Fullerene Rosette: Two-Dimensional Interactive Nanoarchitectonics and Selective Vapor Sensing
Abstract
:1. Introduction
2. Results
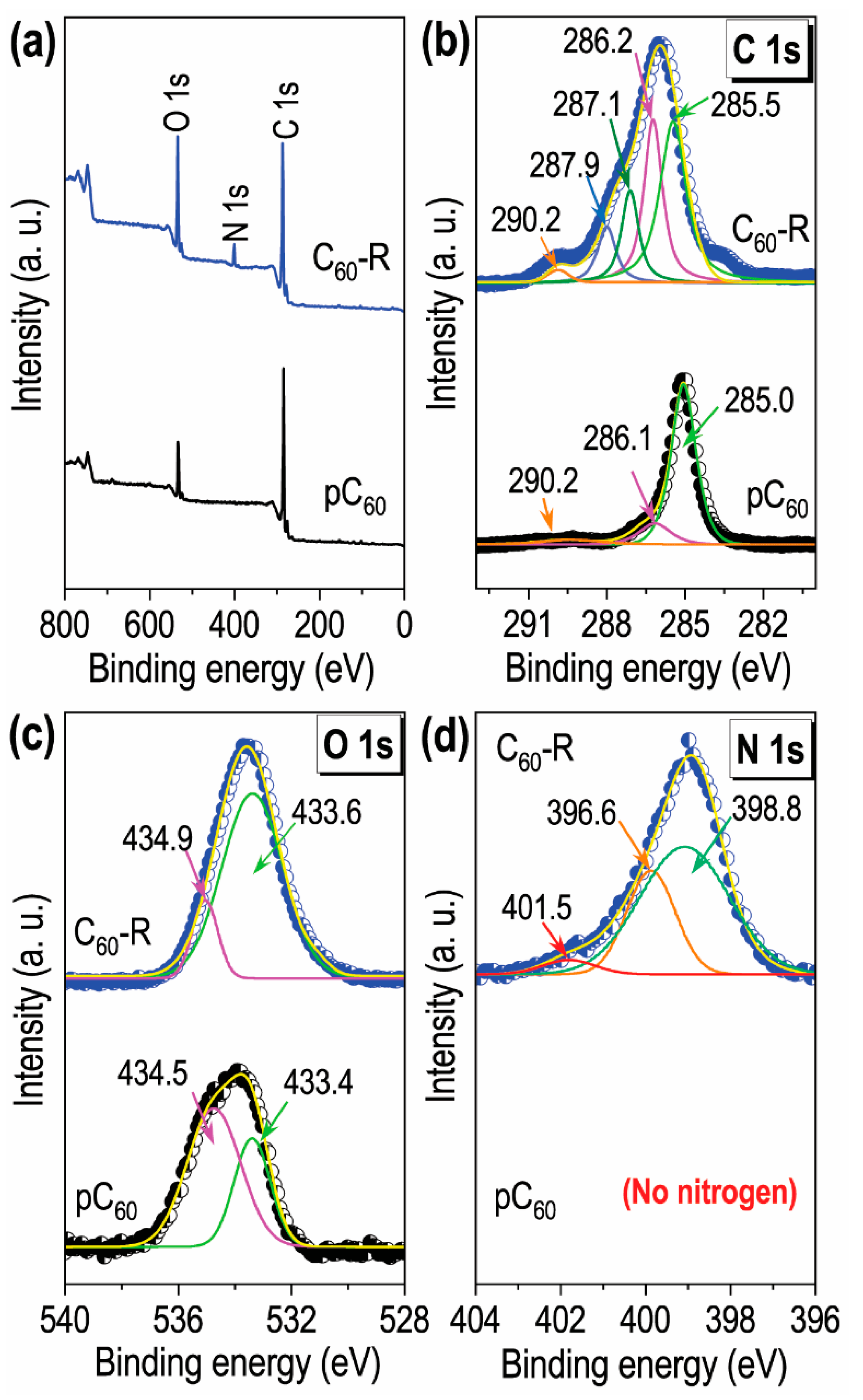
2.1. Characterizations of the Prepared C60 Self-Assembled Structures
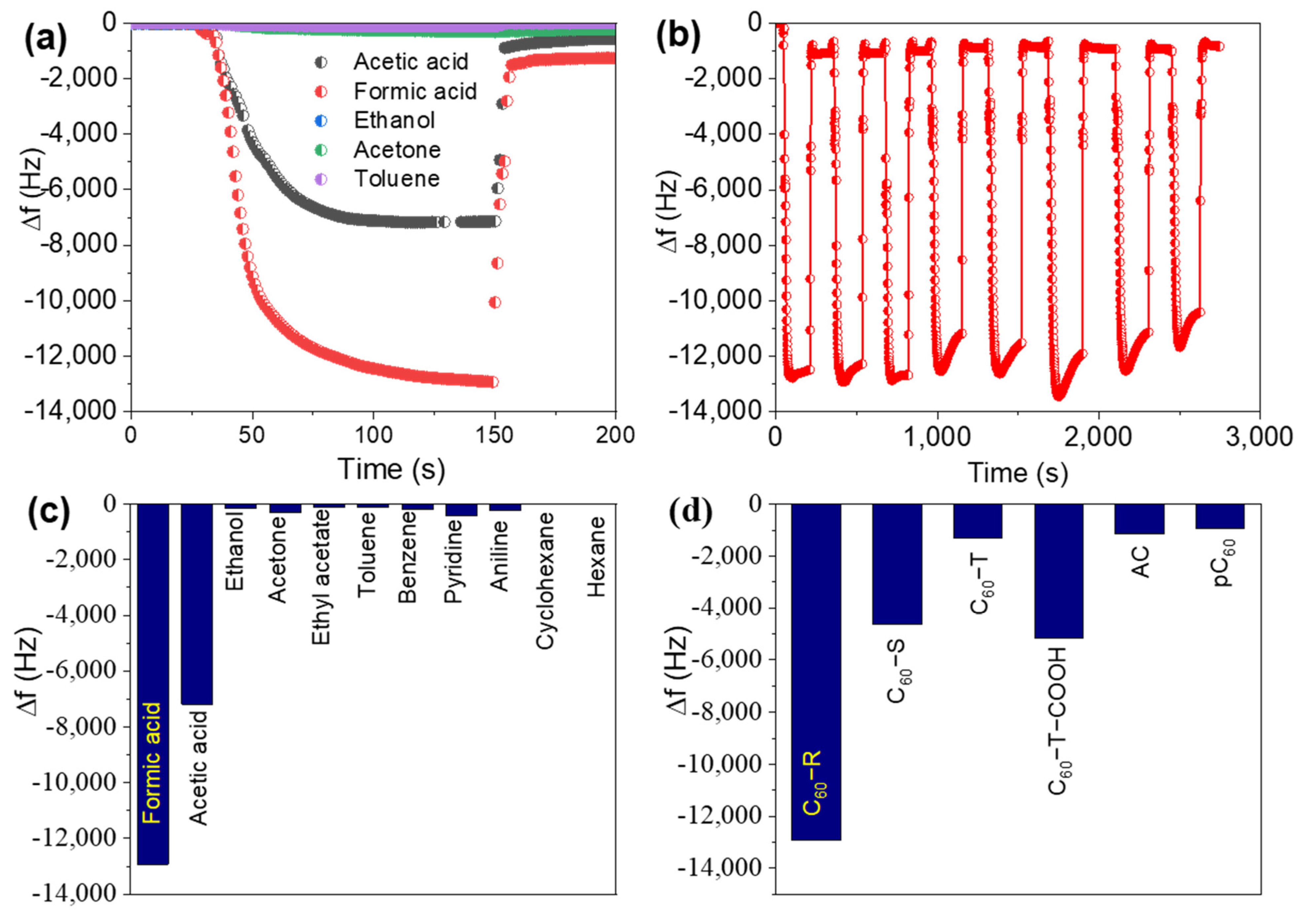
2.2. Sensing of Organic Vapors by Quartz Crystal Microbalance
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of C60 Rosettes
4.3. Characterizations
4.4. Sensing of Vapor by Quartz Crystal Microbalance (QCM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorn, R.A.; Vogl, C. Molecular assembly and structural plasticity of sensory ribbon synapses—A presynaptic perspective. Int. J. Mol. Sci. 2020, 21, 8758. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Mathias, J.I.; Seto, C.T. Self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. Self-assembly strategies for integrating light harvesting and charge separation in artificial photosynthetic systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical self-organizations and emerging functions in architectures, biological and synthetic macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Zero-to-one (or more) nanoarchitectonics: How to produce functional materials from zero-dimensional single-element unit, fullerene. Mater. Adv. 2021, 2, 582–597. [Google Scholar] [CrossRef]
- Chen, G.; Shrestha, L.K.; Ariga, K. Zero-to-two nanoarchitectonics: Fabrication of two-dimensional materials from zero-dimensional fullerene. Molecules 2021, 26, 4636. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for hierarchical fullerene nanomaterials. Nanomaterials 2021, 11, 2146. [Google Scholar] [CrossRef]
- Babu, S.S.; Möhwald, H.; Nakanishi, T. Recent progress in morphology control of supramolecular fullerene assemblies and its applications. Chem. Soc. Rev. 2010, 39, 4021–4035. [Google Scholar] [CrossRef]
- Das, S.; Presselt, M. Progress and development in structural and optoelectronic tunability of supramolecular nonbonded fullerene assemblies. J. Mater. Chem. C 2019, 7, 6194–6216. [Google Scholar] [CrossRef]
- Han, F.; Wang, R.; Feng, Y.; Wang, S.; Liu, L.; Li, X.; Han, Y.; Chen, H. On demand synthesis of hollow fullerene nanostructures. Nat. Commun. 2019, 10, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Guo, J.; Wei, T.; Chen, X.; Yang, Q.; Yang, S. Micron-sized hexagonal single-crystalline rods of metal nitride clusterfullerene: Preparation, characterization, and photoelectrochemical application. Nanoscale 2013, 5, 1993–2001. [Google Scholar] [CrossRef]
- Ji, H.-X.; Hu, J.-S.; Tang, Q.-X.; Song, W.-G.; Wang, C.-R.; Hu, W.-P.; Wan, L.-J.; Lee, S.-T. Controllable preparation of submicrometer single-crystal C60 rods and tubes trough concentration depletion at the surfaces of seeds. J. Phys. Chem. C 2007, 111, 10498–10502. [Google Scholar] [CrossRef]
- Zheng, S.; Cuong, N.T.; Okada, S.; Xu, T.; Shen, W.; Lu, X.; Tsukagoshi, K. Solvent-mediated shape engineering of fullerene (C60) polyhedral microcrystals. Chem. Mater. 2018, 30, 7146–7153. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 013502. [Google Scholar] [CrossRef]
- Minato, J.; Miyazawa, K. Solvated structure of C60 nanowhiskers. Carbon 2005, 43, 2837–2841. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Sasaki, T. Nanoporous fullerene nanowhiskers. Chem. Mater. 2007, 19, 2398–2400. [Google Scholar] [CrossRef]
- Jin, Y.; Curry, R.J.; Sloan, J.; Hatton, R.A.; Chong, L.C.; Blanchard, N.; Stolojan, V.; Kroto, H.W.; Silva, S.R.P. Structural and optoelectronic properties of C60 rods obtained via a rapid synthesis route. J. Mater. Chem. 2006, 16, 3715–3720. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Toward organic thick film solar cells: Three dimensional bulk heterojunction organic thick film solar cell using fullerene single crystal nanorods. Appl. Phys. Lett. 2007, 91, 173503. [Google Scholar] [CrossRef]
- Minato, J.; Miyazawa, K.; Suga, T. Morphology of C60 nanotubes fabricated by the liquid–liquid interfacial precipitation method. Sci. Technol. Adv. Mater. 2005, 6, 272–277. [Google Scholar] [CrossRef]
- Miyazawa, K.; Minato, J.; Yoshii, T.; Fujino, M.; Suga, T. Structural characterization of the fullerene nanotubes prepared by the liquid–liquid interfacial precipitation method. J. Mater. Res. 2005, 20, 688–695. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K. Size-tunable hexagonal fullerene (C60) nanosheets at the liquid−liquid interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, T.; D’Angelo, P.; Miyazawa, K.; Nemoto, Y.; Ito, O.; Tanigaki, N.; Bradley, D.D.C.; Anthopoulos, T.D. Fullerene/cobalt porphyrin hybrid nanosheets with ambipolar charge transporting characteristics. J. Am. Chem. Soc. 2012, 134, 7204–7206. [Google Scholar] [CrossRef]
- Osonoe, K.; Kano, R.; Miyazawa, K.; Tachibana, M. Synthesis of C70 two-dimensional nanosheets by liquid–liquid interfacial precipitation method. J. Cryst. Growth 2014, 401, 458–461. [Google Scholar] [CrossRef]
- Park, C.; Yoon, E.; Kawano, M.; Joo, T.; Choi, H.C. Self-Crystallization of C70 cubes and remarkable enhancement of photoluminescence. Angew. Chem. Int. Ed. 2010, 49, 9670–9675. [Google Scholar] [CrossRef]
- Hill, J.P.; Shrestha, R.G.; Song, J.; Ji, Q.; Ariga, K.; Shrestha, L.K. Monitoring the release of silver from a supramolecular fullerene C60-AgNO3 Nanomaterial. Bull. Chem. Soc. Jpn. 2021, 94, 1347–1354. [Google Scholar] [CrossRef]
- Partheeban, T.; Sathish, M. Selective growth of fullerene octahedra and flower-like particles by a liquid–liquid interfacial precipitation method for super-hydrophobic applications. RSC Adv. 2016, 6, 78791–78794. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Xu, T.; Lu, X. Three-dimensional “star of David”-shaped fullerene (C60) microstructures: Controlled synthesis, photoluminescence, and photoelectrochemical properties. ACS Appl. Electron. Mater. 2020, 2, 2010–2016. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Intentional closing/opening of “hole-in-cube” fullerene crystals with microscopic recognition properties. ACS Nano 2017, 11, 7790–7796. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically structured fullerene C70 cube for sensing volatile aromatic solvent vapors. ACS Nano 2016, 10, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Shrestha, R.G.; Lee, J.; Han, S.A.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Macaroni fullerene crystals-derived mesoporous carbon tubes as a high rate performance supercapacitor electrode material. Bull. Chem. Soc. Jpn. 2021, 94, 1502–1509. [Google Scholar] [CrossRef]
- Tang, Q.; Maji, S.; Jiang, B.; Sun, J.; Zhao, W.; Hill, J.P.; Ariga, K.; Fuchs, H.; Ji, Q.; Shrestha, L.K. Manipulating the structural transformation of fullerene microtubes to fullerene microhorns having microscopic recognition properties. ACS Nano 2019, 13, 14005–14012. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Michinobu, T.; Yoshida, K.; Shirahata, N.; Ariga, K.; Möhwald, H.; Kurth, D.G. Nanocarbon superhydrophobic surfaces created from fullerene-based hierarchical supramolecular assemblies. Adv. Mater. 2008, 20, 443–446. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shen, Y.; Wang, J.; Li, H.; Fernandes, P.; Yoshida, K.; Yagai, S.; Takeuchi, M.; Ariga, K.; Kurth, D.G.; et al. Superstructures and superhydrophobic property in hierarchical organized architectures of fullerenes bearing long alkyl tails. J. Mater. Chem. 2010, 20, 1253–1260. [Google Scholar] [CrossRef]
- Zhou, S.; Burger, C.; Chu, B.; Sawamura, N.; Nagahama, N.; Toganoh, M.; Hackler, U.E.; Isobe, H.; Nakamura, E. Spherical bilayer vesicles of fullerene-based surfactants in water: A laser light scattering study. Science 2001, 291, 1944–1947. [Google Scholar] [CrossRef]
- Neal, E.A.; Nakanishi, T. Alkyl-fullerene materials of tunable morphology and function. Bull. Chem. Soc. Jpn. 2021, 94, 1769–1788. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Hill, J.P.; Nakanishi, W.; Shrestha, L.K.; Liu, C.; Harano, K.; Nakamura, E.; Ariga, K. Supramolecular differentiation for construction of anisotropic fullerene nanostructures by time-programmed control of interfacial growth. ACS Nano 2016, 10, 8796–8802. [Google Scholar] [CrossRef]
- Michinobu, T.; Nakanishi, T.; Hill, J.P.; Funahashi, M.; Ariga, K. Room temperature liquid fullerenes: an uncommon morphology of C60 derivatives. J. Am. Chem. Soc. 2006, 128, 10384–10385. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Hsu, S.-H.; Maji, S.; Chahal, M.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post-assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2020, 7, 787–795. [Google Scholar] [CrossRef]
- Song, J.; Murata, T.; Tsai, K.-C.; Jia, X.; Sciortino, F.; Ma, R.; Yamauchi, Y.; Hill, J.P.; Shrestha, L.K.; Ariga, K. Fullerphene nanosheets: A bottom-Up 2D material for single-carbon-atom-level molecular discrimination. Adv. Mater. Interfaces 2022, 9, 2102241. [Google Scholar] [CrossRef]
- Davis, D.; Kundu, S.; Prabhudesai, V.S.; Sajeev, Y.; Krishnakumar, E. Formation of CO2 from formic acid through catalytic electron channel. J. Chem. Phys. 2018, 149, 064308. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd−Ag nanoparticles within a macroreticular basic resin: An efficient catalyst for hydrogen production from formic acid decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Lu, N.; Gao, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Enhanced formic acid gas-sensing property of WO3 nanorod bundles via hydrothermal method. Sens. Actuators B 2016, 223, 743–749. [Google Scholar] [CrossRef]
- Toh, C.T.; Zhang, H.; Lin, J.; Mayorov, A.S.; Wang, Y.P.; Orofeo, C.M.; Ferry, D.B.; Andersen, H.; Kakenov, N.; Guo, Z.; et al. Synthesis and properties of free-standing monolayer amorphous carbon. Nature 2020, 577, 199–203. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S.A. Preparation and fire behavior of rigid polyurethane foams synthesized from modified urea-melamine-formaldehyde resins. RSC Adv. 2018, 8, 17879–17887. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Park, M.; Kim, H.Y.; Park, S.J. Effects of microporosity and surface chemistry on separation performances of N-containing pitch-based activated carbons for CO2/N2 binary mixture. Sci. Rep. 2016, 6, 23224. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, D.; Zhou, H.; Hou, H.; Zhang, T.; Wu, L.; Cai, L. Polyaniline-modified quartz crystal microbalance sensor for detection of formic acid gas. Water Air Soil Pollut. 2012, 223, 1275–1280. [Google Scholar] [CrossRef]
- Leyva, J.A.M.; de Cisneros, J.L.H.H.; de Barreda, D.G.G.; Becerra, A.J.F. Determination of formic acid vapour using piezoelectric crystals with 4-ethyl-3-thiosemicarbazide and 2,6-diacetylpyridine coatings. Analyst 1993, 118, 175–178. [Google Scholar] [CrossRef]
- Cai, J.; Yan, Y.; Wang, W.; Ma, Y.; Cai, L.; Wu, L.; Zhou, H. Detection of formic acid and acetic acid gases by a QCM sensor coated with an acidified multi-walled carbon nanotube membrane. Environ. Technol. 2021, 1–11, in press. [Google Scholar] [CrossRef]
- Mane, G.P.; Dhawale, D.S.; Anand, C.; Ariga, K.; Ji, Q.; Wahab, M.A.; Mori, T.; Vinu, A. Selective sensing performance of mesoporous carbon nitride with a highly ordered porous structure prepared from 3-amino-1,2,4-triazine. J. Mater. Chem. A 2013, 1, 2913–2920. [Google Scholar] [CrossRef]
- Torad, N.L.; El-Hosainy, H.; Esmat, M.; El-Kelany, K.E.; Tahawy, R.; Na, J.; Ide, Y.; Fukata, N.; Chaikittisilp, W.; Hill, J.P.; et al. Phenyl-modified carbon nitride quantum nanoflakes for ultra-highly selective sensing of formic acid: A combined experimental by QCM and density functional theory study. ACS Appl. Mater. Interfaces 2021, 13, 48595–48610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Xu, X.; Liu, X.; Li, Q.; Guo, J.; Torad, N.L.; Alshehri, S.M.; Ahamad, T.; Hossain, M.S.A.; et al. Assembling well-arranged covalent organic frameworks on MOF-derived graphitic carbon for remarkable formaldehyde sensing. Nanoscale 2020, 12, 15611–15619. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, J.; Ma, R.; Ariga, K.; Shrestha, L.K. Self-assembled corn-husk-shaped fullerene crystals as excellent acid vapor sensors. Chemosens 2022, 10, 16. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, C.; Liu, C.; Liu, J.; Zhu, Y.; Wang, X.; Song, W. Nitrogen-doped hollow carbon spheres derived from amination reaction of fullerene with alkyl diamines as a carbon catalyst for hydrogenation of aromatic nitro compounds. Carbon 2017, 125, 139–145. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous carbon tubes from fullerene crystals as the π-electron carbon source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef]
- Furuuchi, N.; Shrestha, R.G.; Yamashita, Y.; Hirao, T.; Ariga, K.; Shrestha, L.K. Self-assembled fullerene crystals as excellent aromatic Vapor Sensors. Sensors 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Swager, T.M. Carbon nanotube formic acid sensors using a nickel bis(ortho-diiminosemiquinonate) selector. ACS Sens. 2018, 3, 569–573. [Google Scholar] [CrossRef]
- Wang, Z.; Moshman, L.; Kraus, E.; Wilson, B.; Acharya, N.; Diaz, R. A review of the Tawny Crazy Ant, Nylanderia Fulva, an emergent ant invader in the southern united states: Is biological control a feasible management option? Insects 2016, 7, 77. [Google Scholar] [CrossRef]
- Stavrakou, T.; Müller, J.-F.; Peeters, J.; Razavi, A.; Clarisse, L.; Clerbaux, C.; Coheur, P.-F.; Hurtmans, D.; De Mazière, M.; Vigouroux, C.; et al. Satellite evidence for a large source of formic acid from boreal and tropical forests. Nat. Geosci. 2011, 5, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, S.; Labuta, J.; Nakanishi, T.; Tanaka, T.; Kataura, H. Amperometric detection of sub-ppm formaldehyde using single-walled carbon nanotubes and hydroxylamines: A referenced chemiresistive system. ACS Sens. 2017, 2, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- McMartin, K.E.; Ambre, J.J.; Tephly, T.R. Methanol poisoning in human subjects. Role for formic acid accumulation in the metabolic acidosis. Am. J. Med. 1980, 68, 414–418. [Google Scholar] [CrossRef]
- Greenwald, R.; Fitzpatrick, A.M.; Gaston, B.; Marozkina, N.V.; Erzurum, S.; Teague, W.G. Breath formate is a marker of airway S-nitrosothiol depletion in severe asthma. PLoS ONE 2010, 5, e11919. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.; Johnson, B.A.; Hoskins, A.; Dworski, R. Exhaled breath condensate formate after inhaled allergen provocation in atopic asthmatics in vivo. J. Asthma 2013, 50, 619–622. [Google Scholar] [CrossRef]
- Nishikawa, M.; Murata, T.; Ishihara, S.; Shiba, K.; Shrestha, L.K.; Yoshikawa, G.; Minami, K.; Ariga, K. Discrimination of methanol from ethanol in gasoline using a membrane-type surface stress sensor coated with copper(I) complex. Bull. Chem. Soc. Jpn. 2021, 94, 648–654. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef]
- Ariga, K.; Fakhrullin, R. Materials nanoarchitectonics from atom to living cell: A method for everything. Bull. Chem. Soc. Jpn. 2022, in press. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Yamauchi, Y.; Ariga, K. Material evolution with nanotechnology, nanoarchitectonics, and materials informatics: What will be the next paradigm shift in nanoporous materials? Adv. Mater. 2022, 34, 2107212. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; Caseli, L.; Ariga, K. The past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Ariga, K.; Decher, G.; Lvov, Y. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Ruban, A.M.; Davidraj, J.M.; Singh, G.; Al-Muhtaseb, A.H.; Lee, J.M.; Yi, J.; Vinu, A. Single-step synthesis of 2D mesoporous C60/carbon hybrids for supercapacitor and Li-ion battery applications. Bull. Chem. Soc. Jpn. 2021, 94, 133–140. [Google Scholar] [CrossRef]
- Santiago, A.R.P.; Fernandez-Delgado, O.; Gomez, A.; Ahsan, M.A.; Echegoyen, L. Fullerenes as key components for low-dimensional (photo)electrocatalytic nanohybrid materials. Angew. Chem. Int. Ed. 2021, 60, 122–141. [Google Scholar] [CrossRef] [PubMed]
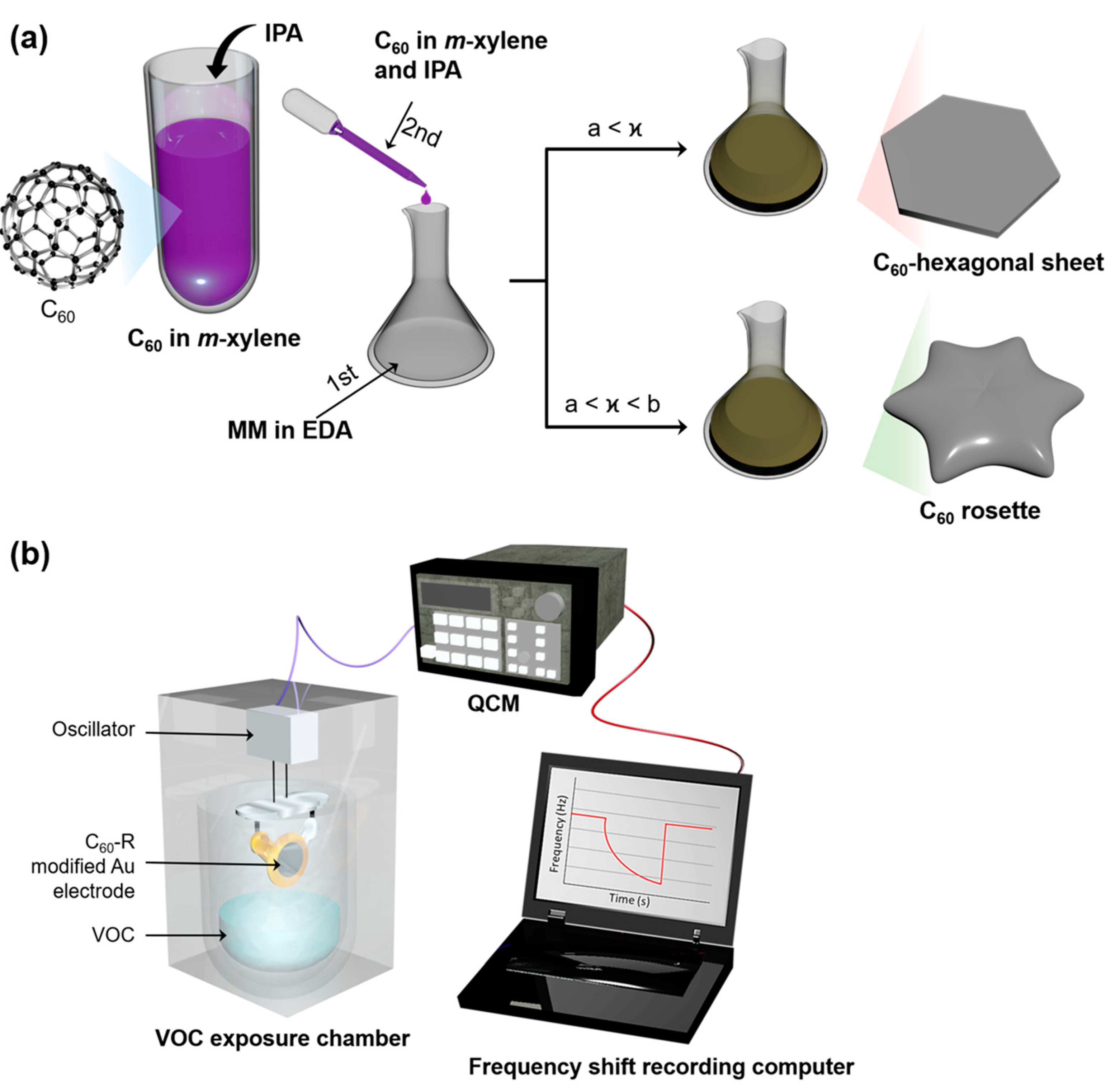
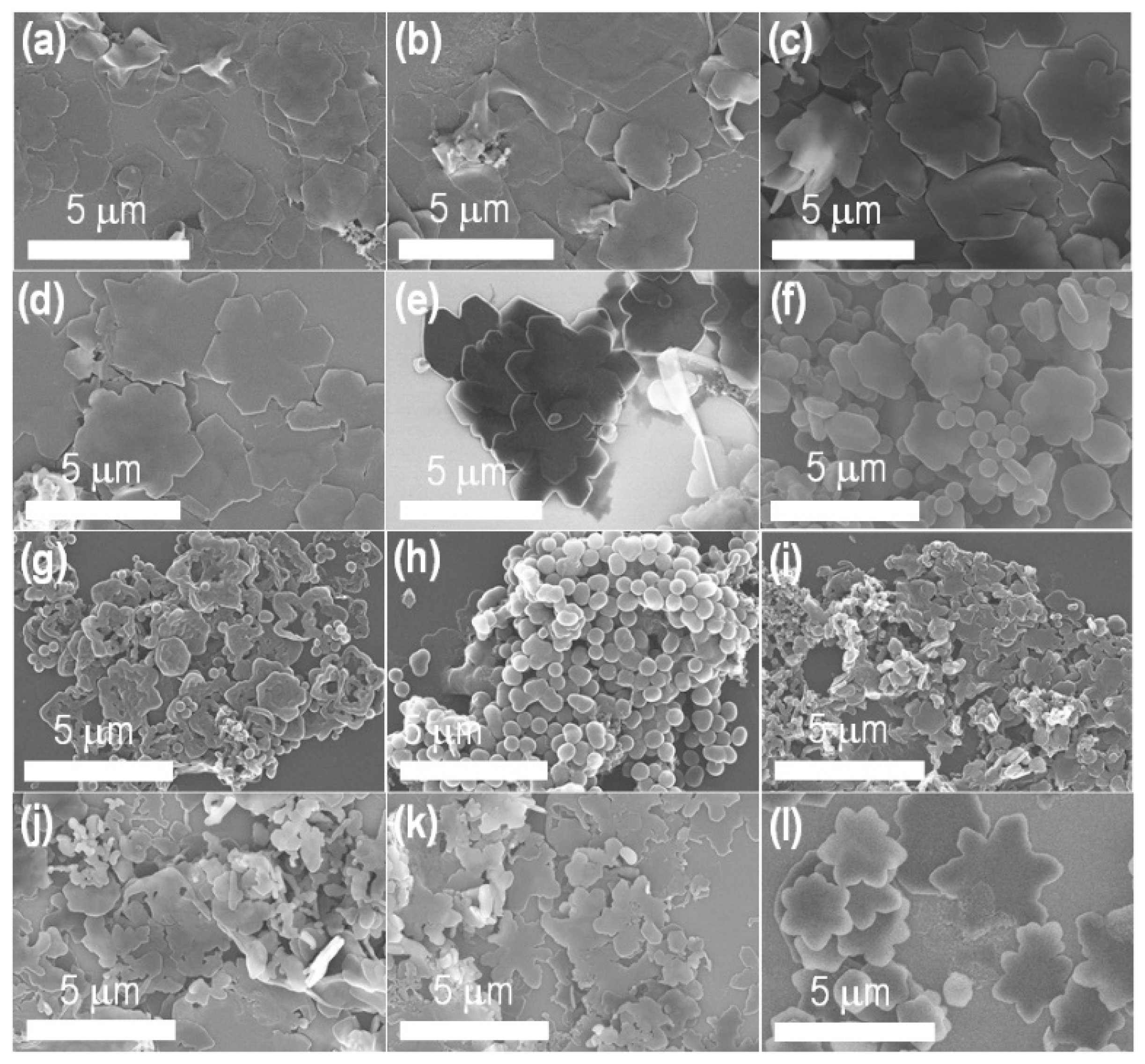
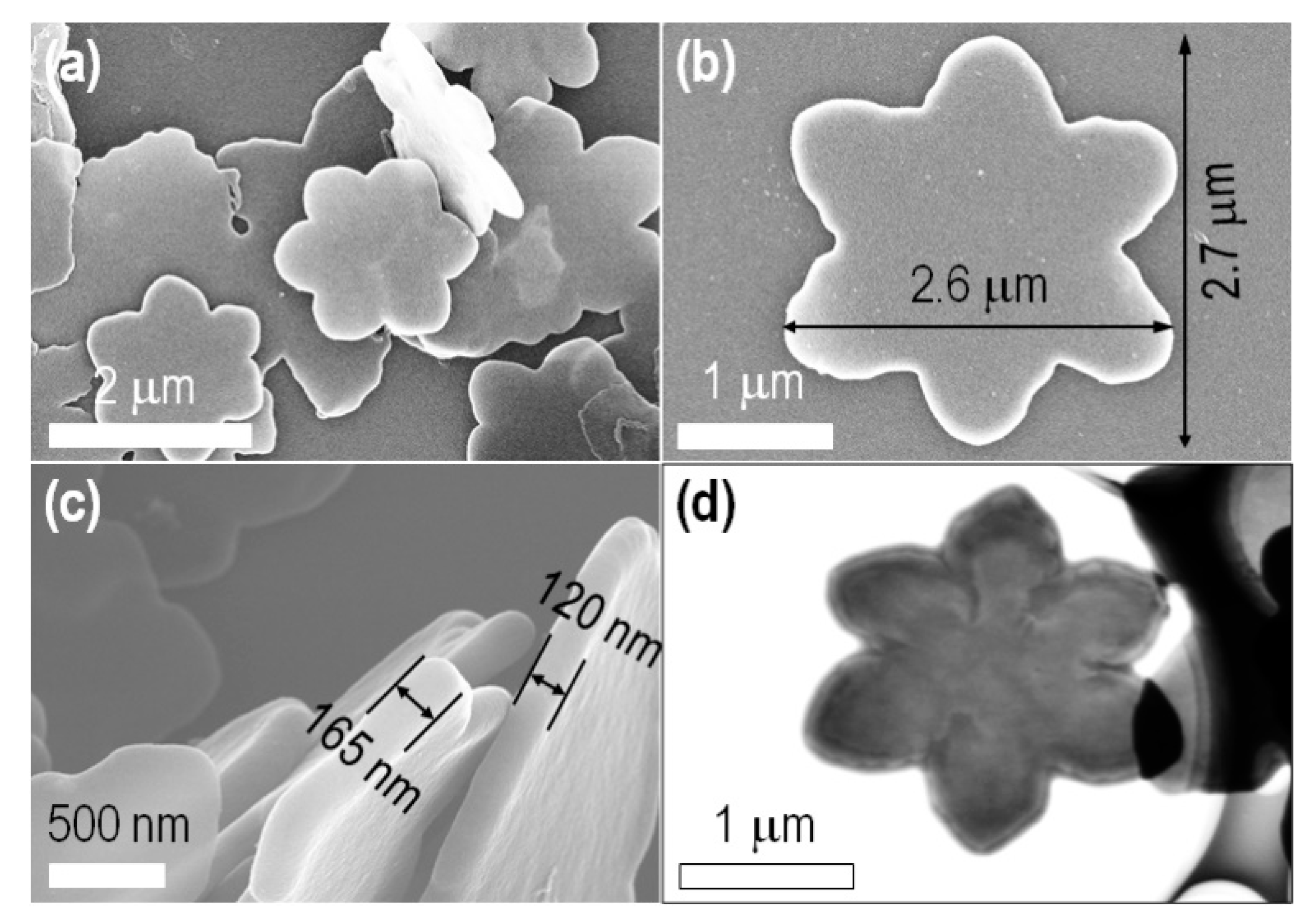
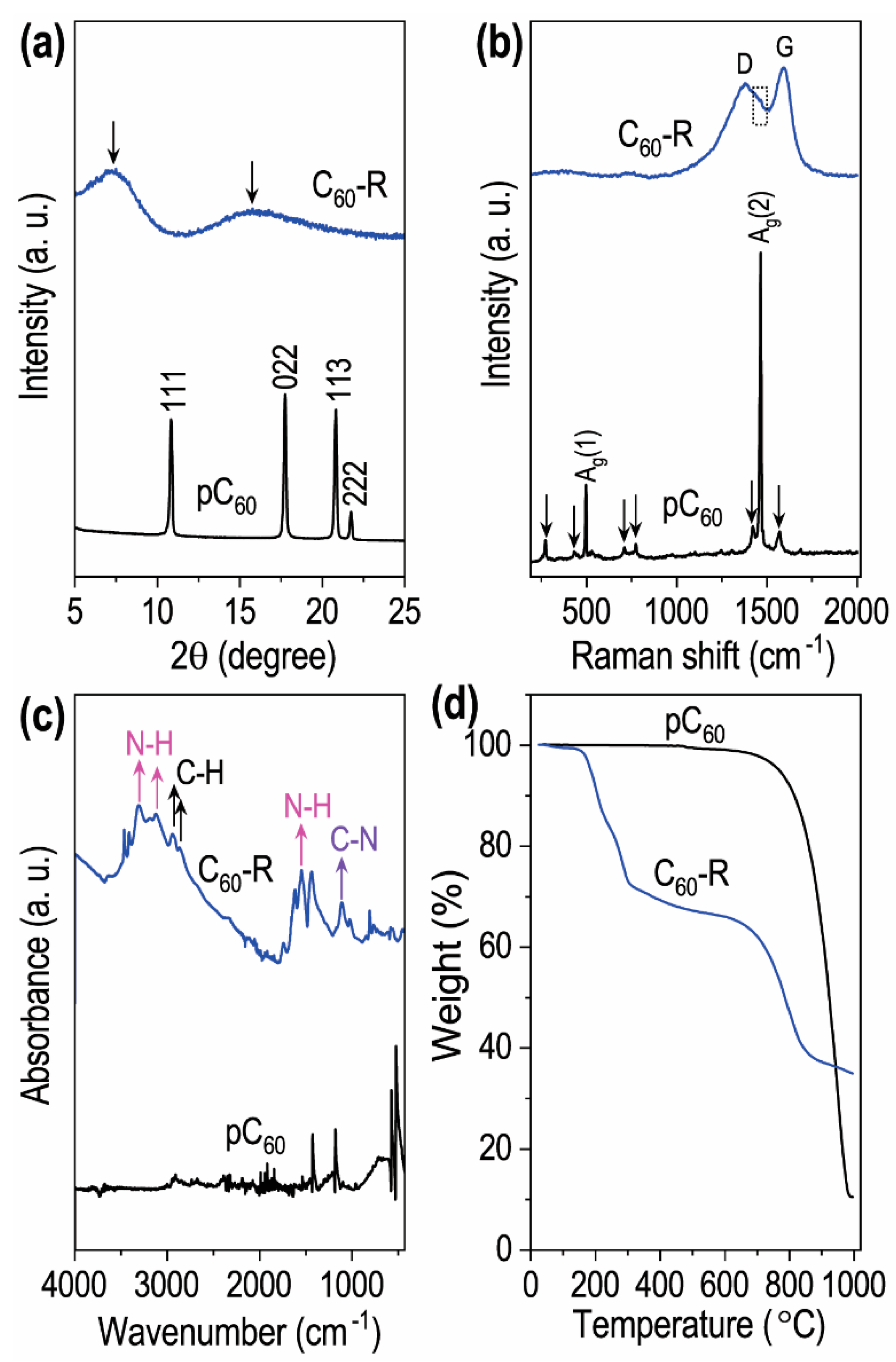
| Entry | C60in
m-xylene (mL) 1 | IPA (mL) | MM in EDA (mL) | Shapes | Remarks |
|---|---|---|---|---|---|
| 1 | 8.0 | 2.0 | 0.5 |  | Edge tends to cleave |
| 2 | 8.0 | 2.0 | 0.6 |  | Edge cleaving started |
| 3 | 8.0 | 2.0 | 0.7 |  | Edge cleaving increases |
| 4 | 8.0 | 2.0 | 0.8 |  | Edge cleaving increases more |
| 5 | 8.0 | 2.0 | 1.0 |  | Beautiful flower shape formed |
| 6 | 8.0 2 | 2.0 | 1.0 | − | No edge cleaving/heterogeneous shapes |
| 7 | 8.0 | 1.0 | 2.0 | − | No edge cleaving/heterogeneous shapes |
| 8 | 8.0 | 1.0 | 2.0 3 | − | Heterogeneous shapes |
| 9 | 8.5 | 1.5 | 0.5 | − | Heterogeneous shapes |
| 10 | 8.5 | 1.5 | 0.6 | − | Heterogeneous shapes with sheet types |
| 11 | 8.5 | 1.5 | 0.7 | − | Heterogeneous flower shapes |
| 12 | 8.5 | 1.5 | 0.8 |  | Most perfect and homogeneous beautiful flower shape formed |
| Materials | ΔF (Hz) | ΔF (Hz) Ratio of FA/PRD (Acid/Base) | ΔF (Hz) Ratio of FA/AA (Acid/Acid) | ΔF (Hz) Ratio of FA/Hydrocarbon (Acid/Neutral) | Reference |
|---|---|---|---|---|---|
| PANI−QCM | 20 | − | − | − | [48] |
| DAP−QCM | 824 | − | − | − | [49] |
| MWCNT | 196 | − | 1.3 | − | [50] |
| MCN−ATN | 1195 | ~4 | 1.16 | 10.5 (toluene) | [51] |
| Ph−g−C3N4 | 13,417 | 15.8 | 3.1 | 106.5 (benzene) | [52] |
| MOF−GC@COF | 98 | ~2 (Et3N) | − | 12.0 (n-hexane) | [53] |
| FNR−EDA | 1758 | ~17 | 1.45 | 14.9 (toluene) | [40] |
| CHFC | 1700 | 7.7 | 0.69 | 30.7 (toluene) | [54] |
| C60−fullerphene | 21,204 | − | 18.8 | 375 (benzene) | [41] |
| C60−R | 12,930 | 31.5 | 1.8 | 112 (toluene) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Bhadra, B.N.; Sutrisno, L.; Shrestha, L.K.; Ariga, K. Fullerene Rosette: Two-Dimensional Interactive Nanoarchitectonics and Selective Vapor Sensing. Int. J. Mol. Sci. 2022, 23, 5454. https://doi.org/10.3390/ijms23105454
Chen G, Bhadra BN, Sutrisno L, Shrestha LK, Ariga K. Fullerene Rosette: Two-Dimensional Interactive Nanoarchitectonics and Selective Vapor Sensing. International Journal of Molecular Sciences. 2022; 23(10):5454. https://doi.org/10.3390/ijms23105454
Chicago/Turabian StyleChen, Guoping, Biswa Nath Bhadra, Linawati Sutrisno, Lok Kumar Shrestha, and Katsuhiko Ariga. 2022. "Fullerene Rosette: Two-Dimensional Interactive Nanoarchitectonics and Selective Vapor Sensing" International Journal of Molecular Sciences 23, no. 10: 5454. https://doi.org/10.3390/ijms23105454
APA StyleChen, G., Bhadra, B. N., Sutrisno, L., Shrestha, L. K., & Ariga, K. (2022). Fullerene Rosette: Two-Dimensional Interactive Nanoarchitectonics and Selective Vapor Sensing. International Journal of Molecular Sciences, 23(10), 5454. https://doi.org/10.3390/ijms23105454








