNA Extraction and qRT-PCR
4.2.3. DNA Constructs
4.2.4. Synthetic mRNA
4.2.5. RNA-Immunoprecipitation (RIP)
4.2.6. Luciferase Reporter Assay
4.2.7. Cytoplasmic and Nuclear Fractionation
4.2.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.A.; Hoffman, L.M.; Beckerle, M.C. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014, 24, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gilmore, T.D. Zyxin and paxillin proteins: Focal adhesion plaque LIM domain proteins go nuclear. Biochim. Biophys. Acta 2003, 1593, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelman, J.D.; Anderson, C.A.; Suarez, C.; Kovar, D.R.; Gardel, M.L. Evolutionarily diverse LIM domain containing proteins bind stressed actin filaments through a conserved mechanism. Proc. Natl. Acad. Sci. USA 2020, 117, 25532–25542. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Focal adhesion: A focal point in current cell biology and molecular medicine. Cell Adh. Migr. 2007, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Phua, D.Y.Z.; Axiotakis, L.; Smith, M.A.; Blankman, E.; Gong, R.; Cail, R.C.; Espinosa de los Reyes, S.; Beckerle, M.C.; Waterman, C.M. Mechanosensing through direct binding of tensed F-actin by LIM domains. Dev. Cell 2020, 55, 468–482. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, D.Y.; Guo, Y.C.; Guo, J. Zyxin: A mechanotransductor to regulate gene expression. Eur. Rev. Med. Pharm. Sci. 2019, 23, 413–425. [Google Scholar] [CrossRef]
- Martynova, N.Y.; Eroshkin, F.M.; Ermolina, L.V.; Ermakova, G.V.; Korotaeva, A.L.; Smurova, K.M.; Gyoeva, F.K.; Zaraisky, A.G. The LIM-domain protein Zyxin binds the homeodomain factor Xanf1/Hesx1 and modulates its activity in the anterior neural plate of Xenopus laevis embryo. Dev. Dyn. 2008, 237, 736–749. [Google Scholar] [CrossRef]
- Martynova, N.Y.; Ermolina, L.V.; Ermakova, G.V.; Eroshkin, F.M.; Gyoeva, F.K.; Baturina, N.S.; Zaraisky, A.G. The cytoskeletal protein Zyxin inhibits Shh signaling during the CNS patterning in Xenopus laevis through interaction with the transcription factor Gli1. Dev. Biol. 2013, 380, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Martynova, N.Y.; Parshina, E.A.; Zaraisky, A.G. Cytoskeletal protein Zyxin in embryonic development: From controlling cell movements and pluripotency to regulating embryonic patterning. FEBS J. 2021. [Google Scholar] [CrossRef]
- Parshina, E.A.; Eroshkin, F.M.; Orlov, E.E.; Gyoeva, F.K.; Shokhina, A.G.; Staroverov, D.B.; Belousov, V.V.; Zhigalova, N.A.; Prokhortchouk, E.B.; Zaraisky, A.G. Cytoskeletal protein zyxin inhibits the activity of genes responsible for embryonic stem cell status. Cell Rep. 2020, 33, 108396. [Google Scholar] [CrossRef]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Stunnenberg, H.G. Mechanisms of transactivation by retinoic acid receptors. Bioessays 1993, 15, 309–315. [Google Scholar] [CrossRef]
- Valcárcel, R.; Holz, H.; Jiménez, C.G.; Barettino, D.; Stunnenberg, H.G. Retinoid-dependent in vitro transcription mediated by the RXR/RAR heterodimer. Genes Dev. 1994, 8, 3068–3079. [Google Scholar] [CrossRef] [Green Version]
- Minucci, S.; Leid, M.; Toyama, R.; Saint-Jeannet, J.P.; Peterson, V.J.; Horn, V.; Ishmael, J.E.; Bhattacharyya, N.; Dey, A.; Dawid, I.B.; et al. Retinoid X receptor (RXR) within the RXR retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol. Cell. Biol. 1997, 17, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Medin, J.A.; Minucci, S.; Driggers, P.H.; Lee, I.J.; Ozato, K. Quantitative increases in DNA binding affinity and positional effects determine 9-cis retinoic acid induced activation of the retinoid X receptor beta homodimer. Mol. Cell. Endocrinol. 1994, 105, 27–35. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Maden, M. Retinoids as endogenous components of the regenerating limb and tail. Wound Repair Regen. 1998, 6, 358–365. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, B.; Mangelsdorf, D.J.; Dyck, J.A.; Bittner, D.A.; Evans, R.M.; De Robertis, E.M. Multiple retinoid-responsive receptors in a single cell: Families of retinoid “X” receptors and retinoic acid receptors in the Xenopus egg. Proc. Natl. Acad. Sci. USA 1992, 89, 2321–2325. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, C.R. The expression of retinoic acid receptors in Xenopus development. Biochem. Soc. Trans. 1994, 22, 575–579. [Google Scholar] [CrossRef]
- Martynova, N.Y.; Parshina, E.A.; Zaraisky, A.G. Using RNA-binding proteins for immunoprecipitation of mRNAs from Xenopus laevis embryos. STAR Protoc. 2021, 2, 100552. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Srivastava, A.R.; Cho-Chung, Y.S.; Longo, D.L. Synergistic effects of retinoic acid and 8-chloro-adenosine 3′,5′-cyclic monophosphate on the regulation of retinoic acid receptor beta and apoptosis: Involvement of mitochondria. Clin. Cancer Res. 1999, 5, 1892–1904. [Google Scholar] [PubMed]
- Barger, P.M.; Kelly, D.P. Identification of a retinoid/chicken ovalbumin upstream promoter transcription factor response element in the human retinoid X receptor gamma2 gene promoter. J. Biol. Chem. 1997, 272, 2722–2728. [Google Scholar] [CrossRef] [Green Version]
- Youn, H.; Kim, E.J.; Um, S.J. Zyxin cooperates with PTOV1 to confer retinoic acid resistance by repressing RAR activity. Cancer Lett. 2013, 331, 192–199. [Google Scholar] [CrossRef]
- Sala, S.; Ampe, C. An emerging link between LIM domain proteins and nuclear receptors. Cell Mol. Life Sci. 2018, 75, 1959–1971. [Google Scholar] [CrossRef]
- Hou, Z.; Peng, H.; White, D.E.; Negorev, D.G.; Maul, G.G.; Feng, Y.; Longmore, G.D.; Waxman, S.; Zelent, A.; Rauscher, F.J. LIM protein Ajuba functions as a nuclear receptor corepressor and negatively regulates retinoic acid signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 2938–2943. [Google Scholar] [CrossRef] [Green Version]
- Martynova, N.Y.; Parshina, E.A.; Zaraisky, A.G. Protocol for separation of the nuclear and the cytoplasmic fractions of Xenopus laevis embryonic cells for studying protein shuttling. STAR Protoc. 2021, 2, 100449. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Eroshkin, F.; Kazanskaya, O.; Martynova, N.; Zaraisky, A. Characterization of cis-regulatory elements of the homeobox gene Xanf-1. Gene 2002, 285, 279–286. [Google Scholar] [CrossRef]
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-Zyxin | A.G. Zaraisky lab, Moscow, Russia | N/A |
| Anti-rabbit IgG, AP-conjugated, produced in goat | Sigma-Aldrich, Missouri, USA | Cat#A3937; RRID:AB_258122 |
| Mouse monoclonal anti-alpha-Tubulin | Sigma-Aldrich, Missouri, USA | Cat#T9026; RRID:AB_477593 |
| Anti-mouse IgG, AP-conjugated | Sigma-Aldrich, Missouri, USA | Cat#A3562; RRID:AB_258091 |
| EZview Red Anti-c-Myc Affinity Gel | Sigma-Aldrich, Missouri, USA | Cat#E6654; RRID:AB_10093201 |
| Bacterial and Virus Strains | ||
| DH5alpha E. coli strain | N/A | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human chorionic gonadotropin | Sigma-Aldrich, Missouri, USA | Cat#CG10 |
| Ethyl 3-aminobenzoate methanesulfonate salt | Sigma-Aldrich, Missouri, USA | Cat#A5040 |
| Western Blue® stabilized substrate for alkaline phosphatase | Promega, Madison, Wisconsin, USA | Cat#S3841 |
| BM Purple AP-substrate | Roche, Basel, Switzerland | Cat#11442074001 |
| Brilliant Blue G solution | Sigma-Aldrich, Missouri, USA | Cat#B8522 |
| Cycloheximide | Sigma-Aldrich, Missouri, USA | Cat#C1988 |
| CNBr-Activated SepharoseTM 4B | GE Healthcare, Chicago, Illinois, USA | N/A |
| Fluorescein Lysine Dextran (FLD) 40 kD | Invitrogen, Waltham, Massachusetts, USA | Cat#D1845 |
| L-Cysteine | Dia-m, Moscow, Russia | Cat#M52904 |
| Ficoll | Dia-m, Moscow, Russia | Cat#PS400 |
| Igepal Nonidet P-40 | Sigma-Aldrich, Missouri, USA | Cat#I-30201 |
| DTT | Fermentas, Waltham, Massachusetts, USA | Cat#RO861 |
| RNase Out RNase inhibitor, 100 units/ml | Invitrogen, Waltham, Massachusetts, USA | Cat#10777-019 |
| Vanadyl ribonucleoside complexes (VRC) | New England Biolabs, Ipswich, Massachusetts, USA | Cat#S1402S |
| Protease Inhibitor Coctail | Sigma-Aldrich, Missouri, USA | Cat#P8340 |
| Bexarotene | LLC “Gurus BioPharm”, Moscow, Russia | N/A |
| Retinoic acid (RA) | Sigma-Aldrich, Missouri, USA | Cat#R2625 |
| Critical Commercial Assays | ||
| mMessage mMachineTM SP6kit | Thermo Fisher, Waltham, Massachusetts, USA | Cat#AM1340 |
| ExtractRNA | Evrogen, Moscow, Russia | Cat# BC032 |
| CleanRNA Standard | Evrogen, Moscow, Russia | Cat# BC033 |
| NucleoSpin RNA purification columns | Macherey Nagel, Germany | Cat#REF 740955.50 |
| MMLV RT | Evrogen, Moscow, Russia | Cat# SK021 |
| qPCRmix-HS SYBR | Evrogen, Moscow, Russia | Cat# PK147L |
| Dual-Luciferase Reporter Assay System | Promega, Madison, Wisconsin, USA | Cat# E1910 |
| Dynabeads mRNA DIRECT Kit | Ambion, Waltham, Massachusetts, USA | Cat# 61011 |
| Experimental Models: Organisms/Strains | ||
| Wild type Xenopus laevis frogs | Nasco, Chicago, USA | Cat#LM00456; RRID:XEP_Xla100 |
| Danio rerio AB/TL strain | N/A | N/A |
| Oligonucleotides | ||
| See Table S1 for all oligonucleotides used | ||
| Recombinant DNA | ||
| See Table S1 for all recombinant DNAs used | ||
| Software and Algorithms | ||
| Excel | Microsoft, USA | N/A |
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| Other | ||
| See Table S1 for all recombinant DNAs and oligonucleotides used | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshina, E.A.; Orlov, E.E.; Zaraisky, A.G.; Martynova, N.Y. The Cytoskeletal Protein Zyxin Inhibits Retinoic Acid Signaling by Destabilizing the Maternal mRNA of the RXRγ Nuclear Receptor. Int. J. Mol. Sci. 2022, 23, 5627. https://doi.org/10.3390/ijms23105627
Parshina EA, Orlov EE, Zaraisky AG, Martynova NY. The Cytoskeletal Protein Zyxin Inhibits Retinoic Acid Signaling by Destabilizing the Maternal mRNA of the RXRγ Nuclear Receptor. International Journal of Molecular Sciences. 2022; 23(10):5627. https://doi.org/10.3390/ijms23105627
Chicago/Turabian StyleParshina, Elena A., Eugeny E. Orlov, Andrey G. Zaraisky, and Natalia Y. Martynova. 2022. "The Cytoskeletal Protein Zyxin Inhibits Retinoic Acid Signaling by Destabilizing the Maternal mRNA of the RXRγ Nuclear Receptor" International Journal of Molecular Sciences 23, no. 10: 5627. https://doi.org/10.3390/ijms23105627
APA StyleParshina, E. A., Orlov, E. E., Zaraisky, A. G., & Martynova, N. Y. (2022). The Cytoskeletal Protein Zyxin Inhibits Retinoic Acid Signaling by Destabilizing the Maternal mRNA of the RXRγ Nuclear Receptor. International Journal of Molecular Sciences, 23(10), 5627. https://doi.org/10.3390/ijms23105627






