Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes
Abstract
:1. Introduction
2. Shared Genetic Background
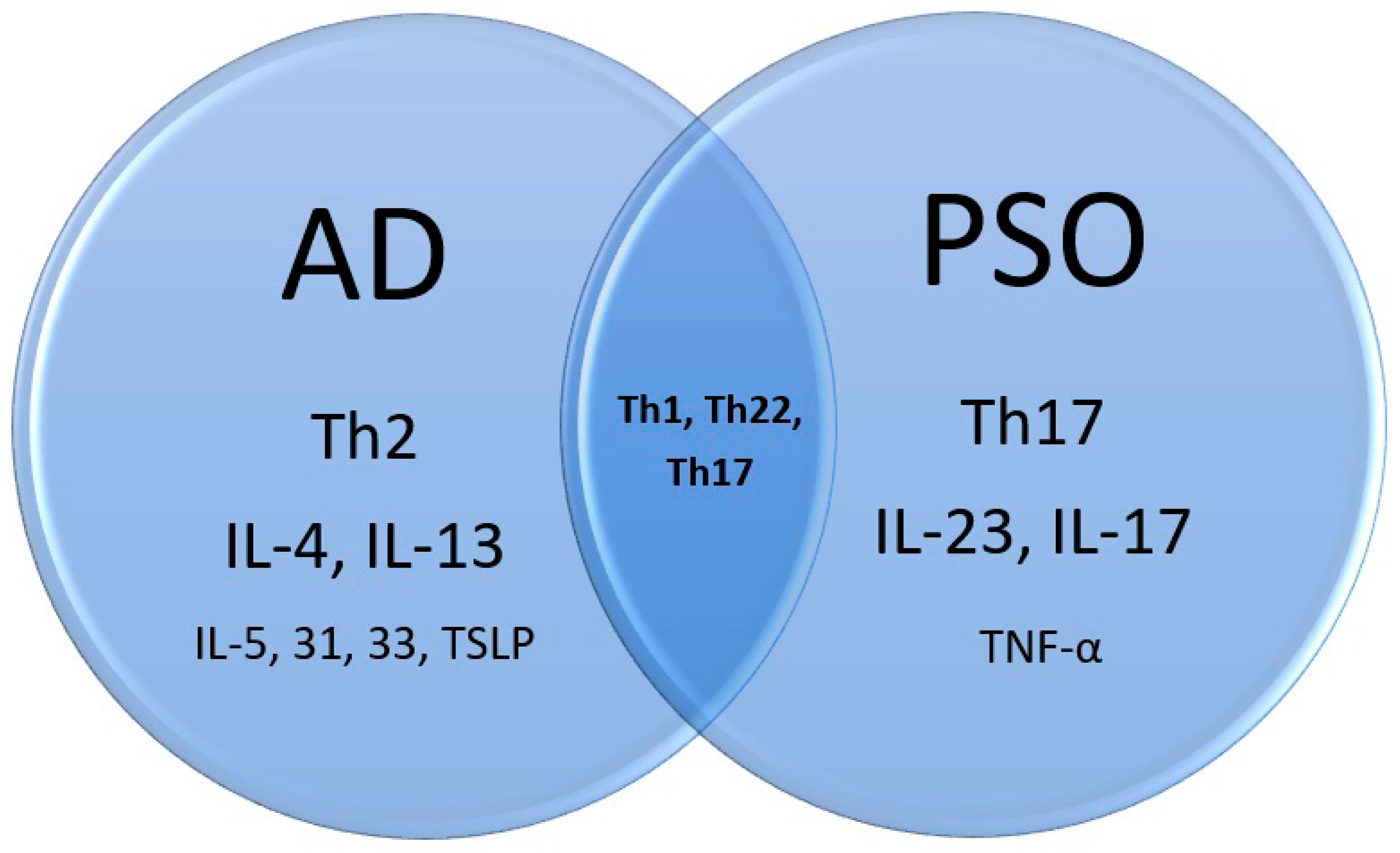
3. Shared Immunopathogenesis
3.1. PSO
3.2. AD
3.3. PSO and AD
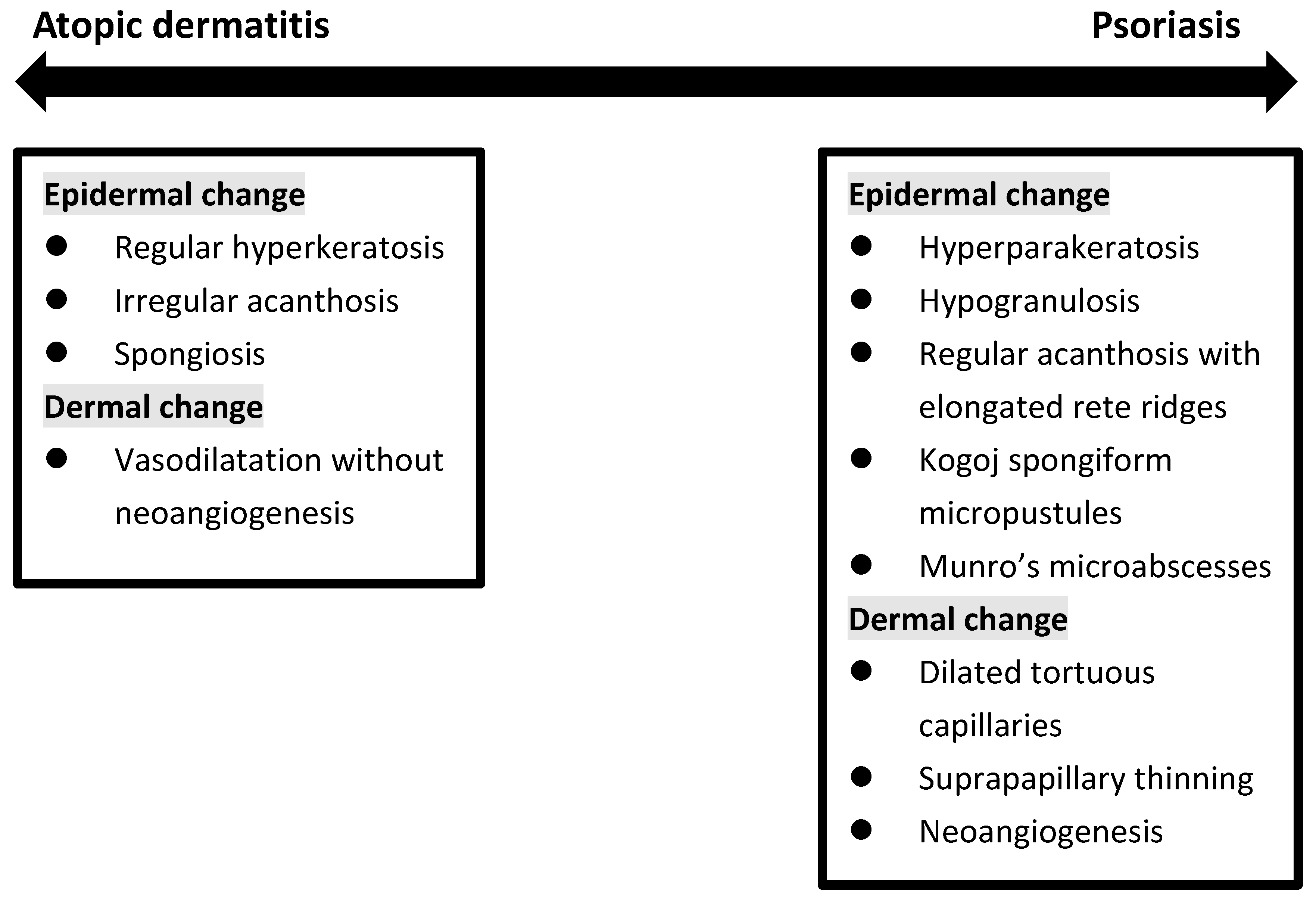
4. Histopathological Findings
5. Shared Comorbidities Focusing on Autoimmune Diseases
5.1. Alopecia Areata
5.2. Vitiligo
5.3. Inflammatory Bowel Disease
5.4. Others
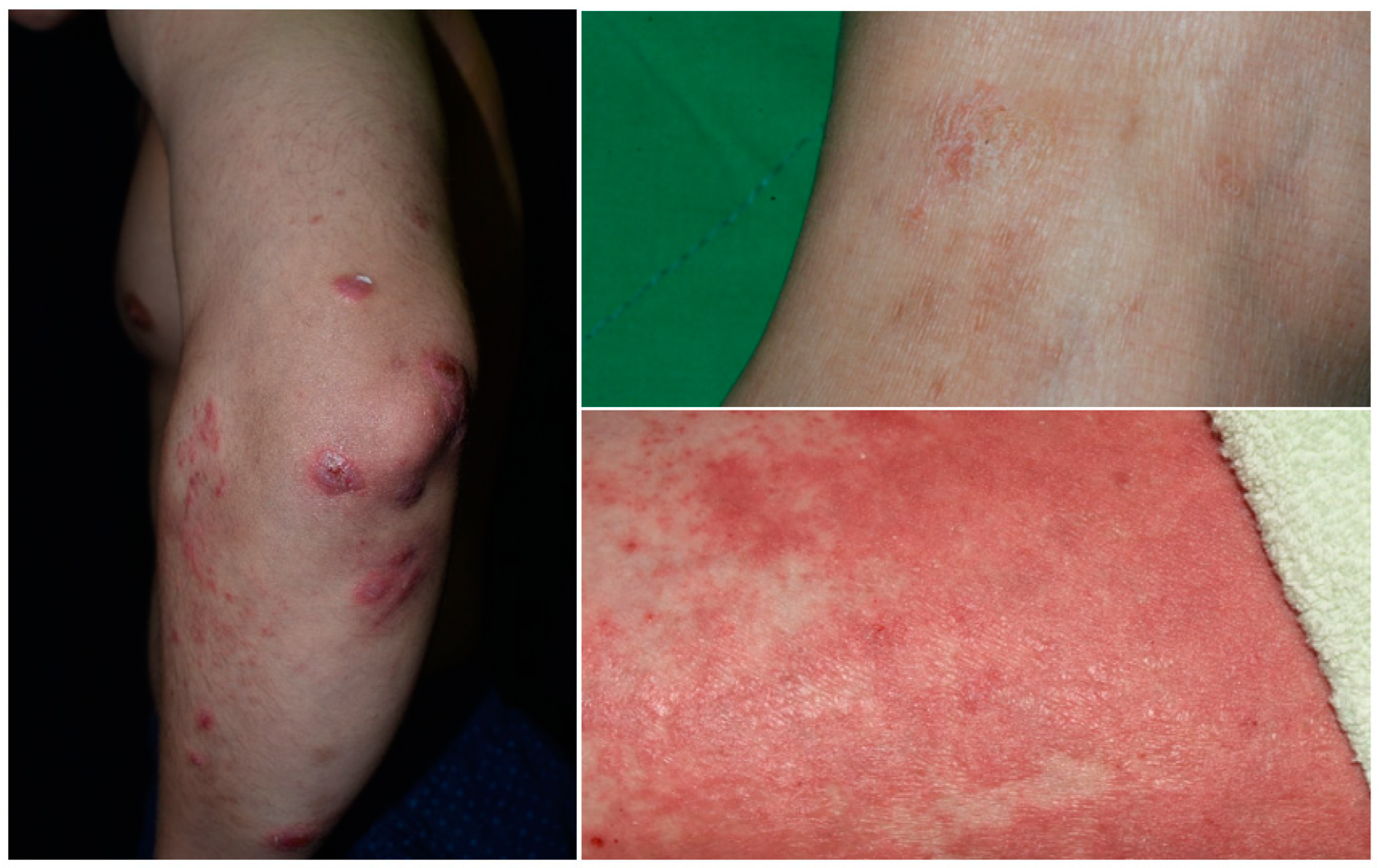
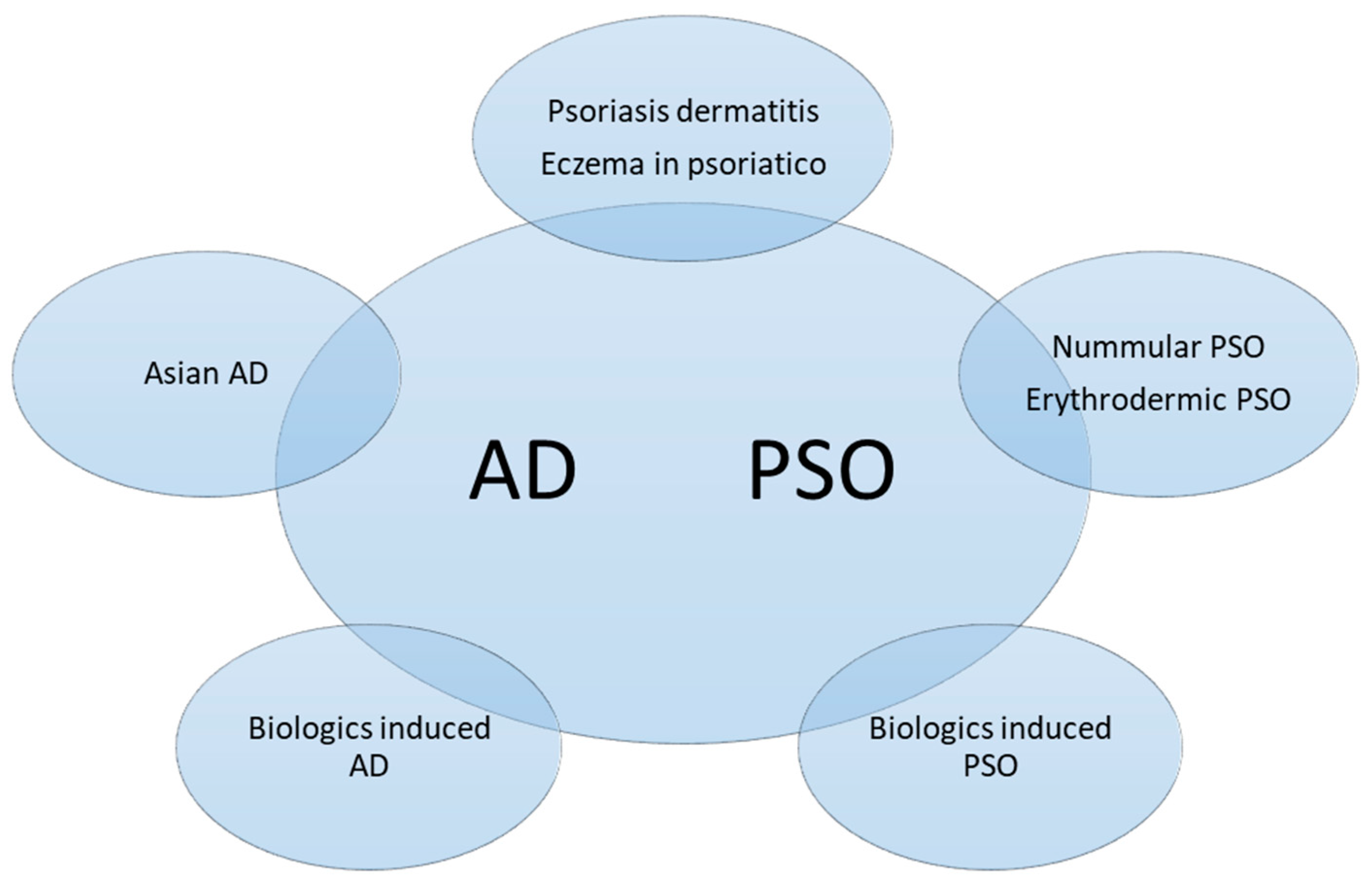
6. Phenotypes of Overlapping Psoriasis and Atopic Dermatitis
- PSO with AD features (Nummular PSO, erythrodermic PSO)
- AD with PSO features (Asian AD)
- Coexisting AD and PSO (Psoriasis dermatitis, PSO-Eczema, PsEma, eczema in psoriatico)
- Development of AD-Like dermatitis during PSO or AD treatment (TNFai, IL-12/23i, IL-17i, IL-23i, IL-4/13i)
- Development of PSO during AD treatment (Dupilumab)
6.1. PSO with AD Features (Nummular PSO, Erythrodermic PSO)
6.2. Asian-Type AD
6.3. Coexisting AD and PSO
6.4. Development of AD-like Dermatitis during PSO or AD Treatment
6.5. Development of PSO during AD Treatment
6.6. Management
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schäbitz, A.; Eyerich, K.; Garzorz-Stark, N. So close, and yet so far away: The dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J. Intern. Med. 2021, 290, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Moldovan, L.I.; Tsoi, L.C.; Ranjitha, U.; Hager, H.; Weidinger, S.; Gudjonsson, J.E.; Kjems, J.; Kristensen, L.S. Characterization of circular RNA transcriptomes in psoriasis and atopic dermatitis reveals disease-specific expression profiles. Exp. Dermatol. 2021, 30, 1187–1196. [Google Scholar] [CrossRef]
- He, H.; Bissonnette, R.; Wu, J.; Diaz, A.; Proulx, E.S.-C.; Maari, C.; Jack, C.; Louis, M.; Estrada, Y.; Krueger, J.G.; et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2021, 147, 199–212. [Google Scholar] [CrossRef]
- Henseler, T.; Christophers, E. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 1995, 32, 982–986. [Google Scholar] [CrossRef]
- Dai, Y.X.; Tai, Y.H.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between psoriasis and atopic dermatitis: A nationwide population-based cohort study. Dermatology 2021, 237, 521–527. [Google Scholar] [CrossRef]
- Barry, K.; Zancanaro, P.; Casseres, R.; Abdat, R.; Dumont, N.; Rosmarin, D. Concomitant atopic dermatitis and psoriasis—A retrospective review. Rev. J. Dermatol. Treat. 2021, 32, 716–720. [Google Scholar] [CrossRef]
- Williams, H.C.; Strachanh, D.P. Psoriasis and Eczema Are Not Mutually Exclusive Diseases. Dermatology 1994, 189, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Beer, W.E.; Smith, A.E.; Kassab, J.Y.; Smith, P.H.; Payne, C.M.R. Concomitance of psoriasis and atopic dermatitis. Dermatology 1992, 184, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, N.; Mohr, N.; Maul, J.T.; Augustin, M. Incidence of atopic conditions in people with psoriasis: A population-based analysis. Eur. J. Dermatol. 2021, 31, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Baurecht, H.; Hotze, M.; Brand, S.; Büning, C.; Cormican, P.; Corvin, A.; Ellinghaus, D.; Ellinghaus, E.; Esparza-Gordillo, J.; Fölster-Holst, R.; et al. Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am. J. Hum. Genet. 2015, 96, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüffmeier, U.; Traupe, H.; Oji, V.; Lascorz, J.; Sta¨nder, M.; Lohmann, J.; Wendler, J.; Burkhardt, H.; Reis, A. Loss-of-function variants of the filaggrin gene are not major susceptibility factors for psoriasis vulgaris or psoriatic arthritis in German patients. J. Investig. Dermatol. 2007, 127, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Terron-Kwiatkowski, A.; Liao, H.; Lee, S.P.; Allen, M.H.; Hull, P.R.; Campbell, L.E.; Trembath, R.C.; Capon, F.; Griffiths, C.E.; et al. Filaggrin null alleles are not associated with psoriasis. J. Investig. Dermatol. 2007, 127, 1878–1882. [Google Scholar] [CrossRef]
- Bergboer, J.G.; Zeeuwen, P.L.; Irvine, A.D.; Weidinger, S.; Giardina, E.; Novelli, G.; Heijer, M.D.; Rodriguez, E.; Illig, T.; Riveira-Munoz, E.; et al. Deletion of Late Cornified Envelope 3B and 3C genes is not associated with atopic dermatitis. J. Investig. Dermatol. 2010, 130, 2057–2061. [Google Scholar] [CrossRef]
- Chang, Y.C.; Wu, W.M.; Chen, C.H.; Hu, C.F.; Hsu, L.A. Association between P478S polymorphism of the filaggrin gene and risk of psoriasis in a Chinese population in Taiwan. Arch. Dermatol. Res. 2008, 300, 133–137. [Google Scholar] [CrossRef]
- Hu, Z.; Xiong, Z.; Xu, X.; Li, F.; Lu, L.; Li, W.; Su, J.; Liu, Y.; Liu, D.; Xie, Z.; et al. Loss-of-function mutations in filaggrin gene associate with psoriasis vulgaris in Chinese population. Hum. Genet. 2012, 131, 1269–1274. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J. Investig. Dermatol. 2019, 139, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Wongpiyabovorn, J.; Suto, H.; Ushio, H.; Izuhara, K.; Mitsuishi, K.; Ikeda, S.; Nakao, A.; Okumura, K.; Ogawa, H. Up-regulation of interleukin-13 receptor α1 on human keratinocytes in the skin of psoriasis and atopic dermatitis. J. Dermatol. Sci. 2003, 33, 31–40. [Google Scholar] [CrossRef]
- Bowes, J.; Eyre, S.; Flynn, E.; Ho, P.; Salah, S.; Warren, R.B.; Marzo-Ortega, H.; Coates, L.; McManus, R.; Ryan, A.W.; et al. Evidence to support IL-13 as a risk locus for psoriatic arthritis but not psoriasis vulgaris. Ann. Rheum. Dis. 2011, 70, 1016–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; Strong, C.D.G.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Schaffer, J.V.; Cerroni, L. Psoriasis. In Dermatology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 140. [Google Scholar]
- Tsai, Y.C.; Tsai, T.F. Anti-interleukin and interleukin therapies for psoriasis: Current evidence and clinical usefulness. Adv. Musculoskelet Dis. 2017, 9, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasumagic-Halilovic, E. Total Serum Immunoglobulin E Levels in Patients with Psoriasis. Mater. Sociomed. 2020, 32, 105–107. [Google Scholar] [CrossRef]
- Ünal, E.S.; Gül, Ü.; Dursun, A.B.; Erkekol, F.Ö. Prediction of atopy via total immunoglobulin E levels and skin prick tests in patients with psoriasis. Turk. J. Med. Sci. 2017, 47, 577–582. [Google Scholar] [CrossRef]
- Furue, K.; Ulzii, D.; Tanaka, Y.; Ito, T.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Pathogenic implication of epidermal scratch injury in psoriasis and atopic dermatitis. J. Dermatol. 2020, 47, 979–988. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Harari, M.; Gruzman, A.; Cohen, G. Pruritus in psoriasis and atopic dermatitis: Current treatments and new perspectives. Pharm. Rep. 2021, 73, 443–453. [Google Scholar] [CrossRef]
- Komiya, E.; Tominaga, M.; Kamata, Y.; Suga, Y.; Takamori, K. Molecular and cellular mechanisms of itch in psoriasis. Int. J. Mol. Sci. 2020, 21, 8406. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Li, X.Y. Inflammatory mediators causing cutaneous chronic itch in some diseases via transient receptor potential channel subfamily V member 1 and subfamily A member 1. J. Dermatol. 2019, 46, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ragaz, A.; Ackerman, A.B. Evolution, maturation, and regression of lesions of psoriasis. New observations and correlation of clinical and histologic findings. Am. J. Dermatopathol. 1979, 1, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Kerr, P.; Grant-Kels, J.M. The histopathologic spectrum of psoriasis. Clin. Dermatol. 2007, 25, 524–528. [Google Scholar] [CrossRef]
- Aslan, C.; Göktay, F.; Mansur, A.T.; Aydıngöz, İ.E.; Güneş, P.; Ekmekçi, T.R. Clinicopathological consistency in skin disorders: A retrospective study of 3949 pathological reports. J. Am. Acad. Dermatol. 2012, 66, 393–400. [Google Scholar] [CrossRef]
- Ibad, S.; Heibel, H.D.; Cockerell, C.J. Specificity of the Histopathologic Diagnosis of Psoriasis. Am. J. Dermatopathol. 2021, 43, 678. [Google Scholar] [CrossRef]
- Helwig, E.B. Pathology of psoriasis. Ann. N. Y. Acad. Sci. 1958, 73, 924–935. [Google Scholar] [CrossRef]
- Chau, T.; Parsi, K.K.; Ogawa, T.; Kiuru, M.; Konia, T.; Li, C.S.; Fung, M.A. Psoriasis or not? Review of 51 clinically confirmed cases reveals an expanded histopathologic spectrum of psoriasis. J. Cutan. Pathol. 2017, 44, 1018–1026. [Google Scholar] [CrossRef]
- Penn, L.; Brinster, N.K. Eosinophils among the histological features of psoriasis. Am. J. Dermatopathol. 2019, 41, 347–349. [Google Scholar] [CrossRef]
- Rosa, G.; Fernandez, A.P.; Schneider, S.; Billings, S.D. Eosinophils are rare in biopsy specimens of psoriasis vulgaris. J. Cutan. Pathol. 2017, 44, 1027–1032. [Google Scholar] [CrossRef]
- Quaade, A.S.; Simonsen, A.B.; Halling, A.S.; Thyssen, J.P.; Johansen, J.D. Prevalence, incidence, and severity of hand eczema in the general population–A systematic review and meta-analysis. Contact Dermat. 2021, 84, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, M.; Franke, I.; Lux, A.; Quist, S.R.; Gollnick, H.P. Eczema in Psoriatico: An Important Differential Diagnosis Between Chronic Allergic Contact Dermatitis and Psoriasis in Palmoplantar Localization. Acta Derm. Venereol. 2018, 98, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, O.; Engin, B.; Oğuz, O.; İlvan, Ş.; Demirkesen, C. Non-pustular palmoplantar psoriasis: Is histologic differentiation from eczematous dermatitis possible? J. Cutan. Pathol. 2008, 35, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Cho, E.B.; Park, E.J.; Park, H.R.; Kim, K.H.; Kim, K.J. The histopathological differentiation between palmar psoriasis and hand eczema: A retrospective review of 96 cases. J. Am. Acad. Dermatol. 2017, 77, 130–135. [Google Scholar] [CrossRef] [PubMed]
- An, M.K.; Yoon, J.H.; Park, E.J.; Park, H.R.; Kim, K.J.; Kim, K.H. Differential histopathological and immunohistochemical findings between palmar psoriasis and chronic hand eczema. Eur. J. Dermatol. 2020, 30, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zeng, N.; Cheng, Y.; Chen, Y.; Li, Y.; Lu, Q.; Xia, Q.; Luo, D. Atopic dermatitis and risk of autoimmune diseases: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 96. [Google Scholar] [CrossRef]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major comorbidities of atopic dermatitis: Beyond allergic disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838. [Google Scholar] [CrossRef]
- Tsai, T.F.; Wang, T.S.; Hung, S.T.; Phiona, I.; Tsai, C.; Schenkel, B.; Zhang, M.; Tang, C.H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Derm. Sci. 2011, 63, 40–46. [Google Scholar] [CrossRef]
- Wei, Y.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between alopecia areata and atopic dermatitis: A population-based cohort study in Taiwan. Clin. Exp. Allergy 2020, 50, 1406–1414. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chen, Y.J.; Tseng, W.C.; Lin, M.W.; Chen, T.J.; Hwang, C.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; et al. Comorbidity profiles among patients with alopecia areata: The importance of onset age, a nationwide population-based study. J. Am. Acad. Dermatol. 2011, 65, 949–956. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, Y.J.; Hwang, C.Y.; Lin, M.W.; Chen, T.J.; Chen, C.C.; Chu, S.Y.; Lee, D.D.; Chang, Y.T.; Liu, H.N. Comorbidity profiles in association with vitiligo: A nationwide population-based study in Taiwan. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.T.; Subramanian, A.; Adderley, N.J.; Zemedikun, D.T.; Gkoutos, G.V.; Nirantharakumar, K. Allergic diseases and long-term risk of autoimmune disorders: Longitudinal cohort study and cluster analysis. Eur. Respir. J. 2019, 54, 1900476. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Thompson, J.M.; Li, W.Q.; Cho, E.; Li, T.; Guttman-Yassky, E.; Qureshi, A.A. Incident alopecia areata and vitiligo in adult women with atopic dermatitis: Nurses’ Health Study 2. Allergy 2017, 72, 831–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of psoriasis with inflammatory bowel disease: A systematic review and meta-analysis. JAMA Derm. 2018, 154, 1417–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.D.; Dreiher, J.; Birkenfeld, S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J. Eur. Acad. Derm. Venereol. 2009, 23, 561–565. [Google Scholar] [CrossRef]
- Birkenfeld, S.; Dreiher, J.; Weitzman, D.; Cohen, A.D. Coeliac disease associated with psoriasis. Br. J. Derm. 2009, 161, 1331–1334. [Google Scholar] [CrossRef]
- Wu, J.J.; Nguyen, T.U.; Poon, K.Y.T.; Herrinton, L.J. The association of psoriasis with autoimmune diseases. J. Am. Acad. Derm. 2012, 67, 924–930. [Google Scholar] [CrossRef]
- Radtke, M.A.; Schäfer, I.; Glaeske, G.; Jacobi, A.; Augustin, M. Prevalence and comorbidities in adults with psoriasis compared to atopic eczema. J. Eur. Acad. Derm. Venereol. 2017, 31, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Y.M.; Egeberg, A.; Gislason, G.H.; Skov, L.; Thyssen, J.P. Autoimmune diseases in adults with atopic dermatitis. J. Am. Acad. Derm. 2017, 76, 274–280. [Google Scholar] [CrossRef]
- Ress, K.; Annus, T.; Putnik, U.; Luts, K.; Uibo, R.; Uibo, O. Celiac disease in children with atopic dermatitis. Pediatr. Derm. 2014, 31, 483–488. [Google Scholar] [CrossRef]
- Hou, Y.C.; Hu, H.Y.; Liu, I.L.; Chang, Y.T.; Wu, C.Y. The risk of autoimmune connective tissue diseases in patients with atopy: A nationwide population-based cohort study. Allergy Asthma Proc. 2017, 38, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kabashima, K. Interaction of psoriasis and bullous diseases. Front. Med. 2018, 5, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kridin, K.; Hammers, C.M.; Ludwig, R.J.; Onn, E.; Schonmann, Y.; Abu-Elhija, A.; Bitan, D.T.; Schmidt, E.; Weinstein, O.; Cohen, A.D. The association of bullous pemphigoid with atopic dermatitis and allergic rhinitis—A population-based study. Dermatitis 2021. [Google Scholar] [CrossRef] [PubMed]
- Ivert, L.U.; Wahlgren, C.F.; Lindelöf, B.; Dal, H.; Bradley, M.; Johansson, E.K. Association between atopic dermatitis and autoimmune diseases: A population-based case–control study. Br. J. Derm. 2021, 185, 335–342. [Google Scholar] [CrossRef]
- Kapila, S.; Hong, E.; Fischer, G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas. J. Derm. 2012, 53, 98–105. [Google Scholar] [CrossRef]
- William, A.; Clay, C.; Lisa, C.S.; Adrian, M.G.; Torsten, E.; Alan, M. PsEma—A hitherto unnamed dermatologic entity with clinical features of both psoriasis and eczema. SKINmed Dermatol. Clin. 2005, 4, 275–281. [Google Scholar] [CrossRef]
- Rebora, A.; Rongioletti, F. Psoriasis; ISED: Brescia, Italy, 1994. [Google Scholar]
- Beylot, C.; Puissant, A.; Bioulac, P.; Saurat, J.H.; Pringuet, R.; Doutre, M.S. Particular clinical features of psoriasis in infants and chidren. Acta Derm. Venereol. Suppl. 1979, 87, 95–97. [Google Scholar]
- Zhang, P.; Chen, H.X.; Duan, Y.Q.; Wang, W.Z.; Zhang, T.Z.; Li, J.W.; Tu, Y.T. Analysis of Th1/Th2 response pattern for erythrodermic psoriasis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 596–601. [Google Scholar] [CrossRef]
- Moy, A.P.; Murali, M.; Kroshinsky, D.; Duncan, L.M.; Nazarian, R.M. Immunologic overlap of helper T-cell subtypes 17 and 22 in erythrodermic psoriasis and atopic dermatitis. JAMA Derm. 2015, 151, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zheng, X.; Duan, Q.; Yang, P.; Zheng, Y. High serum IgE concentration in patients with psoriasis. Clin. Res. Derm. Open Access 2017, 4, 1–4. [Google Scholar]
- Li, L.F.; Sujan, S.A.; Yang, H.; Wang, W.H. Serum immunoglobulins in psoriatic erythroderma. Clin. Exp. Derm. 2005, 30, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.T.; Göktay, F.; Yaşar, Ş.P. Peripheral blood eosinophilia in association with generalized pustular and erythrodermic psoriasis. J. Eur. Acad. Derm. Venereol. 2008, 22, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, E.H.; Park, C.O. Skin barrier-related pathogenesis of atopic dermatitis. In Practical Insights Into Atopic Dermatitis, 1st ed.; Springer: Singapore, 2021; p. 77. [Google Scholar]
- Röcken, M.; Link, C.; Breit, R. The incidence of atopic symptoms in patients with psoriasis. Der Hautarzt Zeitschrift fur Dermatologie Venerologie und Verwandte Gebiete 1991, 42, 684–686. [Google Scholar]
- Welp, K.; Gieler, U.; Ständer, M.; Friederich, H.C. Concomitant psoriasis vulgaris and atopic dermatitis. A study of 1065 patients with psoriasis. Der Hautarzt Zeitschrift fur Dermatologie Venerologie und Verwandte Gebiete 1989, 40, 496–500. [Google Scholar]
- Kouwenhoven, T.A.; Bronckers, I.M.G.J.; Kerkhof, P.C.M.V.D.; Kamsteeg, M.; Seyger, M.M.B. Psoriasis dermatitis: An overlap condition of psoriasis and atopic dermatitis in children. J. Eur. Acad. Derm. Venereol. 2019, 33, e74–e76. [Google Scholar] [CrossRef]
- Eyerich, S.; Onken, A.T.; Weidinger, S.; Franke, A.; Nasorri, F.; Pennino, D.; Grosber, M.; Pfab, F.; Schmidt-Weber, C.B.; Mempel, M. Mutual antagonism of T cells causing psoriasis and atopic eczema. N. Engl. J. Med. 2011, 365, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.J.; Cohen, J.M.; Vesely, M.D.; Damsky, W. Paradoxical eruptions to targeted therapies in dermatology: A systematic review and analysis. J. Am. Acad. Derm. 2020, 86, 1080–1091. [Google Scholar] [CrossRef]
- Wijs, L.E.M.D.; Nguyen, N.T.; Kunkeler, A.C.M.; Nijsten, T.; Damman, J.; Hijnen, D. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: A case series. Br. J. Derm. 2020, 183, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Russo, F.; Rizzo, A.; Santi, F.; Lamberti, A.; Lazzeri, L.; Flori, M.L.; Rubegni, P. A paradoxical head and neck erythema: An adverse event due to dupilumab in adult patients with atopic dermatitis. Int J. Derm. 2021. [Google Scholar] [CrossRef]
- Qi, H.J.; Li, L.F. New Biologics for the Treatment of Atopic Dermatitis: Analysis of Efficacy, Safety, and Paradoxical Atopic Dermatitis Acceleration. Biomed. Res. Int. 2021, 2021, 5528372. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Bowman, S.; Laszik, Z.G.; North, J.P. Clinicopathologic overlap of psoriasis, eczema, and psoriasiform dermatoses: A retrospective study of T helper type 2 and 17 subsets, interleukin 36, and β-defensin 2 in spongiotic psoriasiform dermatitis, sebopsoriasis, and tumor necrosis factor α inhibitor–associated dermatitis. J. Am. Acad. Derm. 2020, 82, 430–439. [Google Scholar] [PubMed]
- Laga, A.C.; Vleugels, R.A.; Qureshi, A.A.; Velazquez, E.F. Histopathologic spectrum of psoriasiform skin reactions associated with tumor necrosis factor-α inhibitor therapy. A study of 16 biopsies. Am. J. Dermatopathol. 2010, 32, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.C.; Tsai, T.F. Switching biologics in psoriasis-practical guidance and evidence to support. Expert Rev. Clin. Pharmacol. 2020, 13, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Heibel, H.D.; Hendricks, A.J.; Foshee, J.P.; Shi, V.Y. Rosacea associated with dupilumab therapy. J. Dermatol. Treat. 2021, 32, 114–116. [Google Scholar] [CrossRef]
- Brumfiel, C.M.; Patel, M.H.; Zirwas, M.J. Development of psoriasis during treatment with dupilumab: A systematic review. J. Am. Acad. Derm. 2022, 86, 708–709. [Google Scholar] [CrossRef]
- Parker, J.J.; Sugarman, J.L.; Silverberg, N.B.; Gonzalez, M.E.; Ramien, M.L.; Teng, J.M.; Paller, A.S. Psoriasiform dermatitis during dupilumab treatment for moderate-to-severe atopic dermatitis in children. Pediatr. Derm. 2021, 38, 1500–1505. [Google Scholar] [CrossRef]
- Uysal, P.I.; Gunhan, O. De novo pustular psoriasis associated with dupilumab therapy in a young male with the diagnosis of atopic dermatitis. Derm. Ther. 2022. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, L.; Lu, Y.; Zhou, P. A case of dupilumab-induced reverse psoriasis in a patient with atopic dermatitis. Derm. Ther. 2022, 35, e15345. [Google Scholar] [CrossRef]
- Elahmed, H.H.; Steinhoff, M. Effectiveness of ustekinumab in patients with atopic dermatitis: Analysis of real-world evidence. J. Dermatol. Treat. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Saeki, H.; Kabashima, K.; Tokura, Y.; Murata, Y.; Shiraishi, A.; Tamamura, R.; Randazzo, B.; Imanaka, K. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: A randomized, double-blind, placebo-controlled, phase II study. Br. J. Derm. 2017, 177, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Comorbidities | Atopic Dermatitis | Psoriasis | |
|---|---|---|---|
| Gastroenterology | Ulcerative colitis | V | Inconsistent data |
| Crohn’s disease | V | V | |
| Celiac disease | V | V | |
| Dermatology | Vitiligo | V | V |
| Alopecia areata | V | V | |
| Atopy | Allergic rhinitis | V | V |
| Asthma | V | V | |
| Musculoskeletal disease | Systemic lupus erythematosus | V | V |
| Rheumatoid arthritis | V | V | |
| Autoimmune bullous disease | Bullous pemphigoid | V | V |
| Pemphigus | Unknown | V | |
| Typical PSO | Typical AD | AD-PSO Overlapping | |
|---|---|---|---|
| Treatment | |||
| Topical agents | Corticosteroids | Corticosteroids | Corticosteroids |
| Vitamin D3 analog | Calcineurin inhibitor | Calcineurin inhibitor | |
| Retinoids | PDE4 inhibitor (crisaborole) JAK inhibitor (ruxolitinib) | ||
| Tar | |||
| Calcineurin inhibitor * | |||
| Conventional oral medications | Methotrexate Acitretin Cyclosporin | Methotrexate * Azathioprine * Cyclosporin | Methotrexate Cyclosporin |
| Phototherapy | NBUVB # | NBUVB # | NBUVB # |
| Biologics | IL-12/23i, IL-17i, IL-23i, TNF-αi | IL-4/13i, IL-13i | IL-12/23i ? |
| Systemic small molecular drugs | PDE4 inhibitor (apremilast) JAK inhibitor (upadacitinib) ** | JAK inhibitor (baricitinib, upadacitinib, abrocitinib) | JAK inhibitor (upadacitinib) ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Tsai, T.-F. Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes. Int. J. Mol. Sci. 2022, 23, 5518. https://doi.org/10.3390/ijms23105518
Tsai Y-C, Tsai T-F. Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes. International Journal of Molecular Sciences. 2022; 23(10):5518. https://doi.org/10.3390/ijms23105518
Chicago/Turabian StyleTsai, Ya-Chu, and Tsen-Fang Tsai. 2022. "Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes" International Journal of Molecular Sciences 23, no. 10: 5518. https://doi.org/10.3390/ijms23105518
APA StyleTsai, Y.-C., & Tsai, T.-F. (2022). Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes. International Journal of Molecular Sciences, 23(10), 5518. https://doi.org/10.3390/ijms23105518







