Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds
Abstract
:1. Introduction
2. Results and Discussion
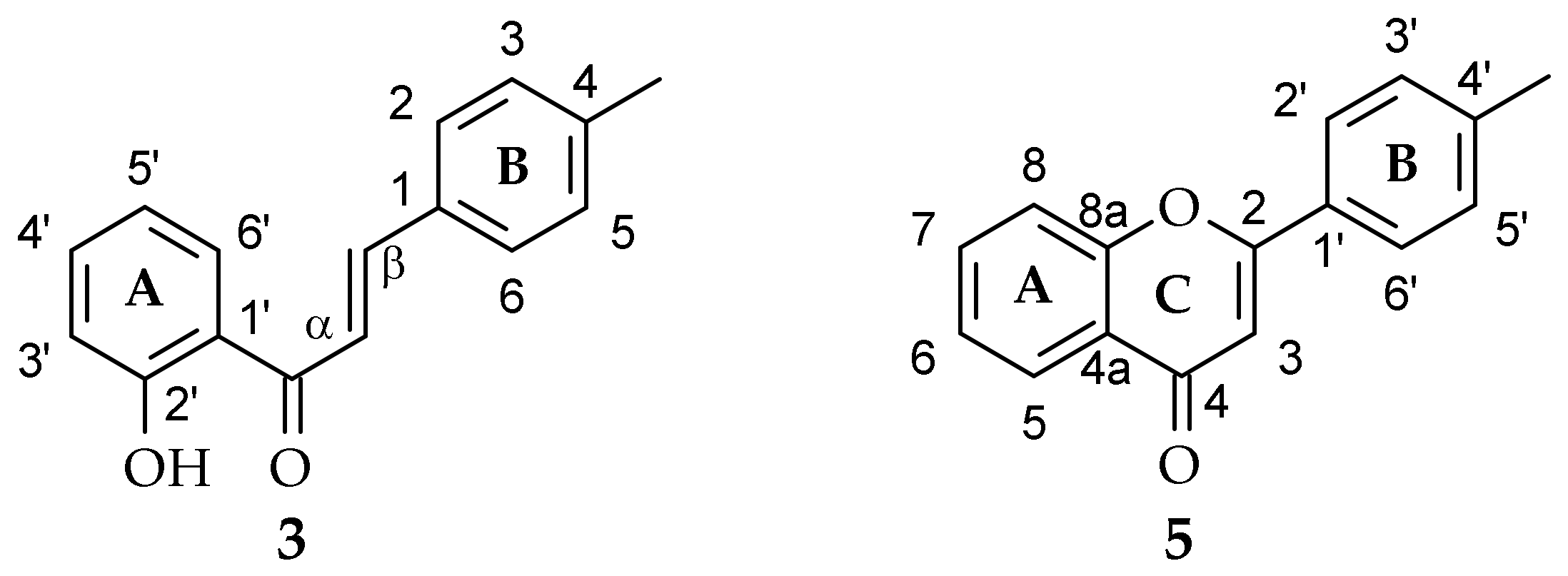
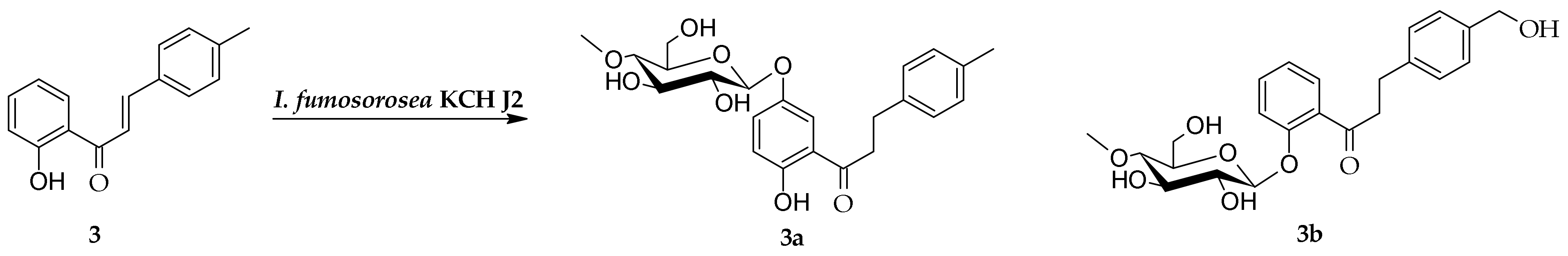
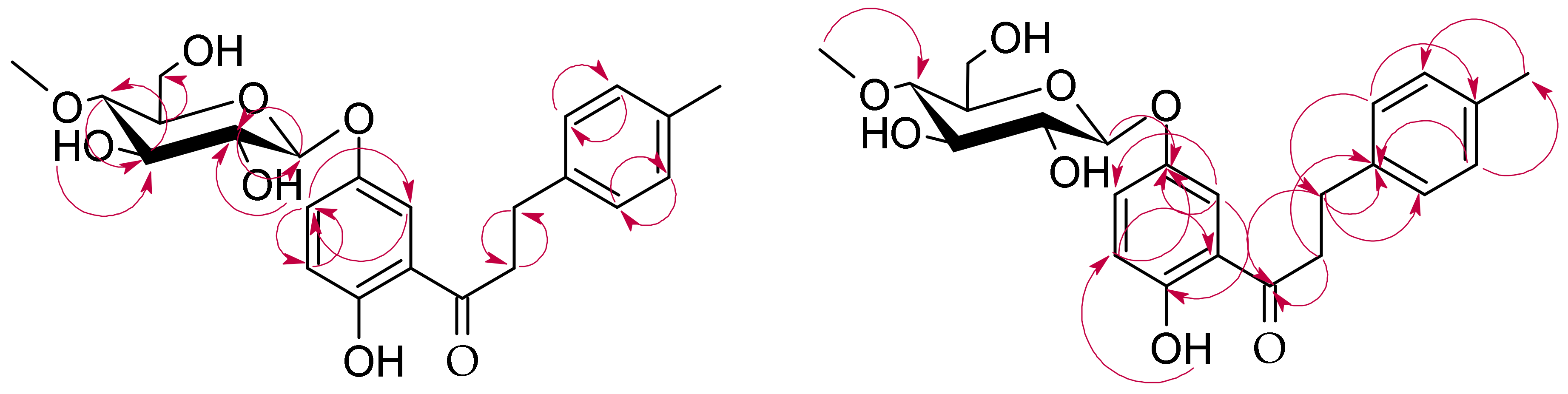
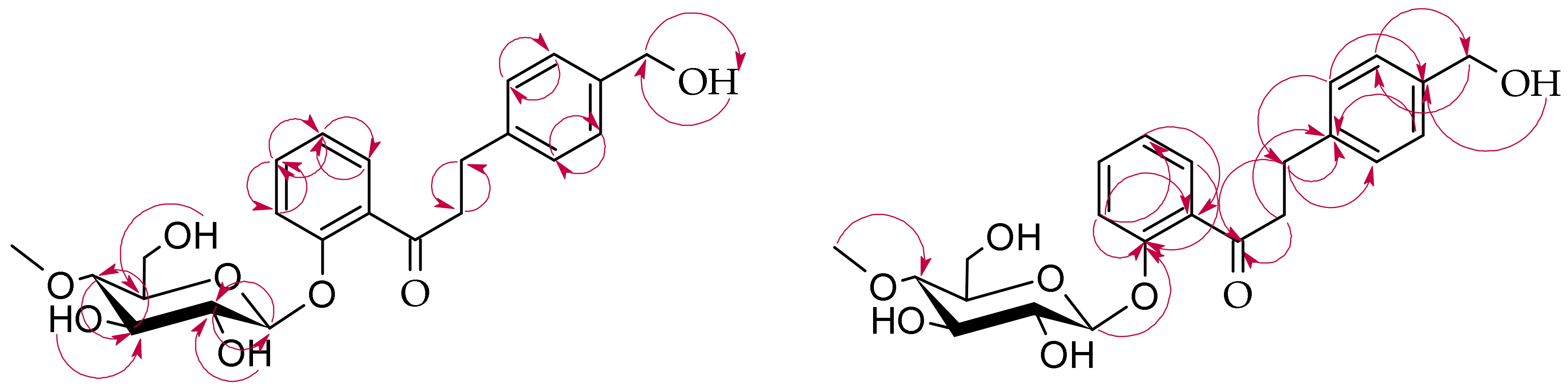
2.1. Biotransformation of 2′-Hydroxy-4-Methylchalcone (3) in the Culture of I. fumosorosea KCH J2
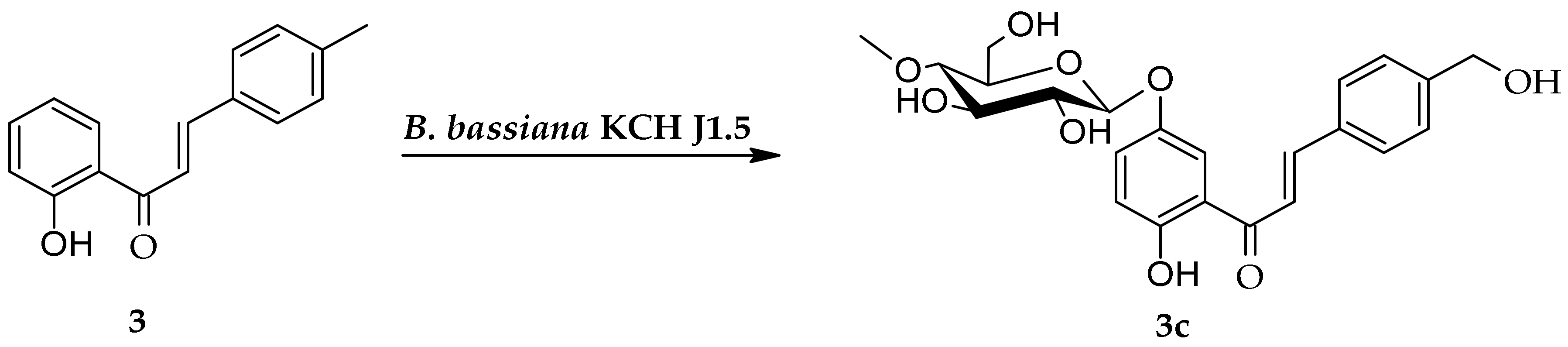
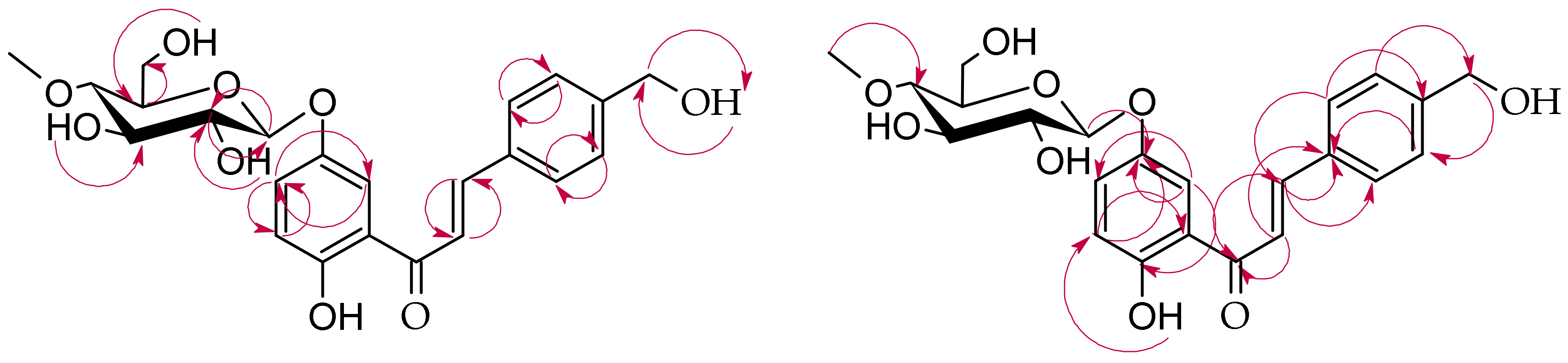
2.2. Biotransformation of 2′-Hydroxy-4-Methylchalcone (3) in the Culture of B. bassiana KCH J1.5
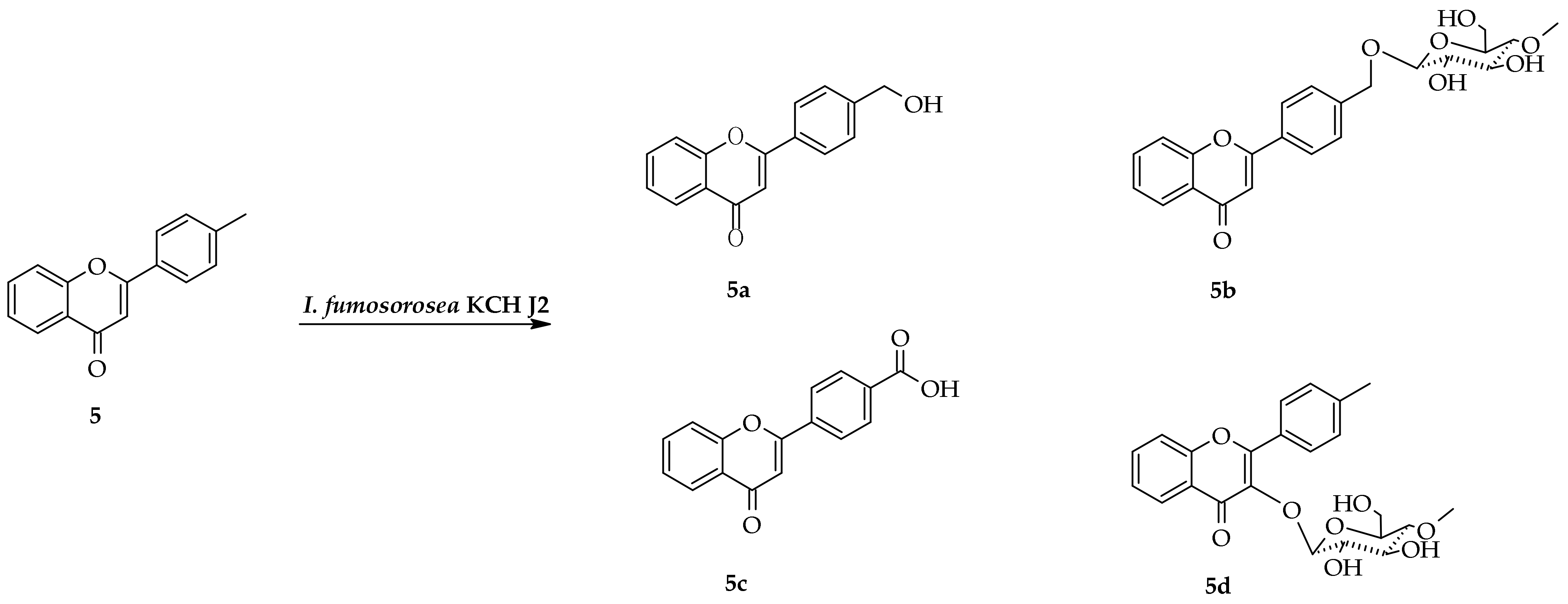
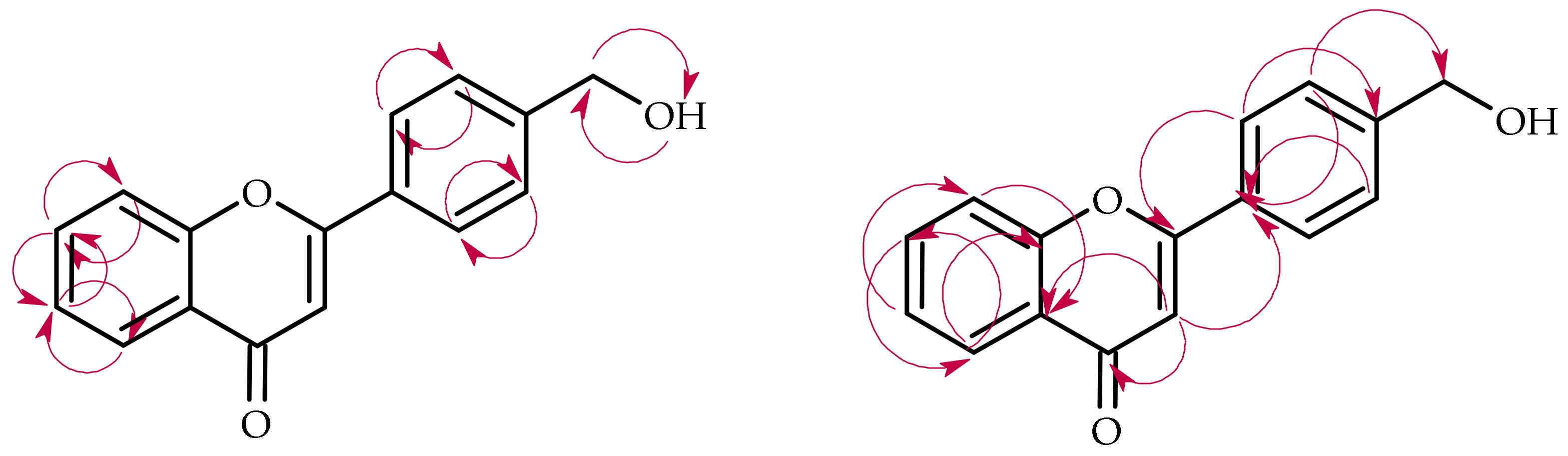
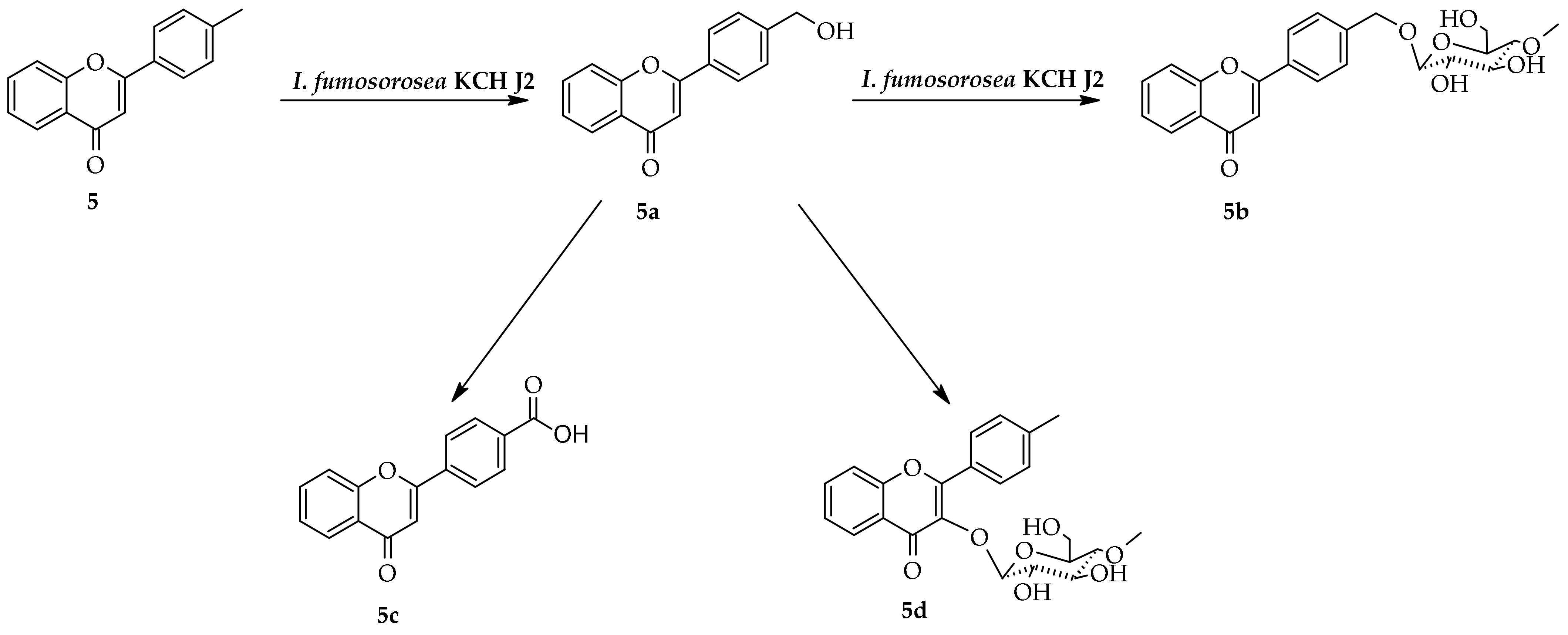
2.3. Biotransformation of 4′-Methylflavone (5) in the Culture of I. fumosorosea KCH J2
2.4. Biotransformation of 4′-Methylflavone (5) in the Culture of B. bassiana KCH J1.5
2.5. Pharmacokinetics, Drug-Likeness, and Biological Activity Prediction
2.5.1. 2′-Hydroxy-4-methylchalcone SwissADME
2.5.2. 2′-Hydroxy-4-methylchalcone Way2Drug Pass Online
2.5.3. 4′-Methylflavone SwissADME
2.5.4. 4′-Methylflavone Way2Drug Pass Online
3. Materials and Methods
3.1. Substrates
3.1.1. 2′-Hydroxy-4-methylchalcone (3)
3.1.2. 4′-Methylflavone (5)
3.2. Microorganisms
3.3. Analysis
3.4. Screening Procedure
3.5. The Semipreparative Biotransformations
3.5.1. 2′-Hydroxy-4-methyldihydrochalcone 5′-O-β-d-(4″-O-methyl)-glucopyranoside (3a)
3.5.2. 4-Hydroxymethyldihydrochalcone 2′-O-β-d-(4″-O-methyl)-glucopyranoside (3b)
3.5.3. 2′-Hydroxy-4-hydroxymethylchalcone 5′-O-β-d-(4″-O-methyl)-glucopyranoside (3c)
3.5.4. 4′-Hydroxymethylflavone (5a)
3.5.5. Flavone 4′-methylene-O-β-d-(4″-O-methyl)-glucopyranoside (5b)
3.5.6. Flavone 4′-carboxylic acid (5c)
3.5.7. 4′-Methylflavone 3-O-β-d-(4″-O-methyl)-glucopyranoside (5d)
3.6. Pharmacokinetics, Drug Nature, Biological Activity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef] [PubMed]
- Erdenetsogt, U.; Nadmid, S.; Paulus, C.; Chanagsuren, G.; Dolgor, E.; Gotov, C.; Dahse, H.M.; Luzhetskyy, A.; Dagvadorj, E. Bioactive Flavonoids from Plant Extract of Pyrethrum pulchrum and Its Acute Toxicity. Nat. Prod. Res. 2021, 35, 5960–5963. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ding, L.; Zhang, N.; Li, W.; Koike, K.; Qiu, F. Flavonoids from Eucommia ulmoides and Their in Vitro Hepatoprotective Activities. Nat. Prod. Res. 2021, 35, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral Activities of Flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Khazeei Tabari, M.A.; Iranpanah, A.; Bahramsoltani, R.; Rahimi, R. Flavonoids as Promising Antiviral Agents against Sars-CoV-2 Infection: A Mechanistic Review. Molecules 2021, 26, 3900. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials 1,2. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, Bioactivity, and Synthesis of Methylated Flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, V.H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of Flavonoids: Chemical Structures, Bioactivities, Progress and Perspectives for Biotechnological Production. Enzyme Microb.Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food. Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Stompor, M.; Kałużny, M.; Żarowska, B. Biotechnological Methods for Chalcone Reduction Using Whole Cells of Lactobacillus, Rhodococcus and Rhodotorula Strains as a Way to Produce New Derivatives. Appl. Microbiol. Biotechnol. 2016, 100, 8371–8384. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, Y.H.; Zhou, Q.; Bai, M.; Chen, G.Y.; Han, C.R.; Song, X.P. A New Dihydrochalcone Glycoside from the Stems of Homalium stenophyllum. Nat. Prod. Res. 2018, 32, 953–958. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Q.; Bao, W.; Ao, W. Structure Elucidation and NMR Assignments of a New Dihydrochalcone from Empetrum nigrum Subsp. asiaticum (Nakai Ex H.Ito) Kuvaev. Nat. Prod. Res. 2020, 34, 930–934. [Google Scholar] [CrossRef]
- Pompermaier, L.; Heiss, E.H.; Alilou, M.; Mayr, F.; Monizi, M.; Lautenschlaeger, T.; Schuster, D.; Schwaiger, S.; Stuppner, H. Dihydrochalcone Glucosides from the Subaerial Parts of Thonningia sanguinea and Their in Vitro PTP1B Inhibitory Activities. J. Nat. Prod. 2018, 81, 2091–2100. [Google Scholar] [CrossRef]
- Lima, E.M.; Fernando, L.M.; Felix, L.P.; de Oliveira Filho, A.A.; Carneiro Neto, A.N.; Moura, R.T.; Teles, Y.C.F. First Complete NMR Data and Theoretical Study of an Antimicrobial Formylated Dihydrochalcone from Psidium guineense Sw. Nat. Prod. Res. 2020, 36, 419–423. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the Biotechnological Glycosylation of Valuable Flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Bai, J.; Yue, Q.; Zhang, M.; Li, J.; Wang, C.; Xu, Y. Methylglucosylation of Phenolic Compounds by Fungal Glycosyltransferase-Methyltransferase Functional Modules. J. Agric. Food Chem. 2019, 67, 8573–8580. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Hu, L.; Lu, H.; Wei, T.; Chen, Q. Metabolic Engineering of Microbial Cell Factories for Biosynthesis of Flavonoids: A Review. Molecules 2021, 26, 4522. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Pląskowska, E.; Stępień, Ł.; Kostrzewa-Susłow, E. Glycosylation of 6-Methylflavone by the Strain Isaria fumosorosea KCH J2. PLoS ONE 2017, 12, e0184885. [Google Scholar] [CrossRef]
- Hyung Ko, J.; Gyu Kim, B.; Joong-Hoon, A. Glycosylation of Flavonoids with a Glycosyltransferase from Bacillus cereus. FEMS Microbiol. Lett. 2006, 258, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Gurung, R.B.; Kim, E.H.; Oh, T.J.; Sohng, J.K. Enzymatic Synthesis of Apigenin Glucosides by Glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol. Cells 2013, 36, 355–361. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts 2020, 10, 1148. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. New Glycosylated Dihydrochalcones Obtained by Biotransformation of 2′-Hydroxy-2-Methylchalcone in Cultures of Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2021, 22, 9619. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Fungal Biotransformation of 2′-Methylflavanone and 2′-Methylflavone as a Method to Obtain Glycosylated Derivatives. Int. J. Mol. Sci. 2021, 22, 9617. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of Methoxylated Flavonoids in the Cultures of Isaria fumosorosea KCH J2. Molecules 2018, 23, 2578. [Google Scholar] [CrossRef] [Green Version]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef] [Green Version]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of Flavones and an Isoflavone (Daidzein) in Cultures of Entomopathogenic Filamentous Fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef] [Green Version]
- Włoch, A.; Strugała-Danak, P.; Pruchnik, H.; Krawczyk-Łebek, A.; Szczecka, K.; Janeczko, T.; Kostrzewa-Susłow, E. Interaction of 4′-Methylflavonoids with Biological Membranes, Liposomes, and Human Albumin. Sci. Rep. 2021, 11, 16003. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, P.; Huszcza, E. Microbial Glycosylation of Flavonoids. Pol. J. Microbiol. 2016, 65, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.; Wang, Z.; Li, G.; Dun, B. Microbial Transformation of Flavonoids by Isaria fumosorosea ACCC 37814. Molecules 2019, 24, 1028. [Google Scholar] [CrossRef] [Green Version]
- Łużny, M.; Tronina, T.; Kozłowska, E.; Dymarska, M.; Popłoński, J.; Łyczko, J.; Kostrzewa-Susłow, E.; Janeczko, T. Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2020, 21, 6121. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, I.S. Microbial Metabolism of the Prenylated Chalcone Xanthohumol. J. Nat. Prod. 2006, 69, 1522–1524. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and Antiproliferative Activity of Glycosides Obtained by Biotransformation of Xanthohumol. Bioorg. Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef]
- Huszcza, E.; Bartmańska, A.; Tronina, T. Glycosylation of Xanthohumol by Fungi. Zeitschrift Naturforschung C 2008, 63, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Overwin, H.; Wray, V.; Hofer, B. Biotransformation of Phloretin by Amylosucrase Yields Three Novel Dihydrochalcone Glucosides. J. Biotechnol. 2015, 211, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Huang, Z.; Ren, S. Production of Cuticle Degrading Enzymes by Isaria fumosorosea and Their Evaluation as a Biocontrol Agent against Diamondback Moth. J. Pest. Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Cito, A.; Barzanti, G.P.; Strangi, A.; Francardi, V.; Zanfini, A.; Dreassi, E. Cuticle-Degrading Proteases and Toxins as Virulence Markers of Beauveria bassiana (Balsamo) Vuillemin. J. Basic Microbiol. 2016, 56, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.; Yu, H.; Wu, P.; Li, S.; Han, L.; Gunatilaka, A.A.L.; et al. Methylglucosylation of Aromatic Amino and Phenolic Moieties of Drug-like Biosynthons by Combinatorial Biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4980–E4989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostrzewa-Susłow, E.; Janeczko, T. Microbial Transformations of 5-Hydroxy- and 5-Methoxyflavone in Aspergillus niger and Penicillium chermesinum Cultures. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 448–452. [Google Scholar]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. 4′-Methylflavanone Glycosides Obtained Using Biotransformation in the Entomopathogenic Filamentous Fungi Cultures as Potential Anticarcinogenic, Antimicrobial, and Hepatoprotective Agents. Int. J. Mol. Sci. 2022, 23, 5373. [Google Scholar] [CrossRef]
- Lynch, T.; Price, A. The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Synthesis and PASS-Assisted in Silico Approach of Some Novel 2-Substituted Benzimidazole Bearing a Pyrimidine-2, 4, 6(Trione) System as Mucomembranous Protector. J. Pharm. Bioallied Sci. 2013, 5, 39–43. [Google Scholar] [CrossRef]
- Nasimullah Qureshi, M.; Stecher, G.; Bonn, G.K. Determination of Total Polyphenolic Compounds and Flavonoids in Juglans regia Leaves. Pak. J. Pharm. Sci 2014, 27, 865–869. [Google Scholar]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma Membrane Integrity: Implications for Health and Disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of Melanogenesis Increase Toxicity of Cyclophosphamide and Lymphocytes against Melanoma Cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, S.; Khan, T.H.; Osborn, H.M.I.; Aba, N.; Williams, O. Tyrosinase Activated Melanoma Prodrugs. Anti-Cancer Agents Med. Chem. 2009, 9, 717–727. [Google Scholar] [CrossRef]
- Buitrago, E.; Hardré, R.; Haudecoeur, R.; Jamet, H.; Belle, C.; Boumendjel, A.; Bubacco, L.; Réglier, M. Are Human Tyrosinase and Related Proteins Suitable Targets for Melanoma Therapy? Curr. Top. Med. Chem. 2016, 16, 3033–3047. [Google Scholar] [CrossRef]
- Debevec, T.; Millet, G.P.; Pialoux, V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front. Physiol. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and Therapeutic Role of Functional Ingredients of Barley Grass for Chronic Diseases in Human Beings. Oxid. Med. Cell. Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef] [Green Version]
- Razuvaeva, Y.G.; Toropova, A.A.; Olennikov, D.N.; Kharzheev, D.v. Antihypoxic Activity of the Dry Extract from Nepeta multifida L. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Noman, M.Z.; Hasmim, M.; Messai, Y.; Terry, S.; Kieda, C.; Janji, B.; Chouaib, S. Hypoxia: A Key Player in Antitumor Immune Response. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Brosius, F.C.; Tuttle, K.R.; Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 2016, 59, 1624–1627. [Google Scholar] [CrossRef]
- Gozgit, J.M.; Bebernitz, G.; Patil, P.; Ye, M.; Parmentier, J.; Wu, J.; Su, N.; Wang, T.; Ioannidis, S.; Davies, A.; et al. Effects of the JAK2 Inhibitor, AZ960, on Pim/BAD/BCL-XL Survival Signaling in the Human JAK2 V617F Cell Line SET-2. J. Biol. Chem. 2008, 283, 32334–32343. [Google Scholar] [CrossRef] [Green Version]
- Harry, B.L.; Eckhardt, S.G.; Jimeno, A. JAK2 Inhibition for the Treatment of Hematologic and Solid Malignancies. Expert Opin. Investig. Drugs 2012, 21, 637–655. [Google Scholar] [CrossRef]
- Judd, L.M.; Menheniott, T.R.; Ling, H.; Jackson, C.B.; Howlett, M.; Kalantzis, A.; Priebe, W.; Giraud, A.S. Inhibition of the JAK2/STAT3 Pathway Reduces Gastric Cancer Growth in Vitro and in Vivo. PLoS ONE 2014, 9, e95993. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Moretti, L.; Giacalone, N.J.; Schleicher, S.; Speirs, C.K.; Carbone, D.P.; Lu, B. Inhibition of JAK2 Signaling by TG101209 Enhances Radiotherapy in Lung Cancer Models. J. Thorac. Oncol. 2011, 6, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Meredith, T.; Swoboda, J.; Walker, S. Staphylococcus aureus and Bacillus subtilis W23 Make Polyribitol Wall Teichoic Acids Using Different Enzymatic Pathways. Chem. Biol. 2010, 17, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, P.-J.; van Strijp, J. Anaphylatoxins: Their Role in Bacterial Infection and Inflammation. Immunol. Res. 2007, 37, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Byrne, N.M.; al Jamal, W.; Coulter, J.A. Exploiting Current Understanding of Hypoxia Mediated Tumour Progression for Nanotherapeutic Development. Cancers 2019, 11, 1989. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Zhang, Q. Understanding the Oxygen-Sensing Pathway and Its Therapeutic Implications in Diseases. Am. J. Clin. Pathol. 2020, 190, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Mahadevan, D. A Comprehensive Review of Protein Kinase Inhibitors for Cancer Therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Anantram, A.; Joshi, U.J.; Srivastava, S.; Govil, G. Effect of Methyl Substitution in Flavones on Its Localization and Interaction with DPPC Model Membrane: Implications for Anti-Proliferative Activity. Int. J. Curr. Pharm. Res. 2015, 7, 43–50. [Google Scholar]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Lv, D.; Cheng, X.; Tang, L.; Jiang, M. The Cardioprotective Effect of Total Flavonoids on Myocardial Ischemia/Reperfusion in Rats. Biomed. Pharmacother. 2017, 88, 277–284. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective Effects of Different Flavonoids against Myocardial Ischaemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]

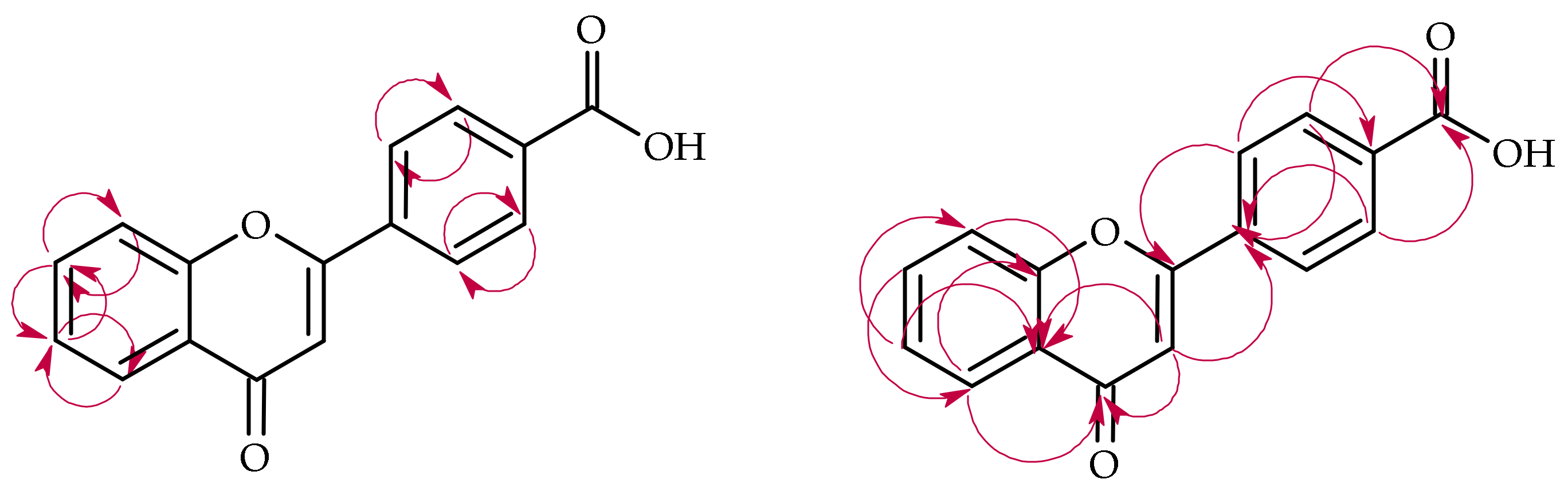
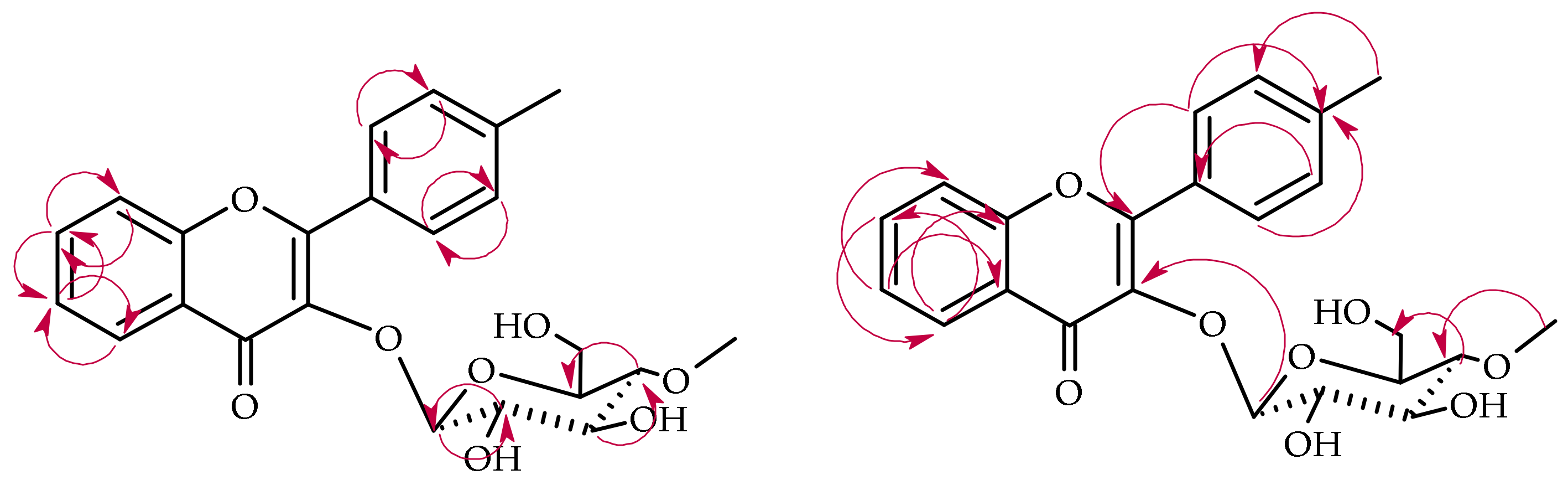
| Proton | Compound | |||
|---|---|---|---|---|
| 3 | 3a | 3b | 3c | |
| H-α | 7.93 (d) J = 15.4 | 3.42 (m) | 3.41 (m) | 7.97 (d) J = 15.4 |
| H-β | 8.02 (d) J = 15.4 | 3.00 (t) J = 7.5 | 2.97 (t) J = 7.5 | 7.93 (d) J = 15.4 |
| H-2 | 7.79 (d) J = 8.0 | 7.19 (d) J = 8.0 | 7.26 (d) J = 8.3 | 7.89 (d) J = 8.2 |
| H-3 | 7.31 (d) J = 7.9 | 7.09 (d) J = 7.8 | 7.23 (d) J = 8.3 | 7.48 (d) J = 8.2 |
| H-5 | 7.31 (d) J = 7.9 | 7.09 (d) J = 7.8 | 7.23 (d) J = 8.3 | 7.48 (d) J = 8.2 |
| H-6 | 7.79 (d) J = 8.0 | 7.19 (d) J = 8.0 | 7.26 (d) J = 8.3 | 7.89 (d) J = 8.2 |
| H-3′ | 6.99 (t) J = 8.4 | 6.87 (d) J = 9.0 | 7.29 (dd) J = 8.4, J = 0.7 | 6.92 (d) J = 9.0 |
| H-4′ | 7.57 (m) | 7.28 (dd) J = 9.0 J = 3.0 | 7.47 (ddd) J = 8.4, J = 7.3, J = 1.8 | 7.33 (dd) J = 9.0 J = 2.9 |
| H-5′ | 6.99 (t) J = 8.4 | - | 7.10 (td) J = 7.6 J = 1.0 | - |
| H-6′ | 8.27 (d) J = 8.2 | 7.64 (d) J = 2.9 | 7.57 (dd) J = 7.7 J = 1.8 | 7.91 (d) J = 3.0 |
| H-1″ | - | 4.83 (d) J = 7.8 | 5.06 (d) J = 7.8 | 4.92 (d) J = 7.8 |
| H-2″ | - | 3.42 (m) | 3.50 (m) | 3.47 (m) |
| H-3″ | - | 3.59 (m) | 3.63 (td) J = 9.0, J = 4.3 | 3.64 (m) |
| H-4″ | - | 3.15 (dd) J = 9.7, J = 8.9 | 3.21 (dd) J = 9.6, J = 9.1 | 3.15 (m) |
| H-5″ | - | 3.47 (m) | 3.50 (m) | 3.60 (ddd) J = 9.6, J = 6.1, J = 2.1 |
| H-6” | - | 3.84 (m) 3.67 (m) | 3.84 (ddd) J = 11.6 J = 5.3 J = 2.2 3.68 (m) | 3.93 (ddd) J = 11.5, J = 5.3, J = 1.5 3.73 (dt) J = 11.9 J = 6.2 |
| C4″-OCH3 | - | 3.55 (s) | 3.56 (s) | 3.57 (s) |
| C2′-OH | 12.94 (s) | 11.95 (s) | - | 12.60 (s) |
| C4-CH3 | 2.39 (s) | 2.27 (s) | - | - |
| 2″-OH | - | 4.62 (d) J = 2.6 | 4.60 (d) J = 4.2 | 4.65 (d) J = 3.8 |
| 3″-OH | - | 4.39 (d) J = 3.5 | 4.50 (d) J = 4.4 | 4.45 (d) J = 3.6 |
| 6″-OH | - | 3.84 (m) | 3.74 (dd) J = 6.8 J = 5.4 | 4.05 (m) |
| 4-CH2- | - | - | 4.58 (d) J = 5.7 | 4.70 (m) |
| 4-CH2-OH | - | - | 4.08 (t) J = 5.8 | 4.39 (m) |
| Carbon | Compound | |||
|---|---|---|---|---|
| 3 | 3a | 3b | 3c | |
| C-α | 120.4 | 40.4 | 45.7 | 120.8 |
| C-β | 146.4 | 30.4 | 30.6 | 146.5 |
| C-1 | 142.4 | 138.8 | 140.9 | 134.2 |
| C-2 | 130.0 | 129.3 | 127.6 | 130.1 |
| C-3 | 130.6 | 129.8 | 129.1 | 127.8 |
| C-4 | 133.0 | 136.1 | 141.2 | 147.0 |
| C-5 | 130.6 | 129.8 | 129.1 | 127.8 |
| C-6 | 130.0 | 129.3 | 127.6 | 130.1 |
| C-1′ | 120.9 | 119.9 | 130.7 | 120.4 |
| C-2′ | 164.5 | 158.5 | 157.1 | 159.9 |
| C-3′ | 118.9 | 119.4 | 117.1 | 119.5 |
| C-4′ | 137.4 | 127.5 | 133.9 | 127.2 |
| C-5′ | 119.8 | 150.8 | 123.1 | 150.9 |
| C-6′ | 131.3 | 118.2 | 130.3 | 117.9 |
| C-1″ | - | 102.8 | 102.2 | 102.9 |
| C-2″ | - | 74.9 | 75.0 | 75.0 |
| C-3″ | - | 78.0 | 78.1 | 78.1 |
| C-4″ | - | 80.4 | 80.1 | 80.6 |
| C-5″ | - | 77.2 | 77.2 | 77.2 |
| C-6″ | - | 62.3 | 62.1 | 62.5 |
| 4″-OCH3 | - | 60.6 | 60.6 | 60.6 |
| C=O | 195.1 | 206.9 | 202.4 | 194.7 |
| 4-CH3 | 21.5 | 21.0 | - | - |
| 4-CH2- | - | - | 64.6 | 64.3 |
| Proton | Compound | ||||
|---|---|---|---|---|---|
| 5 | 5a | 5b | 5c | 5d | |
| H-3 | 6.82 (s) | 6.86 (s) | 6.88 (s) | 6.99 (s) | - |
| H-5 | 8.12 (dd) J = 7.9, J = 1.6 | 8.13 (dd) J = 7.9, J = 1.6 | 8.13 (dd) J = 7.8, J = 1.7 | 8.14 (dd) J = 7.9, J = 1.6 | 8.19 (dd) J = 8.0, J = 1.7 |
| H-6 | 7.48 (m) | 7.50 (m) | 7.50 (m) | 7.52 (m) | 7.53 (ddd) J = 8.0, J = 7.1, J = 0.9 |
| H-7 | 7.81 (ddd) J = 8.7, J = 7.1 J = 1.7 | 7.82 (ddd) J = 8.6, J = 7.1 J = 1.7 | 7.83 (ddd) J = 8.7, J = 7.1, J = 1.7 | 7.85 (ddd) J = 8.6, J = 7.1 J = 1.7 | 7.85 (ddd) J = 8.7, J = 7.1 J = 1.7 |
| H-8 | 7.73 (d) J = 8.4 | 7.75 (d) J = 8.4 | 7.75 (dd) J = 8.4, J = 0.8 | 7.79 (dd) J = 8.4, J = 0.6 | 7.74 (d) J = 8.4 |
| H-2′ | 7.99 (d) J = 8.3 | 8.08 (d) J = 8.4 | 8.09 (d) J = 8.4 | 8.26 (m) | 8.17 (d) J = 8.3 |
| H-3′ | 7.41 (d) J = 8.0 | 7.59 (d) J = 8.6 | 7.65 (d) J = 8.4 | 8.23 (m) | 7.37 (d) J = 8.1 |
| H-5′ | 7.41 (d) J = 8.0 | 7.59 (d) J = 8.6 | 7.65 (d) J = 8.4 | 8.23 (m) | 7.37 (d) J = 8.1 |
| H-6′ | 7.99 (d) J = 8.3 | 8.08 (d) J = 8.4 | 8.09 (d) J = 8.4 | 8.26 (m) | 8.17 (d) J = 8.3 |
| H-1″ | - | - | 4.42 (d) J = 7.8 | - | 5.22 (d) J = 7.9 |
| H-2″ | - | - | 3.29 (m) | - | 3.40 (m) |
| H-3″ | - | - | 3.52 (m) | - | 3.58 (m) |
| H-4″ | - | - | 3.13 (dd) J = 9.5, J = 9.0 | - | 3.11 (m) |
| H-5″ | - | - | 3.29 (m) | - | 3.20 (ddd) J = 9.7, J = 5.0, J = 2.4 |
| H-6″ | - | - | 3.83 (d) J = 10.5 3.69 (m) | - | 3.55 (m) 3.48 (dd) J = 7.0, J = 4.9 |
| C4″-OCH3 | - | - | 3.54 (s) | - | 3.51 (s) |
| C4′-CH3 | 2.43 (s) | - | - | - | 2.43 (s) |
| 2″-OH | - | - | - | - | 5.33 (d) J = 2.3 |
| 3″-OH | - | - | - | - | 4.36 (d) J = 3.5 |
| 6″-OH | - | - | 3.66 (d) J = 4.6 | - | 3.55 (m) |
| 4′-CH2- | - | 4.76 (d) J = 5.8 | 5.00 (d) J = 13.0 4.76 (d) J = 13.0 | - | - |
| 4′-CH2-OH | - | 4.47 (t) J = 5.8 | - | - | - |
| Carbon | Compound | ||||
|---|---|---|---|---|---|
| 5 | 5a | 5b | 5c | 5d | |
| C-2 | 164.1 | 164.0 | 163.8 | 162.8 | 158.5 |
| C-3 | 107.4 | 107.6 | 107.8 | 109.2 | 138.3 |
| C-4 | 177.9 | 177.9 | 177.9 | 177.9 | 175.6 |
| C-4a | 124.9 | 124.9 | 124.9 | 124.9 | 124.5 |
| C-5 | 126.0 | 126.0 | 126.0 | 126.0 | 126.2 |
| C-6 | 126.1 | 126.1 | 126.2 | 126.4 | 126.0 |
| C-7 | 134.7 | 134.8 | 134.8 | 135.1 | 135.1 |
| C-8 | 119.2 | 119.3 | 119.3 | 119.4 | 119.2 |
| C-8a | 157.2 | 157.2 | 157.1 | 157.2 | 156.3 |
| C-1′ | 123.0 | 131.2 | 131.7 | 136.8 | 129.1 |
| C-2′ | 127.2 | 127.1 | 127.1 | 127.4 | 130.3 |
| C-3′ | 130.6 | 127.8 | 128.9 | 131.1 | 129.6 |
| C-4′ | 143.1 | 147.6 | 143.4 | 134.0 | 142.2 |
| C-5′ | 130.6 | 127.8 | 128.9 | 131.1 | 129.6 |
| C-6′ | 127.2 | 127.1 | 127.1 | 127.4 | 130.3 |
| C-1″ | - | - | 103.3 | - | 104.7 |
| C-2″ | - | - | 75.2 | - | 75.6 |
| C-3″ | - | - | 78.1 | - | 78.3 |
| C-4″ | - | - | 80.5 | - | 79.9 |
| C-5″ | - | - | 77.0 | - | 77.2 |
| C-6″ | - | - | 62.4 | - | 62.2 |
| 4″-OCH3 | - | - | 60.5 | - | 60.5 |
| 4′-CH3 | 21.4 | - | - | - | 21.5 |
| 4′-CH2- | - | 64.1 | 70.3 | - | - |
| 4′-COOH | - | - | - | 166.9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds. Int. J. Mol. Sci. 2022, 23, 5558. https://doi.org/10.3390/ijms23105558
Krawczyk-Łebek A, Dymarska M, Janeczko T, Kostrzewa-Susłow E. Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds. International Journal of Molecular Sciences. 2022; 23(10):5558. https://doi.org/10.3390/ijms23105558
Chicago/Turabian StyleKrawczyk-Łebek, Agnieszka, Monika Dymarska, Tomasz Janeczko, and Edyta Kostrzewa-Susłow. 2022. "Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds" International Journal of Molecular Sciences 23, no. 10: 5558. https://doi.org/10.3390/ijms23105558
APA StyleKrawczyk-Łebek, A., Dymarska, M., Janeczko, T., & Kostrzewa-Susłow, E. (2022). Glycosylation of Methylflavonoids in the Cultures of Entomopathogenic Filamentous Fungi as a Tool for Obtaining New Biologically Active Compounds. International Journal of Molecular Sciences, 23(10), 5558. https://doi.org/10.3390/ijms23105558






