CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro?
Abstract
:1. Introduction
2. Results
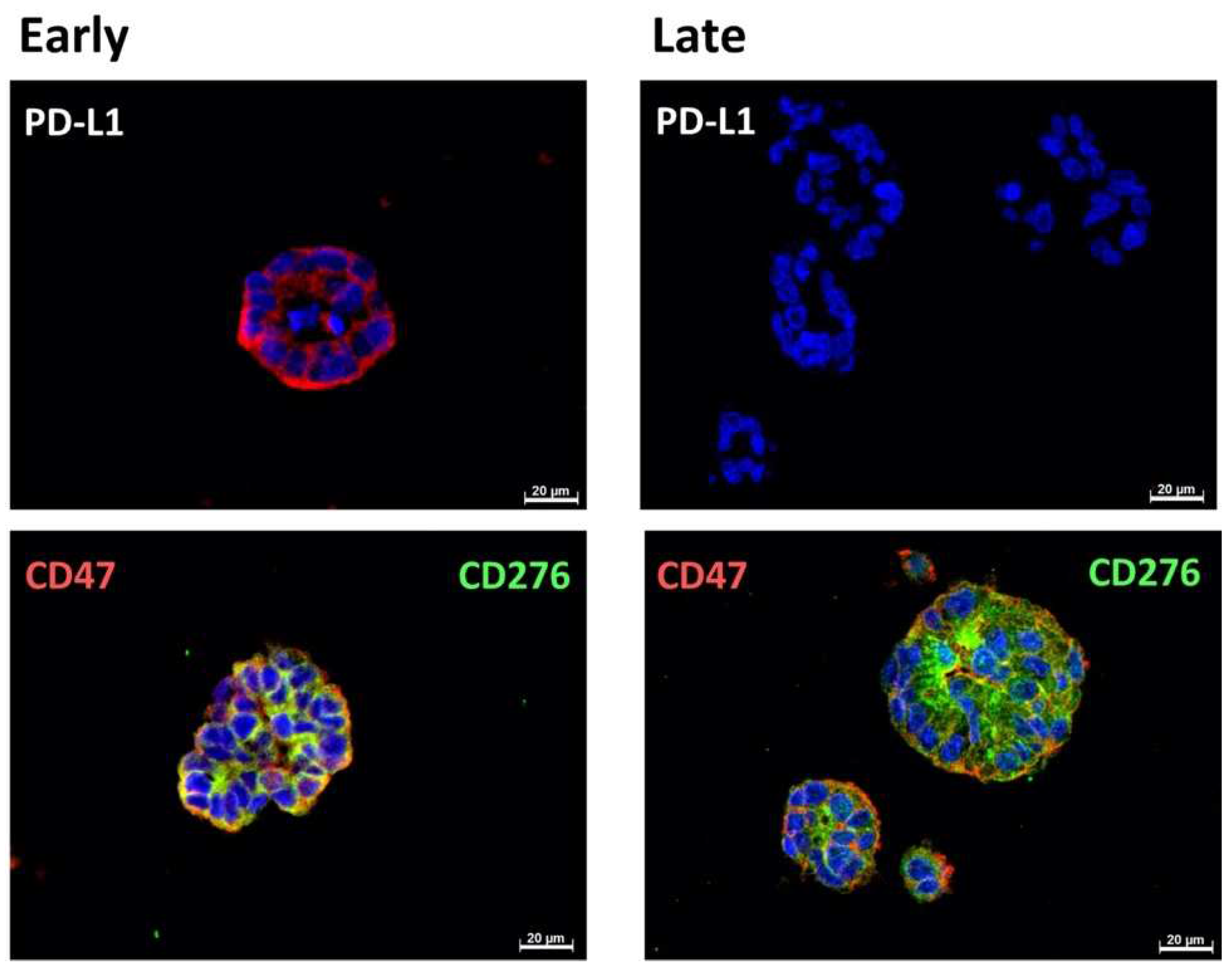
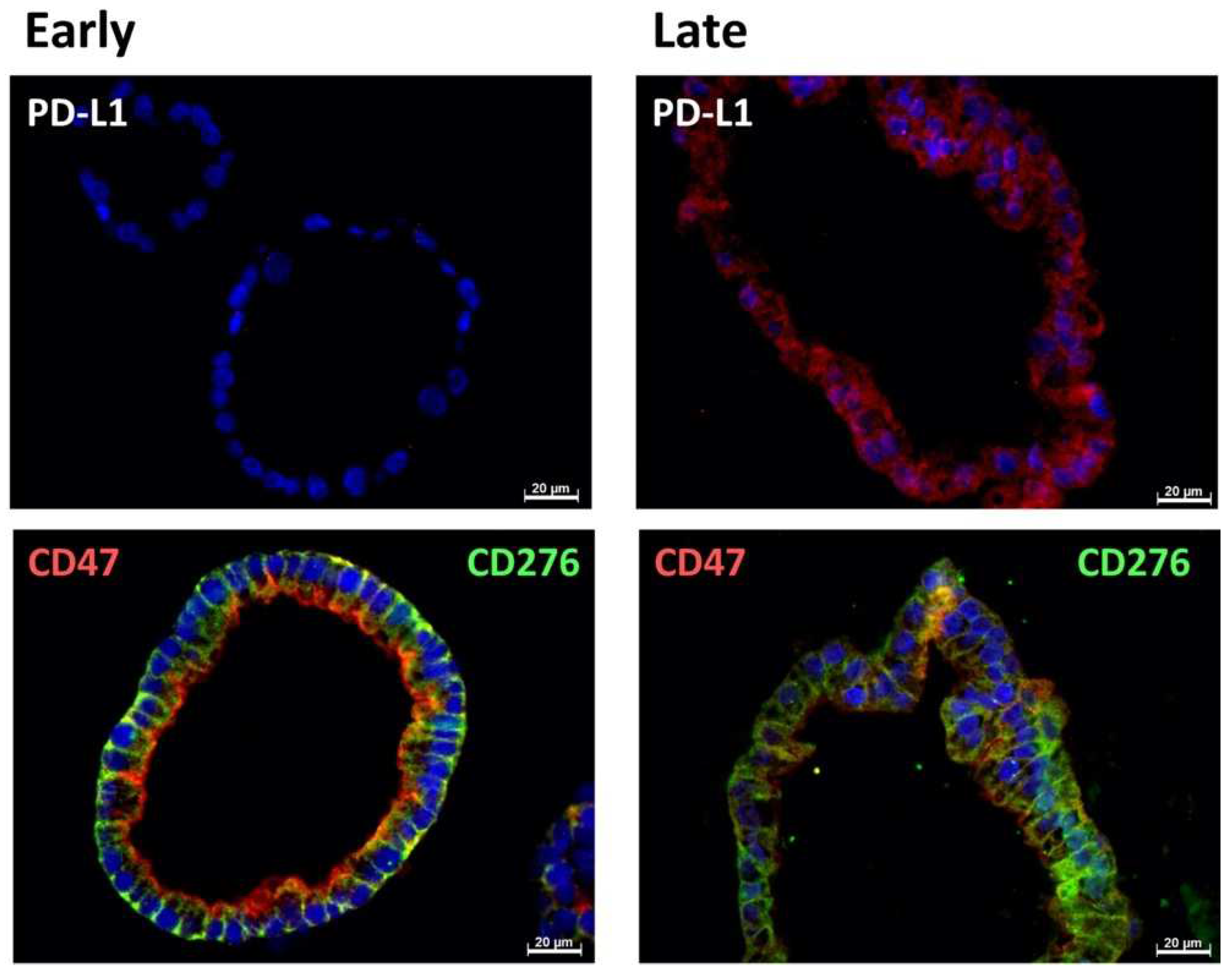
2.1. Expression of Immune Checkpoint Antigens on Early and Late Passage 3D Organoids
2.2. Expression of Bladder Cancer Stem-Cell Markers CD24 and CD44 on Early and Late Passage 3D Organoids
2.3. Transcripts Encoding the Immune Modulatory Factors and Stem-Cell Markers
2.4. Expression Marker Proteins on Tissue Samples of the Corresponding Bladder Cancer
3. Discussion
4. Materials and Methods
4.1. Bladder Cancer Organoids
4.2. Immunofluorescence and Immunohistochemistry
4.3. Transcript Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a.t. | ambient temperature |
| BC | bladder cancer |
| BCO | bladder cancer organoids |
| BSA | bovine serum albumin |
| CSC | cancer stem cell(s) |
| DN | double negative |
| DP | double positive |
| IMA | immune modulatory antigen(s) |
| f.c. | final concentration |
| o.n. | overnight |
Appendix A
Appendix A1. Isolation of Cells
- Prewarm media and PBS to 37 °C, prewarm collagenase/hyaluronidase to a.t.
- Check weight of petri dish
- Transfer the piece of tissue to the Petri dish: Remove the transport medium, aspirate the tissue with a pipette and place it in the Petri dish—aspirate the excess medium—put the lid on the Petri dish and weigh the tissue
- Pour 1 mL of the working medium into the Petri dish
- Cut the tissue into approx. 1 mm pieces by aid of 2 sterile scalpels
- Resuspend the minced tissue in 5 mL working medium and transfer minced tissue in a 15 mL tube using a pipette. Rinse the Petri dish with fresh working medium and transfer it to the 15 mL tube as well
- Centrifugation at 480× g, 10 min, a.t., aspirate the supernatant
- Resuspend the pellet in DPBS using 1000 µL DPBS per 100 mg wet weight of tissue
- Add 15 µL collagenase / hyaluronidase per 1000 µL DPBS or 100 mg tissue to the 15 mL tube
- Place the tube in the incubator (37 °C, 5% CO2, 30 min) and take it out in between and mix gently
- After 30 min, add the same amount of collagenase/hyaluronidase to the 15 mL tube and incubate for additional 30 min under the same conditions, occasionally mix the samples
- Filter digested tissue through a 70 µm cell strainer into a 50 mL tube. If the sieve is clogged, filter the residual material with a new sieve (2–3 times if necessary). Rinse clogged filters with DPBS if needed.
- Collect filtrates in a 15 mL tube
- Centrifugation 150 g, 7 min, a.t., aspirate the supernatant, leave approx. 200 µL pellet and suspension in the tube
- Add 10 mL RBC Lysis Buffer, incubate 10 min on ice
- Centrifugation 150 g, 7 min, a.t., aspirate the supernatant
- Resuspend the cell pellet in 1000 µL working medium
- Mix but well by gentle pipetting, transfer an aliquot (10 µL) into a 96-well plate to determine the yield and viability of cells by trypan blue by exclusion in a Neubauer counting chamber
- Dilute the samples to the cell density of 2 × 106 cells/mL, corresponding to 10,000 cells/dome, put diluted cells on wet ice
- Calculate the required amount of Matrigel (15 µL BME per 5 µL cell suspension and well).
- Mix 5 µL per well of cell suspension with 15 mL of cold Matrigel. Avoid air bubbles
- Collect the 48-well plate from the incubator, immediately plate out Matrigel domes (20 µL/dome)
- Turn over the plate and place it up-side down in a cell culture incubator (37 °C, 5% CO2, humidified atmosphere) for 45 min
- Collect the plate and turn it over again. Tilt the plate slightly, slowly pipette 250 µL of BCO culture medium into the lower edge
- Incubate the dome at 37 °C, 5% CO2, humidified atmosphere in a cell culture incubator
Appendix A2. Splitting of Organoids
- Carefully aspirate BCO culture medium, avoid destruction/removal of organoids
- Add 250 µL DPBS + 50 µL Dispase II per well, incubate 60 min in a cell culture incubator (37 °C, 5% CO2, humidified atmosphere)
- Add 200 µL TrypLE by pipette, resuspend carefully and transfer the suspension in 1.4 mL centrifugation tube
- Digest the Matrigel scaffold by incubation on a rotating shaker for exactly 15 min at 37 °C and 1400 rpm
- Resuspend the sample well by gentle pipetting, transfer in to a 15 mL tube and add 1 mL of stop medium.
- Centrifugation (150 g, 7 min, a.t.), then carefully remove the supernatant. Add BCO culture medium to split the cultures in new domes.
Appendix B
| Antibody | Isotype | Host | Dilution (C.S.) | Dilution (P.S.) | Company | Catalog Number |
|---|---|---|---|---|---|---|
| Anti−PD−1 | Ig | Mouse | 1:100 | 1:50 | Sigma | SAB3500122 |
| Anti−PD−L1 | IgG1k | Mouse | 1:50 | 1:50 | Invitrogen | 14−5983−82 |
| Anti−CD 276 | IgG | Rabbit | 1:100 | 1:50 | Abcam | Ab226256 |
| Anti−CTLA−4 | IgG2a Kappa | Mouse | 1:100 | 1:50 | Abnova | H00001493-M06 |
| Anti−CD 47 | IgG1 | Mouse | 1:50 | 1:50 | Invitrogen | 14−0479−82 |
| Anti−CD 24 | IgG | Rabbit | 1:50 | 1:50 | Abcam | Ab202073 |
| Anti−CD 44 | IgG1 | Mouse | 1:100 | 1:50 | Abcam | Ab9524 |
| Isotype control IgG | IgG | Rabbit | 1:100 | 1:50 | Abcam | Ab172730 |
| Isotype control IgG1 | IgG1 | Mouse | 1:100 | 1:50 | Abcam | Ab281291 |
| DAPI | 1:1000 | 1:1000 | DAKO | S 3023 | ||
| Alexa Fluor® 488 | IgG (H + L) | Goat anti−rabbit | 1:1000 | 1:1000 | Jackson Imm.Res. | 111−545−045 |
| CyTM3 | IgG (H + L) | Goat anti−mouse | 1:1000 | 1:1000 | Jackson Imm.Res. | 115−165−166 |
Appendix C
| Primer | Product Size (bp) | Forward | Reverse | Citation |
|---|---|---|---|---|
| PD−L1 | 204 | GTACCTTGGCTTTGCCACAT | CCAACACCACAAGGAGGAGT | Primer−BLAST |
| CD 276 | 224 | TTTCCTTTCCCCTCCTTCCTCC | TGTGACCAGCACATGCTTCC | Primer−BLAST |
| CD 47 | 213 | GGAGCCATTCTTTTCGTCCCA | ACACGCCGCAATACAGAGAC | Primer−BLAST |
| CD 24 | 208 | TGAAGAACATGTGAGAGGTTTGAC | GAAAACTGAATCTCCATTCCACAA | Primer−BLAST |
| CD 44 | 377 | CACAATCCAGGCAACTCCTA | TACTCTGCTGCGTTGTCATT | Primer−BLAST |
| GAPDH | 346 | GCTCTCCAGAACATCATCCCTGCC | CGTTGTCATACCAGGAAATGAGCTT | Primer−BLAST |
| ß−actin | 276 | AACTGGGACGACATGGAGAA | ATACCCCTCGTAGATGGGCA | Primer−BLAST |
References
- van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and progression of disease in non-muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Dobosz, P.; Stempor, P.A.; Roszik, J.; Herman, A.; Layani, A.; Berger, R.; Avni, D.; Sidi, Y.; Leibowitz-Amit, R. Checkpoint Genes at the Cancer Side of the Immunological Synapse in Bladder Cancer. Transl. Oncol. 2020, 13, 193–200. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Rothman, N.; Garcia-Closas, M.; Chatterjee, N.; Malats, N.; Wu, X.; Figueroa, J.D.; Real, F.X.; Van Den Berg, D.; Matullo, G.; Baris, D.; et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat. Genet. 2010, 42, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Lim, J.S.; Jeon, B.H. Chapter 3—Pathophysiology of Bladder Cancer. In Bladder Cancer; Ku, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Cortina, C.; Turon, G.; Stork, D.; Hernando-Momblona, X.; Sevillano, M.; Aguilera, M.; Tosi, S.; Merlos-Suarez, A.; Stephan-Otto Attolini, C.; Sancho, E.; et al. A genome editing approach to study cancer stem cells in human tumors. EMBO Mol. Med. 2017, 9, 869–879. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; KMortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Takeishi, S.; Nakayama, K.I. To wake up cancer stem cells, or to let them sleep, that is the question. Cancer Sci. 2016, 107, 875–881. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yu, J.; Shi, J.; Wang, C.; Fu, W.; Chen, Z.; Yang, J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012, 322, 70–77. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamawaki, H.; Usui, T.; Sasaki, K. Emerging Roles of Cancer Stem Cells in Bladder Cancer Progression, Tumorigenesis, and Resistance to Chemotherapy: A Potential Therapeutic Target for Bladder Cancer. Cells 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Aghaalikhani, N.; Rashtchizadeh, N.; Shadpour, P.; Allameh, A.; Mahmoodi, M. Cancer stem cells as a therapeutic target in bladder cancer. J. Cell Physiol. 2019, 234, 3197–3206. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Cui, S.; Reichner, J.S.; Mateo, R.B.; Albina, J.E. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 1994, 54, 2462–2467. [Google Scholar]
- Noman, M.Z.; Van Moer, K.; Marani, V.; Gemmill, R.M.; Tranchevent, L.O.-C.; Azuaje, F.; Muller, A.; Chouaib, S.; Thiery, J.P.; Berchem, G.; et al. CD47 is a direct target of SNAI1 and ZEB1 and its blockade activates the phagocytosis of breast cancer cells undergoing EMT. OncoImmunology 2018, 7, e1345415. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.S.; Volkmer, J.P.; Weissman, I. Cancer stem cells in bladder cancer: A revisited and evolving concept. Curr. Opin. Urol. 2010, 20, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Hofner, T.; Macher-Goeppinger, S.; Klein, C.; Schillert, A.; Eisen, C.; Wagner, S.; Rigo-Watermeier, T.; Baccelli, I.; Vogel, V.; Trumpp, A.; et al. Expression and prognostic significance of cancer stem cell markers CD24 and CD44 in urothelial bladder cancer xenografts and patients undergoing radical cystectomy. Urol. Oncol. 2014, 32, 678–686. [Google Scholar] [CrossRef]
- Manhas, J.; Bhattacharya, A.; Agrawal, S.K.; Gupta, B.; Das, P.; Deo, S.V.S.; Pal, S.; Sen, S. Characterization of cancer stem cells from different grades of human colorectal cancer. Tumor Biol. 2016, 37, 14069–14081. [Google Scholar] [CrossRef]
- Ren, F.; Sheng, W.-Q.; Du, X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef]
- Bentivegna, A.; Conconi, D.; Panzeri, E.; Sala, E.; Bovo, G.; Vigano, P.; Brunelli, S.; Bossi, M.; Tredici, G.; Strada, G.; et al. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010, 101, 416–424. [Google Scholar] [CrossRef]
- Yin, B.; Zeng, Y.; Liu, G.; Wang, X.; Wang, P.; Song, Y. MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 2934–2941. [Google Scholar]
- Verma, A.; Kapoor, R.; Mittal, R.D. Genetic Variation in CD166 Gene and Its Association with Bladder Cancer Risk in North Indian Population. Indian J. Clin. Biochem. 2017, 32, 292–300. [Google Scholar] [CrossRef]
- Ooki, A.; VandenBussche, C.J.; Kates, M.; Hahn, N.M.; Matoso, A.; McConkey, D.J.; Bivalacqua, T.J.; Hoque, M.O. CD24 regulates cancer stem cell (CSC)-like traits and a panel of CSC-related molecules serves as a non-invasive urinary biomarker for the detection of bladder cancer. Br. J. Cancer 2018, 119, 961–970. [Google Scholar] [CrossRef]
- Friederichs, J.; Zeller, Y.; Hafezi-Moghadam, A.; Grone, H.J.; Ley, K.; Altevogt, P. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res. 2000, 60, 6714–6722. [Google Scholar]
- Overdevest, J.B.; Thomas, S.; Kristiansen, G.; Hansel, D.E.; Smith, S.C.; Theodorescu, D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011, 71, 3802–3811. [Google Scholar] [CrossRef] [Green Version]
- Farid, R.M.; Sammour, S.A.-E.; Shehab ElDin, Z.A.; Salman, M.I.; Omran, T.I. Expression of CD133 and CD24 and their different phenotypes in urinary bladder carcinoma. Cancer Manag. Res. 2019, 11, 4677–4690. [Google Scholar] [CrossRef] [Green Version]
- Hacek, J.; Brisuda, A.; Babjuk, M.; Zamecnik, J. Expression of cancer stem cells markers in urinary bladder urothelial carcinoma and its precursor lesions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2021, 165, 316–321. [Google Scholar] [CrossRef]
- Krause, D.S.; Lazarides, K.; von Andrian, U.H.; Van Etten, R.A. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 2006, 12, 1175–1180. [Google Scholar] [CrossRef]
- Huang, L.-R.; Hsu, H.-C. Cloning and Expression of <em>CD24</em> Gene in Human Hepatocellular Carcinoma: A Potential Early Tumor Marker Gene Correlates with <em>p53</em> Mutation and Tumor Differentiation. Cancer Res. 1995, 55, 4717. [Google Scholar] [PubMed]
- Abramson, C.S.; Kersey, J.H.; LeBien, T.W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J. Immunol. 1981, 126, 83. [Google Scholar] [PubMed]
- Senner, V.; Sturm, A.; Baur, I.; Schrell, U.H.; Distel, L.; Paulus, W. CD24 promotes invasion of glioma cells in vivo. J. Neuropathol. Exp. Neurol. 1999, 58, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.C.; Oxford, G.; Wu, Z.; Nitz, M.D.; Conaway, M.; Frierson, H.F.; Hampton, G.; Theodorescu, D. The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res. 2006, 66, 1917–1922. [Google Scholar] [CrossRef] [Green Version]
- Bretz, N.P.; Salnikov, A.V.; Perne, C.; Keller, S.; Wang, X.; Mierke, C.T.; Fogel, M.; Erbe-Hofmann, N.; Schlange, T.; Moldenhauer, G.; et al. CD24 controls Src/STAT3 activity in human tumors. Cell. Mol. Life Sci. 2012, 69, 3863–3879. [Google Scholar] [CrossRef]
- Kristiansen, G.; Sammar, M.; Altevogt, P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J. Mol. Histol. 2004, 35, 255–262. [Google Scholar] [CrossRef]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef] [Green Version]
- Vachon, P.H. Integrin signaling, cell survival, and anoikis: Distinctions, differences, and differentiation. J. Signal Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef] [Green Version]
- Wantoch von Rekowski, K.; König, P.; Henze, S.; Schlesinger, M.; Zawierucha, P.; Januchowski, R.; Bendas, G. The Impact of Integrin-Mediated Matrix Adhesion on Cisplatin Resistance of W1 Ovarian Cancer Cells. Biomolecules 2019, 9, 788. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Jin, W.; Xiao, W.; Deng, B.; Wu, D.; Zhi, J.; Wu, K.; Cao, X.; Chen, S.; Ding, Y.; et al. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am. J. Cancer Res. 2019, 9, 2774–2788. [Google Scholar]
- Sasaki, N.; Toyoda, M.; Yoshimura, H.; Matsuda, Y.; Arai, T.; Takubo, K.; Aida, J.; Ishiwata, T. H19 long non-coding RNA contributes to sphere formation and invasion through regulation of CD24 and integrin expression in pancreatic cancer cells. Oncotarget 2018, 9, 34719–34734. [Google Scholar] [CrossRef] [Green Version]
- Santini, M.T.; Rainaldi, G.; Indovina, P.L. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit. Rev. Oncol./Hematol. 2000, 36, 75–87. [Google Scholar] [CrossRef]
- Green, M.; Filippou, A.; Sant, G.; Theoharides, T.C. Expression of intercellular adhesion molecules in the bladder of patients with interstitial cystitis. Urology 2004, 63, 688–693. [Google Scholar] [CrossRef]
- Coskun, U.; Sancak, B.; Sen, I.; Bukan, N.; Tufan, M.A.; Gulbahar, O.; Sozen, S. Serum P-selectin, soluble vascular cell adhesion molecule-I (s-VCAM-I) and soluble intercellular adhesion molecule-I (s-ICAM-I) levels in bladder carcinoma patients with different stages. Int. Immunopharmacol. 2006, 6, 672–677. [Google Scholar] [CrossRef]
- Parlato, M.; Souza-Fonseca-Guimaraes, F.; Philippart, F.O.; Misset, B.T.; Captain Study, G.; Adib-Conquy, M.; Cavaillon, J.-M. CD24-Triggered Caspase-Dependent Apoptosis via Mitochondrial Membrane Depolarization and Reactive Oxygen Species Production of Human Neutrophils Is Impaired in Sepsis. J. Immunol. 2014, 192, 2449. [Google Scholar] [CrossRef] [Green Version]
- Runz, S.; Mierke, C.T.; Joumaa, S.; Behrens, J.; Fabry, B.; Altevogt, P. CD24 induces localization of β1 integrin to lipid raft domains. Biochem. Biophys. Res. Commun. 2008, 365, 35–41. [Google Scholar] [CrossRef]
- Yang, S.H.; Hu, M.H.; Wu, C.C.; Chen, C.W.; Sun, Y.H.; Yang, K.C. CD24 expression indicates healthier phenotype and less tendency of cellular senescence in human nucleus pulposus cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3021–3028. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.J.; Fleming, J.M.; Ali, M.A.; Pesesky, M.W.; Ginsburg, E.; Vonderhaar, B.K. Dynamic regulation of CD24 and the invasive, CD44posCD24neg phenotype in breast cancer cell lines. Breast Cancer Res. 2009, 11, R82. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Amend, B.; Todenhöfer, T.; Lipke, N.; Aicher, W.K.; Montes-Mojarro, I.A.; Fend, F.; Stenzl, A.; Harland, N. Rothelial carcinoma cells organoids reveal eminent differences in drug sensitivities towards Cisplatin, VTX, and S63 when compared to two-dimensional culture systems. Int. J. Mol. Sci. 2022. submitted. [Google Scholar]
- Overdevest, J.B.; Knubel, K.H.; Duex, J.E.; Thomas, S.; Nitz, M.D.; Harding, M.A.; Smith, S.C.; Frierson, H.F.; Conaway, M.; Theodorescu, D. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl. Acad. Sci. USA 2012, 109, E3588–E3596. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, Y.; Gao, J.; Lian, X.; Wang, Y. The clinicopathological and prognostic value of CD44 expression in bladder cancer: A study based on meta-analysis and TCGA data. Bioengineered 2020, 11, 572–581. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Vanderlinden, L.; Joshi, M.; Chauca-Diaz, A.; Owens, C.; Hansel, D.E.; Sempeck, C.; Ghosh, D.; Theodorescu, D. Androgen Receptor Regulates CD44 Expression in Bladder Cancer. Cancer Res. 2021, 81, 2833–2846. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Mori, T.; Uchide, T.; Azakami, D.; Fukushima, R.; Yoshida, T.; et al. Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture. Sci. Rep. 2020, 10, 9393. [Google Scholar] [CrossRef]
- Elbadawy, M.; Usui, T.; Mori, T.; Tsunedomi, R.; Hazama, S.; Nabeta, R.; Uchide, T.; Fukushima, R.; Yoshida, T.; Shibutani, M.; et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019, 110, 2806–2821. [Google Scholar] [CrossRef]
- Schneider, A.K.; Chevalier, M.F.; Derre, L. The multifaceted immune regulation of bladder cancer. Nat. Rev. Urol. 2019, 16, 613–630. [Google Scholar] [CrossRef]
- Morimoto, J.; Matsumoto, M.; Miyazawa, R.; Yoshida, H.; Tsuneyama, K.; Matsumoto, M. Aire suppresses CTLA-4 expression from the thymic stroma to control autoimmunity. Cell Rep. 2022, 38, 110384. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef] [Green Version]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef] [Green Version]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Elbadawy, M.; Sato, Y.; Mori, T.; Goto, Y.; Hayashi, K.; Yamanaka, M.; Azakami, D.; Uchide, T.; Fukushima, R.; Yoshida, T.; et al. Anti-tumor effect of trametinib in bladder cancer organoid and the underlying mechanism. Cancer Biol. Ther. 2021, 22, 357–371. [Google Scholar] [CrossRef]
- Medle, B.; Sjjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M.; Bernardo, C. Patient-Derived Bladder Cancer Organoid Models in Tumor Biology and Drug Testing: A Systematic Review. Cancers 2022, 14, 2062. [Google Scholar] [CrossRef]
- Sawodny, O.; Stenzl, A. Intraoperative Multisensory Tissue Differnetiation in Oncology. Available online: https://www.grk2543.uni-stuttgart.de/en/ (accessed on 1 April 2022).
- Hoffmann, M.J.; Koutsogiannouli, E.; Skowron, M.A.; Pinkerneil, M.; Niegisch, G.; Brandt, A.; Stepanow, S.; Rieder, H.; Schulz, W.A. The New Immortalized Uroepithelial Cell Line HBLAK Contains Defined Genetic Aberrations Typical of Early Stage Urothelial Tumors. Bladder Cancer 2016, 2, 449–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, I.M.; Dimke, H.; Frische, S. A single simple procedure for dewaxing, hydration and heat-induced epitope retrieval (HIER) for immunohistochemistry in formalin fixed paraffin-embedded tissue. Eur. J. Histochem. 2015, 59, 2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Short Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Rasmussen, R.; Morrison, T.; Herrmann, M.; Wittwer, C. Quantitative PCR by contiuous fluorescence monitoring of a double strand DNA specific binding dye. Biochimica 1998, 2, 8–11. [Google Scholar]
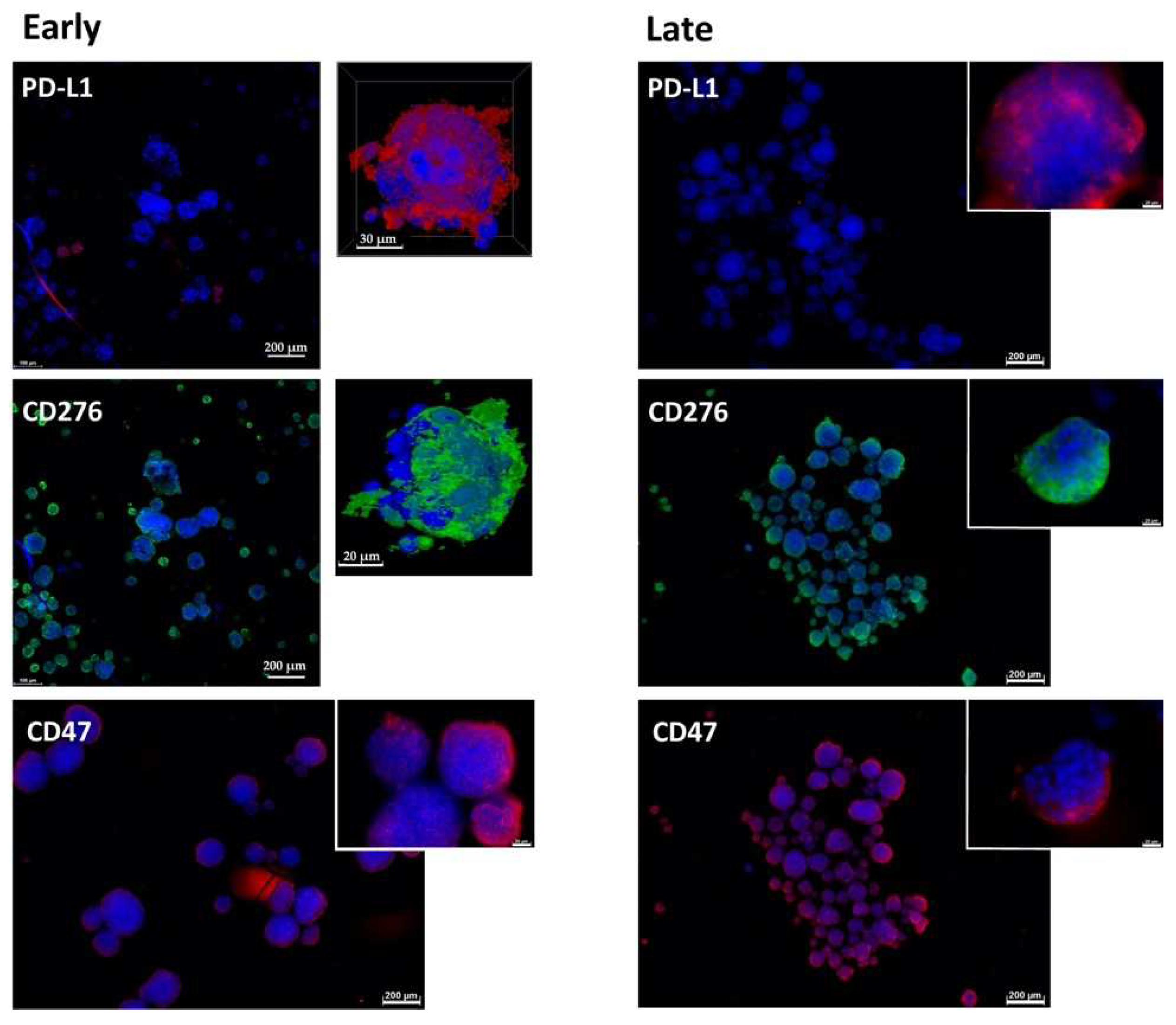
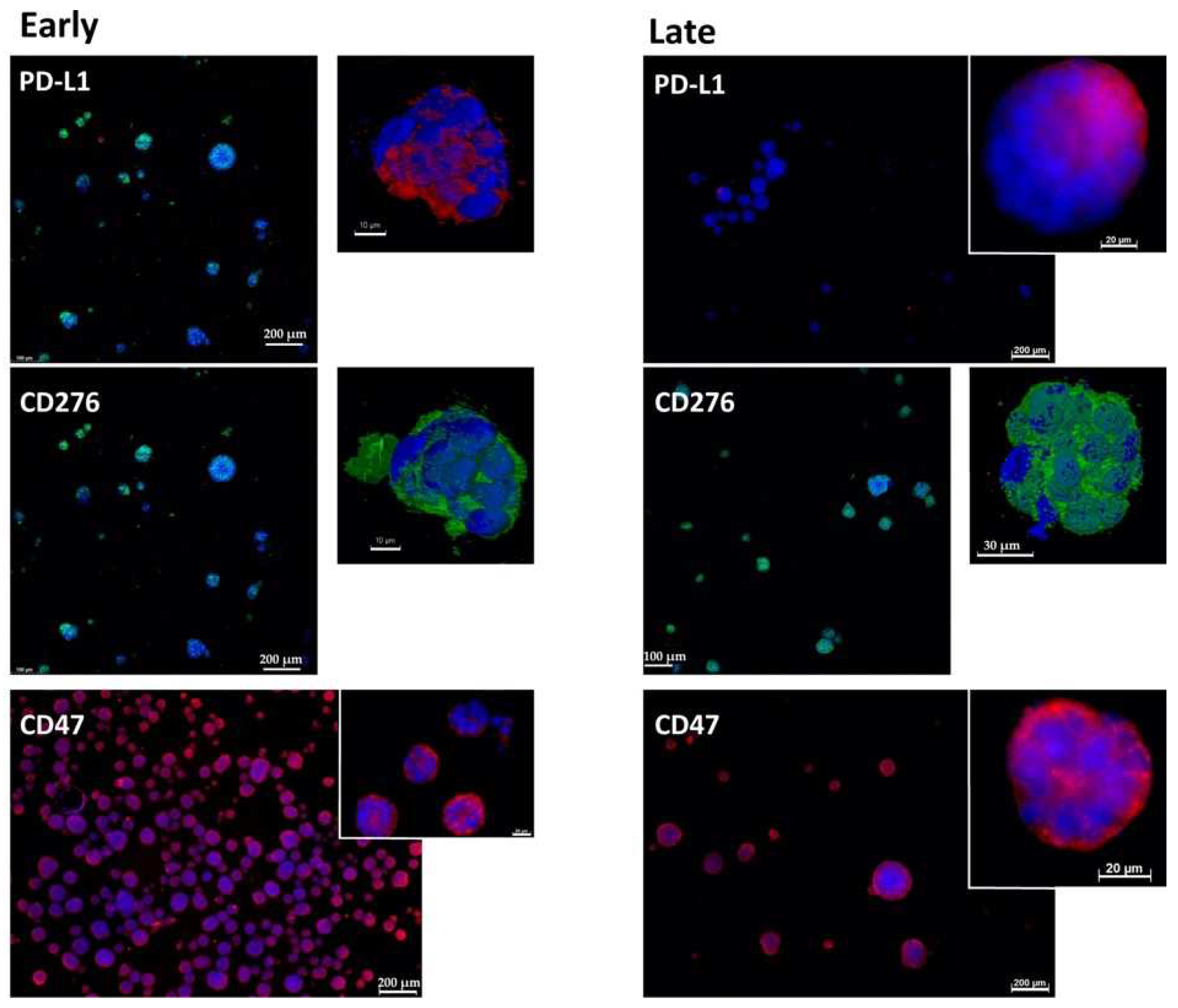
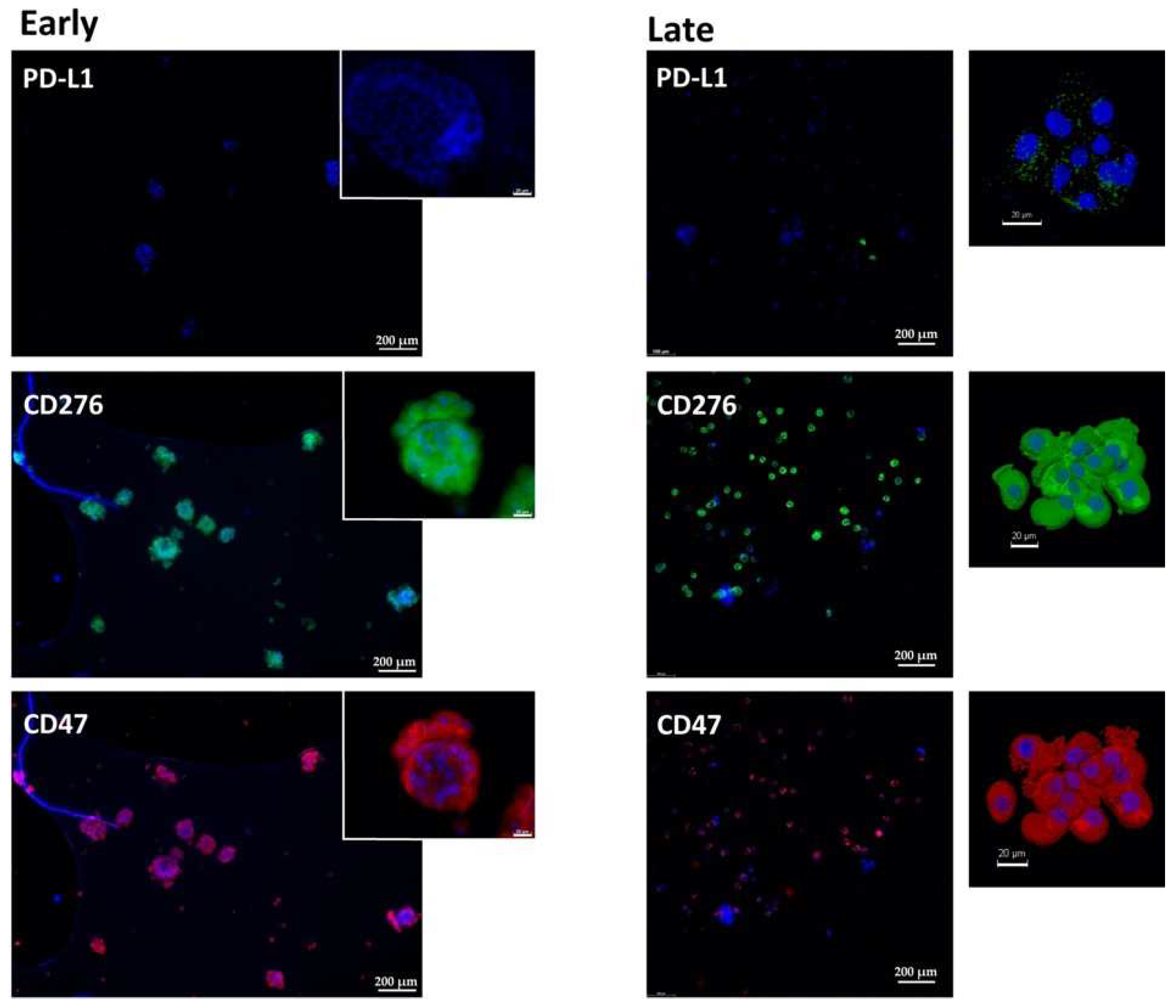
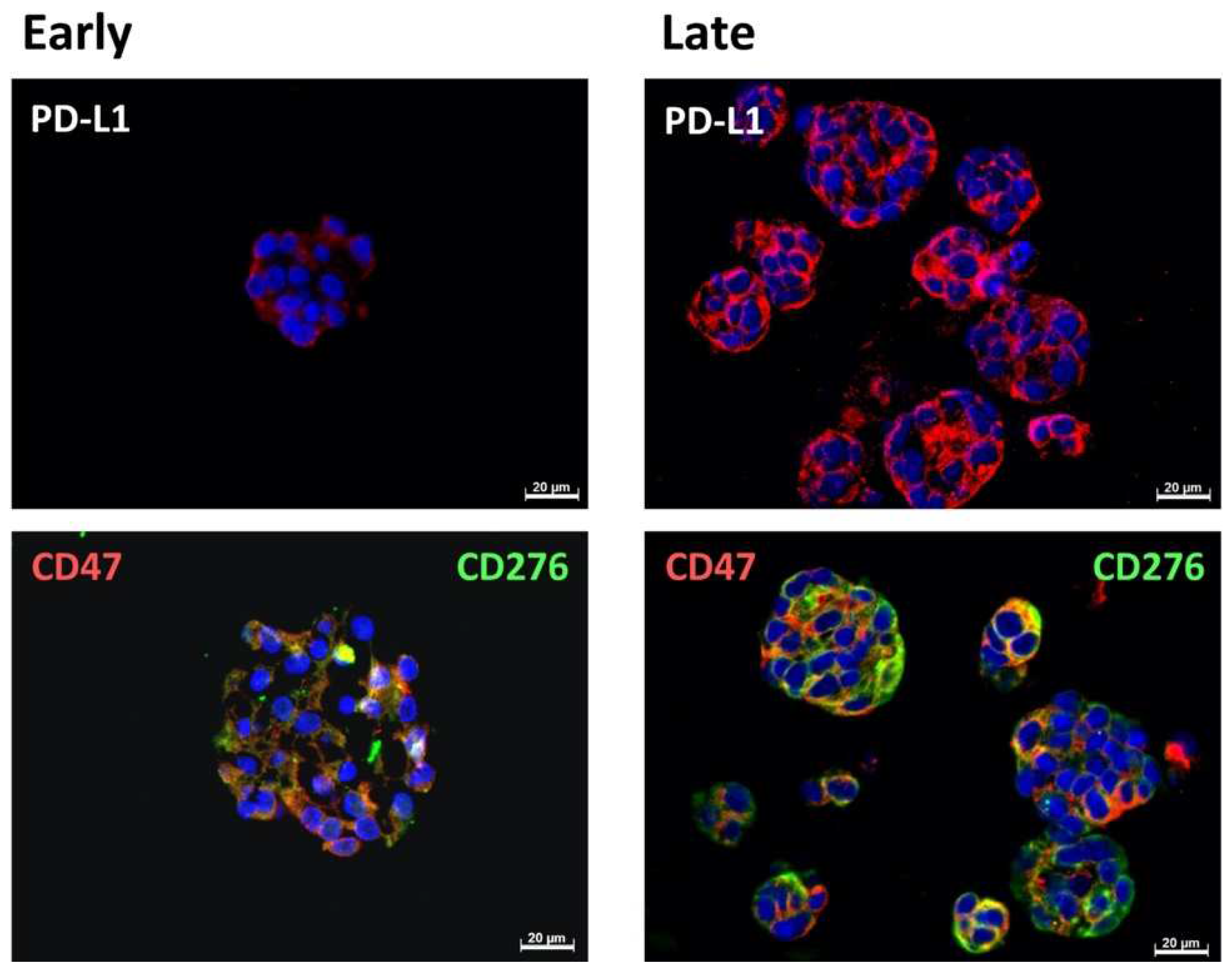
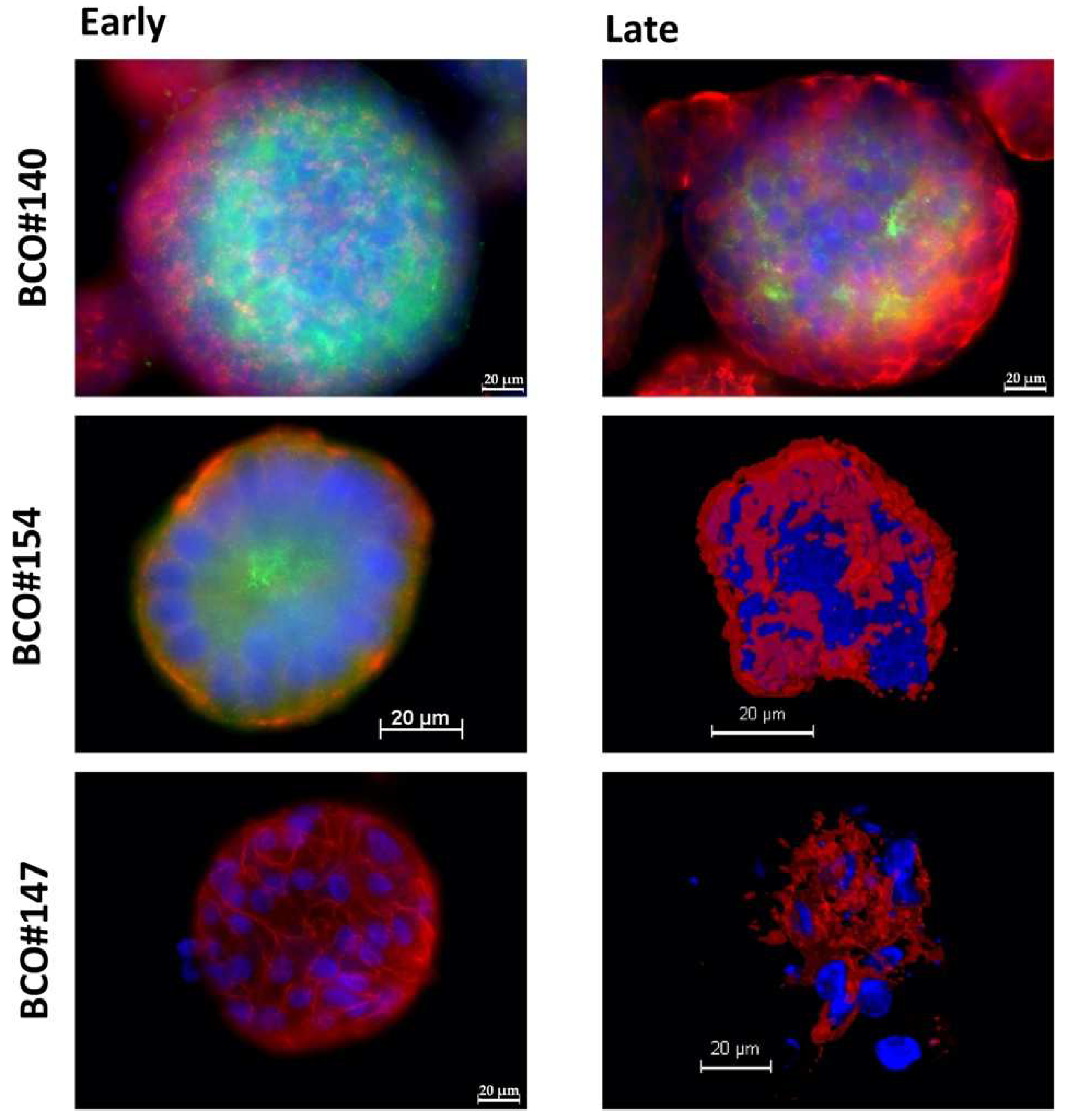
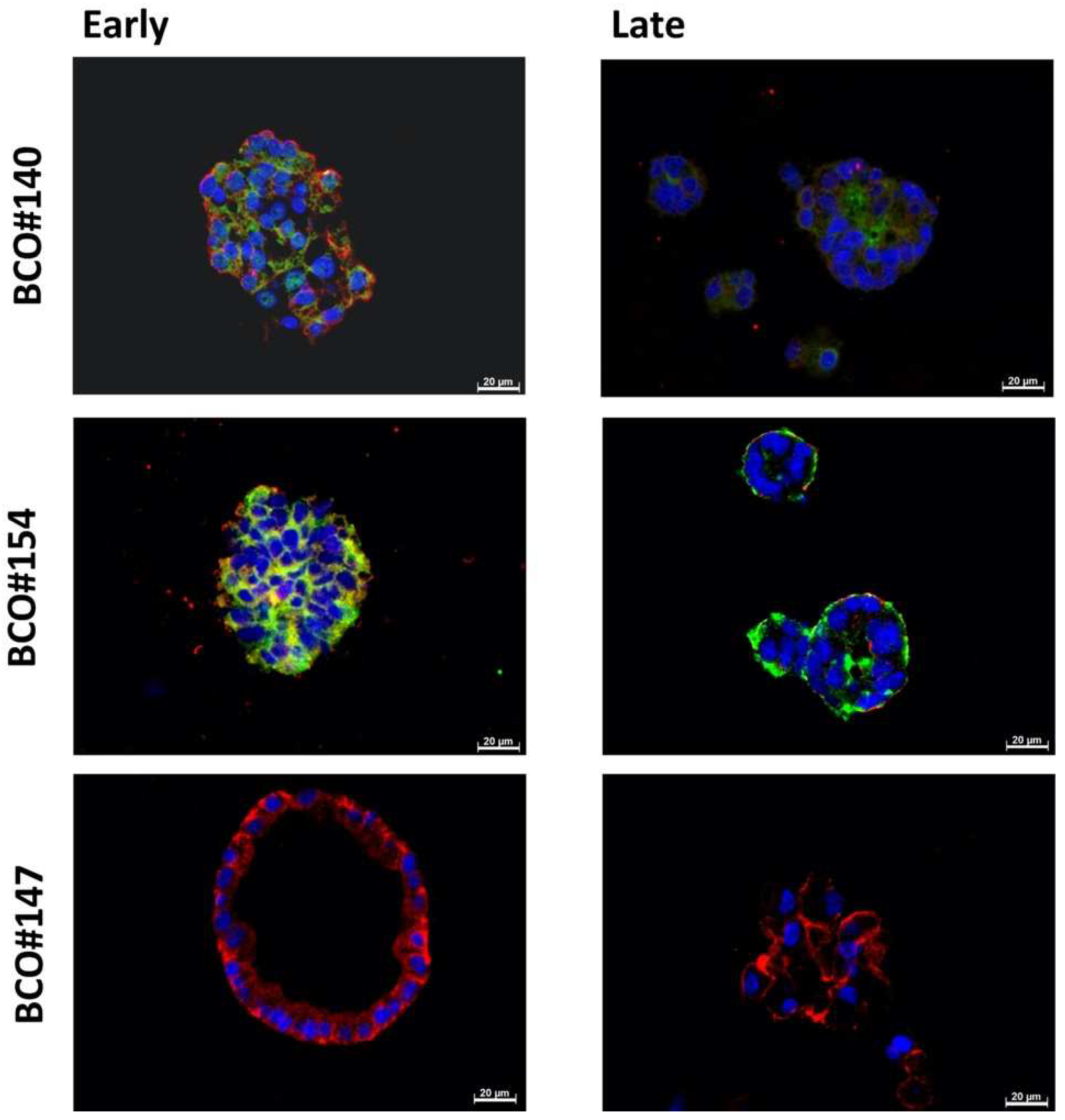
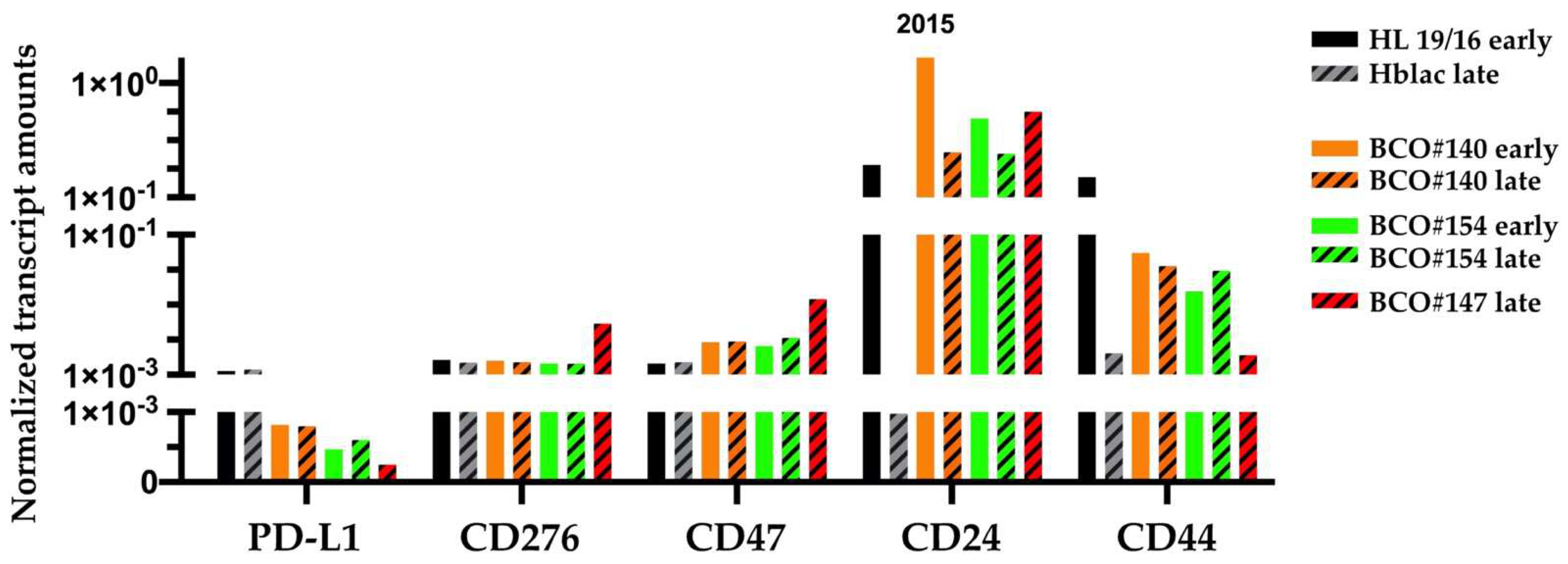
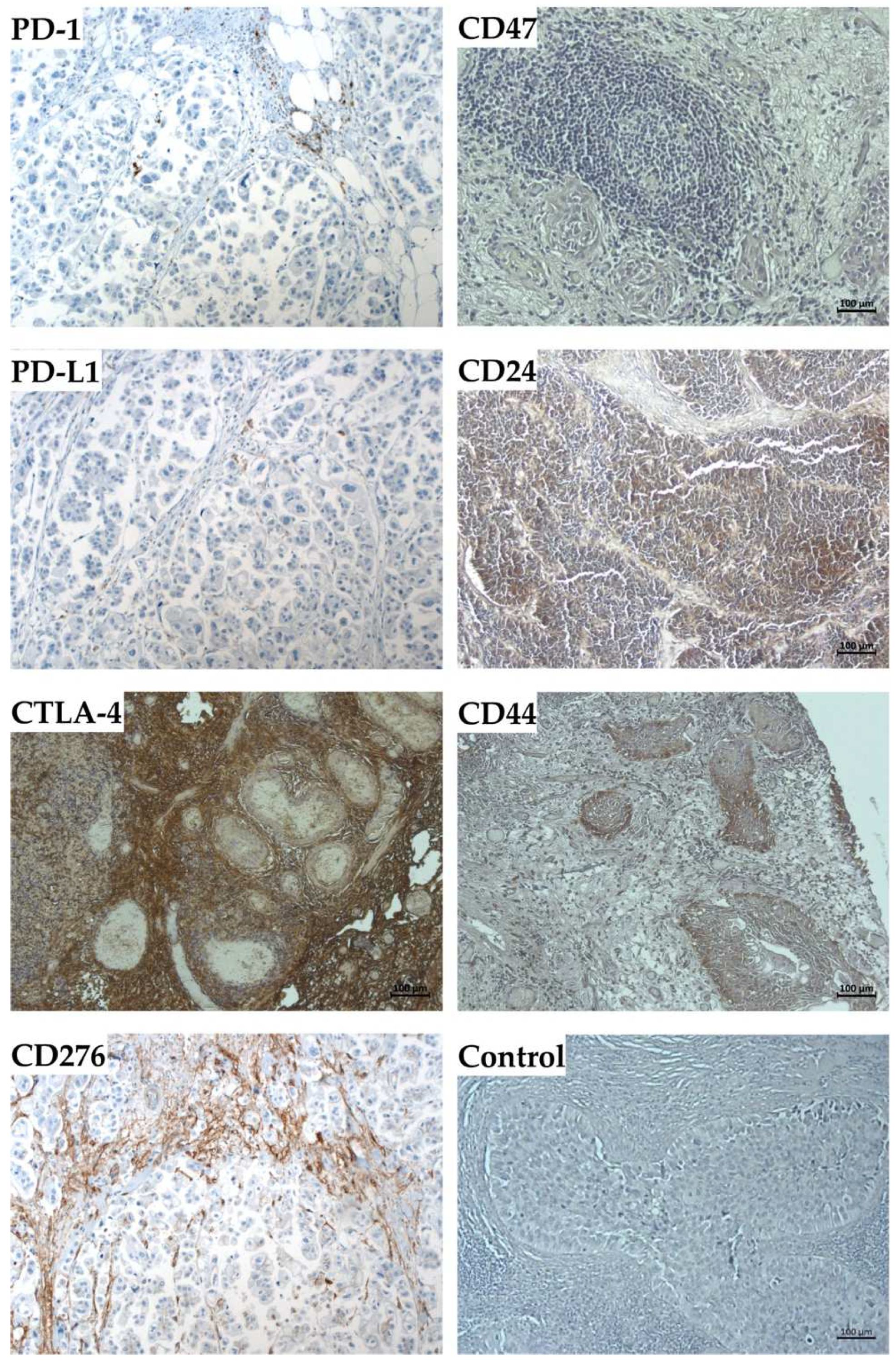
| Patient | #140 | #147 | #154 |
|---|---|---|---|
| Age (years) | 64 | 61 | 64 |
| Gender | male | male | male |
| Localization | bladder | renal pelvis | bladder |
| Tumor Stage (pT) Tumor Grade (G) | pT1 G3, high grade | pT4 G3 high grade | pT2 G3 high grade |
| Surgery | TURBT 1 | RNU 1 | RC 1 |
| Histology | Small cell carcinoma | Urothelial carcinoma | Urothelial carcinoma |
| Cell | Passage | PD-L1 | CD 276 | CD 47 |
|---|---|---|---|---|
| BCO#140 | Early | + | 3+ | 3+ |
| Late | +/− | 3+ | 3+ | |
| BCO#154 | Early | +/− | 3+ | 3+ |
| Late | +/− | 3+ | 3+ | |
| BCO#147 | Early | − | 3+ | 3+ |
| Late | +/− | 3+ | 3+ |
| Cell | Passage | PD-L1 | CD 276 | CD 47 |
|---|---|---|---|---|
| BCO#140 | Early | pos | pos | pos |
| Late | pos | pos | pos | |
| BCO#154 | Early | pos | pos | pos |
| Late | neg | pos | pos | |
| BCO#147 | Early | neg | pos | pos |
| Late | pos | pos | pos |
| Cell | Passage | CD 24 | CD 44 |
|---|---|---|---|
| BCO#140 | Early | 2+ | 3+ |
| Late | 2+ | 3+ | |
| BCO#154 | Early | + | 3+ |
| Late | − | 3+ | |
| BCO#147 | Early | − | 3+ |
| Late | − | 3+ |
| Cell | Passage | CD 24 | CD 44 |
|---|---|---|---|
| BCO#140 | Early | pos | pos |
| Late | pos | pos | |
| BCO#154 | Early | pos | pos |
| Late | pos | pos | |
| BCO#147 | Early | neg | pos |
| Late | neg | pos |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, R.; Harland, N.; Montes-Mojarro, I.A.; Fend, F.; Aicher, W.K.; Stenzl, A.; Amend, B. CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro? Int. J. Mol. Sci. 2022, 23, 5453. https://doi.org/10.3390/ijms23105453
Geng R, Harland N, Montes-Mojarro IA, Fend F, Aicher WK, Stenzl A, Amend B. CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro? International Journal of Molecular Sciences. 2022; 23(10):5453. https://doi.org/10.3390/ijms23105453
Chicago/Turabian StyleGeng, Ruizhi, Niklas Harland, Ivonne A. Montes-Mojarro, Falko Fend, Wilhelm K. Aicher, Arnulf Stenzl, and Bastian Amend. 2022. "CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro?" International Journal of Molecular Sciences 23, no. 10: 5453. https://doi.org/10.3390/ijms23105453
APA StyleGeng, R., Harland, N., Montes-Mojarro, I. A., Fend, F., Aicher, W. K., Stenzl, A., & Amend, B. (2022). CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro? International Journal of Molecular Sciences, 23(10), 5453. https://doi.org/10.3390/ijms23105453










