Interactions between Macrophages and Mast Cells in the Female Reproductive System
Abstract
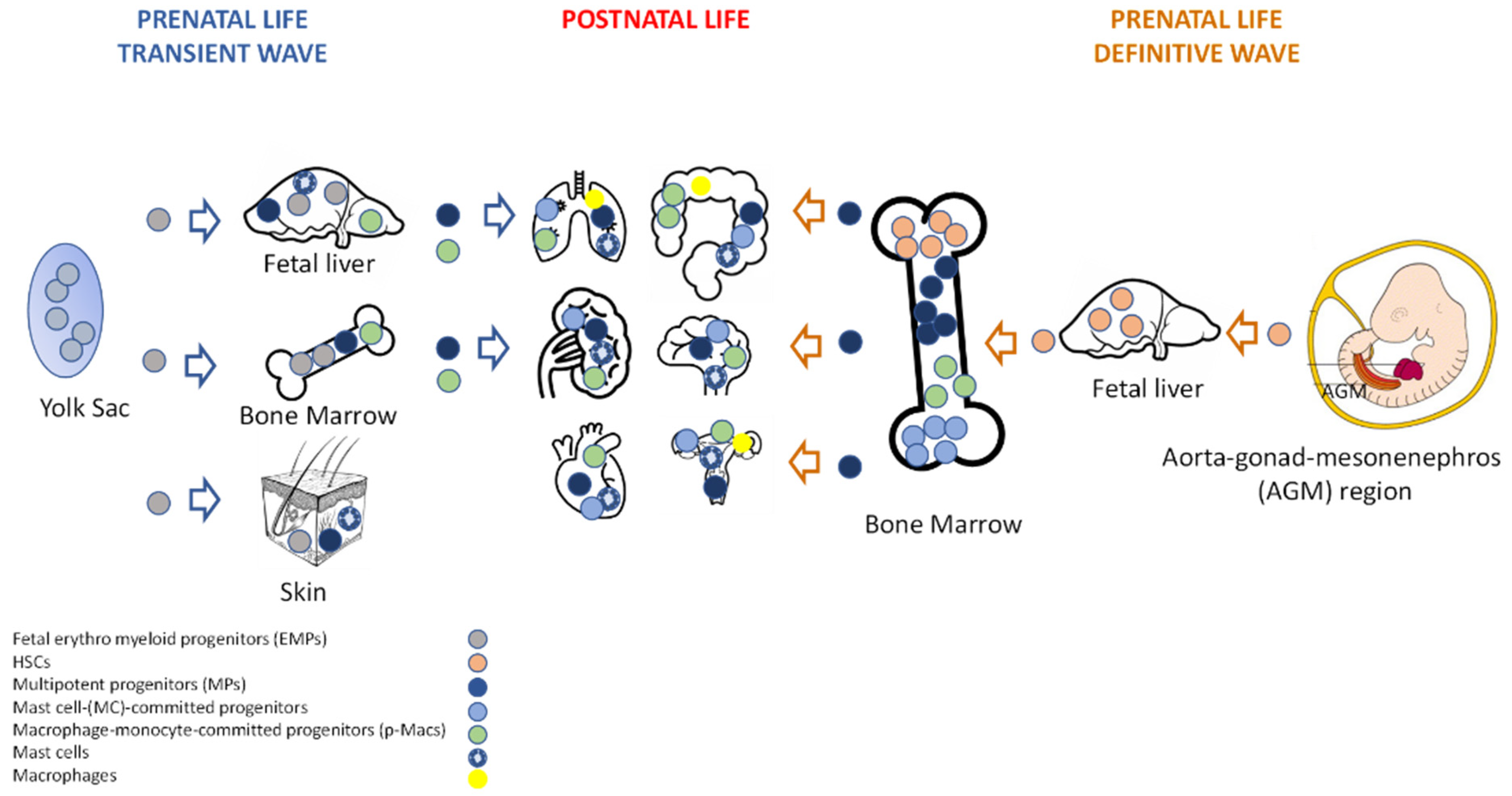
1. Development and Tissue Distribution
2. General Biological Functions
3. Physiological Role in Omentum
3.1. Peritoneum
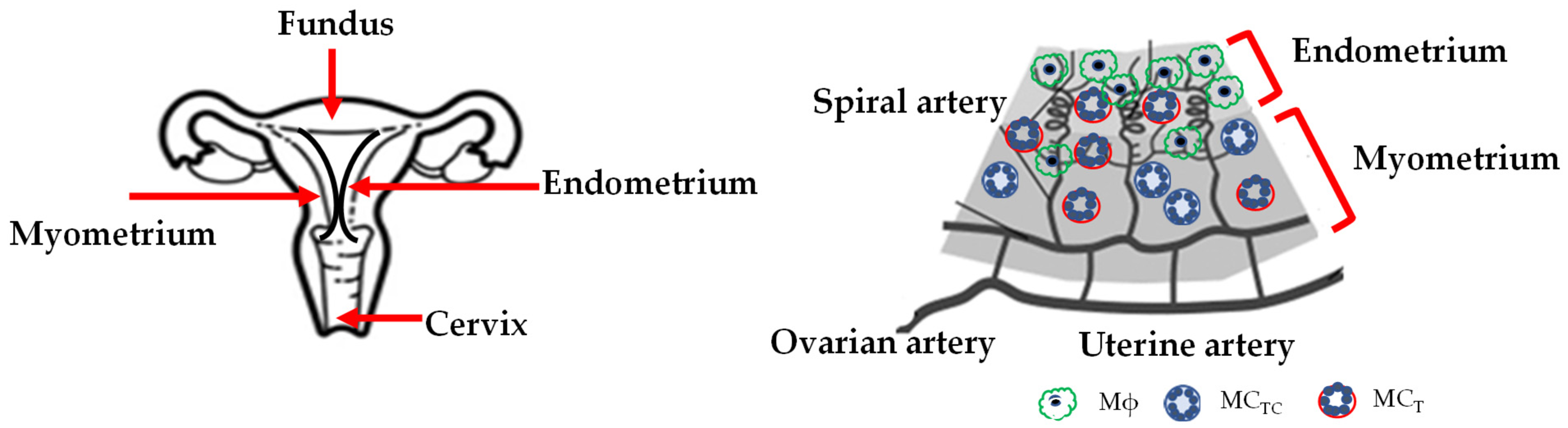
3.2. Uterus
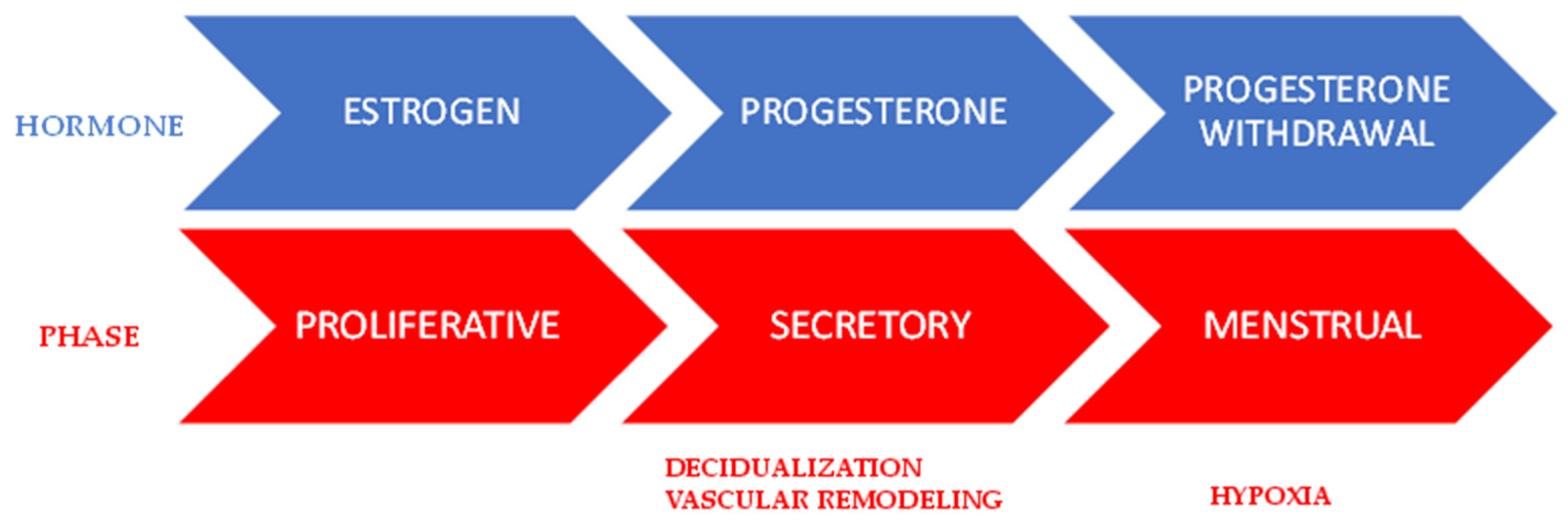
3.2.1. Proliferative Phase
| Phase | Cell | Molecules/Status | Ref. |
|---|---|---|---|
| Proliferative | uMϕ | TNF-α; IL-1β; IL-10; IL-1RA; VEGF; FGF2; PDFG; phagocytosis; CD69; CD71; CD54 | [69,70,71] |
| Proliferative | uMC | Steady state | [68] |
| Secretory | uMϕ | IL-11; activin; CD163 (70%) | [71,74,75,76] |
| Mid-late secretory | uMC | Tryptase; chymase; TNF-α; histamine; heparin | [68] |
| Menstrual repair | uMϕ | TNF-α; MMP-9; MMP-12; MMP-14 Phagocytosis; TIMP; VEGF | [64,77,78] [78] |
| Menstrual | mMC | Tryptase; chymase | [68] |
3.2.2. Secretory Phase
3.2.3. Menstrual Phase
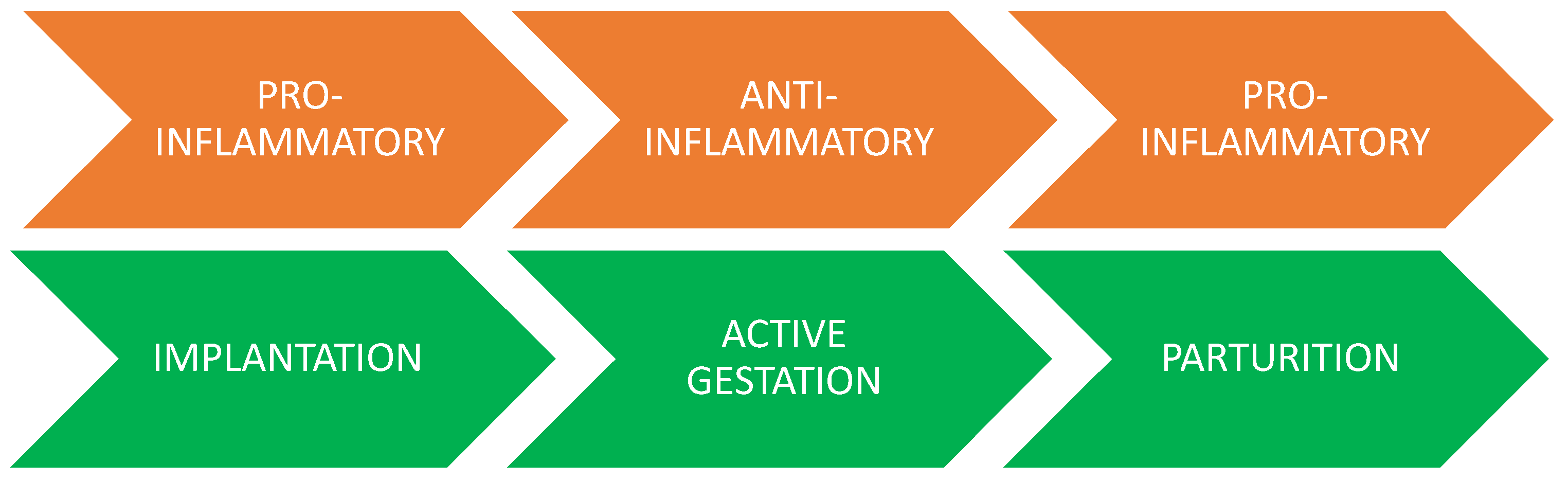
3.2.4. The Pregnant Uterus
3.3. Placenta
4. Inflammatory Roles in the Omentum
4.1. Peritoneum
4.2. Uterus
4.2.1. Endometriosis
4.2.2. Preeclampsia
5. Conclusions
6. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast Cells as Protectors of Health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed]
- Presta, I.; Donato, A.; Zaffino, P.; Spadea, M.F.; Mancuso, T.; Malara, N.; Chiefari, E.; Donato, G. Does a Polarization State Exist for Mast Cells in Cancer? Med. Hypotheses 2019, 131, 109281. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef] [PubMed]
- Tober, J.; Koniski, A.; Mcgrath, K.E.; Vemishetti, R.; Emerson, R.; de Mesy-Bentley, K.K.L.; Waugh, R.; Palis, J. The Megakaryocyte Lineage Originates from Hemangioblast Precursors and Is an Integral Component Both of Primitive and of Definitive Hematopoiesis. Blood 2006, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Guan Ng, L.; Richard Stanley, E.; et al. Supporting Online Material Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Proc. Natl. Acad. Sci. USA 2009, 136, 1168. [Google Scholar] [CrossRef]
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Günther, P.; Crozet, L.; Jacome-Galarza, C.E.; Händler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of Tissue-Resident Macrophages during Organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, Mesenchymal Stem Cells and Bone Regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef]
- Mass, E.; Gentek, R. Fetal-Derived Immune Cells at the Roots of Lifelong Pathophysiology. Front. Cell Dev. Biol. 2021, 9, 356. [Google Scholar] [CrossRef]
- Cox, N.; Pokrovskii, M.; Vicario, R.; Geissmann, F. Origins, Biology, and Diseases of Tissue Macrophages. Annu. Rev. Immunol. 2021, 39, 313–344. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.S.; Hallgren, J. Mast Cell Progenitors: Origin, Development and Migration to Tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-Resident Macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Tagliani, E.; Shi, C.; Nancy, P.; Tay, C.S.; Pamer, E.G.; Erlebacher, A. Coordinate Regulation of Tissue Macrophage and Dendritic Cell Population Dynamics by CSF-1. J. Exp. Med. 2011, 208, 1901–1916. [Google Scholar] [CrossRef]
- Akula, S.; Paivandy, A.; Fu, Z.; Thorpe, M.; Pejler, G.; Hellman, L. How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells? A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells. Cells 2020, 9, 2118. [Google Scholar] [CrossRef]
- Hou, F.; Xiao, K.; Tang, L.; Xie, L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front. Immunol. 2021, 12, 753940. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Kalesnikoff, J.; Galli, S.J. New Developments in Mast Cell Biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast Cells as a Unique Hematopoietic Lineage and Cell System: From Paul Ehrlich’s Visions to Precision Medicine Concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]
- Azzolina, A.; Bongiovanni, A.; Lampiasi, N. Substance P Induces TNF-α and IL-6 Production through NFκB in Peritoneal Mast Cells. Biochim. Biophys. Acta-Mol. Cell Res. 2003, 1643, 75–83. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Lin, T.J.; Marshall, J.S. Toll-like Receptor 4-Mediated Activation of Murine Mast Cells. J. Leukoc. Biol. 2001, 70, 977–984. [Google Scholar] [PubMed]
- Yoshioka, M.; Fukuishi, N.; Iriguchi, S.; Ohsaki, K.; Yamanobe, H.; Inukai, A.; Kurihara, D.; Imajo, N.; Yasui, Y.; Matsui, N.; et al. Lipoteichoic Acid Downregulates FcεRI Expression on Human Mast Cells through Toll-like Receptor 2. J. Allergy Clin. Immunol. 2007, 120, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, K.; Port, F.; Grochowy, G.; Kalis, C.; Bessler, W.; Galanos, C.; Krystal, G.; Freudenberg, M.; Huber, M. Stimulation of Mast Cells via FcɛR1 and TLR2: The Type of Ligand Determines the Outcome. Mol. Immunol. 2007, 44, 2087–2094. [Google Scholar] [CrossRef]
- Saluja, R.; Delin, I.; Nilsson, G.P.; Adner, M. FceR 1-Mediated Mast Cell Reactivity Is Amplified through Prolonged Toll-Like Receptor-Ligand Treatment. PLoS ONE 2012, 7, e43547. [Google Scholar] [CrossRef]
- Yang, C.; Mo, X.; Lv, J.; Liu, X.; Yuan, M.; Dong, M.; Li, L.; Luo, X.; Fan, X.; Jin, Z.; et al. Lipopolysaccharide Enhances FcεRI-Mediated Mast Cell Degranulation by Increasing Ca2+ Entry through Store-Operated Ca2+ Channels: Implications for Lipopolysaccharide Exacerbating Allergic Asthma. Exp. Physiol. Exp. Physiol. 2012, 97, 1315–1327. [Google Scholar] [CrossRef]
- Qiao, H.; Andrade, M.V.; Lisboa, F.A.; Morgan, K.; Beaven, M.A. FcR1 and Toll-like Receptors Mediate Synergistic Signals to Markedly Augment Production of Inflammatory Cytokines in Murine Mast Cells. Blood 2006, 107, 610–618. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage Polarization Comes of Age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Ebert, R.; Cumbana, R.; Lehmann, C.; Kutzner, L.; Toewe, A.; Ferreirós, N.; Parnham, M.J.; Schebb, N.H.; Steinhilber, D.; Kahnt, A.S. Long-Term Stimulation of Toll-like Receptor-2 and -4 Upregulates 5-LO and 15-LO-2 Expression Thereby Inducing a Lipid Mediator Shift in Human Monocyte-Derived Macrophages. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158702. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Gustafsson, C.; Mjö Sberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Ran Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene Expression Profiling of Human Decidual Macrophages: Evidence for Immunosuppressive Phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef]
- Mezouar, S.; Mege, J.-L. Gene Expression Profiling of Placenta from Normal to Pathological Pregnancies. In Placenta; IntechOpen: London, UK, 2018. [Google Scholar]
- Sun, J.; Ramnath, R.D.; Tamizhselvi, R.; Bhatia, M. Role of Protein Kinase C and Phosphoinositide 3-Kinase-Akt in Substance P-Induced Proinflammatory Pathways in Mouse Macrophages. FASEB J. Res. Commun. 2008, 23, 997–1010. [Google Scholar] [CrossRef]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2021, 8, 617879. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishiharat, T.; Kogat, T.; Martins, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone-marrow derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Library, W.O.; Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage Plasticity and Polarization in Tissue Repair and Remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 Macrophage Polarization and Potentially Therapeutic Naturally Occurring Compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.S.; Glim, J.E.; Stavenuiter, A.W.D.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H.J. Human Macrophage Polarization in Vitro: Maturation and Activation Methods Compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.C.; Soldano, S.; Contini, P.; Tomatis, V.; Ruaro, B.; Paolino, S.; Brizzolara, R.; Montagna, P.; Sulli, A.; Pizzorni, C.; et al. A Circulating Cell Population Showing Both M1 and M2 Monocyte/Macrophage Surface Markers Characterizes Systemic Sclerosis Patients with Lung Involvement. Respir. Res. 2018, 19, 186. [Google Scholar] [CrossRef]
- Mezouar, S.; Katsogiannou, M.; Ben Amara, A.; Bretelle, F.; Mege, J.L. Placental Macrophages: Origin, Heterogeneity, Function and Role in Pregnancy-Associated Infections. Placenta 2021, 103, 94–103. [Google Scholar] [CrossRef]
- Montana, G.; Lampiasi, N. Substance P Induces HO-1 Expression in RAW 264.7 Cells Promoting Switch towards M2-like Macrophages. PLoS ONE 2016, 11, e0167420. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Iwasaki, N.; Terawaki, S.; Shimizu, K.; Oikawa, D.; Sakamoto, H.; Sunami, K.; Tokunaga, F. Th2 Cells and Macrophages Cooperatively Induce Allergic Inflammation through Histamine Signaling. PLoS ONE 2021, 16, e0248158. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast Cell and Macrophage Chemokines CXCL1/CXCL2 Control the Early Stage of Neutrophil Recruitment during Tissue Inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Xu, W.; Schlagwein, N.; Roos, A.; van den Berg, T.K.; Daha, M.R.; van Kooten, C. Human Peritoneal Macrophages Show Functional Characteristics of M-CSF-Driven Anti-Inflammatory Type 2 Macrophages. Eur. J. Immunol. 2007, 37, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible Differentiation of Pro- and Anti-Inflammatory Macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Hawley, C.A.; Garner, H.; Scott, C.L.; Schridde, A.; Steers, N.J.; Mack, M.; Joshi, A.; Guilliams, M.; Mowat, A.M.I.; et al. Long-Lived Self-Renewing Bone Marrow-Derived Macrophages Displace Embryo-Derived Cells to Inhabit Adult Serous Cavities. Nat. Commun. 2016, 7, ncomms11852. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Enerback, L. Immature Peritoneal Mast Cells in Neonatal Rats Express the CTMC Phenotype, as Well as Functional IgE Receptors. APMIS 1999, 107, 957–965. [Google Scholar] [CrossRef]
- Malbec, O.; Roget, K.; Schiffer, C.; Iannascoli, B.; Dumas, A.R.; Arock, M.; Daëron, M. Peritoneal Cell-Derived Mast Cells: An In Vitro Model of Mature Serosal-Type Mouse Mast Cells. J. Immunol. 2007, 178, 6465–6475. [Google Scholar] [CrossRef]
- Cocchiara, R.; Albeggiani, G.; di Trapani, G.; Azzolina, A.; Lampiasi, N.; Rizzo, F.; Geraci, D. Modulation of rat peritoneal mast cell and human basophil histamine release by estrogens. Int. Arch. Allergy Appl. Immunol. 1990, 93, 192–197. [Google Scholar] [CrossRef]
- Pepe, G.; Braga, D.; Renzi, T.A.; Villa, A.; Bolego, C.; D’Avila, F.; Barlassina, C.; Maggi, A.; Locati, M.; Vegeto, E. Self-Renewal and Phenotypic Conversion Are the Main Physiological Responses of Macrophages to the Endogenous Estrogen Surge. Sci. Rep. 2017, 7, 44270. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Hämmerling, G.J. Cellular Regulation of the Uterine Microenvironment That Enables Embryo Implantation. Front. Immunol. 2015, 6, 321. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-Cell Transcriptomic Atlas of the Human Endometrium during the Menstrual Cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed, R.; Salazar Garcia, M.D.; Skariah, A.; Dambaeva, S.; Fernandez, E.; Germain, A.; Gilman-Sachs, A.; et al. Mast Cell–Induced Immunopathology in Recurrent Pregnancy Losses. Am. J. Reprod. Immunol. 2019, 82, e13128. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A. Inflammation, Leukocytes and Menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Zhang, J.; Brasted, M. Leukocyte Networks and Human Endometrial Remodelling. J. Reprod. Immunol. 2002, 57, 95–108. [Google Scholar] [CrossRef]
- Zierau, O.; Zenclussen, A.C.; Jensen, F. Role of Female Sex Hormones, Estradiol and Progesterone, in Mast Cell Behavior. Front. Immunol. 2012, 3, 2010–2013. [Google Scholar] [CrossRef]
- Levier, R.R.; Spaziani, E. The Effects of Estradiol on the Occurrence of Mast Cells in the Rat Uterus. Exp. Cell Res. 1966, 41, 244–252. [Google Scholar] [CrossRef]
- De Leo, B.; Esnal-Zufiaurre, A.; Collins, F.; Critchley, H.O.D.; Saunders, P.T.K. Immunoprofiling of Human Uterine Mast Cells Identifies Three Phenotypes and Expression of ERβ and Glucocorticoid Receptor. F1000Research 2017, 6, 667. [Google Scholar] [CrossRef]
- Eidukaite, A. Vytas Tamosiunas Endometrial and Peritoneal Macrophages: Expression of Activation and Adhesion Molecules. Am. J. Reprod. Immunol. 2004, 52, 113–117. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. Structural Changes in Endometrial Basal Glands during Menstruation. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1175–1185. [Google Scholar] [CrossRef]
- Jensen, A.L.; Collins, J.; Shipman, E.P.; Wira, C.R.; Guyre, P.M.; Pioli, P.A. A Subset of Human Uterine Endometrial Macrophages Is Alternatively Activated. Am. J. Reprod. Immunol. 2012, 68, 374–386. [Google Scholar] [CrossRef]
- Jensen, F.; Woudwyk, M.; Teles, A.; Woidacki, K.; Taran, F.; Costa, S.; Malfertheiner, S.F.; Zenclussen, A.C. Estradiol and Progesterone Regulate the Migration of Mast Cells from the Periphery to the Uterus and Induce Their Maturation and Degranulation. PLoS ONE 2010, 5, e14409. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, R.; Albeggiani, G.; Trapani, G.D.; Azzolina, A.; Lampiasi, N.; Rizzo, F.; Diotallevi, L.; Gianaroli, L.; Geraci, D. Oestradiol Enhances in Vitro the Histamine Release Induced by Embryonic Histamine-Releasing Factor (EHRF) from Uterine Mast Cells. Hum. Reprod. 1992, 7, 1036–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leung, P.H.Y.; Salamonsen, L.A.; Findlay, J.K. Immunolocalization of Inhibin and Activin Subunits in Human Endometrium across the Menstrual Cycle. Hum. Reprod. 1998, 13, 3469–3477. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, A.; Gaide Chevronnay, H.P.; Lemoine, P.; Courtoy, P.J.; Marbaix, E.; Henriet, P. Matrix Metalloproteinase-27 Is Expressed in CD163 1/CD206 1 M2 Macrophages in the Cycling Human Endometrium and in Superficial Endometriotic Lesions. Mol. Hum. Reprod. 2014, 20, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The Endometrial Immune Environment of Women with Endometriosis. Hum. Reprod. Update 2019, 25, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage Expression in Endometrium of Women with and without Endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Stewart, J.A.; Bulmer, J.N.; Murdoch, A.P. Endometrial Leucocytes: Expression of Steroid Hormone Receptors. J. Clin. Pathol. 1998, 51, 121–126. [Google Scholar] [CrossRef]
- Wang, H.; Critchley, H.O.; Kelly, R.W.; Shen, D.; Baird, D.T. Progesterone Receptor Subtype B Is Differentially Regulated in Human Endometrial Stroma. Mol. Hum. Reprod. 1998, 4, 407–412. [Google Scholar] [CrossRef]
- Carlino, C.; Stabile, H.; Morrone, S.; Bulla, R.; Soriani, A.; Agostinis, C.; Bossi, F.; Mocci, C.; Sarazani, F.; Tedesco, F.; et al. Recruitment of Circulating NK Cells through Decidual Tissues: A Possible Mechanism Controlling NK Cell Accumulation in the Uterus during Early Pregnancy. Blood 2008, 111, 3108–3115. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Kelly, R.W.; Brenner, R.M.; Baird, D.T. The Endocrinology of Menstruation—A Role for the Immune System. Clin. Endocrinol. 2001, 55, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.S.; Miller, L.; Roby’, K.F.; Huang, J.; Platt, J.S.; Debrot, B.L. Female Steroid Hormones Regulate Production of Pro-Inflammatory Molecules in Uterine Leukocytes. J. Reprod. Immunol. 1997, 35, 87–99. [Google Scholar] [CrossRef]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible Involvement of Inflammatory/Reparative Processes in the Development of Uterine Fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, G.; Jian, W.; Woolley, D.E.; Salamonsen, L.A. Mast Cell Regulation of Human Endometrial Matrix Metalloproteinases: A Mechanism Underlying Menstruation. Biol. Reprod. 1998, 59, 693–703. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Krikun, G.; Hausknecht, V.A.; Papp, C.; Schatz, F. Matrix Metalloproteinase and Matrix Metalloproteinase Inhibitor Expression in Endometrial Stromal Cells during Progestin-Initiated Decidualization and Menstruation-Related Progestin Withdrawal. Endocrinology 1998, 139, 4607–4613. [Google Scholar] [CrossRef][Green Version]
- Woidacki, K.; Jensen, F.; Zenclussen, A.C.; Blank, U.; Varin-Blank, N. Mast Cells as Novel Mediators of Reproductive Processes. Front. Immunol. 2013, 4, 29. [Google Scholar] [CrossRef]
- Trabucchi, E.; Radaelli, E.; Marazzi, M.; Foschi, D.; Musazzi, M.; Veronesi, A.M.; Montorsi, W. The role of mast cells in wound healing. Int. J. Tissue React. 1988, 10, 367–372. [Google Scholar]
- Critchley, H.O.D.; Osei, J.; Henderson, T.A.; Boswell, L.; Sales, K.J.; Jabbour, H.N.; Hirani, N. Hypoxia-Inducible Factor-1 Expression in Human Endometrium and Its Regulation by Prostaglandin E-Series Prostanoid Receptor 2 (EP2). Endocrinology 2006, 147, 744–753. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast Cell Proteases as Protective and Inflammatory Mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef]
- Aplin, J.D.; Charlton, A.K.; Ayad, S. An Immunohistochemical Study of Human Endometrial Extracellular Matrix during the Menstrual Cycle and First Trimester of Pregnancy. Cell Tissue Res. 1988, 253, 231–240. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Godbole, S. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF May Contribute to Macrophage Recruitment and M2 Polarization in the Decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef]
- Cousins, F.L.; Kirkwood, P.M.; Saunders, P.T.K.; Gibson, D.A. Evidence for a Dynamic Role for Mononuclear Phagocytes during Endometrial Repair and Remodelling. Sci. Rep. 2016, 6, 36748. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Ovarian Steroid Hormones Regulate Granulocyte-Macrophage Colony-Stimulating Factor Synthesis by Uterine Epithelial Cells in the Mouse. Biol. Reprod. 1996, 54, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, R.; Lampiasi, N.; Albeggiani, G.; Azzolina, A.; Bongiovanni, A.; Gianaroli, L.; di Blasi, F.; Geraci, D. A Factor Secreted by Human Embryo Stimulates Cytokine Release by Uterine Mast Cell. MHR Basic Sci. Reprod. Med. 1996, 2, 781–791. [Google Scholar] [CrossRef][Green Version]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, Á.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. CCL2 Shapes Macrophage Polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-Dependent Gene Expression Profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the Fetal–Maternal Interface Express Markers of Alternative Activation and Are Induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef]
- Helige, C.; Ahammer, H.; Hammer, A.; Huppertz, B.; Frank, H.G.; Dohr, G. Trophoblastic Invasion in Vitro and in Vivo: Similarities and Differences. Hum. Reprod. 2008, 23, 2282–2291. [Google Scholar] [CrossRef]
- Helige, C.; Ahammer, H.; Moser, G.; Hammer, A.; Dohr, G.; Huppertz, B.; Sedlmayr, P. Distribution of Decidual Natural Killer Cells and Macrophages in the Neighbourhood of the Trophoblast Invasion Front: A Quantitative Evaluation. Hum. Reprod. 2014, 29, 8–17. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Innate Immune Cells in the Placental Bed in Healthy Pregnancy and Preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two Unique Human Decidual Macrophage Populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, J.; Heikkinen, J.; Möttönen, M.; Komi, J.; Alanen, A.; Lassila, O. Phenotypic Characterization of Human Decidual Macrophages. Clin. Exp. Immunol. 2003, 131, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Postovit, L.M.; Macdonald-Goodfellow, S.K.; McDonald, G.T.; Caldwell, J.D.; Graham, C.H. Activated Macrophages Inhibit Human Cytotrophoblast Invasiveness in vitro. Biol. Reprod. 2005, 73, 237–243. [Google Scholar] [CrossRef]
- Nicola, C.; Chirpac, A.; Lala, P.K.; Chakraborty, C. Roles of Rho Guanosine 5’-Triphosphatase A, Rho Kinases, and Extracellular Signal Regulated Kinase (1/2) in Prostaglandin E2-Mediated Migration of First-Trimester Human Extravillous Trophoblast. Endocrinology 2008, 149, 1243–1251. [Google Scholar] [CrossRef]
- Bosquiazzo, V.L.; Ramos, J.G.; Varayoud, J.; Muñoz-de-Toro, M.; Luque, E.H. Mast Cell Degranulation in Rat Uterine Cervix during Pregnancy Correlates with Expression of Vascular Endothelial Growth Factor MRNA and Angiogenesis. Reproduction 2007, 133, 1045–1055. [Google Scholar] [CrossRef]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for Immune Cell Involvement in Decidual Spiral Arteriole Remodeling in Early Human Pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Alvin Jose, M. Anti-implantation activity of H2 receptor blockers and meloxicam, a COX-inhibitor, in albino Wistar rats. Eur. J. Contracept. Reprod. Health Care 2009, 14, 444–450. [Google Scholar] [CrossRef]
- Meyer, N.; Woidacki, K.; Knöfler, M.; Meinhardt, G.; Nowak, D.; Velicky, P.; Pollheimer, J.; Zenclussen, A.C. Chymase-Producing Cells of the Innate Immune System Are Required for Decidual Vascular Remodeling and Fetal Growth. Sci. Rep. 2017, 7, 45106. [Google Scholar] [CrossRef]
- Olmos-ortiz, A.; Flores-espinosa, P.; Mancilla-Herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor—Dependent Responses at the Maternal—Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654. [Google Scholar] [CrossRef]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Latifi, Z.; Fattahi, A.; Ranjbaran, A.; Nejabati, H.R.; Imakawa, K. Potential Roles of Metalloproteinases of Endometrium-Derived Exosomes in Embryo-Maternal Crosstalk during Implantation. J. Cell. Physiol. 2018, 233, 4530–4545. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Uterine NK Cells and Macrophages in Pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Guo, Y.; So, K.-H.; Vijayan, M.; Guo, Y.; Wong, V.H.H.; Yao, Y.; Lee, K.-F.; Chiu, P.C.N.; Yeung, W.S.B. Soluble Human Leukocyte Antigen G5 Polarizes Differentiation of Macrophages toward a Decidual Macrophage-like Phenotype. Hum. Reprod. 2015, 30, 2263–2274. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Haidl, G. Significance of Mast Cells in Spermatogenesis, Implantation, Pregnancy, and Abortion: Cross Talk and Molecular Mechanisms. Am. J. Reprod. Immunol. 2020, 83, e13228. [Google Scholar] [CrossRef]
- Matsuno, T.; Toyoshima, S.; Sakamoto-Sasaki, T.; Kashiwakura, J.-I.; Matsuda, A.; Watanabe, Y.; Azuma, H.; Kawana, K.; Yamamoto, T.; Okayama, Y. Characterization of Human Decidual Mast Cells and Establishment of a Culture System. Allergol. Int. 2018, 67, S18–S24. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Osakabe, M.; Sugimoto, A.; Ushirokawa, A.; Shibata, Y.; Nakamura, H.; Shibuya, R.; Minamiguchi, S.; Sugai, T.; et al. Decidualization of Stromal Cells Promotes Involvement of Mast Cells in Successful Human Pregnancy by Increasing Stem Cell Factor Expression. Front. Immunol. 2022, 13, 779574. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Sugimoto, A.; Iemura, Y.; Minamiguchi, S.; Nomura, T.; Haga, H. Possible Involvement of Human Mast Cells in the Establishment of Pregnancy via Killer Cell Ig-Like Receptor 2DL4. Am. J. Pathol. 2018, 188, 1497–1508. [Google Scholar] [CrossRef]
- Von Wolff, M.; Wang, X.; Gabius, H.-J.; Strowitzki, T. Galectin Fingerprinting in Human Endometrium and Decidua during the Menstrual Cycle and in Early Gestation. Mol. Hum. Reprod. 2004, 11, 189–194. [Google Scholar] [CrossRef]
- Fischer, I.; Redel, S.; Hofmann, S.; Kuhn, C.; Friese, K.; Walzel, H.; Jeschke, U. Stimulation of Syncytium Formation in Vitro in Human Trophoblast Cells by Galectin-1. Placenta 2010, 31, 825–832. [Google Scholar] [CrossRef]
- Woidacki, K.; Popovic, M.; Metz, M.; Schumacher, A.; Linzke, N.; Teles, A.; Poirier, F.; Fest, S.; Jensen, F.; Rabinovich, G.A.; et al. Mast Cells Rescue Implantation Defects Caused by C-Kit Deficiency. Cell Death Dis. 2013, 4, e462. [Google Scholar] [CrossRef] [PubMed]
- McIntire, R.H.; Hunt, J.S. Antigen Presenting Cells and HLA-G—A Review. Placenta 2005, 26, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.S.; Morales, P.J.; Pace, J.L.; Fazleabas, A.T.; Langat, D.K. A Commentary on Gestational Programming and Functions of HLA-G in Pregnancy. Placenta 2007, 28, S57–S63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunt, J.S.; Langat, D.L. HLA-G: A Human Pregnancy-Related Immunomodulator. Curr. Opin. Pharmacol. 2009, 9, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Houser, B.L.; Nicotra, M.L.; Strominger, J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5767–5772. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Furuhata, A.; Suzuki, E.; Toi, M.; Tsuruyama, T.; Okayama, Y.; Haga, H. The Killer Cell Ig-like Receptor 2DL4 Expression in Human Mast Cells and Its Potential Role in Breast Cancer Invasion. Cancer Immunol. Res. 2015, 3, 871–880. [Google Scholar] [CrossRef]
- Mezouar, S.; Ben Amara, A.; Chartier, C.; Gorvel, L.; Mege, J.-L. A fast and reliable method to isolate human placental macrophages. Curr. Protoc. Immunol. 2019, 125, e77. [Google Scholar] [CrossRef]
- Ribatti, D.; Belloni, A.S.; Nico, B.; Salà, G.; Longo, V.; Mangieri, D.; Crivellato, E.; Nussdorfer, G.G. Tryptase- and Leptin-Positive Mast Cells Correlate with Vascular Density in Uterine Leiomyomas. Am. J. Obstet. Gynecol. 2007, 196, e1–e470. [Google Scholar] [CrossRef]
- Böckle, B.C.; Sölder, E.; Kind, S.; Romani, N.; Sepp, N.T. DC-SIGN+ CD163+ Macrophages Expressing Hyaluronan Receptor LYVE-1 Are Located within Chorion Villi of the Placenta. Placenta 2008, 29, 187–192. [Google Scholar] [CrossRef]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The Role of Decidual Immune Cells on Human Pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Vachek, Z. Derivation and Ultrastructure of the Stroma Cells of the Human Chorionic Villus. Folia Morphol. 1970, 18, 1–13. [Google Scholar]
- Kaufmann, P.; Stark, J.; Stegner, H.E. The Villous Stroma of the Human Placenta, I. The Ultrastructure of Fixed Connective Tissue Cells. Cell Tissue Res. 1977, 177, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Wolfe, B.; Golos, T.; Reyes, L.; Wolfe, B.; Golos, T. Hofbauer Cells: Placental Macrophages of Fetal Origin. Macrophages 2017, 62, 45–60. [Google Scholar] [CrossRef]
- Reyes, L.; Golos, T.G. Hofbauer Cells: Their Role in Healthy and Complicated Pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef] [PubMed]
- Ramhorst, R.; Grasso, E.; Paparini, D.; Hauk, V.; Gallino, L.; Calo, G.; Vota, D.; Leirós, C.P.; Erez Leir, C.P. Cell Adhesion & Migration Decoding the Chemokine Network That Links Leukocytes with Decidual Cells and the Trophoblast during Early Implantation Decoding the Chemokine Network That Links Leukocytes with Decidual Cells and the Trophoblast during Early Implantation. Cell Adhes. Migr. 2016, 10, 197–207. [Google Scholar] [CrossRef]
- Luque-Martin, R.; Mander, P.K.; Leenen, P.J.M.; Winther, M.P.J. Classic and New Mediators for in Vitro Modelling of Human Macrophages. J. Leukoc. Biol. 2021, 109, 549–560. [Google Scholar] [CrossRef]
- Muñoz-Garcia, J.; Cochonneau, D.; Télétchéa, S.; Moranton, E.; Lanoe, D.; Brion, R.; Lézot, F.; Heymann, M.F.; Heymann, D. The Twin Cytokines Interleukin-34 and CSF-1: Masterful Conductors of Macrophage Homeostasis. Theranostics 2021, 11, 1568–1593. [Google Scholar] [CrossRef]
- Freuchet, A.; Salama, A.; Remy, S.; Guillonneau, C.; Anegon, I. IL-34 and CSF-1, Deciphering Similarities and Differences at Steady State and in Diseases. J. Leukoc. Biol. 2021, 110, 771–796. [Google Scholar] [CrossRef]
- Boulakirba, S.; Pfeifer, A.; Mhaidly, R.; Obba, S.; Goulard, M.; Schmitt, T.; Chaintreuil, P.; Calleja, A.; Furstoss, N.; Orange, F.; et al. IL-34 and CSF-1 Display an Equivalent Macrophage Differentiation Ability but a Different Polarization Potential. Sci. Rep. 2018, 8, 256. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef]
- Norström, A.; Vukas Radulovic, N.; Bullarbo, M.; Ekerhovd, E. Mast Cell Involvement in Human Cervical Ripening. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.C.H.; Vong, J.S.L.; Ji, L.; Poon, L.C.Y.; Jiang, P.; Lui, K.O.; Ni, Y.B.; To, K.F.; Cheng, Y.K.Y.; Chiu, R.W.K.; et al. Integrative Single-Cell and Cell-Free Plasma RNA Transcriptomics Elucidates Placental Cellular Dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, E7786–E7795. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, M.R.; Li, M.; Wang, H. Three Macrophage Subsets Are Identified in the Uterus during Early Human Pregnancy. Cell. Mol. Immunol. 2018, 15, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Lindau, R.; Vondra, S.; Spreckels, J.; Solders, M.; Svensson-Arvelund, J.; Berg, G.; Pollheimer, J.; Kaipe, H.; Jenmalm, M.C.; Ernerudh, J. Decidual Stromal Cells Support Tolerance at the Human Foetal-Maternal Interface by Inducing Regulatory M2 Macrophages and Regulatory T-Cells. J. Reprod. Immunol. 2021, 146, 103330. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Ribatti, D. Mast Cell-Mediated Mechanistic Pathways in Organ Transplantation. Eur. J. Pharmacol. 2019, 857, 172458. [Google Scholar] [CrossRef] [PubMed]
- Marx, L.; Arck, P.; Kieslich, C.; Mitterlechner, S.; Kapp, M.; Dietl, J. American Journal of Reproductive Immunology Decidual Mast Cells Might Be Involved in the Onset of Human First-Trimester Abortion. Am. J. Reprod. Immunol. 1999, 41, 34–40. [Google Scholar] [CrossRef]
- Davies, L.C.; Rosas, M.; Smith, P.J.; Fraser, D.J.; Jones, S.A.; Taylor, P.R. A Quantifiable Proliferative Burst of Tissue Macrophages Restores Homeostatic Macrophage Populations after Acute Inflammation. Eur. J. Immunol. 2011, 41, 2155–2164. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional Plasticity of Macrophages: Reversible Adaptation to Changing Microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef]
- Dahdah, A.; Gautier, G.; Attout, T.; Fiore, F.; Lebourdais, E.; Msallam, R.; Daëron, M.; Monteiro, R.C.; Benhamou, M.; Charles, N.; et al. Mast Cells Aggravate Sepsis by Inhibiting Peritoneal Macrophage Phagocytosis. J. Clin. Investig. 2014, 124, 4577–4589. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Das, A.M.; Virág, L.; Szabó, C.; Perretti, M. Regulation of Macrophage Inflammatory Protein-1α Expression and Function by Endogenous Interleukin-10 in a Model of Acute Inflammation. Biochem. Biophys. Res. Commun. 1999, 255, 279–282. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Lelito, M.; Kozakiewicz, E.; van Rooijen, N.; Plytycz, B.; Arnold, B. Resident Peritoneal Leukocytes Are Important Sources of MMP-9 during Zymosan Peritonitis: Superior Contribution of Macrophages over Mast Cells. Immunol. Lett. 2007, 113, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Laurin, L.-P.; Brissette, M.-J.; Lepage, S.; Cailhier, J.F. Regulation of Experimental Peritonitis: A Complex Orchestration. Nephron Exp. Nephrol. 2012, 120, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Finlay, C.M.; Cunningham, K.T.; Doyle, B.; Mills, K.H.G. IL-33–Stimulated Murine Mast Cells Polarize Alternatively Activated Macrophages, Which Suppress T Cells That Mediate Experimental Autoimmune Encephalomyelitis. J. Immunol. 2020, 205, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- de Zuani, M.; Paolicelli, G.; Zelante, T.; Renga, G.; Romani, L.; Arzese, A.; Pucillo, C.E.M.; Frossi, B. Mast Cells Respond to Candida Albicansinfections and Modulate Macrophages Phagocytosis of the Fungus. Front. Immunol. 2018, 9, 2829. [Google Scholar] [CrossRef]
- Crisafulli, C.; Cuzzocrea, S. The Role of Endogenous and Exogenous Ligands for the Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α) in the Regulation of Inflammation in Macrophages. Shock 2009, 32, 62–73. [Google Scholar] [CrossRef]
- Crisafulli, C.; Galuppo, M.; Cuzzocrea, S. Effects of Genetic and Pharmacological Inhibition of TNF-α in the Regulation of Inflammation in Macrophages. Pharmacol. Res. 2009, 60, 332–340. [Google Scholar] [CrossRef]
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression Profiling of Endometrium from Women with Endometriosis Reveals Candidate Genes for Disease-Based Implantation Failure and Infertility. Endocrinology 2003, 144, 2870–2881. [Google Scholar] [CrossRef]
- Bunis, D.G.; Wang, W.; Vallvé-Juanico, J.; Houshdaran, S.; Sen, S.; ben Soltane, I.; Kosti, I.; Vo, K.C.; Irwin, J.C.; Giudice, L.C.; et al. Whole-Tissue Deconvolution and ScRNAseq Analysis Identify Altered Endometrial Cellular Compositions and Functionality Associated with Endometriosis. Front. Immunol. 2022, 12, 788315. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Burney, R.O.; Talbi, S.; Hamilton, A.E.; Vo, K.C.; Nyegaard, M.; Nezhat, C.R.; Lessey, B.A.; Giudice, L.C. Gene Expression Analysis of Endometrium Reveals Progesterone Resistance and Candidate Susceptibility Genes in Women with Endometriosis. Endocrinology 2007, 148, 3814–3826. [Google Scholar] [CrossRef]
- Berkkanoglu, M.; Arici, A. Immunology and Endometriosis. Am. J. Reprod. Immunol. 2003, 50, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kapranov, P.; Wessels, J.M.; Chen, S.; Zhong, Q.; Yang, F.; Chen, X.; Li, J.; Zhong, C. Patterns of Immune Infiltration in Endometriosis and Their Relationship to R-AFS Stages. Front. Genet. 2021, 1, 631715. [Google Scholar] [CrossRef]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The Role of Peritoneal Macrophages in Endometriosis. Int. J. Mol. Sci. 2021, 22, 792. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Darcha, C.; Fukaya, T.; Yajima, A.; Bruhat, M.A. Increased Mast Cell Density in Peritoneal Endometriosis Compared with Eutopic Endometrium with Endometriosis. Am. J. Reprod. Immunol. 1998, 40, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.O.; Pullen, N.A. Molecular Sciences Beyond IgE: Alternative Mast Cell Activation across Different Disease States. Int. J. Mol. Sci. 2020, 21, 1498. [Google Scholar] [CrossRef]
- Osuga, Y.; Koga, K.; Tsutsumi, O.; Igarashi, T.; Okagaki, R.; Takai, Y.; Matsumi, H.; Fujiwara, H.H.; Momoeda, M.; Yano, T.; et al. Stem Cell Factor (SCF) Concentrations in Peritoneal Fluid of Women with or without Endometriosis. Am. J. Reprod. Immunol. 2000, 44, 231–235. [Google Scholar] [CrossRef]
- Orlova, Y.A.; Hromova, A.M.; Kaidashev, I.P.; Shlykova, O.A.; Izmailova, O.V.; Martynenko, V.B. Pathogenetic role of macrophage colony-stimulating factor (csf-1) in predicting endometrioid disease. Wiadomosci Lek. 2021, 74, 1939–1944. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen Receptor β Upregulates CCL2 via NF-κ B Signaling in Endometriotic Stromal Cells and Recruits Macrophages to Promote the Pathogenesis of Endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef]
- Anaf, V.; Chapron, C.; el Nakadi, I.; de Moor, V.; Simonart, T.; Noël, J.C. Pain, Mast Cells, and Nerves in Peritoneal, Ovarian, and Deep Infiltrating Endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef]
- Godin, S.K.; Wagner, J.; Huang, P.; Bree, D. The Role of Peripheral Nerve Signaling in Endometriosis. FASEB BioAdv. 2021, 3, 802–813. [Google Scholar] [CrossRef]
- Zhu, T.H.; Ding, S.J.; Li, T.T.; Zhu, L.B.; Huang, X.F.; Zhang, X.M. Estrogen Is an Important Mediator of Mast Cell Activation in Ovarian Endometriomas. Reproduction 2018, 155, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Guo, X.; Yu, Q.; Ding, S.; Xu, X.; Peng, Y.; Zhu, L.; Zou, G.; Zhang, X. Possible Involvement of Crosstalk between Endometrial Cells and Mast Cells in the Development of Endometriosis via CCL8/CCR1. Biomed. Pharmacother. 2020, 129, 110476. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, X.; Li, T.; Yu, Q.; Wang, J.; Chen, Y.; Ding, S.; Zhu, L.; Zou, G.; Zhang, X. NLRP3 Inflammasome Activation of Mast Cells by Estrogen via the Nuclear-Initiated Signaling Pathway Contributes to the Development of Endometriosis. Front. Immunol. 2021, 12, 3909. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Akiyama, I.; Sato, E.; Ito, M.; Kobayashi, M.; Nakashima, A.; Wada, S.; Onda, T.; et al. IL-33 Exacerbates Endometriotic Lesions via Polarizing Peritoneal Macrophages to M2 Subtype. Reprod. Sci. 2020, 27, 869–876. [Google Scholar] [CrossRef]
- Miller, J.E.; Lingegowda, H.; Symons, L.K.; Bougie, O.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. IL-33 Activates Group 2 Innate Lymphoid Cell Expansion and Modulates Endometriosis. JCI Insight 2021, 6, e149699. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Placental Oxidative Stress: From Miscarriage to Preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Szukiewicz, A.; Maslinska, D.; Gujski, M.; Poppe, P.; Mazurek-Kantor, J. Mast Cell Number, Histamine Concentration and Placental Vascular Response to Histamine in Preeclampsia. Inflamm. Res. 1999, 48, 39–40. [Google Scholar] [CrossRef]
- Szewczyk, G.; Pyzlak, M.; Klimkiewicz, J.; Śmiertka, W.; Miedzińska-Maciejewska, M.; Szukiewicz, D. Mast Cells and Histamine: Do They Influence Placental Vascular Network and Development in Preeclampsia? Mediat. Inflamm. 2012, 2012, 307189. [Google Scholar] [CrossRef]
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the Immune Response and Inflammation by Histamine and Histamine Receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef]
- Schonkeren, D.; van der Hoorn, M.L.; Khedoe, P.; Swings, G.; van Beelen, E.; Claas, F.; van Kooten, C.; de Heer, E.; Scherjon, S. Differential Distribution and Phenotype of Decidual Macrophages in Preeclamptic versus Control Pregnancies. Am. J. Pathol. 2011, 178, 709–717. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.C.; Gao, P.J. Immune Imbalance Is Associated with the Development of Preeclampsia. Medicine 2019, 98, e15080. [Google Scholar] [CrossRef] [PubMed]
- Nizyaeva, N.V.; Kulikova, G.V.; Nagovitsyna, M.N.; Shchegolev, A.I. Peculiarities of the Expression of TLR4 and Inhibitor of TLR-Cascade Tollip in the Placenta in Earlyand Late-Onset Preeclampsia. Bull. Exp. Biol. Med. 2019, 166, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular Immune Responses in the Pathophysiology of Preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine Mapping of Sera from Women with Preeclampsia and Normal Pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and Inflammatory Markers (TNF-α, IL-6 and IL-8) in Pre-Eclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef]
- Broekhuizen, M.; Hitzerd, E.; van den Bosch, T.P.P.; Dumas, J.; Verdijk, R.M.; van Rijn, B.B.; Danser, A.H.J.; van Eijck, C.H.J.; Reiss, I.K.M.; Mustafa, D.A.M. The Placental Innate Immune System Is Altered in Early-Onset Preeclampsia, but Not in Late-Onset Preeclampsia. Front. Immunol. 2021, 12, 780043. [Google Scholar] [CrossRef] [PubMed]
- Mitani, R.; Maeda, K.; Fukui, R.; Endo, S.; Saijo, Y.; Shinohara, K.; Kamada, M.; Irahara, M.; Yamano, S.; Nakaya, Y.; et al. Production of Human Mast Cell Chymase in Human Myometrium and Placenta in Cases of Normal Pregnancy and Preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 155–160. [Google Scholar] [CrossRef]
- Murata, H.; Tanaka, S.; Okada, H. Clinical Medicine Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef]
- Bezemer, R.E.; Schoots, M.H.; Timmer, A.; Scherjon, S.A.; Erwich, J.J.H.M.; van Goor, H.; Gordijn, S.J.; Prins, J.R. Altered Levels of Decidual Immune Cell Subsets in Fetal Growth Restriction, Stillbirth, and Placental Pathology. Front. Immunol. 2020, 11, 1898. [Google Scholar] [CrossRef]
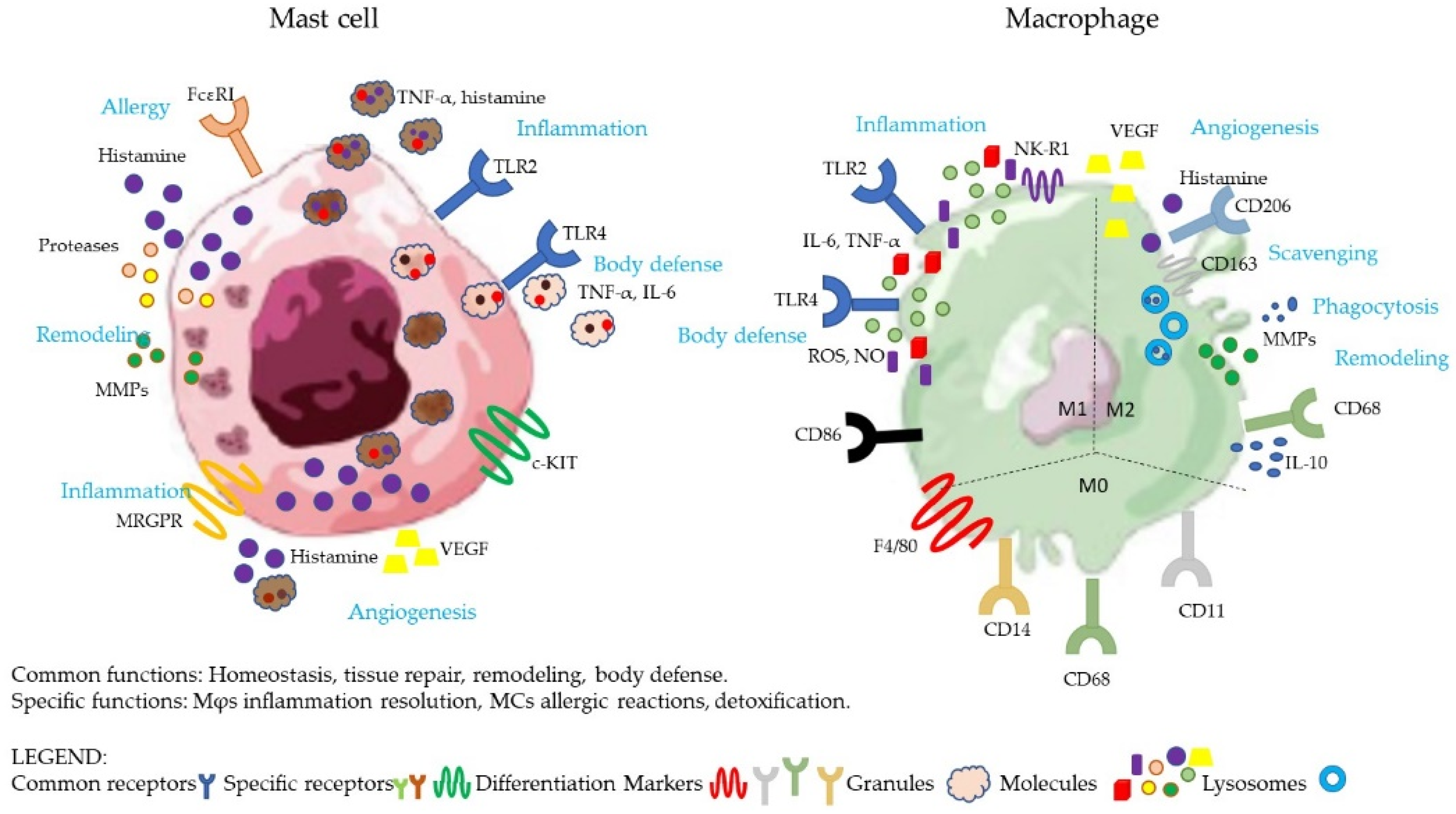
| Receptor | Ligand | Expression | Function |
|---|---|---|---|
| TLR2 | Bacterial, viral, fungal and parasites lipids, proteins, polysaccharides | MC and M1 M2 | Pro-inflammatory cytokines production, degranulation [28,29,30], IL-10, 15-LO [30] |
| TLR4 | Bacterial and viral proteins | MC and M1 M2 | Pro-inflammatory cytokines production, degranulation [25,31,32,33,34], IL-10, 15-LO, 15-HETE lipoxins [34] |
| FcεRI | Allergens | MC | Allergic reaction [13,17,35] |
| CD14 | Endotoxin receptor | Mɸ | Pro-inflammatory cytokine production [36,37] |
| F4/80 | Marker mouse | Mɸ | Differentiation, induction of CD8+ T regulatory cells [10] |
| c-KIT | SCF | MCs | Migration, differentiation, and survival [12,13,21] |
| NK-R1 | Substance P | Mɸ | Pro-inflammatory cytokines, NO, ROS, production. Induction of COX-2 activity [38] |
| MRGPR | Substance P | MC | Pro-inflammatory cytokine production, histamine and protease release [23] |
| Time | Phenotype | Function | Production | References |
|---|---|---|---|---|
| Pre-implantation/human, mouse | M1 | ECM digestion, engulf apoptotic cells | MMP-7; MMP-9; Gelatinase | [102,108,111,112] |
| Pre-implantation | M2 | Remodeling | VEGF, IGF, Fn1, MMPs | [102] |
| Pre-implantation/human, murine | MCs | Remodeling angiogenesis trophoblast growth, syncytium formation | Histamine, chymase, VEGF, Gal-1 | [72,87,107,109,110] |
| Implantation | M1 | Favor implantation, vascular remodeling, angiogenesis | TNF-α, VEGF, PDGF, FGF, MMP3, MMP9; IL-1β | [111] |
| Implantation/human, mouse | MCC | Vascular remodeling, fetal growth, migration and proliferation of extravillous | MMPs, VEGF, FGF, histamine, tryptase, leptin, cytokines | [63,73,97,110,113] |
| Post-implantation | M2 | Maternal–fetal tolerance | IL-10, IL-1β, Il-6, TNF-α; PGE2 | [36,69,99,103,112,114,115] |
| Post-implantation | MCTC | Maternal– fetal tolerance | TGF-β IL-10, LIF | [116] |
| Early pregnancy | MCTC | Placenta formation | Histamine, Gal-1 | [117] |
| Time | Phenotype | Function | Production | References |
|---|---|---|---|---|
| 1st–2nd trimester | M1 | Maternal–fetal tolerance | TNF-α, IL-6, IL-12, IL-23, IFN-γ, IL-18 | [37] |
| 1st–2nd trimester | M2 | Maternal–fetal tolerance | IL-4, IL-10, VEGF, IGF, FN | [36] |
| 2nd–3rd trimester | M2 | Maternal–fetal tolerance | VEGF, IL-6, IL-10 | [36] |
| End of pregnancy | M1 | Favor labor, remove apoptotic cells | IL-6, IL-10, IL-1, TNF-α, IFN-γ | [36,37,141] |
| End of pregnancy | MC | Favor labor | Histamine | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampiasi, N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. Int. J. Mol. Sci. 2022, 23, 5414. https://doi.org/10.3390/ijms23105414
Lampiasi N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. International Journal of Molecular Sciences. 2022; 23(10):5414. https://doi.org/10.3390/ijms23105414
Chicago/Turabian StyleLampiasi, Nadia. 2022. "Interactions between Macrophages and Mast Cells in the Female Reproductive System" International Journal of Molecular Sciences 23, no. 10: 5414. https://doi.org/10.3390/ijms23105414
APA StyleLampiasi, N. (2022). Interactions between Macrophages and Mast Cells in the Female Reproductive System. International Journal of Molecular Sciences, 23(10), 5414. https://doi.org/10.3390/ijms23105414






