Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes
Abstract
:1. Introduction
2. Results
2.1. Effects of pCBs on Viability, Apoptosis and Cell Cycle of Human HaCaT Cells
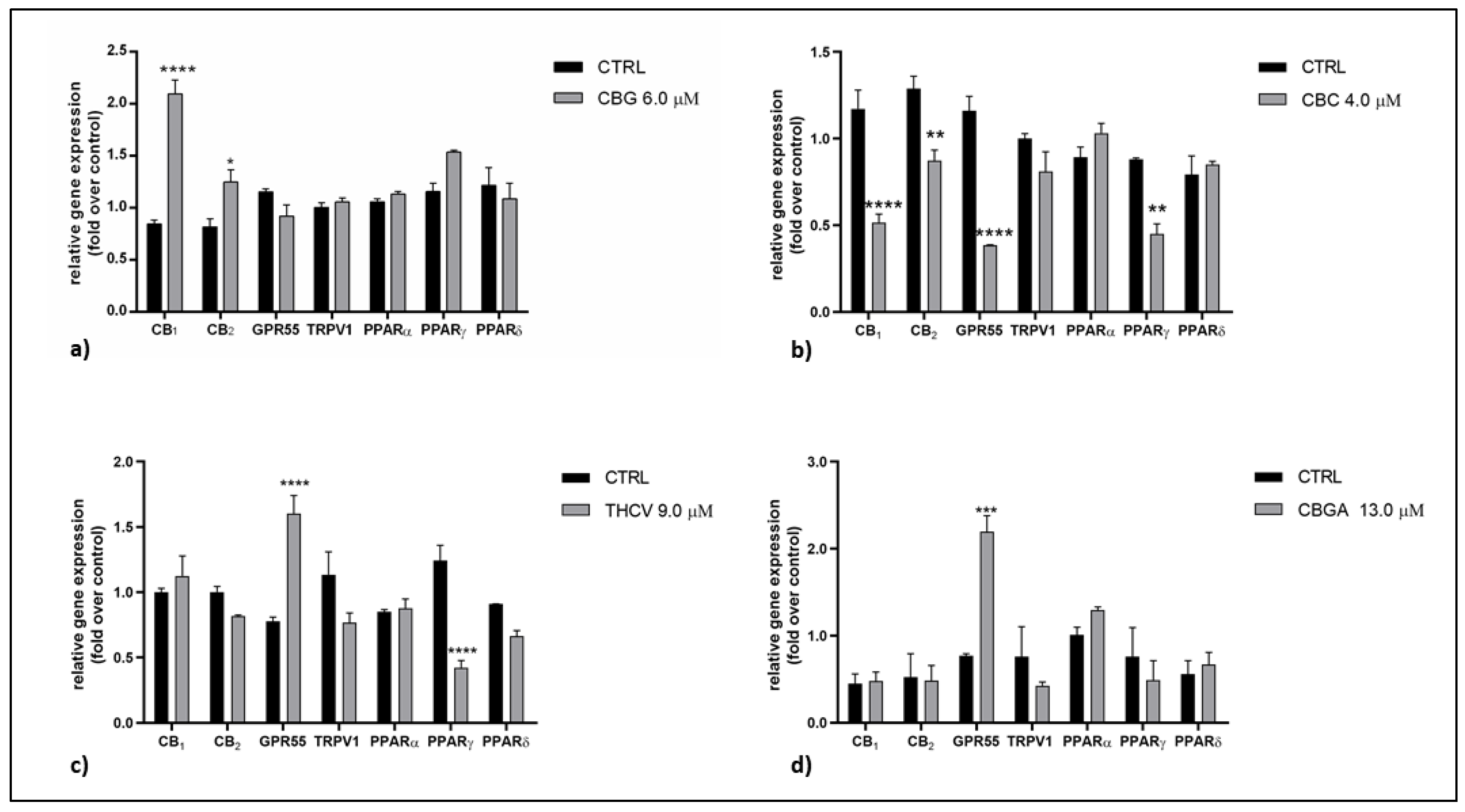
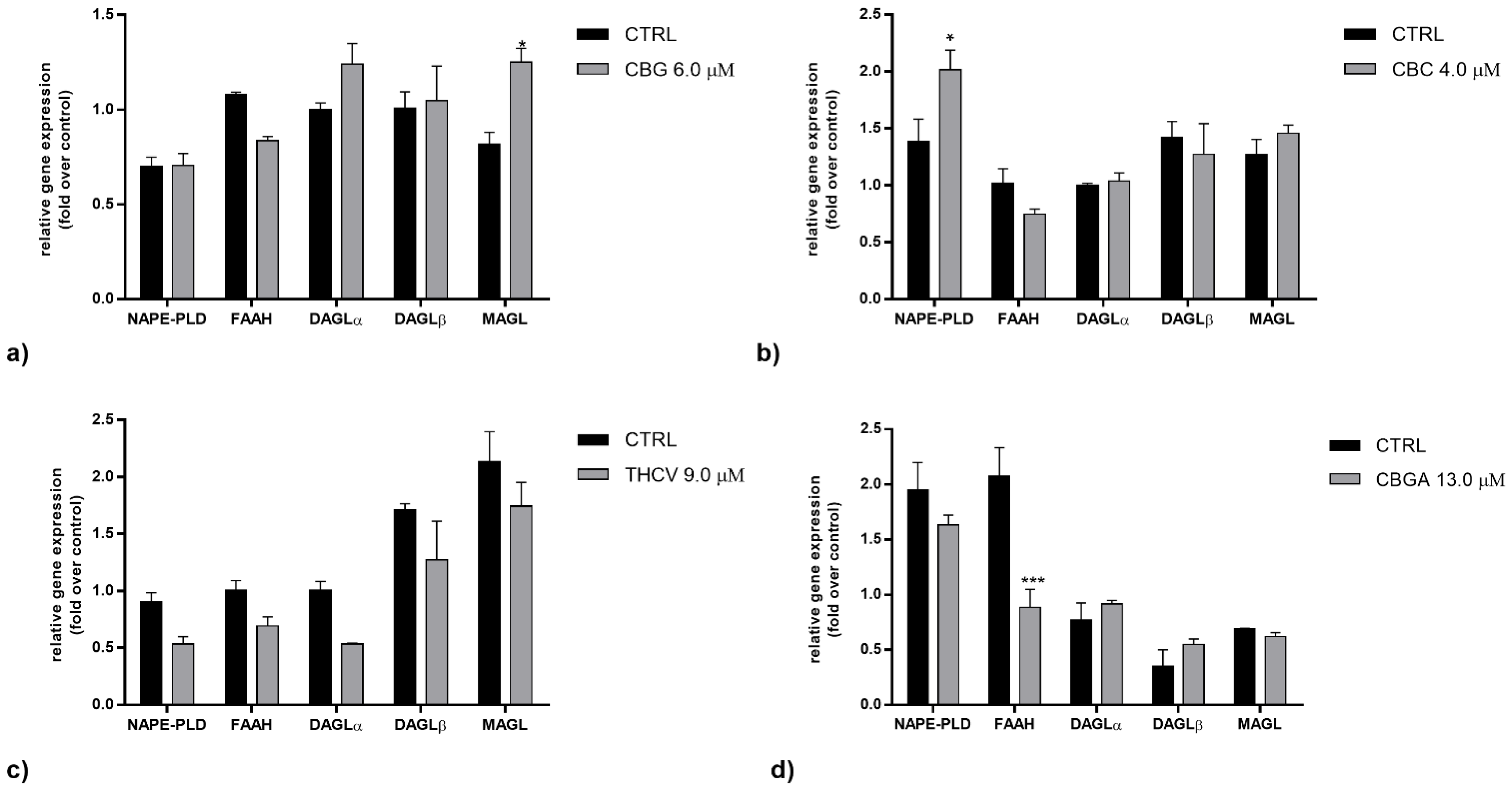
2.2. Effects of pCBs on Gene Expression of ECS Elements in HaCaT Cells
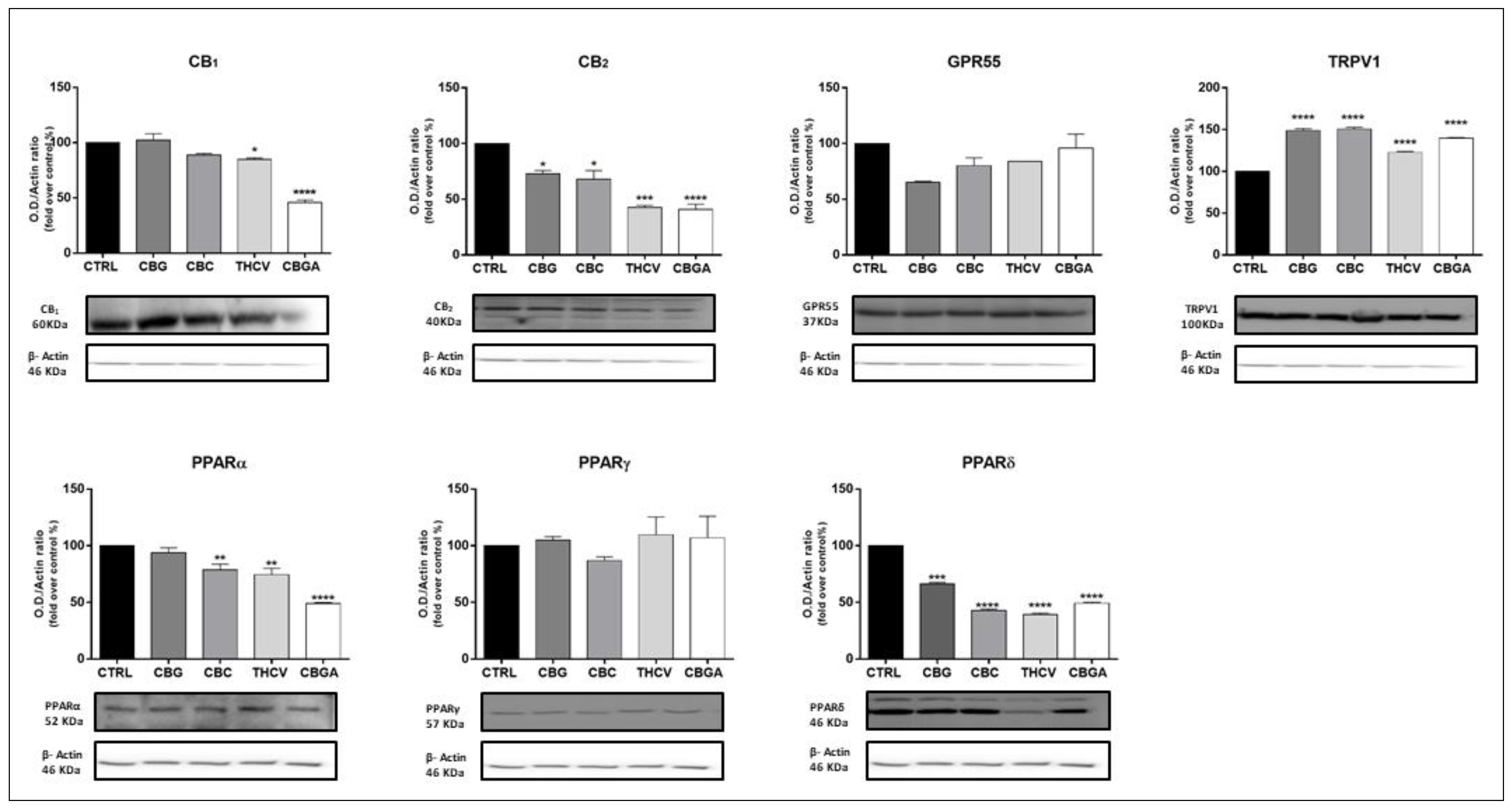
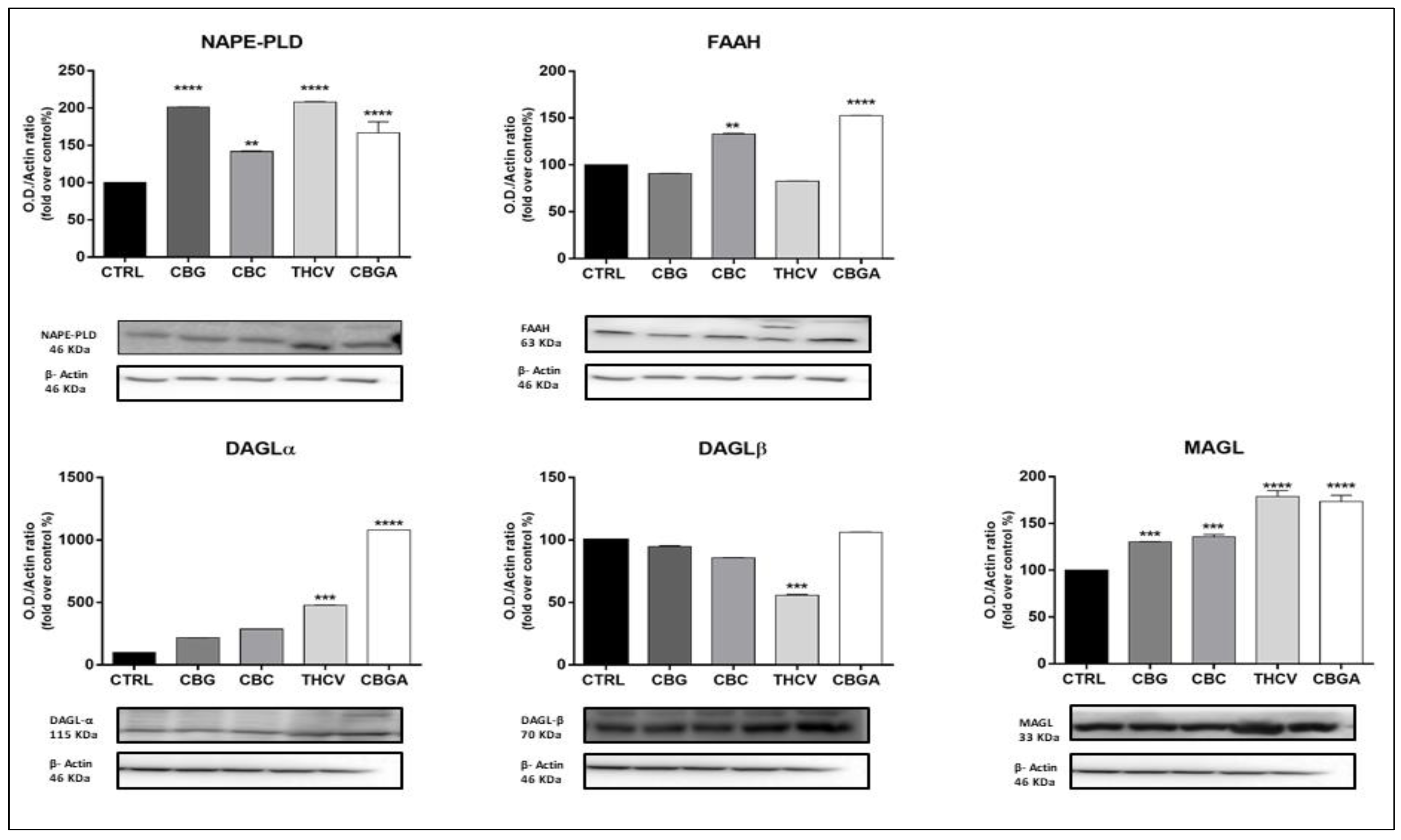
2.3. Effects of pCBs on Protein Expression of ECS Elements in HaCaT Cells
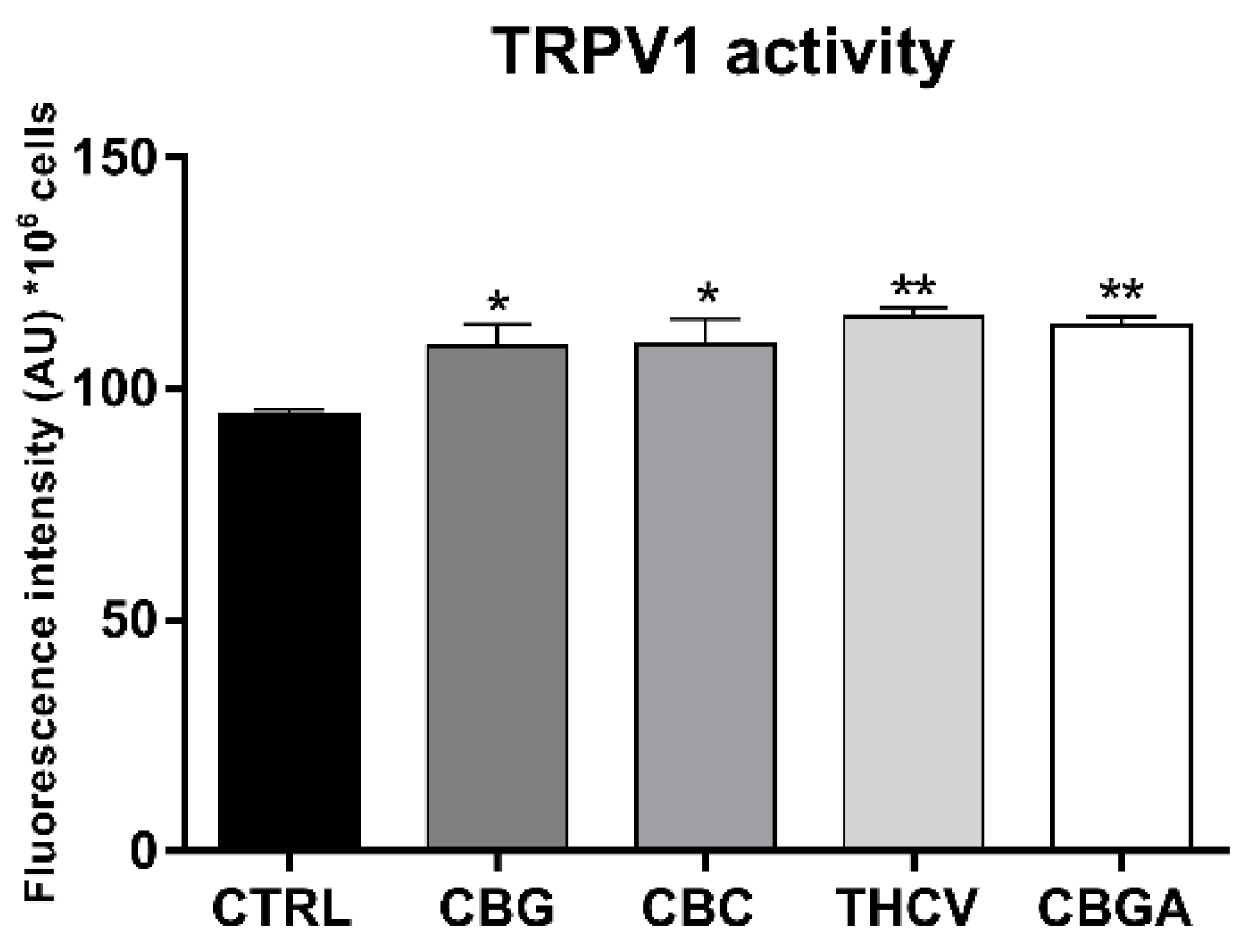
2.4. Effects of pCBs on Functional Activity of ECS Elements in HaCaT Cells
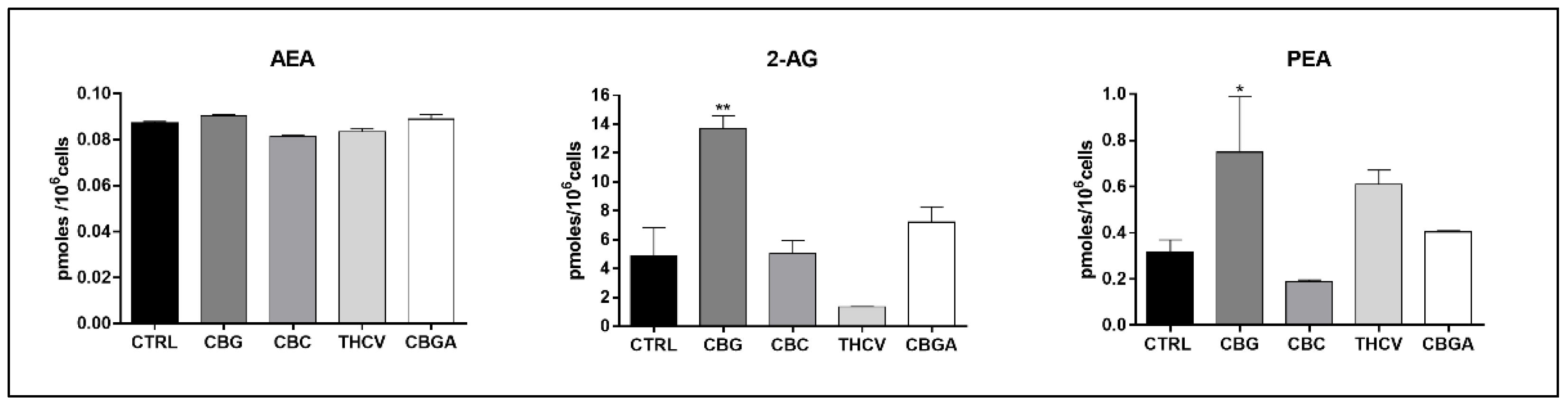
2.5. Endogenous Content of eCBs in HaCaT Cells Treated with pCBs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line and Treatment
4.3. MTT Cytotoxicity Assay
4.4. Determination of Apoptosis
4.5. Cell Cycle Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Western Blotting
4.8. Receptor Binding Assay on Adherent Living Cells
4.9. TRPV1 Calcium Assay
4.10. Enzyme Assays
4.11. Quantitation of Endogenous Levels of eCBs and PEA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElSohly, M.A.; Radwan, M.M.; Waseem, G.; Suman, C.; Ahmed, G. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [PubMed]
- Radwan, M.M.; Suman, C.; Shahbaz, G.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, F.; Nanda, S.; Mohanty, S.; Dalai, A.K.; Kumar, V.; Ponnusamy, S.K.; Naik, S. Cannabis: Chemistry, extraction and therapeutic applications. Chemosphere 2022, 289, 133012. [Google Scholar] [CrossRef]
- Protti, M.; Brighenti, V.; Battaglia, M.R.; Anceschi, L.; Pellati, F.; Mercolini, L. Cannabinoids from Cannabis sativa L. A New Tool Based on HPLC-DAD-MS/MS for a Rational Use in Medicinal Chemistry. ACS Med. Chem. Lett. 2019, 10, 539–544. [Google Scholar] [CrossRef]
- Menon, S. All the Highs and Lows on Marijuana. The Economic Times, 13 December 2020. [Google Scholar]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Maccarrone, M. Missing pieces to the endocannabinoid puzzle. Trends Mol. Med. 2020, 26, 263–272. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Banerjee, R.; Erridge, S.; Salazar, O.; Mangal, N.; Couch, D.; Pacchetti, B.; Sodergren, M.H. Real World Evidence in Medical Cannabis Research. Ther. Innov. Regul. Sci. 2022, 56, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Volk, J.; Stehle, F.; Kayser, O.; Warzecha, H. Subcellular localization defines modification and production of Δ9-tetrahydrocannabinolic acid synthase in transiently transformed Nicotiana benthamiana. Biotechnol. Lett. 2018, 40, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Wilson, S.; Corey, W.; Dvorakova, M.; Bosquez, T.; Tracey, J.; Wilkowski, C.; Ho, K.; Wager-Miller, J.; Mackie, K. An evaluation of understudied phytocannabinoids and their effects in two neuronal models. Molecules 2021, 26, 5352. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, R.K.; Zou, S.; Kadhim, S.; Padania, S.; Hsu, E.; Kumar, U. Cannabinol modulates neuroprotection and intraocular pressure: A potential multi-target therapeutic intervention for glaucoma. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166325. [Google Scholar] [CrossRef] [PubMed]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindila, L.; Lutz, B. Extraction and Simultaneous Quantification of Endocannabinoids and Endocannabinoid-Like Lipids in Biological Tissues. Methods Mol. Biol. 2016, 1412, 9–18. [Google Scholar]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; De Petrocellis, L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2012, 204, 267–276. [Google Scholar] [CrossRef]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Tubaro, A.; Giangaspero, A.; Sosa, S.; Negri, R.; Grassi, G.; Casano, S.; Della Loggia, R.; Appendino, G. Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia 2010, 81, 816–819. [Google Scholar] [CrossRef]
- Oddi, S.; Maccarrone, M. Phytocannabinoids and skin disorders. In Handbook of Cannabis; Pertwee, R.G., Ed.; Oxford Scholarship Online: Oxford, UK, 2014; pp. 1–19. [Google Scholar]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [Green Version]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Zákány, N.; Tiede, S.; Purba, T.; Harries, M.; Tsuruta, D.; Bíró, T.; Paus, R. Human epithelial stem cell survival within their niche requires “tonic” cannabinoid receptor 1-signalling-Lessons from the hair follicle. Exp. Dermatol. 2021, 30, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, N.; Oddi, S.; Malaponti, M.; Maccarrone, M. Regulation of gene transcription and keratinocyte differentiation by anandamide. Vitam. Horm. 2009, 81, 441–467. [Google Scholar] [PubMed]
- Szabó, I.L.; Lisztes, E.; Béke, G.; Tóth, K.F.; Paus, R.; Oláh, A.; Bíró, T. The pPhytocannabinoid (-)-cannabidiol operates as a complex, differential modulator of human hair growth: Anti-inflammatory submicromolar versus hair growth inhibitory micromolar effects. J. Investig. Dermatol. 2020, 140, 484–488.e5. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis sativa and skin health: Dissecting the role of phytocannabinoids. Planta Med. 2021. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.-J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Rapino, C.; Castellucci, A.; Lizzi, A.R.; Sabatucci, A.; Angelucci, C.B.; Tortolani, D.; Rossi, G.; D’Andrea, G.; Maccarrone, M. Modulation of Endocannabinoid-Binding Receptors in Human Neuroblastoma Cells by Tunicamycin. Molecules 2019, 24, 1432. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allara, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.; Pirazzi, V.; Pasquariello, N.; Maccarrone, M. Endocannabinoid signaling and epidermal differentiation. Eur. J. Dermatol. 2011, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Szöllősi, A.G.; Tóth, B.I.; Demaude, J.; Bouez, C.; Breton, L.; Bíró, T. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J. Investig. Dermatol. 2012, 132, 1967–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci. Rep. 2021, 11, 22494. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Glanemann, C.; Loos, A.; Gorret, N.; Willis, L.B.; O’Brien, X.M.; Lessard, P.A.; Sinskey, A.J. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: Implications for DNA microarray analysis. Appl. Microbiol. Biotechnol. 2003, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Rusconi, F.; Rubino, T.; Cattaneo, A.; Martegani, E.; Parolaro, D.; Bachi, A.; Zippel, R. Transcriptomicand proteomic analyses of mouse cerebellum reveals alter-ations in RasGRF1 expression following in vivo chronictreatment with delta 9-tetra-hydrocannabinol. J. Mol. Neurosci. 2009, 37, 111–122. [Google Scholar] [CrossRef]
- Bari, M.; Tedesco, M.; Battista, N.; Pasquariello, N.; Pucci, M.; Gasperi, V.; Scaldaferri, M.L.; Farini, D.; De Felici, M.; Maccarrone, M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 139–147. [Google Scholar] [CrossRef]
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB 1, CB 2 and CB 1/CB 2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bort, A.; Alvarado-Vazquez, P.A.; Moracho-Vilrriales, C.; Virga, K.G.; Gumina, G.; Romero-Sandoval, A.; Asbill, S. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol. Pain. 2017, 13, 1744806916688220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Caterina, M.J. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem. Neurosci. 2014, 5, 1107–1116. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savil, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, V.; Evangelista, D.; Oddi, S.; Florenzano, F.; Chiurchiù, V.; Avigliano, L.; Catani, M.V.; Maccarrone, M. Regulation of inflammation and proliferation of human bladder carcinoma cells by type-1 and type-2 cannabinoid receptors. Life Sci. 2015, 138, 41–51. [Google Scholar] [CrossRef]
- Lyon, E. Mutation detection using fluorescent hybridization probes and melting curve analysis. Expert Rev. Mol. Diagn. 2001, 1, 92–101. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, B.; Diamanti, E.; Pizzirani, D.; Tardia, P.; Maccesi, M.; Realini, N.; Magotti, P.; Garau, G.; Bakkum, T.; Rivara, S.; et al. Synthesis and characterization of the first inhibitor of Nacylphosphatidylethanolamine phospholipase D (NAPE-PLD). Chem. Commun. 2017, 53, 12814–12817. [Google Scholar] [CrossRef] [PubMed]
- Darrow, A.L.; Olson, M.W.; Xin, H.; Burke, S.L.; Smith, C.; Schalk-Hihi, C.; Williams, R.; Bayoumy, S.S.; Deckman, I.C.; Todd, M.J.; et al. A novel fluorogenic substrate for the measurement of endothelial lipase activity. J. Lipid Res. 2011, 52, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddi, S.; Bari, M.; Battista, N.; Barsacchi, D.; Cozzani, I.; Maccarrone, M. Confocal microscopy and biochemical analysis reveal spatial and functional separation between anandamide uptake and hydrolysis in human keratinocytes. Cell. Mol. Life Sci. 2005, 62, 386–395. [Google Scholar] [CrossRef]
- Oddi, S.; Caporali, P.; Dragotto, J.; Totaro, A.; Maiolati, M.; Scipioni, L.; Angelucci, C.B.; Orsini, C.; Canterini, S.; Rapino, C.; et al. The endocannabinoid system is affected by cholesterol dyshomeostasis: Insights from a murine model of Niemann Pick type C disease. Neurobiol. Dis. 2019, 130, 104531. [Google Scholar] [CrossRef]
- Sergi, M.; Battista, N.; Montesano, C.; Curini, R.; Maccarrone, M.; Compagnone, D. Determination of the two major endocannabinoids in human plasma by µ-SPE by HPLC-MS/MS. Anal. Bioanal. Chem. 2012, 405, 785–793. [Google Scholar] [CrossRef]
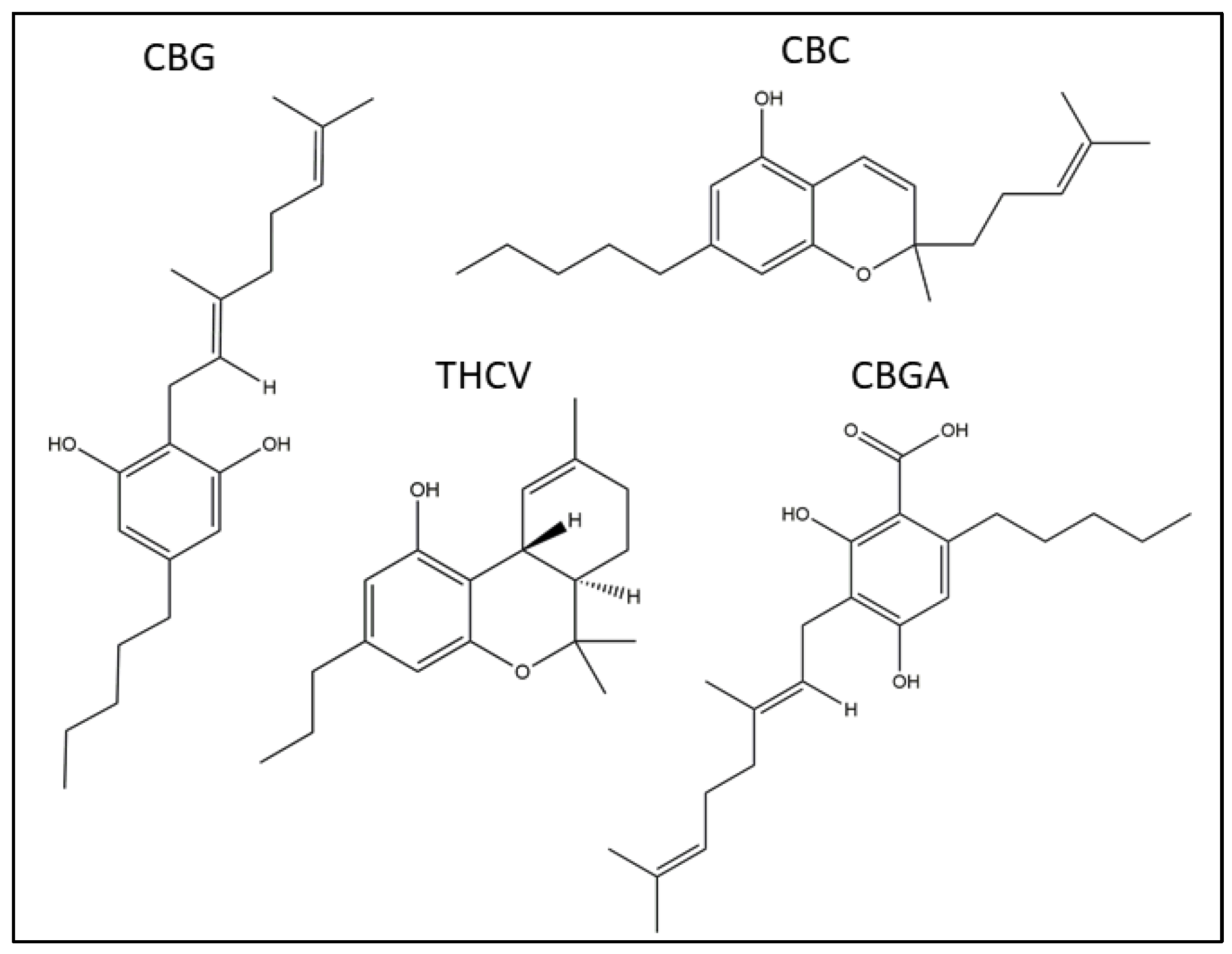

| pCB | Time Point | IC50 (µM) |
|---|---|---|
| CBG | 6 h 12 h 24 h | 13.5 13.0 11.7 |
| CBC | 6 h 12 h 24 h | 15.3 15.6 8.2 |
| THCV | 6 h 12 h 24 h | 14.3 22.4 18.6 |
| CBGA | 6 h 12 h 24 h | >25.0 24.5 25.3 |
| pCBs | Gene Expression | Protein Expression | ||
|---|---|---|---|---|
| Receptors | Enzymes | Receptors | Enzymes | |
| CBG | CB1 ↑++++ CB2 ↑+ | MAGL ↑+ | CB2 ↓+ TRPV1 ↑++++ PPARδ ↓+++ | NAPE-PLD ↑++++ MAGL ↑+++ |
| CBC | CB1 ↓++++ CB2 ↓++ GPR55 ↓++++ PPARγ ↓++ | NAPE-PLD ↑+ | CB2 ↓+ TRPV1 ↑++++ PPARα ↓++ PPARδ ↓++++ | NAPE-PLD ↑++ MAGL ↑+++ FAAH ↑++ |
| THCV | GPR55 ↑++++ PPARγ ↓++++ | CB1 ↓+ CB2 ↓+++ TRPV1 ↑++++ PPARα ↓++ PPARδ↓++++ | NAPE-PLD ↑++++ DAGLα ↑+++ DAGLβ ↓+++ MAGL ↑++++ | |
| CBGA | GPR55 ↑+++ | FAAH ↓+++ | CB1 ↓++++ CB2 ↓++++ TRPV1 ↑++++ PPARα ↓++++ PPARδ ↓++++ | NAPE-PLD ↑++++ FAAH ↑++++ DAGLα ↑++++ MAGL ↑++++ |
| HaCaT Cell Treatment | CB1/2 Binding Activity (pmol/mg of Protein) |
|---|---|
| Control | 4.033 ± 0.516 |
| CBG | 8.027 ± 0.681 * |
| CBC | 10.380 ± 0.750 ** |
| THCV | 8.187 ± 0.302 * |
| CBGA | 8.373 ± 0.410 * |
| pCB | NAPE-PLD/FAAH Activity Ratio (Mean ± SEM) | DAGLα,β/MAGL Activity Ratio (Mean ± SEM) |
|---|---|---|
| Control | 1.00 ± 0.00 | 1.00 ± 0.00 |
| CBG (6.0 µM) | 0.97 ± 0.36 | 1.33 ± 0.13 |
| CBC (4.0 µM) | 0.41 ± 0.21 | 2.09 ± 0.23 *** |
| THCV (9.0 µM) | 0.71 ± 0.30 | 1.56 ± 0.01 * |
| CBGA (13.0 µM) | 2.51 ± 1.62 | 0.40 ± 0.06 * |
| Gene | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) |
|---|---|---|
| cnr1 | CCTTTTGCTGCCTAAATCCAC | CCACTGCTCAAACATCTGAC |
| cnr2 | TCAACCCTGTCATCTATGCTC | AGTCAGTCCCAACACTCATC |
| gpr55 | ATCTACATGATCAACCTGGC | ATGAAGCAGATGGTGAAGACGC |
| trpv1 | TCACCTACATCCTCCTGCTC | AAGTTCTTCCAGTGTCTGCC |
| pparα | TGGGAAGGCAGCGTTGATTA | CTGTGTCCTTCCCACTCTCG |
| pparγ | TGATGTCTTGACTCATGGGTGT | CACGGAGCTGATCCCAAAGT |
| pparδ | AGGTTCCCCAAGAGGGAAGA | CAGGAGGAGACAGTTCCAACC |
| napepld | TTGTGAATCCGTGGCCAACATGG | TACTGCCATGGTGAAGCACG |
| Faah | CCCAATGGCTTAAAGGACTG | ATGAACCGCAGACACAAC |
| daglα | AATGGCTATCATCTGGCTGAGC | TTCCGAGGGTGACATTCTTAGC |
| daglβ | GCGCAAAGTAAACGGCAAGA | CTGCAGCTTGGGCTTTTCAT |
| Mgll | ATGCAGAAAGACTACCCTGGGC | TTATTCCGAGAGAGCACGC |
| Actb | TGACCCAGATCATGTTTGAG | TTAATGTCACGCACGATTTCC |
| gapdh | CAGCCTCAAGATCATCAGCA | TGTGGTCATGAGTCCTTCCA |
| Antibody | Diluition | Brand |
|---|---|---|
| CB1 receptor rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| CB2 receptor rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| GPR55 receptor rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| TRPV1 rabbit polyclonal | 1:1000 | OriGene (Rockville, MD, USA) |
| PPAR α rabbit polyclonal | 1:1000 | Sigma-Aldrich (St. Louis, MO, USA) |
| PPAR γ rabbit monoclonal | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) |
| PPAR δ rabbit polyclonal | 1:750 | Invitrogen (Waltham, MA, USA) |
| NAPE-PLD rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| FAAH rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| DAGL-α rabbit polyclonal | 1:1000 | Invitrogen (Waltham, MA, USA) |
| DAGL- β rabbit monoclonal | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) |
| MAGL rabbit polyclonal | 1:200 | Cayman Chemical (Ann Arbor, MI, USA) |
| β-Actin rabbit monoclonal | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meo, C.; Tortolani, D.; Standoli, S.; Angelucci, C.B.; Fanti, F.; Leuti, A.; Sergi, M.; Kadhim, S.; Hsu, E.; Rapino, C.; et al. Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. Int. J. Mol. Sci. 2022, 23, 5430. https://doi.org/10.3390/ijms23105430
Di Meo C, Tortolani D, Standoli S, Angelucci CB, Fanti F, Leuti A, Sergi M, Kadhim S, Hsu E, Rapino C, et al. Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. International Journal of Molecular Sciences. 2022; 23(10):5430. https://doi.org/10.3390/ijms23105430
Chicago/Turabian StyleDi Meo, Camilla, Daniel Tortolani, Sara Standoli, Clotilde Beatrice Angelucci, Federico Fanti, Alessandro Leuti, Manuel Sergi, Salam Kadhim, Eric Hsu, Cinzia Rapino, and et al. 2022. "Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes" International Journal of Molecular Sciences 23, no. 10: 5430. https://doi.org/10.3390/ijms23105430
APA StyleDi Meo, C., Tortolani, D., Standoli, S., Angelucci, C. B., Fanti, F., Leuti, A., Sergi, M., Kadhim, S., Hsu, E., Rapino, C., & Maccarrone, M. (2022). Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. International Journal of Molecular Sciences, 23(10), 5430. https://doi.org/10.3390/ijms23105430









