The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Generation and Purification of the Monoclonal Antibodies Specific for LHBS Pre-S and S Regions
2.3. Determination of Antibody Sensitivities
2.4. DNA Sequencing of the Antibody Variable Genes
2.5. The Sandwich ELISA for LHBS Detection
2.6. Detection of HBV Markers
2.7. Immunohistochemical Staining and Interpretation
2.8. Statistical Analysis
3. Results
3.1. Establishment of the Chemiluminescence ELISA Kit for LHBS Quantification in Serum
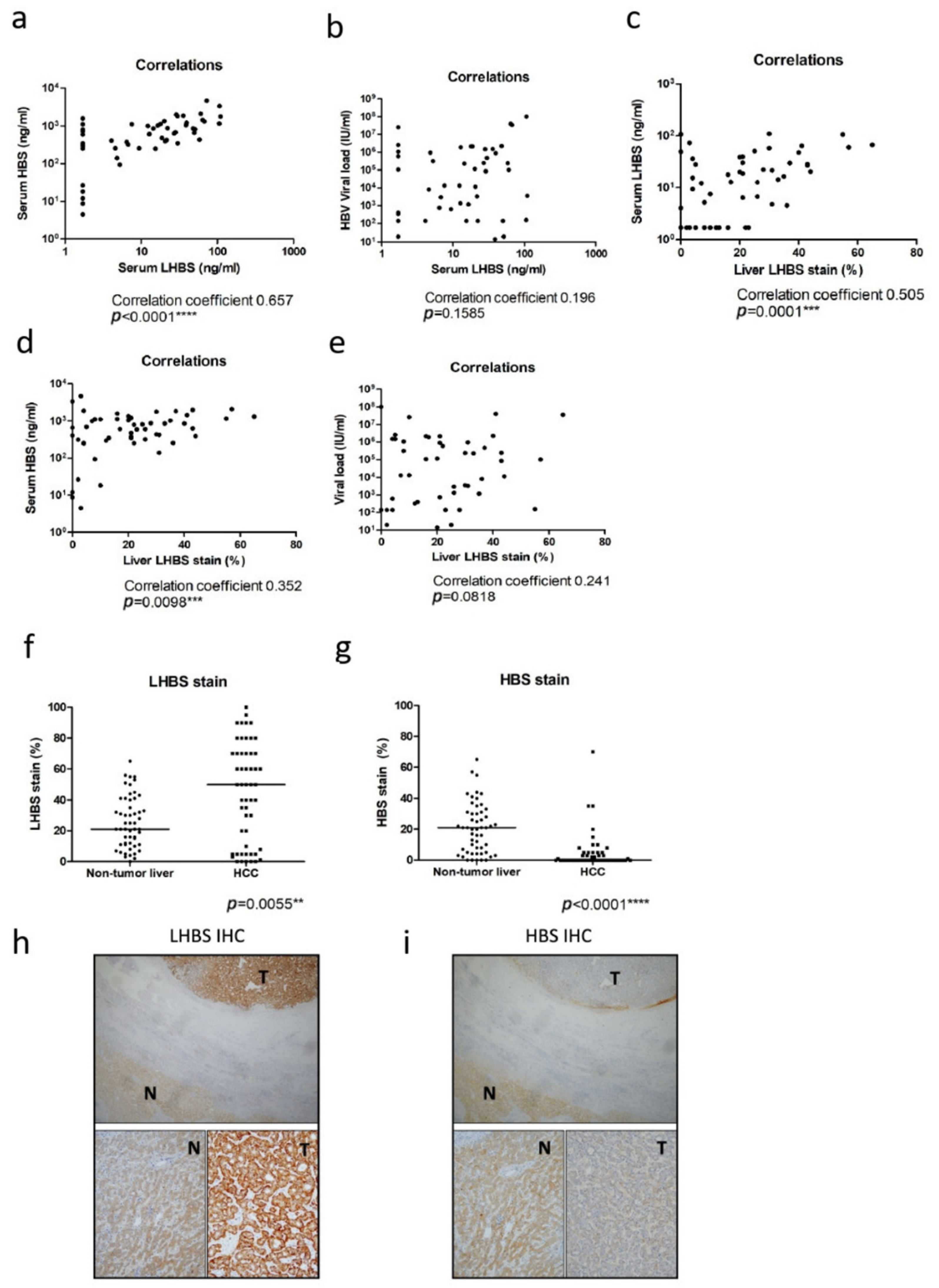
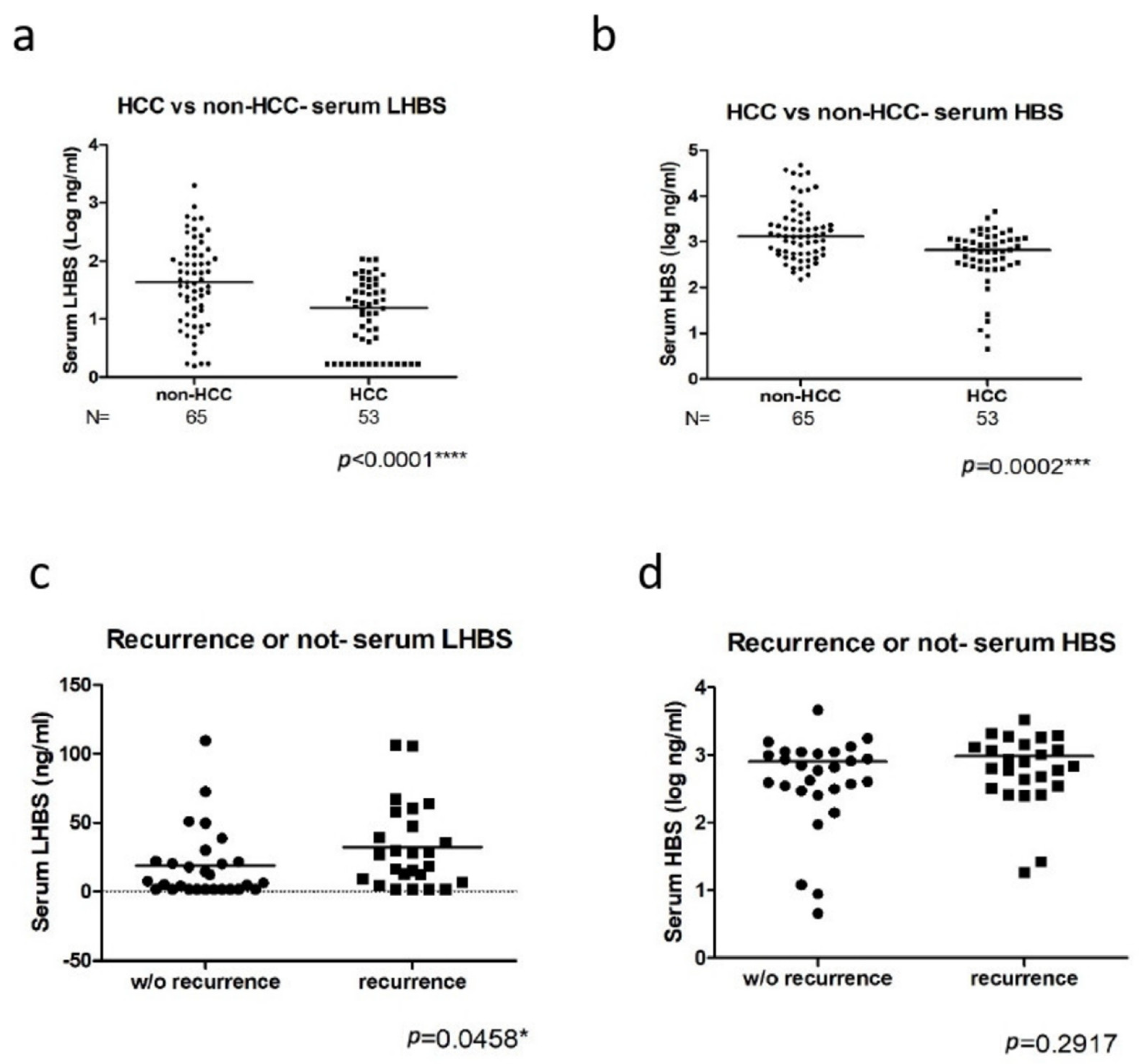
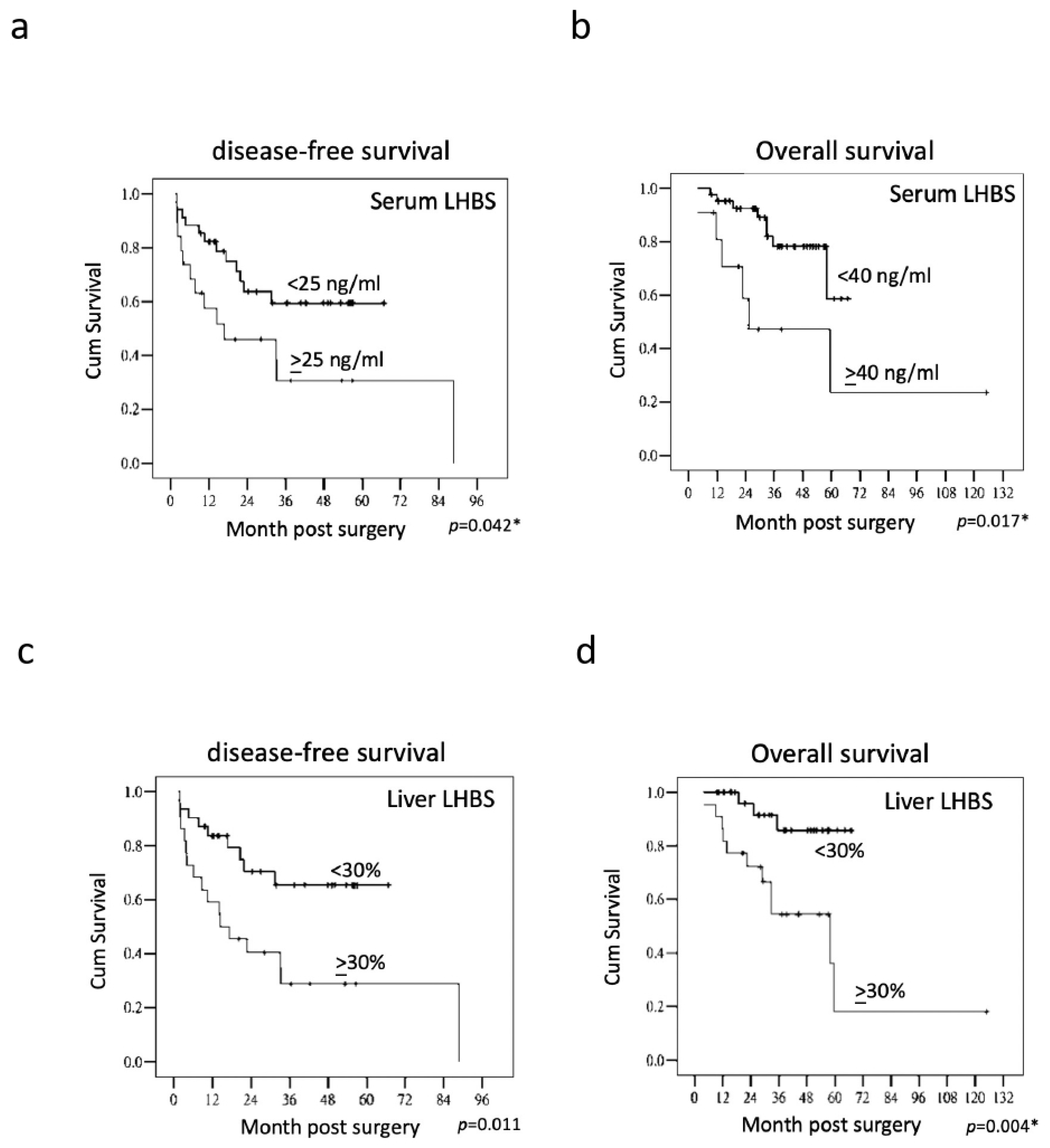
3.2. The Association of LHBS Expression with HBV Replication and HCC Prognosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CDR | complementarity-determining region |
| CHB | chronic hepatitis B |
| DFS | disease-free survival |
| ER | endoplasmic reticulum |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| JAB1 | c-Jun activation domain binding protein 1 |
| LHBS | large HBV surface protein |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LOL | limit of linearity |
| NTCP | sodium taurocholate co-transporting polypeptide |
| OS | overall survival |
| RFA | radiofrequency ablation |
| TACE | transarterial chemoembolization |
References
- Arbuthnot, P.; Kew, M. Hepatitis B virus and hepatocellular carcinoma. Int. J. Exp. Pathol. 2001, 82, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Borras, J. Epidemiology of primary liver cancer. Semin. Liver Dis. 1999, 19, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Prevention of hepatitis B virus-related hepatocellular carcinoma. Hepatoma Res. 2021, 7, 9. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of hepatocellular cancer after resection: Patterns, treatments, and prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Pazgan-Simon, M.; Simon, K.A.; Jarowicz, E.; Rotter, K.; Szymanek-Pasternak, A.; Zuwała-Jagiełło, J. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin. Exp. Hepatol. 2017, 4, 210–216. [Google Scholar] [CrossRef]
- Cougot, D.; Wu, Y.; Cairo, S.; Caramel, J.; Renard, C.A.; Lévy, L.; Buendia, M.A.; Neuveut, C. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J. Biol. Chem. 2007, 282, 4277–4287. [Google Scholar] [CrossRef] [Green Version]
- Henkler, F.F.; Koshy, R. Hepatitis B virus transcriptional activators: Mechanisms and possible role in oncogenesis. J. Viral. Hepat. 1996, 3, 109–121. [Google Scholar] [CrossRef]
- Yen, C.-J.; Yang, S.-T.; Chen, R.-Y.; Huang, W.; Chayama, K.; Lee, M.-H.; Yang, S.-J.; Lai, H.-S.; Yen, H.-Y.; Hsiao, Y.-W.; et al. Hepatitis B virus X protein (HBx) enhances centrosomal P4.1-associated protein (CPAP) expression to promote hepatocarcinogenesis. J. Biomed. Sci. 2019, 26, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yan, Q.; Gong, L.; Xu, H.; Liu, B.; Fang, X.; Yu, D.; Li, L.; Wei, T.; Wang, Y.; et al. C-terminal truncated HBx initiates hepatocarcinogenesis by downregulating TXNIP and reprogramming glucose metabolism. Oncogene 2021, 40, 1147–1161. [Google Scholar] [CrossRef]
- Ali, A.; Abdel-Hafiz, H.; Suhail, M.; Al-Mars, A.; Zakaria, M.K.; Fatima, K.; Ahmad, S.; Azhar, E.; Chaudhary, A.; Qadri, I. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 10238–10248. [Google Scholar] [CrossRef] [PubMed]
- Churin, Y.; Roderfeld, M.; Roeb, E. Hepatitis B virus large surface protein: Function and fame. Hepatobiliary Surg. Nutr. 2015, 4, 1–10. [Google Scholar] [PubMed]
- Li, W. The hepatitis B virus receptor. Annu. Rev. Cell Dev. Biol. 2015, 31, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Watashi, K.; Urban, S.; Li, W.; Wakita, T. NTCP and beyond: Opening the door to unveil hepatitis B virus entry. Int. J. Mol. Sci. 2014, 15, 2892–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.-S.; Ai, Y.-L.; Tsai, H.-W.; Chan, S.-H.; Cheng, K.-H.; Lee, Y.-P.; Kao, C.-W.; Wang, Y.-C.; Chen, Y.-L.; Lin, C.-H.; et al. Hepatitis B virus surface gene pre-S2 mutant as a high-risk serum marker for hepatoma recurrence after curative hepatic resection. Hepatology 2018, 19, 280–286. [Google Scholar]
- Li, T.-N.; Wu, Y.-J.; Tsai, H.-W.; Sun, C.-P.; Wu, H.-L.; Pei, Y.-N.; Lu, K.-Y.; Yen, T.T.-C.; Chang, C.-W.; Chan, H.-L.; et al. Intrahepatic hepatitis B virus large surface antigen induces hepatocyte hyperploidy via failure of cytokinesis. J. Pathol. 2018, 245, 502–513. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, S.Y.; Kim, B.J. Naturally occurring hepatitis B virus mutations leading to endoplasmic reticulum stress and their contribution to the progression of hepatocellular carcinoma. Int. J. Mol. Sci. 2019, 20, 597. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.N.; Chisari, F.V. Strong, sustained hepatocellular proliferation precedes hepatocarcinogenesis in hepatitis B surface antigen transgenic mice. Hepatology 1995, 21, 620–626. [Google Scholar]
- Schulze, A.; Schieck, A.; Ni, Y.; Mier, W.; Urban, S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 2010, 84, 1989–2000. [Google Scholar] [CrossRef] [Green Version]
- Sankhyan, A.; Sharma, C.; Dutta, D.; Sharma, T.; Chosdol, K.; Wakita, T.; Watashi, K.; Awasthi, A.; Acharya, S.K.; Khanna, N.; et al. Inhibition of preS1-hepatocyte interaction by an array of recombinant human antibodies from naturally recovered individuals. Sci. Rep. 2016, 6, 21240. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar] [PubMed]
- Tseng, T.-C.; Liu, C.-J.; Yang, H.-C.; Su, T.-H.; Wang, C.; Chen, C.; Kuo, S.F.; Liu, C.-H.; Chen, P.-J.; Chen, D.-S.; et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012, 142, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.T.; Poovorawan, K.; Charoen, P.; Soonthornworasiri, N.; Nontprasert, A.; Kittitrakul, C.; Phumratanaprapin, W.; Tangkijvanich, P. Association between hepatitis B surface antigen levels and the risk of hepatocellular carcinoma in patients with chronic hepatitis B infection: Systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2019, 20, 2239–2246. [Google Scholar]
- Japp, N.C.; Souchek, J.J.; Sasson, A.R.; Hollingsworth, M.A.; Batra, S.K.; Junker, W.M. Tumor biomarker in-solution quantification, standard production, and multiplex detection. J. Immunol. Res. 2021, 2021, 9942605. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Lu, Y.; Luo, S.; Zhang, J.; Zhu, P. Cryo-EM structure of native spherical subviral particles isolated from HBV carriers. Virus Res. 2019, 259, 90–96. [Google Scholar] [CrossRef]
- Patient, R.; Hourioux, C.; Sizaret, P.Y.; Trassard, S.; Sureau, C.; Roingeard, P. Hepatitis B virus subviral envelope particle morphogenesis and intracellular trafficking. J. Virol. 2007, 81, 3842–3851. [Google Scholar] [CrossRef] [Green Version]
- Bruss, V.; Gerhardt, E.; Vieluf, K.; Wunderlich, G. Functions of the large hepatitis B virus surface protein in viral particle morphogenesis. Intervirology 1996, 39, 23–31. [Google Scholar] [CrossRef]
- Pfefferkorn, M.; Böhm, S.; Schott, T.; Deichsel, D.; Bremer, C.M.; Schröder, K.; Gerlich, W.H.; Glebe, D.; Berg, T.; Van Bömmel, F. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut 2018, 67, 2045–2053. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.J.; Ahn, S.H.; Lee, J.H.; Park, Y.J.; Kim, H.S. Correlation between quantitative serum HBsAg and HBV DNA test in Korean patients who showed high level of HBsAg. J. Clin. Pathol. 2010, 63, 1027–1031. [Google Scholar] [CrossRef]
- Nie, J.J.; Kang, L.; Pian, Y.; Gao, Z.X.; Hu, J.H. Prevalence of hepatitis B surface antigen in coastal area of Tianjin, China. Future Virol. 2020, 15, 91–99. [Google Scholar] [CrossRef]
- Lee, P.L.; Chen, J.J.; Tung, H.D.; Cheng, C.T.; Chuang, T.W.; Wang, S.J.; Wu, H.-C. Serum hepatitis B surface antigen level might predict cirrhosis and hepatocellular carcinoma in older patients with chronic hepatitis B. Adv. Dig. Med. 2015, 2, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.L.; Hung, J.H.; Huang, W. Association of the hepatitis B virus large surface protein with viral infectivity and endoplasmic reticulum stress-mediated liver carcinogenesis. Cells 2020, 9, 2052. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Su, I.J.; Lei, H.Y.; Wang, H.C.; Lin, W.C.; Chang, W.T.; Huang, W.; Chang, W.; Chang, Y.; Chen, C.-H.; et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004, 279, 46384–46392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-C.; Teng, C.-F.; Wu, H.-C.; Tsai, H.-W.; Chuang, H.-C.; Tsai, T.-F.; Hsu, Y.-H.; Huang, W.; Wu, L.-W.; Su, I.-J. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology 2009, 49, 1962–1971. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Su, I.-J.; Wang, H.-C.; Chang, W.-W.; Lei, H.-Y.; Lai, M.-D.; Huang, W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis 2004, 25, 2023–2032. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-H.; Su, I.-J.; Wang, H.-C.; Tsai, J.-H.; Huang, Y.-J.; Chang, W.-W.; Lai, M.-D.; Lei, H.-Y.; Huang, W. Hepatitis B virus pre-S2 mutant surface antigen induces degradation of cyclin-dependent kinase inhibitor p27Kip1 through c-Jun activation domain-binding protein 1. Mol. Cancer Res. 2007, 5, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-H.; Chang, Y.-Y.; Su, I.-J.; Yen, C.-J.; Liu, Y.-R.; Liu, R.-J.; Hsieh, W.-C.; Tsai, H.-W.; Wang, L.H.-C.; Huang, W. Hepatitis B virus pre-S2 mutant large surface protein inhibits DNA double-strand break repair and leads to genome instability in hepatocarcinogenesis. J. Pathol. 2015, 236, 337–347. [Google Scholar] [CrossRef]
- Liu, W.; Cao, Y.; Wang, T.; Xiang, G.; Lu, J.; Zhang, J.; Hou, P. The N-glycosylation modification of LHBs (large surface proteins of HBV) effects on endoplasmic reticulum stress, cell proliferation and its secretion. Hepat. Mon. 2013, 13, e12280. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Wu, H.C.; Chen, C.F.; Fausto, N.; Lei, H.Y.; Su, I.J. Different types of ground glass hepatocytes in chronic hepatitis B virus infection contain specific pre-S mutants that may induce endoplasmic reticulum stress. Am. J. Pathol. 2003, 163, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-W.; Lin, Y.-J.; Lin, P.-W.; Wu, H.-C.; Hsu, K.-H.; Yen, C.-J.; Chan, S.-H.; Huang, W.; Su, I.-J. A clustered ground-glass hepatocyte pattern represents a new prognostic marker for the recurrence of hepatocellular carcinoma after surgery. Cancer 2011, 117, 2951–2960. [Google Scholar] [CrossRef]
- Tsai, H.-W.; Lin, Y.-J.; Wu, H.C.; Chang, T.-T.; Wu, I.-C.; Cheng, P.-N.; Yen, C.-J.; Chan, S.-H.; Huang, W.; Su, I.-J. Resistance of ground glass hepatocytes to oral antivirals in chronic hepatitis B patients and implication for the development of hepatocellular carcinoma. Oncotarget 2016, 7, 27724–27734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
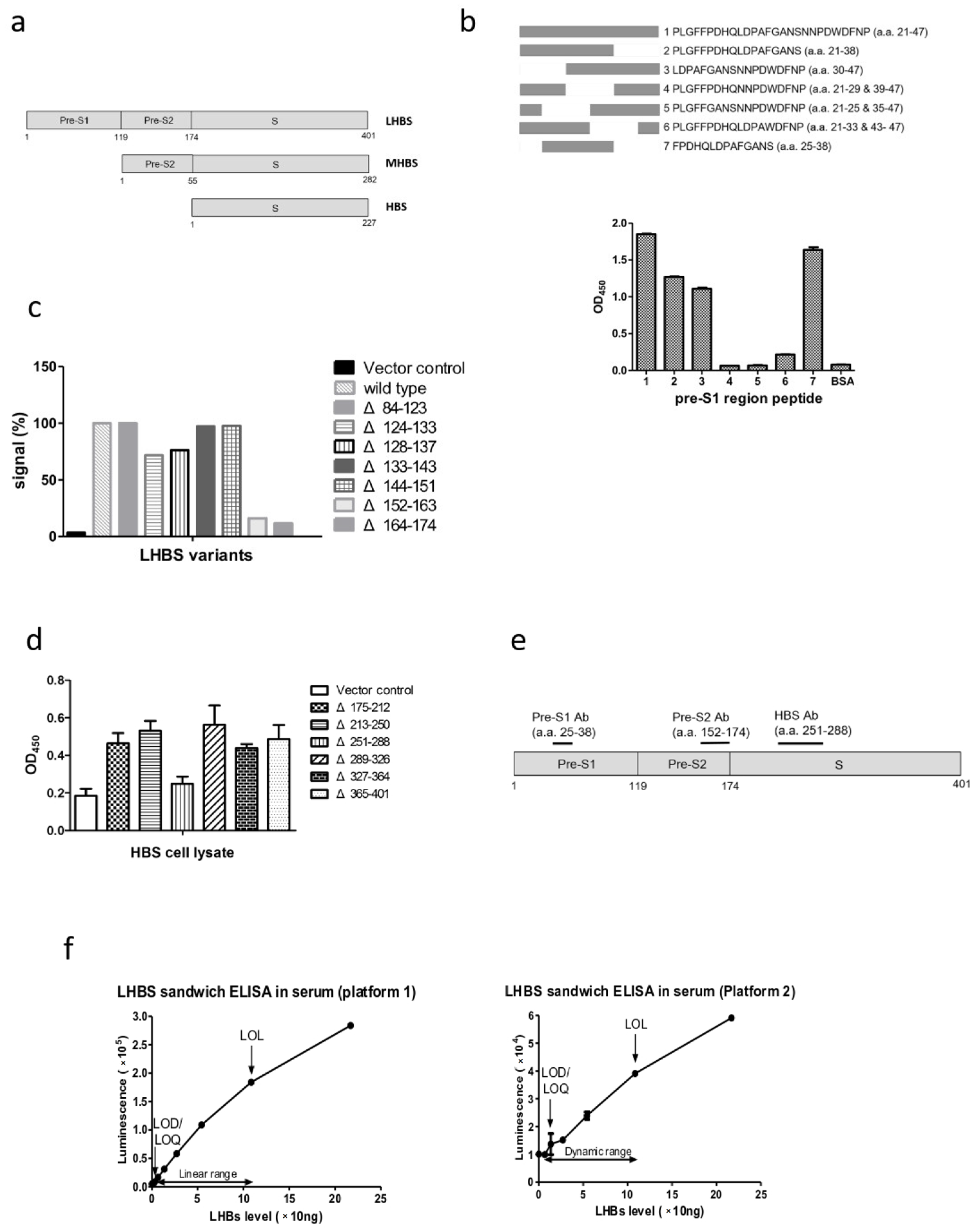
| Antibody | CDR Sequences |
|---|---|
| Pre-S1 | Heavy chain GYSITSDYA (CDR1)….IRYSGTT (CDR2)….ARGGTGLTY (CDR3) |
| Kappa light chain ENVGTY (CDR1)….GAS (CDR2)….GQTYNYPFT (CDR3) | |
| Pre-S2 | Heavy chain GYTFTSYW (CDR1)….INPSNGIT (CDR2)….TIGYDYGSNYEAMDF (CDR3) |
| Kappa light chain KSLLHSNGITY (CDR1)….QMS (CDR2)….AQNLELPWT (CDR3) | |
| HBS | Heavy chain GYTLTDYVIG (CDR1)….EVYPGSVYTSYNEKFKG (CDR2)….AYDGYSPFDY (CDR3) |
| Kappa light chain KASENVGTYVS (CDR1)….GASNRYI (CDR2)….GQSYNYPHT (CDR3) |
| Factors | Group | Serum LHBS (ng/mL) Mean (S.D.) | p Value | Serum HBS (ng/mL) Mean (S.D.) | p Value |
|---|---|---|---|---|---|
| Age | <60 years | 20.57 (20.33) | 0.506 | 778.94 (559.90) | 0.970 |
| ≥60 years | 33.04 (38.35) | 1030.32 (1199.85) | |||
| Sex | Male | 26.00 (27.74) | 0.442 | 929.93 (890.06) | 0.275 |
| Female | 20.90 (32.44) | 607.32 (568.75) | |||
| Cirrhosis | Absent | 14.78 (25.65) | 0.005 * | 725.67 (807.28) | 0.153 |
| Present | 30.77 (28.63) | 949.18 (864.96) | |||
| HBeAg | Absent | 26.81 (28.46) | 0.238 | 926.86 (935.48) | 0.732 |
| Present | 20.10 (28.79) | 708.05 (502.38) | |||
| Serum AFP (ng/mL) | <200 | 25.06 (29.25) | 0.687 | 868.08 (874.88) | 0.742 |
| ≥200 | 24.96 (24.98) | 874.53 (691.82) | |||
| Viral load (IU/mL) | <20,000 | 21.80 (29.65) | 0.157 | 674.75 (871.87) | 0.061 |
| ≥20,000 | 28.96 (26.96) | 1103.84 (761.18) | |||
| Tumor differentiation | W | 17.84 (9.49) | 0.705 | 634.77 (550.53) | 0.471 |
| M-P | 26.32 (30.48) | 910.71 (884.07) | |||
| Multifocal tumors | Absent | 23.07 (26.56) | 0.294 | 784.99 (664.80) | 0.359 |
| Present | 38.01 (38.54) | 1421.50 (1564.14) | |||
| Satellite nodule | Absent | 25.12 (30.22) | 0.566 | 818.02 (875.01) | 0.117 |
| Present | 24.71 (20.14) | 1088.51 (689.43) | |||
| Tumor size | <5 cm | 23.63 (28.09) | 0.684 | 766.13 (844.84) | 0.098 |
| ≥5 cm | 27.78 (29.67) | 1069.19 (828.50) | |||
| Vascular invasion | Absent | 19.34 (23.03) | 0.238 | 684.03 (516.76) | 0.242 |
| Present | 31.82 (33.29) | 1098.15 (1084.99) | |||
| AJCC stage | I-II | 14.79 (16.59) | 0.010 * | 588.15 (470.62) | 0.010 * |
| IIIA-C | 37.42 (34.63) | 1208.49 (1058.58) | |||
| Pre-S deletion | Absent | 28.88 (32.94) | 0.833 | 806.16 (832.59) | 0.445 |
| Present | 22.52 (25.27) | 910.33 (861.86) |
| Factors | Group | Liver LHBS (%) Mean (S.D.) | p Value | Tumor LHBS (%) Mean (S.D.) | p Value |
|---|---|---|---|---|---|
| Age | <60 years | 26.85 (16.48) | 0.383 | 41.29 (32.13) | 0.264 |
| ≥60 years | 23.42 (17.63) | 50.68 (31.47) | |||
| Sex | Male | 26.85 (16.48) | 0.142 | 41.29 (32.13) | 0.205 |
| Female | 23.42 (17.63) | 50.68 (31.47) | |||
| Cirrhosis | Absent | 18.89 (14.03) | 0.044 * | 61.31 (27.52) | 0.007 * |
| Present | 29.38 (17.25) | 35.35 (30.71) | |||
| HBeAg | Absent | 25.89 (17.20) | 0.936 | 43.17 (34.52) | 0.578 |
| Present | 24.85 (16.29) | 48.78 (23.80) | |||
| Serum AFP (ng/mL) | <200 | 24.22 (15.68) | 0.297 | 44.37 (32.34) | 0.893 |
| ≥200 | 33.50 (21.70) | 46.25 (31.48) | |||
| Viral load (IU/mL) | <20,000 | 21.93 (14.76) | 0.116 | 42.96 (31.74) | 0.641 |
| ≥20,000 | 30.08 (18.33) | 46.70 (32.69) | |||
| Tumor differentiation | W | 27.87 (11.24) | 0.412 | 43.12 (24.63) | 0.932 |
| M-P | 25.22 (17.69) | 44.93 (33.27) | |||
| Multifocal tumors | Absent | 24.86 (16.45) | 0.478 | 45.47 (32.32) | 0.634 |
| Present | 30.57 (19.67) | 39.28 (30.88) | |||
| Satellite nodule | Absent | 23.95 (17.10) | 0.086 | 41.67 (31.33) | 0.138 |
| Present | 32.80 (14.07) | 57.50 (32.85) | |||
| Tumor size | <5 cm | 24.71 (15.05) | 0.792 | 46.77 (30.67) | 0.509 |
| ≥5 cm | 27.38 (20.16) | 40.55 (34.76) | |||
| Vascular invasion | Absent | 22.37 (16.48) | 0.061 | 34.14 (31.28) | 0.009 * |
| Present | 30.66 (16.28) | 57.50 (28.59) | |||
| AJCC stage | I-II | 23.93 (16.62) | 0.442 | 43.93 (30.54) | 0.809 |
| IIIA-C | 27.66 (17.18) | 45.54 (34.15) | |||
| Pre-S deletion | Absent | 24.19 (16.14) | 0.696 | 58.09 (29.47) | 0.052 |
| Present | 26.56 (17.43) | 35.84 (30.76) |
| Factors | Group | Liver HBS (%) Mean (S.D.) | p Value | Tumor HBS (%) Mean (S.D.) | p Value |
|---|---|---|---|---|---|
| Age | <60 years | 22.70 (16.56) | 0.228 | 3.50 (8.57) | 0.177 |
| ≥60 years | 17.05 (15.71) | 7.10 (16.11) | |||
| Sex | Male | 22.90 (16.67) | 0.056 | 3.69 (7.82) | 0.980 |
| Female | 11.10 (10.93) | 9.50 (22.13) | |||
| Cirrhosis | Absent | 17.21 (13.68) | 0.358 | 7.26 (17.45) | 0.611 |
| Present | 22.61 (17.54) | 3.41 (6.95) | |||
| HBeAg | Absent | 21.05 (16.76) | 0.920 | 2.61 (6.51) | 0.055 |
| Present | 19.64 (15.65) | 10.85 (19.50) | |||
| Serum AFP (ng/mL) | <200 | 18.91 (14.61) | 0.179 | 4.37 (11.90) | 0.223 |
| ≥200 | 30.62 (22.55) | 7.12 (11.78) | |||
| Viral load (IU/mL) | <20,000 | 17.34 (14.87) | 0.113 | 2.13 (4.54) | 0.077 |
| ≥20,000 | 24.70 (17.42) | 8.00 (16.45) | |||
| Tumor differentiation | W | 26.75 (11.96) | 0.142 | 10.25 (24.30) | 0.687 |
| M-P | 19.60 (16.88) | 3.82 (8.03) | |||
| Multifocal tumors | Absent | 19.95 (16.11) | 0.431 | 3.82 (7.93) | 0.787 |
| Present | 25.42 (18.35) | 11.14 (26.12) | |||
| Satellite nodule | Absent | 18.41 (15.98) | 0.045 * | 5.72 (12.94) | 0.112 |
| Present | 30.40 (14.87) | 0.80 (1.75) | |||
| Tumor size | <5 cm | 19.34 (15.09) | 0.547 | 5.34 (13.22) | 0.488 |
| ≥5 cm | 23.27 (18.71) | 3.72 (8.67) | |||
| Vascular invasion | Absent | 16.74 (14.39) | 0.055 | 5.66 (14.62) | 0.707 |
| Present | 25.87 (17.55) | 4.20 (8.38) | |||
| AJCC stage | I-II | 18.55 (15.73) | 0.287 | 4.51 (9.43) | 0.562 |
| IIIA-C | 23.25 (17.01) | 5.12 (14.38) | |||
| Pre-S deletion | Absent | 22.00 (17.40) | 0.696 | 7.90 (16.56) | 0.207 |
| Present | 19.81 (15.83) | 2.75 (6.80) |
| Factor | Group | Disease-Free Survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Age | <60/≥60 years | 0.517 (0.205–1.305) | 0.163 | |||
| Sex | Male/female | 0.538 (0.160–1.805) | 0.315 | |||
| Cirrhosis | −/+ | 1.121 (0.479–2.621) | 0.793 | |||
| Serum AFP | <200/≥200 ng/mL | 1.570 (0.585–4.213) | 0.371 | |||
| Viral load | <20,000/≥20,000 IU/mL | 2.354 (1.028–5.394) | 0.043 * | NS | ||
| Differentiation | W/M-P | 1.709 (0.508–5.748) | 0.419 | |||
| Multifocal tumor | −/+ | 2.056 (0.699–6.044) | 0.190 | |||
| Satellite nodule | −/+ | 1.945 (0.768–4.930) | 0.161 | |||
| Tumor size | <5/≥5 cm | 4.140 (1.833–9.352) | 0.001 * | 3.979 (1.750–9.048) | 0.001 * | |
| Vascular invasion | −/+ | 2.317 (1.023–5.248) | 0.044 * | 2.590 (1.157–5.797) | NS | |
| AJCC stage | I-II/IIIA-C | 2.762 (1.142–6.681) | 0.024 * | NS | ||
| HBeAg | −/+ | 1.173 (0.486–2.833) | 0.723 | |||
| Pre-S deletion | −/+ | 0.852 (0.379–1.916) | 0.698 | |||
| Serum HBS | <1000/≥1000 ng/mL | 1.806 (0.798–4.088) | 0.156 | |||
| Serum LHBS | <25/≥25 ng/mL | 2.246 (1.007–5.010) | 0.048 * | 2.267 (1.001–5.137) | NS | |
| Small HBS stain | <30/≥30% | 1.686 (0.748–3.802) | 0.208 | |||
| LHBS stain | <30/≥30% | 2.811 (1.229–6.433) | 0.014 * | 2.650 (1.154–6.087) | 0.022 * | |
| Factor | Group | Overall Survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Age | <60/≥60 years | 0.453 (0.126–1.624) | 0.224 | |||
| Sex | Male/female | 0.035 (0.000–15.883) | 0.283 | |||
| Cirrhosis | −/+ | 1.533 (0.480–4.897) | 0.471 | |||
| Serum AFP | <200/≥200 ng/mL | 0.800 (0.179–3.577) | 0.770 | |||
| Viral load | <20,000/≥20,000 IU/mL | 2.188 (0.730–6.557) | 0.162 | |||
| Differentiation | W/M-P | 1.609 (0.357–7.249) | 0.535 | |||
| Multifocal tumor | −/+ | 5.266 (1.549–17.899) | 0.008 * | 27.399 (4.053–185.239) | 0.001 * | |
| Satellite nodule | −/+ | 1.996 (0.621–6.416) | 0.246 | |||
| Tumor size | <5/≥5 cm | 4.195 (1.398–12.591) | 0.011 * | 19.935 (3.387–117.335) | 0.001 * | |
| Vascular invasion | −/+ | 2.892 (1.114–7.513) | 0.029 * | NS | ||
| AJCC stage | I-II/IIIA-C | 4.750 (1.314–17.173) | 0.017 * | NS | ||
| HBeAg | −/+ | 1.225 (0.376–3.992) | 0.736 | |||
| Pre-S deletion | −/+ | 0.911 (0.317–2.615) | 0.863 | |||
| Serum HBS | <1000/≥1000 ng/mL | 2.745 (0.921–8.179) | 0.070 | |||
| Serum LHBS | <40/≥40 ng/mL | 3.508 (1.183–10.406) | 0.024 * | 6.432 (1.276–32.422) | 0.024 * | |
| Small HBS stain | <30/≥30% | 2.046 (0.713–5.876) | 0.183 | |||
| LHBS stain | <30/≥30% | 5.431 (1.513–19.497) | 0.009 * | 5.250 (1.295–21.278) | 0.020 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-W.; Lee, Y.-P.; Yen, C.-J.; Cheng, K.-H.; Huang, C.-J.; Huang, W. The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery. Int. J. Mol. Sci. 2022, 23, 5376. https://doi.org/10.3390/ijms23105376
Tsai H-W, Lee Y-P, Yen C-J, Cheng K-H, Huang C-J, Huang W. The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery. International Journal of Molecular Sciences. 2022; 23(10):5376. https://doi.org/10.3390/ijms23105376
Chicago/Turabian StyleTsai, Hung-Wen, Yun-Ping Lee, Chia-Jui Yen, Kuang-Hsiung Cheng, Chien-Jung Huang, and Wenya Huang. 2022. "The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery" International Journal of Molecular Sciences 23, no. 10: 5376. https://doi.org/10.3390/ijms23105376
APA StyleTsai, H.-W., Lee, Y.-P., Yen, C.-J., Cheng, K.-H., Huang, C.-J., & Huang, W. (2022). The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery. International Journal of Molecular Sciences, 23(10), 5376. https://doi.org/10.3390/ijms23105376






