β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice
Abstract
:1. Introduction
2. Results
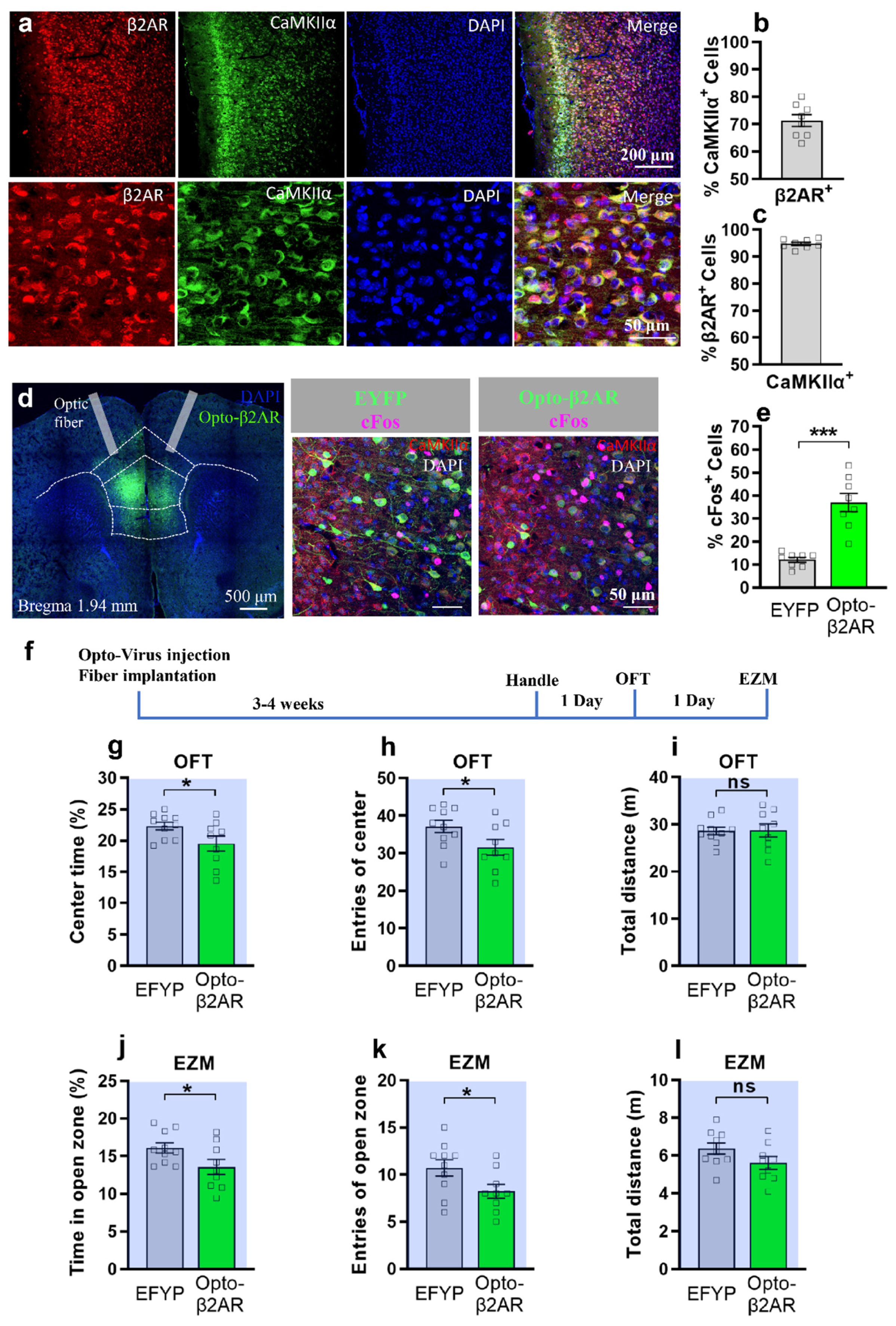
2.1. Optogenetic Activation of β2-AR in mPFC CaMKIIα Neurons Induced Anxiety in Open-Field Test and Elevated Zero Maze
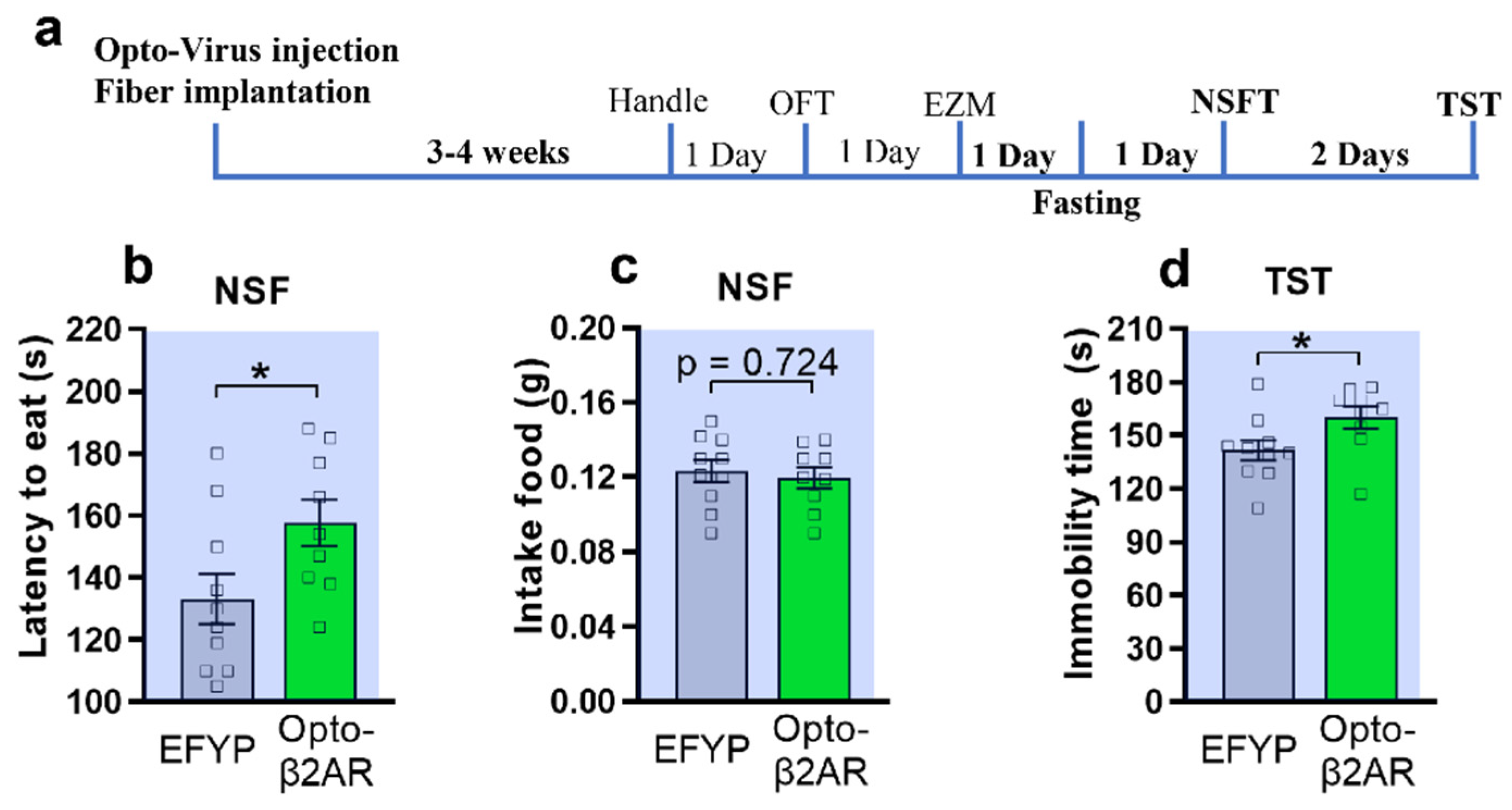
2.2. Optogenetic Activation of β2-AR within mPFC CaMKIIα Neurons Induced Anxiety-like Behavior in Novelty-Suppressed Feeding Test and Depression-like Behavior in Tail-Suspension Test
2.3. Optogenetic Activation of β2-AR within mPFC CaMKIIα Neurons Was Sufficient to Reduce Social Interaction
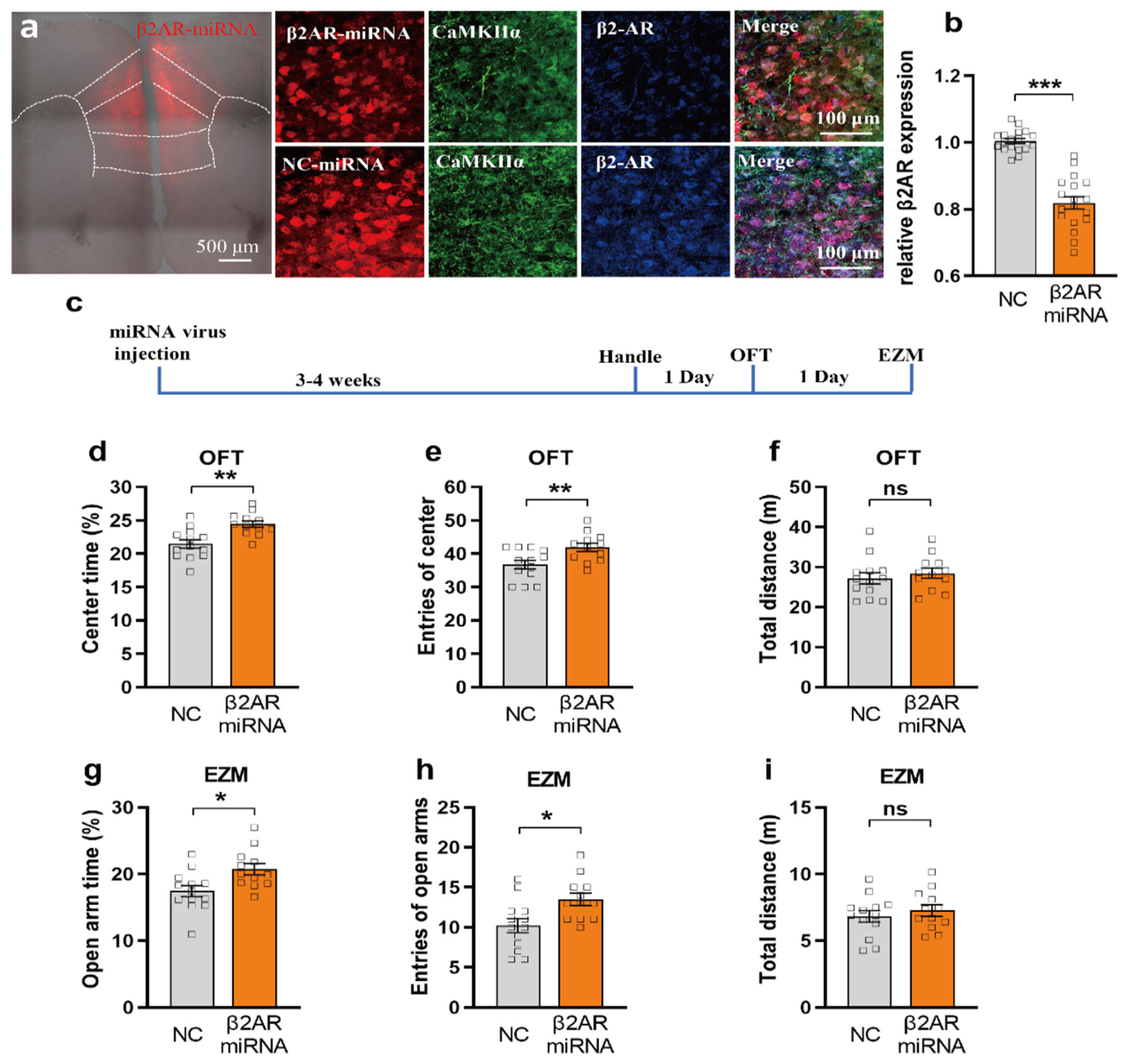
2.4. miRNA Silencing of β2-AR within mPFC CaMKIIα Neurons Reduced Anxiety-like Behavior
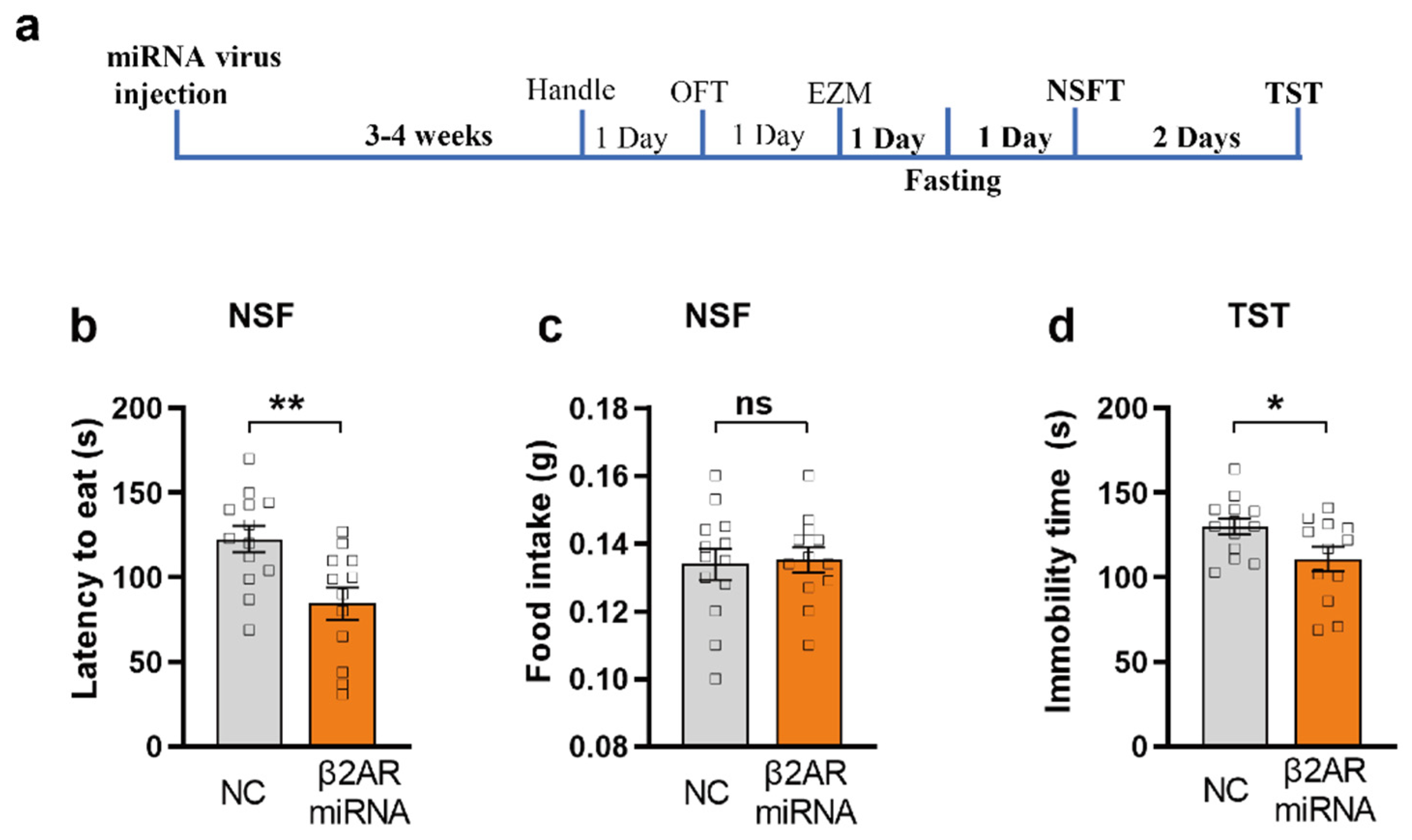
2.5. miRNA Silencing of β2-AR within mPFC CaMKIIα Neurons Induced Anxiolytic-like Behavior in NSFT and Antidepression-like Behavior in TST
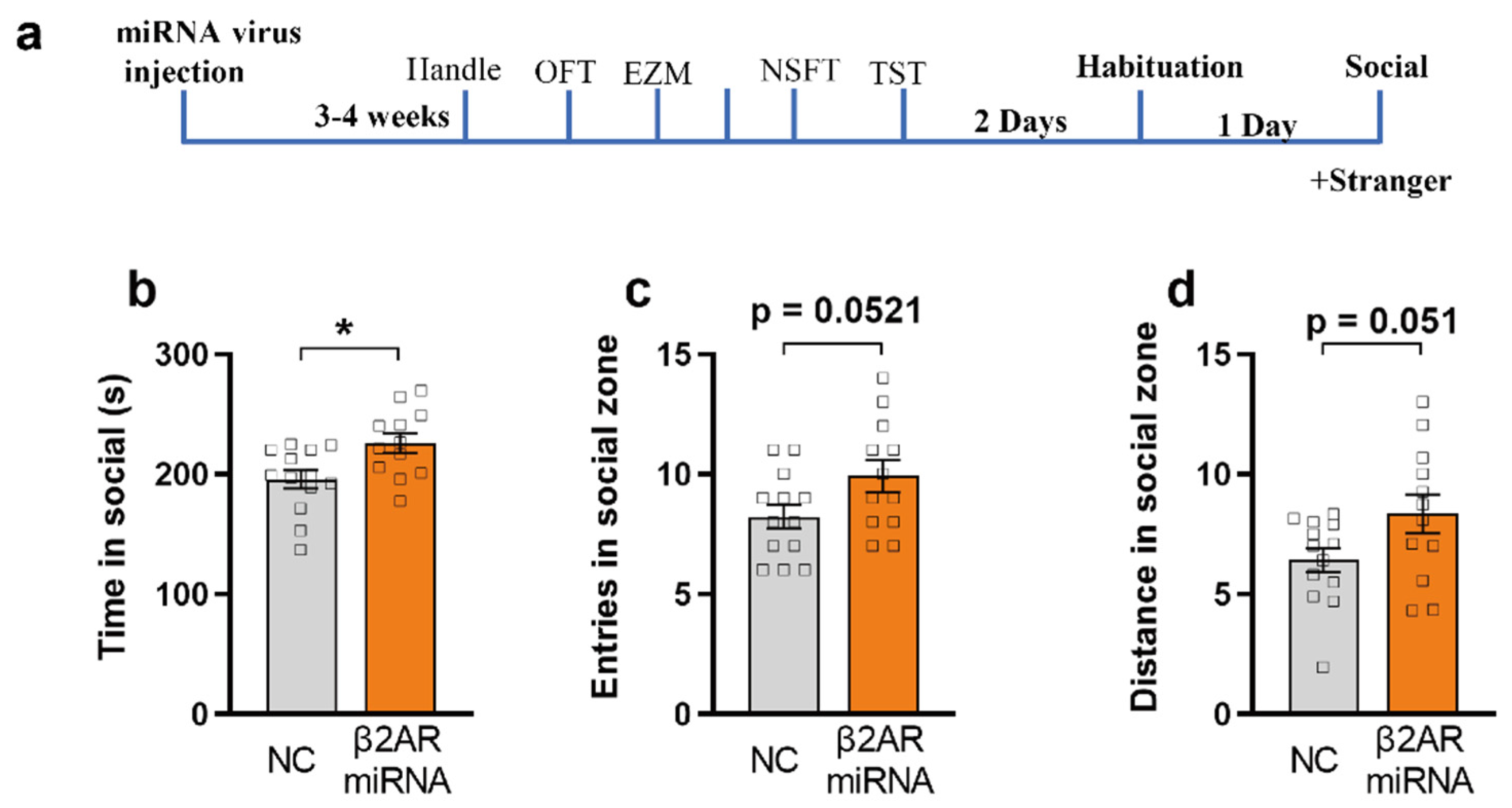
2.6. miRNA Silencing of β2-AR within mPFC CaMKIIα Neurons Promotes Social Interaction
3. Discussion
4. Materials and Methods
4.1. Animal and Ethical Consideration
4.2. AAVs Generation
4.3. Stereotactic Surgery and Virus Injection
4.4. Immunohistochemistry
4.5. Behavior
4.5.1. Open-Field Test (OFT)
4.5.2. Elevated Zero Maze (EZM)
4.5.3. Novelty-Suppressed Feeding Test (NSFT)
4.5.4. Tail-Suspension Test (TST)
4.5.5. Social Interaction Behaviors
4.6. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, A.; Lerner, T.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.A.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Airan, R.D.; Thompson, K.R.; Fenno, L.E.; Bernstein, H.; Deisseroth, K. Temporally precise in vivo control of in-tracellular signalling. Nature 2009, 458, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Hill, S.; Summers, R.J. Evolution of β-blockers: From anti-anginal drugs to ligand-directed signalling. Trends Pharmacol. Sci. 2011, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyan, N.; Rayapati, D.K.; Puttiah, R.H.; Tavane, P.; Singh, S.E.; Rangan, V.; Kalakunta, P.R. Evalu-ation of Anxiety Induced Cardiovascular Response in known Hypertensive Patients Undergoing Exodontia—A Prospective Study. J. Clin. Diagn. Res. 2016, 10, ZC123–ZC127. [Google Scholar] [PubMed]
- Berg, L.; Eckardt, J.; Masseck, O.A. Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS ONE 2019, 14, e0210949. [Google Scholar] [CrossRef]
- Bi, L.-L.; Wang, J.; Luo, Z.-Y.; Chen, S.-P.; Geng, F.; Chen, Y.; Li, S.-J.; Yuan, C.; Lin, S.; Gao, T.-M. Enhanced ex-citability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 2013, 72, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, R.J.; McKittrick, C.R.; Blanchard, D. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol. Behav. 2001, 73, 261–271. [Google Scholar] [CrossRef]
- Bortolotto, V.; Bondi, H.; Cuccurazzu, B.; Rinaldi, M.; Canonico, P.L.; Grilli, M. Salmeterol, a β2 Adrenergic Agonist, Promotes Adult Hippocampal Neurogenesis in a Region-Specific Manner. Front. Pharmacol. 2019, 10, 1000. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.; Kelly, C.; McGilloway, S.; Gilliland, A. β2-Adrenoceptor antagonism in anxiety. Eur. Neuropsychopharmacol. 1990, 1, 75–77. [Google Scholar] [CrossRef]
- Cooper, S.; Gilliland, A.; Kelly, C.; McGilloway, S. A comparison of a β2-adrenoceptor antagonist (ICI 118,551), diazepam and placebo in the treatment of acute anxiety. J. Psychopharmacol. 1991, 5, 155–159. [Google Scholar] [CrossRef]
- Dixsaut, L.; Gräff, J. The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams. Int. J. Mol. Sci. 2021, 22, 12113. [Google Scholar] [CrossRef]
- Dong, J.-H.; Wang, Y.-J.; Cui, M.; Wang, X.-J.; Zheng, W.-S.; Ma, M.-L.; Yang, F.; He, D.-F.; Hu, Q.-X.; Zhang, D.-L.; et al. Adaptive Activation of a Stress Response Pathway Improves Learning and Memory Through Gs and β-Arrestin-1–Regulated Lactate Metabolism. Biol. Psychiatry 2017, 81, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Famularo, R.; Kinscherff, R.; Fenton, T. Propranolol Treatment for Childhood Posttraumatic Stress Disorder, Acute Type. Am. J. Dis. Child. 1988, 142, 1244–1247. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Burgos-Robles, A.; Bhagat, N.D.; Leppla, C.A.; Tye, K.M. Bidirectional modulation of anxie-ty-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 2016, 321, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, C.J.; Knox, D.; Liberzon, I. Inactivation of the prelimbic cortex enhances freezing induced by trime-thylthiazoline, a component of fox feces. Behav. Brain Res. 2011, 221, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Silva, B.A.; Perova, Z.; Marrone, L.; Masferrer, M.E.; Zhan, Y.; Kaplan, A.; Greetham, L.; Verrechia, V.; Halman, A.; et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat. Neurosci. 2017, 20, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchikami, M.; Thomas, A.; Liu, R.; Wohleb, E.S.; Land, B.B.; DiLeone, R.J.; Aghajanian, G.K.; Duman, R.S. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. USA 2015, 112, 8106–8111. [Google Scholar] [CrossRef] [Green Version]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic β 2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Hen, R. The developmental origins of anxiety. Nat. Rev. Neurosci. 2004, 5, 545–552. [Google Scholar] [CrossRef]
- Hara, M.R.; Sachs, B.D.; Caron, M.G.; Lefkowitz, R.J. Pharmacological blockade of a β2AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 2013, 12, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Hare, B.D.; Duman, R.S. Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Mol. Psychiatry 2020, 25, 2742–2758. [Google Scholar] [CrossRef]
- Hirschberg, S.; Li, Y.; Randall, A.; Kremer, E.J.; Pickering, A.E. Functional dichotomy in spinal- vs prefron-tal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 2017, 6, e29808. [Google Scholar] [CrossRef] [PubMed]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Huntley, G.W.; Benson, D. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: Comparison with GAD-67 expression. J. Neurosci. 1994, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- King, D.J.; Devaney, N.M.; Gilbert, J.K. A Double-Blind Placebo Controlled Trial of a Selective beta 2 Adre-noceptor Antagonist (ICI 118551) in Chronic Anxiety. Int. Clin. Psychopharmacol. 1987, 2, 191–200. [Google Scholar] [CrossRef]
- Laricchiuta, D.; Sciamanna, G.; Gimenez, J.; Termine, A.; Fabrizio, C.; Caioli, S.; Balsamo, F.; Panuccio, A.; De Bardi, M.; Saba, L.; et al. Optogenetic Stimulation of Prelimbic Pyramidal Neurons Maintains Fear Memories and Modulates Amygdala Pyramidal Neuron Transcriptome. Int. J. Mol. Sci. 2021, 22, 810. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Zhou, Z.; Zhang, H.; Shi, Q.; Lei, Z.; Shen, P.; Yang, Q.; Zhao, B.; Chen, S.; et al. Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Curr. Biol. 2018, 28, 859–871.e5. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, I.; King, A.P.; Ressler, K.J.; Almli, L.M.; Zhang, P.; Ma, S.T.; Cohen, G.H.; Tamburrino, M.B.; Calabrese, J.R.; Galea, S. Interaction of theADRB2Gene Polymorphism with Childhood Trauma in Predicting Adult Symptoms of Posttraumatic Stress Disorder. JAMA Psychiatry 2014, 71, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liang, X.; Ren, W.-W.; Li, B.-M. Expression of β1- and β2-adrenoceptors in different subtypes of interneurons in the medial prefrontal cortex of mice. Neuroscience 2014, 257, 149–157. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Pereira, I.S.; Bravo, L.; Camarena-Delgado, C.; García, L.B.; Mico, J.-A.; Berrocoso, E. Chemogenetic Silencing of the Locus Coeruleus–Basolateral Amygdala Pathway Abolishes Pain-Induced Anxiety and Enhanced Aversive Learning in Rats. Biol. Psychiatry 2019, 85, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef] [Green Version]
- McCall, J.G.; Siuda, E.R.; Bhatti, D.L.; Lawson, L.A.; McElligott, Z.A.; Stuber, G.D.; Bruchas, M.R. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 2017, 6, e18247. [Google Scholar] [CrossRef] [PubMed]
- Nabi, H.; Hall, M.; Koskenvuo, M.; Singh-Manoux, A.; Oksanen, T.; Suominen, S.; Kivimäki, M.; Vahtera, J. Psy-chological and Somatic Symptoms of Anxiety and Risk of Coronary Heart Disease: The Health and Social Support Prospective Cohort Study. Biol. Psychiatry 2010, 67, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Nawreen, N.; Cotella, E.M.; Morano, R.; Mahbod, P.; Dalal, K.S.; Fitzgerald, M.; Martelle, S.; Packard, B.A.; Franco-Villanueva, A.; Moloney, R.D.; et al. Chemogenetic Inhibition of Infralimbic Prefrontal Cortex GABAergic Parvalbumin Interneu-rons Attenuates the Impact of Chronic Stress in Male Mice. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.P.; Colgan, L.; Nou, E.; Arnsten, A.F. β2 adrenergic agonist, clenbuterol, enhances working memory performance in aging animals. Neurobiol. Aging 2008, 29, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehsia, N.S.; Dhalla, N.S. Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp. Clin. Cardiol. 2010, 15, e86–e95. [Google Scholar]
- Roest, A.; Martens, E.J.; de Jonge, P.; Denollet, J. Anxiety and Risk of Incident Coronary Heart Disease: A Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and ter-ritories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Schiff, H.C.; Johansen, J.P.; Hou, M.; Bush, D.E.A.; Smith, E.K.; Klein, J.A.E.; LeDoux, J.E.; Sears, R.M. β-Adrenergic Receptors Regulate the Acquisition and Consolidation Phases of Aversive Memory Formation Through Distinct, Temporally Regulated Signaling Pathways. Neuropsychopharmacology 2017, 42, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, L.A.; Miyamichi, K.; Gao, X.J.; Beier, K.T.; Weissbourd, B.; Deloach, K.E.; Ren, J.; Ibanes, S.; Malenka, R.C.; Kremer, E.J.; et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 2015, 524, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.-O.; Zhang, E.T.; Piantadosi, S.C.; Marcus, D.J.; Motard, L.E.; Kan, B.K.; Gomez, A.M.; Nguyen, T.K.; Xia, L.; Bruchas, M.R. A locus coeruleus to dentate gyrus noradrenergic circuit modulates aversive contextual processing. Neuron 2021, 109, 2116–2130.e6. [Google Scholar] [CrossRef] [PubMed]
- Siuda, E.R.; Mccall, J.G.; Al-hasani, R.; Shin, G.; Park, S., II; Schmidt, M.J.; Anderson, S.L.; Planer, W.J.; Rogers, J.A.; Bruchas, M.R. Optodynamic simulation of b-adrenergic receptor signalling. Nat. Commun. 2015, 6, 8480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siuda, E.R.; Al-Hasani, R.; McCall, J.G.; Bhatti, D.; Bruchas, M.R. Chemogenetic and Optogenetic Activation of Gαs Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacology 2016, 41, 2011–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenen, S.A.; van Wijk, A.J.; van der Heijden, G.J.M.G.; van Westrhenen, R.; de Lange, J.; de Jongh, A. Pro-pranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2016, 30, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Saitoh, A.; Ohashi, M.; Yamada, M.; Oka, J.-I.; Yamada, M. The infralimbic and prelimbic medial pre-frontal cortices have differential functions in the expression of anxiety-like behaviors in mice. Behav. Brain Res. 2016, 304, 120–124. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
- Wang, G.-Q.; Cen, C.; Li, C.; Cao, S.; Wang, N.; Zhou, Z.; Liu, X.-M.; Xu, Y.; Tian, N.; Zhang, Y.; et al. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat. Commun. 2015, 6, 7660. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tu, J.; Cao, B.; Mu, L.; Yang, X.; Cong, M.; Ramkrishnan, A.S.; Chan, R.H.; Wang, L.; Li, Y. Astrocytic l -Lactate Signaling Facilitates Amygdala-Anterior Cingulate Cortex Synchrony and Decision Making in Rats. Cell Rep. 2017, 21, 2407–2418. [Google Scholar] [CrossRef]
- Williams, S.E.; Carroll, D.; Veldhuijzen van Zanten, J.J.C.S.; Ginty, A.T. Anxiety symptom interpretation: A potential mechanism explaining the cardiorespiratory fitness–anxiety relationship. J. Affect. Disord. 2016, 193, 151–156. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. β-Adrenergic Receptor Antagonism Prevents Anxiety-Like Behavior and Microglial Reactivity Induced by Repeated Social Defeat. J. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Norton, J.; Carrière, I.; Ritchie, K.; Chaudieu, I.; Ryan, J.; Ancelin, M.-L. Preliminary evidence for a role of the adrenergic nervous system in generalized anxiety disorder. Sci. Rep. 2017, 7, 42676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.C.; Sun, Y.Y.; Cai, W.; He, X.T.; Yi, F.; Li, B.M.; Zhang, X.H. Activation of β2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learn. Mem. 2013, 20, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Liu, Z.; Zhou, Y.; Yin, X.; Xu, B.; Ma, L.; Liu, X. Lack of β2-AR increases anxiety-like behaviors and re-warding properties of cocaine. Front. Behav. Neurosci. 2017, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Zuend, M.; Saab, A.S.; Wyss, M.T.; Ferrari, K.D.; Hösli, L.; Looser, Z.J.; Stobart, J.L.; Duran, J.; Guinovart, J.J.; Barros, L.F.; et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020, 2, 179–191. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Z.; Lam, Y.; Li, C.; Fu, Z.; Ramkrishnan, A.S.; Liu, S.; Li, Y. β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice. Int. J. Mol. Sci. 2022, 23, 5578. https://doi.org/10.3390/ijms23105578
Lei Z, Lam Y, Li C, Fu Z, Ramkrishnan AS, Liu S, Li Y. β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice. International Journal of Molecular Sciences. 2022; 23(10):5578. https://doi.org/10.3390/ijms23105578
Chicago/Turabian StyleLei, Zhuogui, Yukyan Lam, Cheukhin Li, Zhongqi Fu, Aruna S. Ramkrishnan, Shu Liu, and Ying Li. 2022. "β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice" International Journal of Molecular Sciences 23, no. 10: 5578. https://doi.org/10.3390/ijms23105578
APA StyleLei, Z., Lam, Y., Li, C., Fu, Z., Ramkrishnan, A. S., Liu, S., & Li, Y. (2022). β2-Adrenoceptors in the Medial Prefrontal Cortex Excitatory Neurons Regulate Anxiety-like Behavior in Mice. International Journal of Molecular Sciences, 23(10), 5578. https://doi.org/10.3390/ijms23105578





