Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae
Abstract
1. Introduction
2. Experiment Results
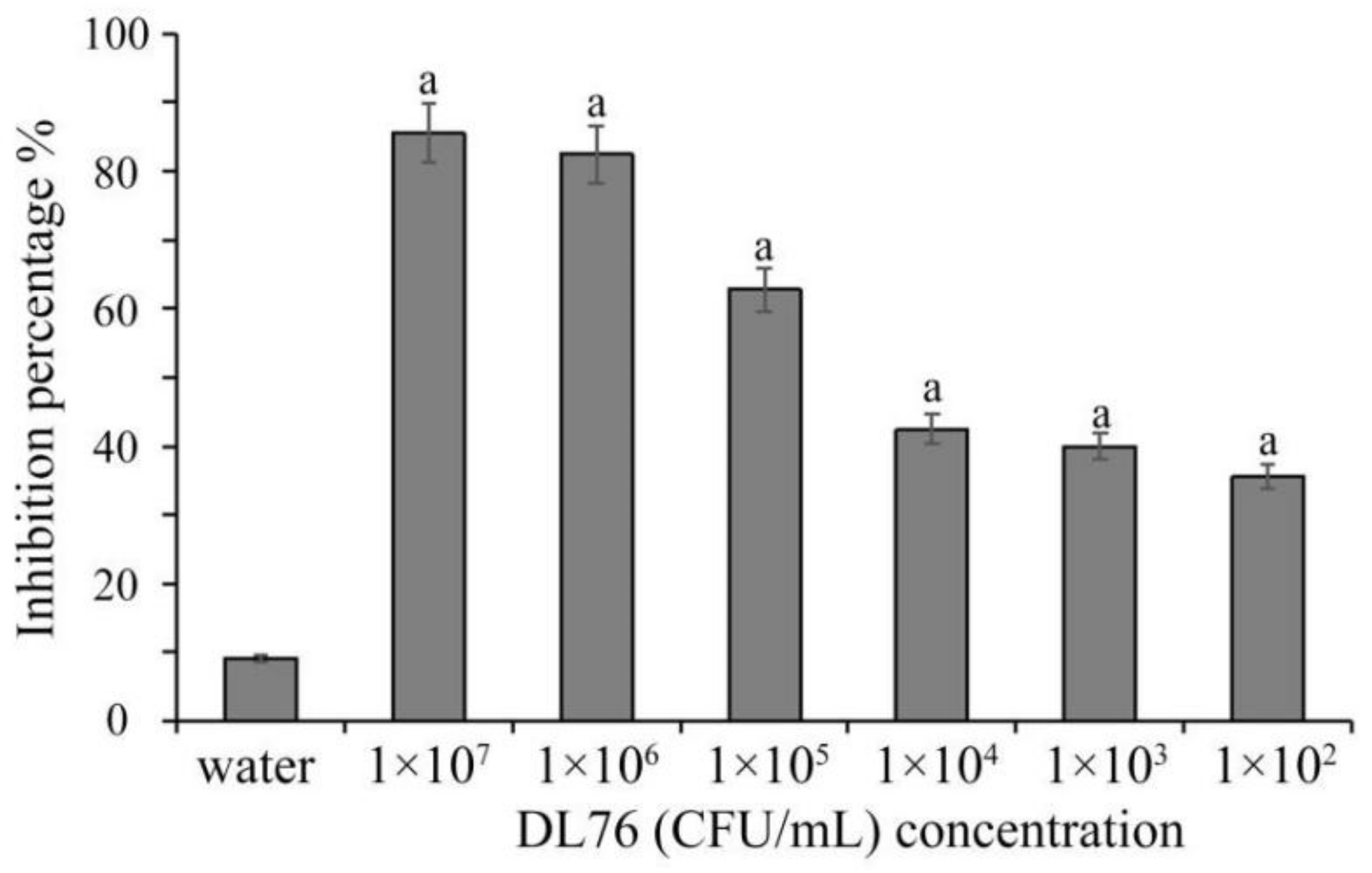
2.1. The Antifungal Activities of the Culture Filtrates DL76 at Different Concentration
2.2. Stability of Antifungal Metabolites
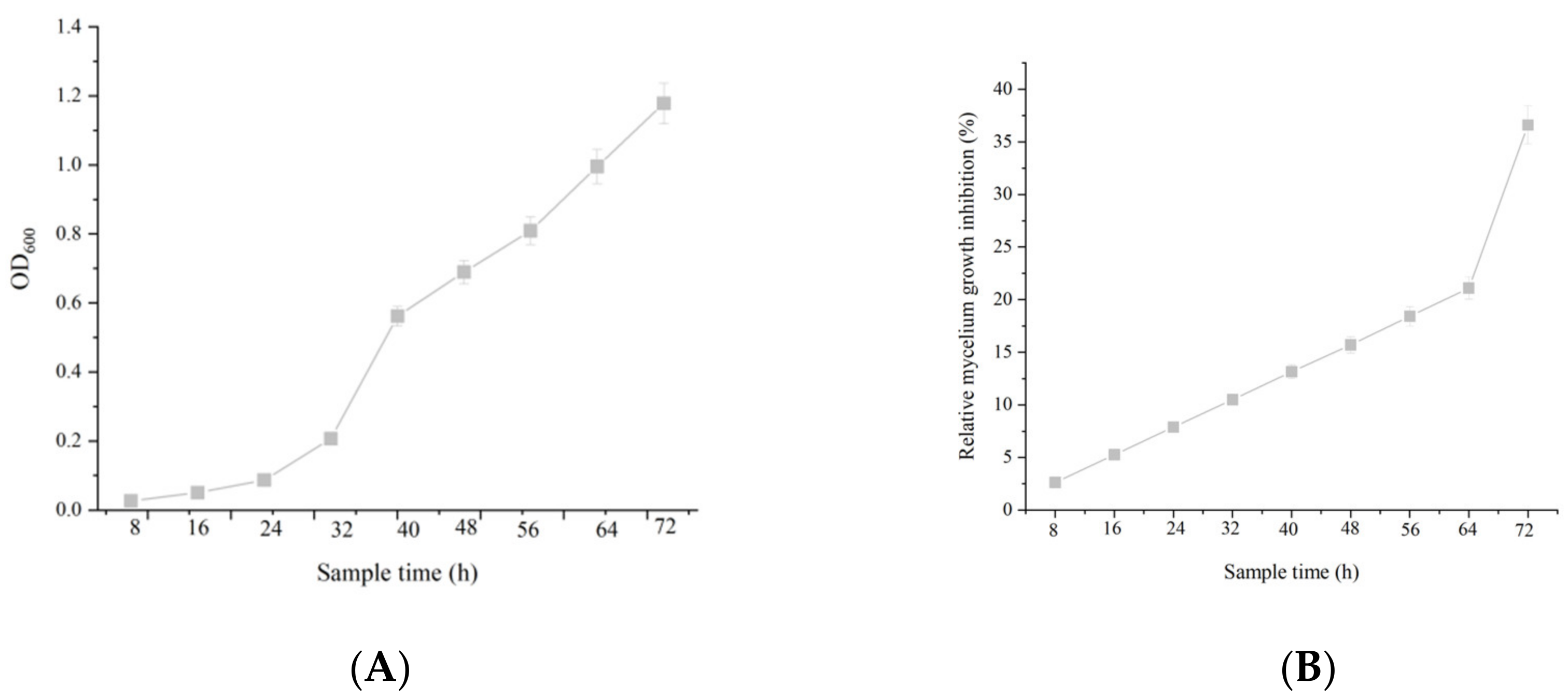
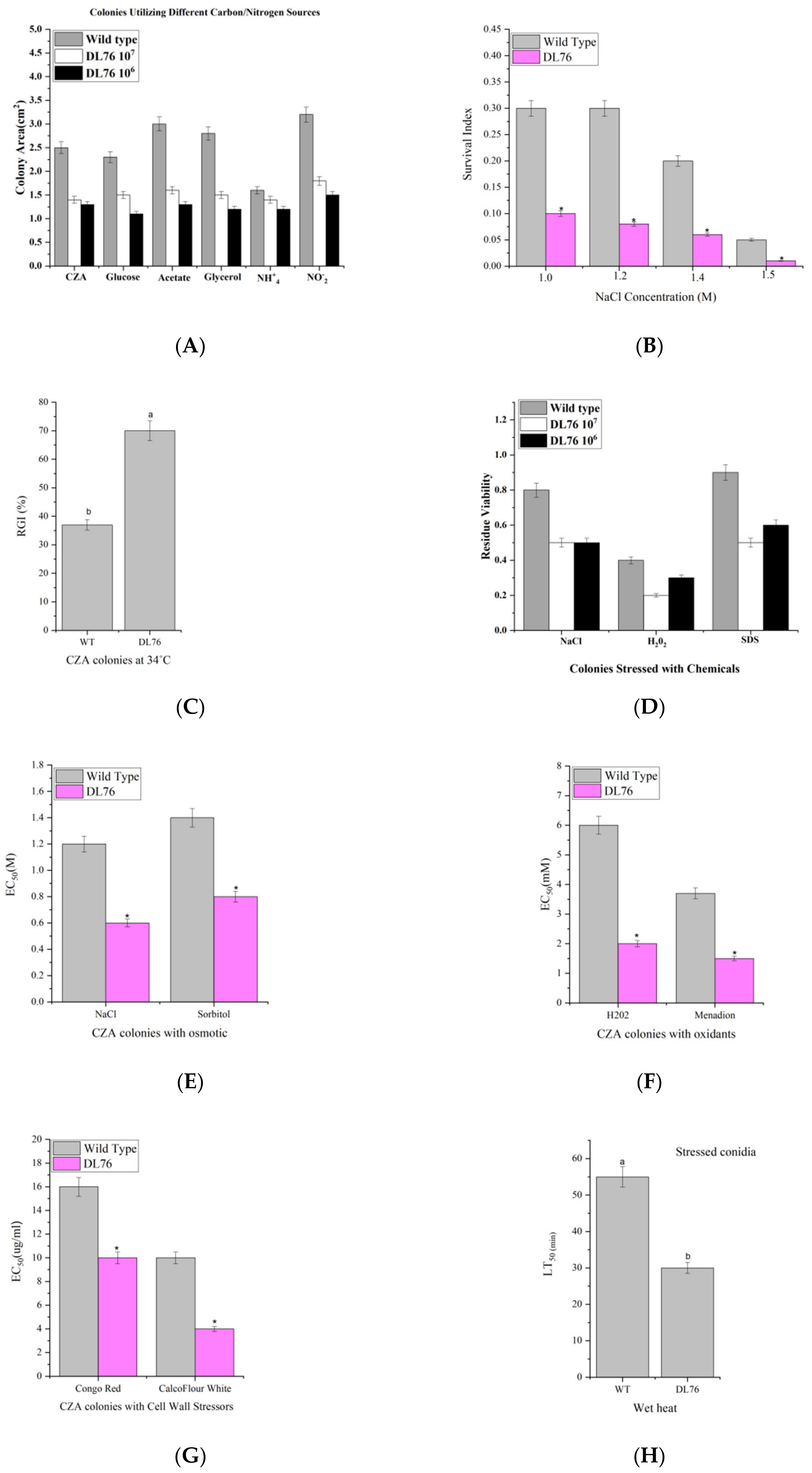
2.3. The Correlation between Cell Growth and Anti-Fungal Activity
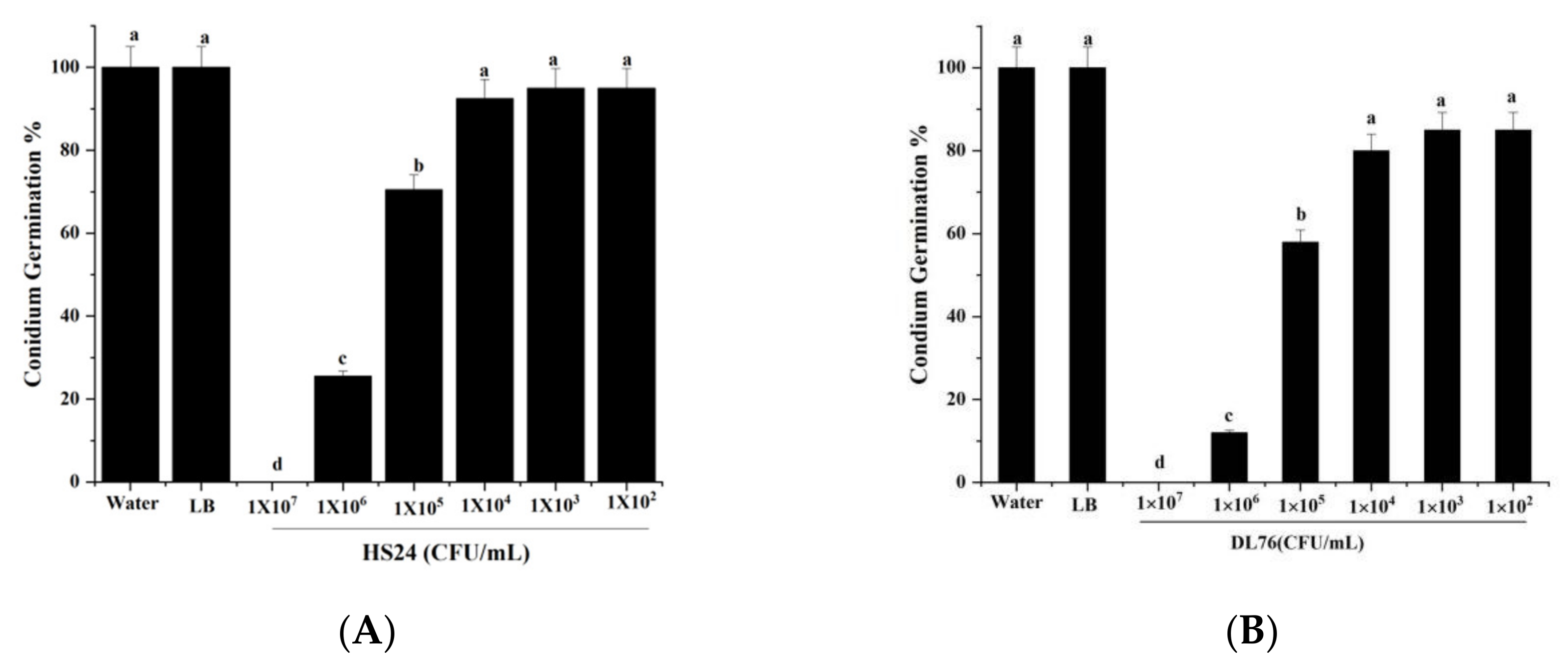
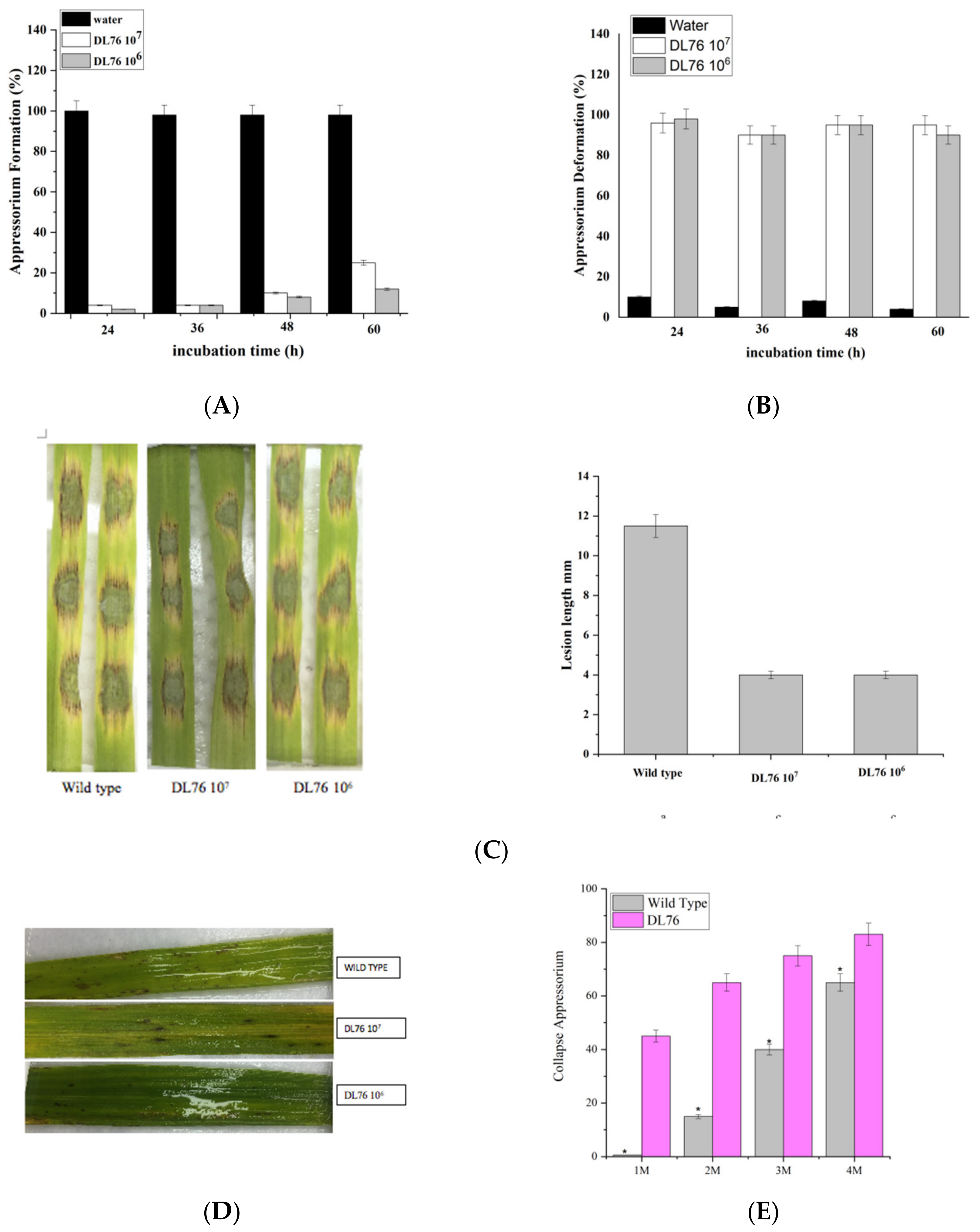
2.4. Effect of DL76 on Conidium Attachment
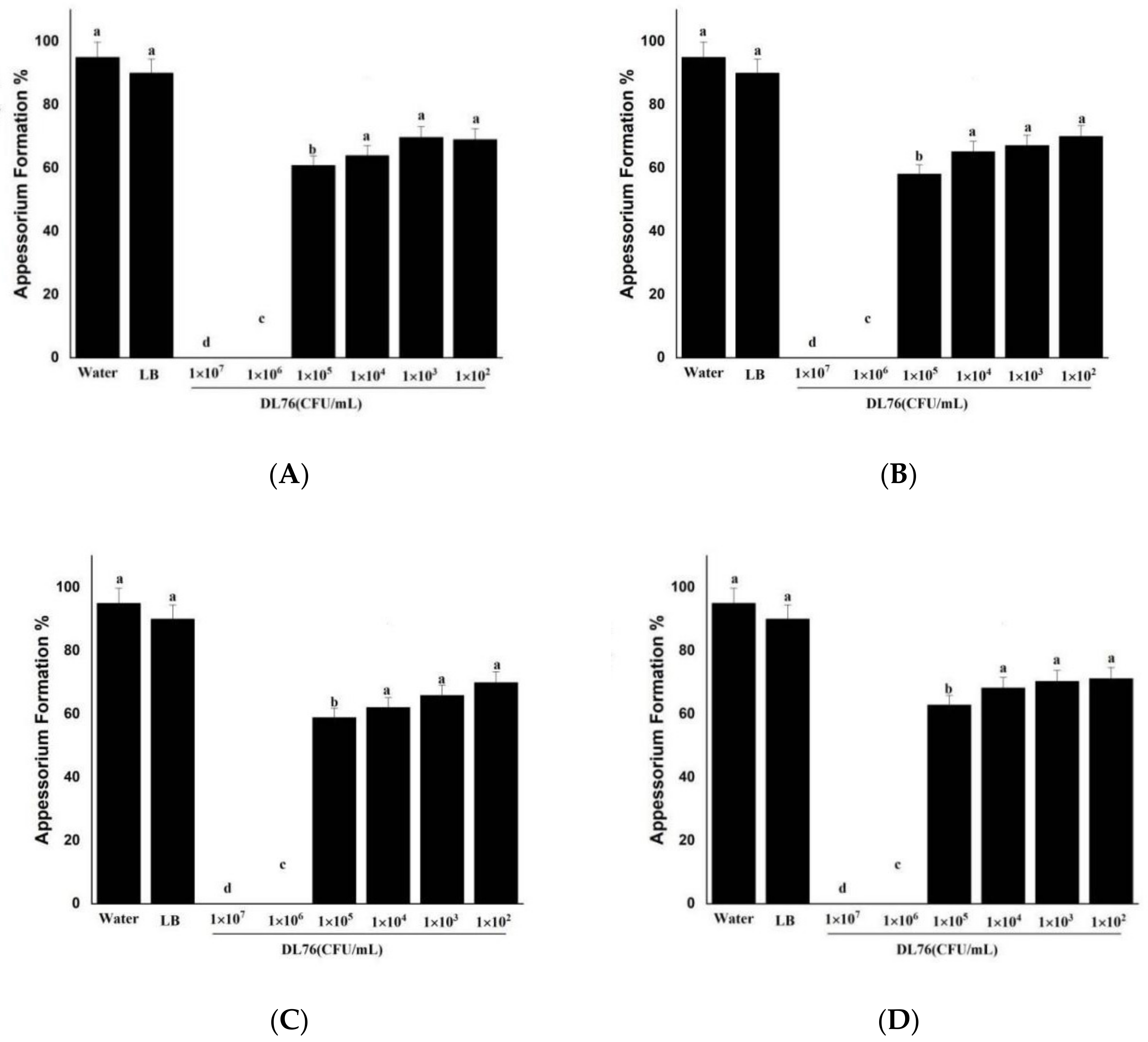
2.5. The DL76 Suppresses the Formation of Appressorium
2.6. Differentiation of Appressoria
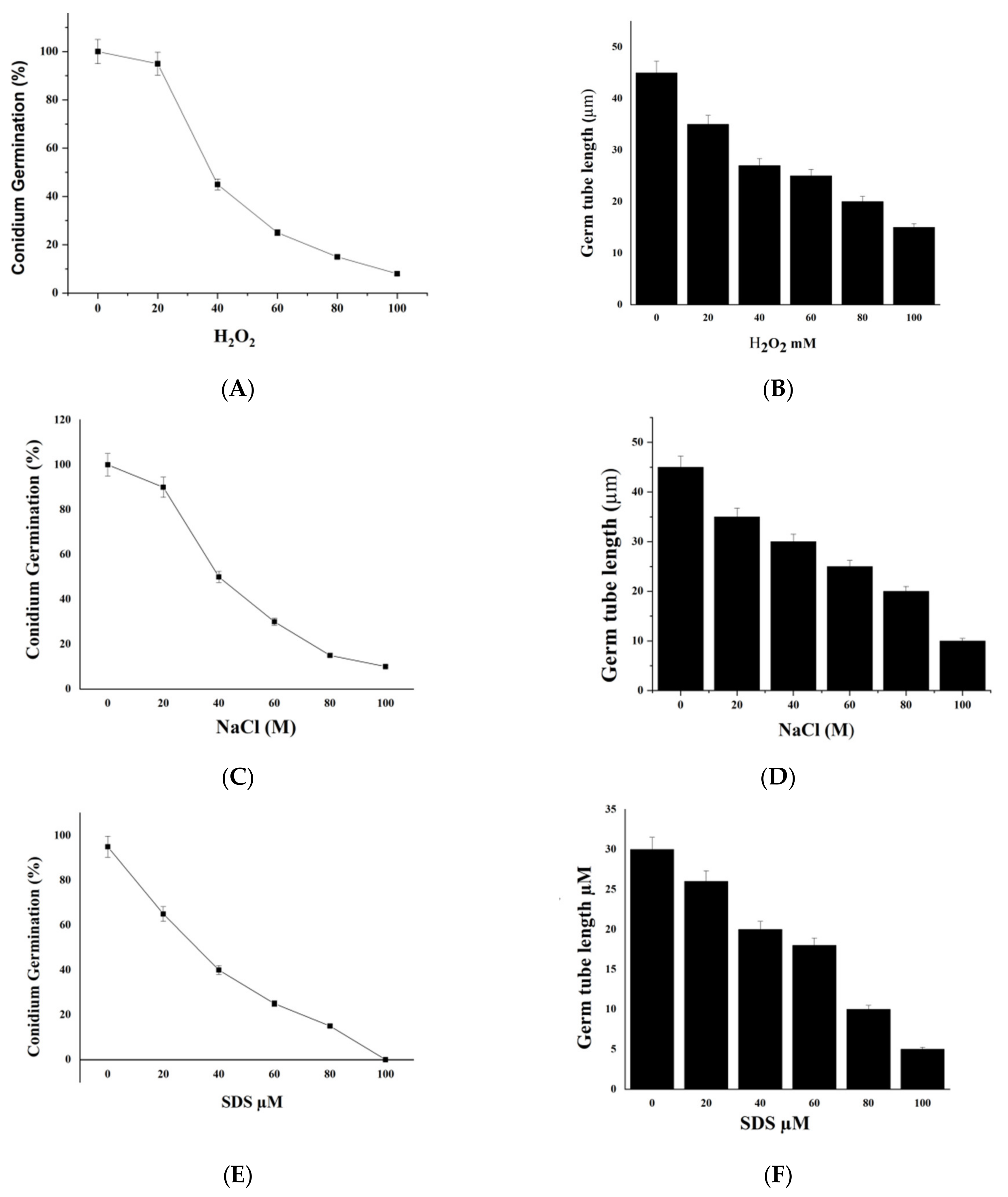
2.7. We Treated M. oryzae Conidia with Environmental Stress Inhibitors
2.8. DL76 Reduced M. oryzae Adaptation to the Environment and Contributed to Cellular Response and Abiotic Stresses
2.9. DL76 Is Essential for Pathogenic Development in M. oryzae and Responsible for Appressorium-like Structure Formation
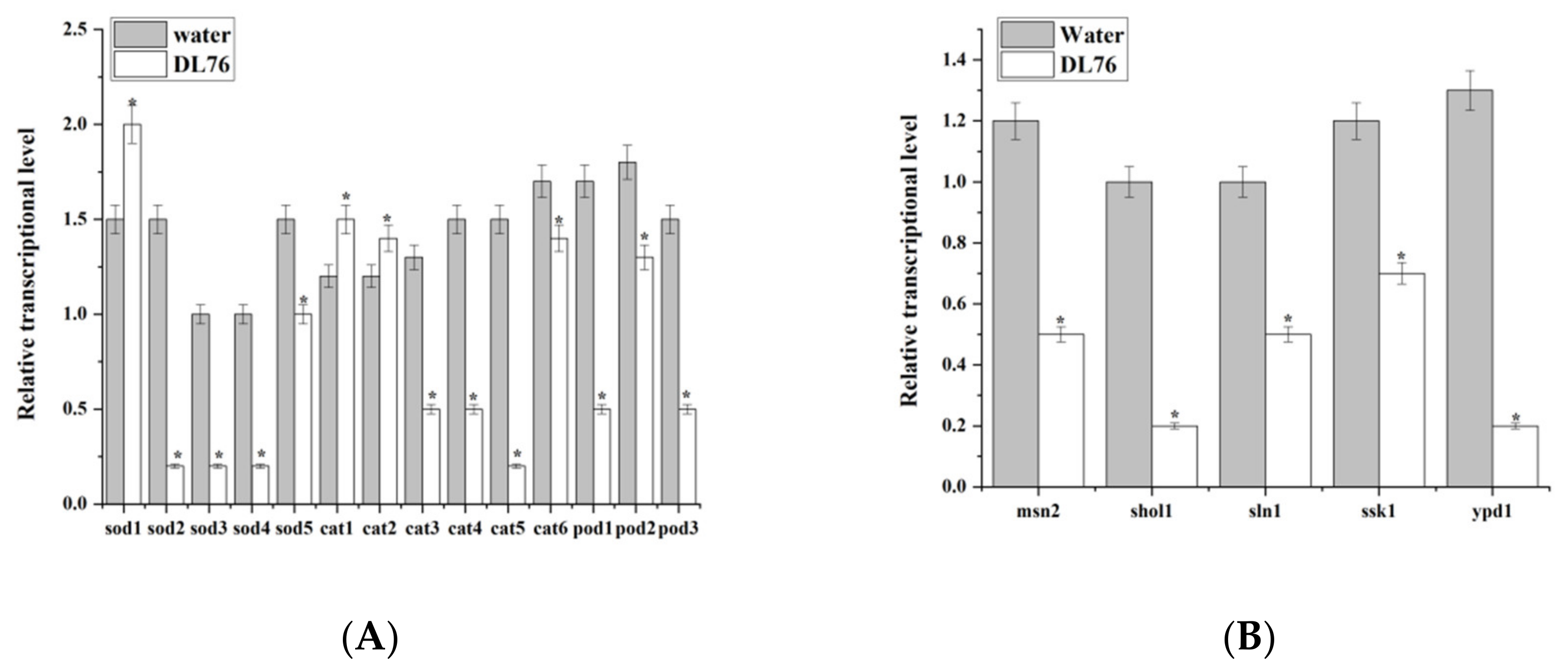
2.10. DL76 Is Involved in Down-Regulating the Expression of Oxidation and Osmotic Genes in M. oryzae
3. Discussion
4. Materials and Methods
4.1. The Evaluation of Antifungal Activity
4.2. The Stability of PH and Temperature of Filtrate on the Growth Suppression of M. oryzae
4.3. Correlation between Cell Growth and Anti-Fungal Activity
4.4. Effect of the Supernatant at Different Concentrations on Conidium Germination, Appressorium Formation, Germ Tube Length, Turgor Pressure, Infection Process, Vegetative Growth and Conidiation, and Cell Activity
4.4.1. The Conidia Germination Test
4.4.2. The Appressorium Formation
4.5. Environmental Stresses at Different Concentrations of SDS, H2O2, Glycerol, and NaCl (0, 20, 40, 60, 80, and 100 µΜ) Were Added to the Conidial Suspension
4.6. Measurement of Growth and Conidiation Parameters
4.7. Assaying Cellular Responses to Chemical and Environmental Stresses
4.8. Assays for Stress Tolerance of Antisense Transformants
4.9. Experiment on Isolated Leaves
4.10. RNA Extraction and Transcripts of Stress-Responsive Gene Transcription Analysis
4.11. Statistics and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.X.; Zhang, X.X.; Shen, S.H. Proteome Analysis for Antifungal Effects of Bacillus subtilis KB-1122 on Magnaporthe grisea P131. World J. Microbiol. Biotchnol. 2014, 30, 1763–1774. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Tu, X.R.; Wei, S.J.; Huang, L.; Li, X.H.; Lu, H. In Vitro Antifungal Activity of Antifungalmycin 702, A New Polyene Macrolide Antibiotic, Against the Rice Blast Fungus Magnaporthe-grisea. Biotechnol. Lett. 2013, 35, 1475. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Y.; Ning, P.; Zheng, L.; Huang, J.; Li, G. Suppression of Magnaporthe oryzae by Culture Filtrates of Streptomyces globisporus JK-1. Biol. Control 2011, 58, 139–148. [Google Scholar] [CrossRef]
- Ohtaka, N.; Kawamata, H.; Narisawa, K. Suppression of Rice Blast Using Freeze-Killed Mycelia of Biocontrol Fungal Candidate MKP5111B. J. Gen. Plant Pathol. 2008, 74, 101–108. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of Biological Control Agents and Physical Treatments in Maintaining the Quality of Fresh and Minimally-Processed Fruit and Vegetables. Crit. Rev. Food Sci. Nutr. 2019, 60, 2837–2855. [Google Scholar] [CrossRef]
- Moraes, B.J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.M.; Kupper, K.C.; Augusto, F.; De Carvalho, J.E.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Kohl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Hyakumachi, M.; Takahashi, H.; Matsubara, Y.; Someya, N.; Shimizu, M.; Kobayashi, K. Recent Studies on Biological Control of Plant Diseases in Japan. J. Gen. Plant Pathol. 2014, 80, 287–302. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Rabindran, R.; Muthamilan, M.; Nayar, K.; Rajappan, K.; Subramanian, N. Development of a Powder Formulation of Pseudomonas fluorescens for Control of Rice Blast. Plant Pathol. 2010, 46, 291–297. [Google Scholar] [CrossRef]
- Sha, Y.; Wang, Q.; Li, Y. Suppression of Magnaporthe oryzae and Interaction between Bacillus subtilis and Rice Plants in the Control of Rice Blast. SpringerPlus 2016, 5, 1238. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Gnanamanickam, S.S. Biological Control of Setaria Blast (Magnaporthe grisea) with Bacterial Strains. Crop Prot. 2008, 27, 263–267. [Google Scholar] [CrossRef]
- Zarandi, M.E.; Bonjar, G.H.S.; Dehkaei, F.P.; Moosavi, S.A.A.; Farokhi, P.R.; Aghighi, S. Biological Control of Rice Blast (Magnaporthe oryzae) By Use of Streptomyces sindeneusis Isolate 263 in Greenhouse. Am. J. Appl. Sci. 2009, 6, 194–199. [Google Scholar] [CrossRef]
- Anand, T.; Chandrasekaran, A.; Kuttalam, S.; Senthilraja, G.; Samiyappan, R. Integrated Control of Fruit Rot and Powdery Mildew of Chilli Using the Biocontrol Agent Pseudomonas fluorescens and A Chemical Fungicide. Biol. Control 2010, 52, 1–7. [Google Scholar] [CrossRef]
- Yan, Q.H.; Zhou, J.X.; Li, H.Z.; Zhi, Q.Q.; Zhou, X.P.; He, Z.M. Coexistence of and Interaction Relationships between Anaflatoxin-Producing Fungus and a Bacterium. Fungal Biol. 2015, 119, 605–614. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Miller, M.E.; Sudini, H.; Reddy, E.C.S.; Zhou, X.G.; Groth, D.E. Ultrastructural Studies on The Interaction Between Bacillus subtilis MBI 600 (Integral) and the Rice Sheath Blight Pathogen, Rhizoctonia solani. Afr. J. Microbiol. Res. 2013, 7, 2078–2086. [Google Scholar]
- Strunnikova, O.K.; Vishnevskaya, N.A.; Ruchiy, A.S.; Shakhnazarova, V.Y.; Vorobyov, N.I.; Chebotar, V.K. The Influence of Soils with Different Textures on Development, Colonization Capacity, and Interactions Between Fusarium culmorum and Pseudomonas fluorescens in Soil and on Barley Roots. Plant Soil 2015, 389, 131–144. [Google Scholar] [CrossRef]
- Grover, M.; Nain, L.; Singh, S.B.; Saxena, A.K. Molecular and Biochemical Approaches for Characterization of Antifungal Trait of a Potent Biocontrol Agent Bacillus subtilis RP24. Curr. Microbiol. 2010, 60, 99–106. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural Products from Endophytic Microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of Endophytic Bacillus velezensis ZSY-1 Strain and Antifungal Activity of Its Volatile Compounds Against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Ali, G.S.; El-Sayed, A.; Patel, J.S.; Green, K.B.; Ali, M.; Brennan, M.; Norman, D. Ex Vivo Application of Secreted Metabolites Produced by Soil-Inhabiting Bacillus Spp. Efficiently Controls Foliar Diseases Caused by Alternaria Spp. Appl. Environ. Microbiol. 2015, 82, 478–490. [Google Scholar] [CrossRef]
- Howard, R.J.; Ferrari, M.A.; Roach, D.H.; Money, N.P. Penetration of Hard Substrates by a Fungus Employing Enormous Turgor Pressures. Proc. Natl. Acad. Sci. USA 1991, 88, 11281–11284. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. On The Trail of A Cereal Killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.A.; Koiv, V.; Norman-Setterblad, C.; Pirhonen, M. Role of Rpos in Virulence and Stress Tolerance of the Plant Pathogen Erwinia Carotovora Subsp. Carotovora. Microbiology 1999, 145, 3547–3556. [Google Scholar] [CrossRef][Green Version]
- Guo, M.; Chen, Y.; Du, Y.; Dong, Y.H.; Guo, W.; Zhai, S.; Zhang, H.F.; Dong, S.M.; Zhang, Z.G.; Wang, Y.C.; et al. The Bzip Transcription Factor Moap1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae. PLoS Pathog. 2011, 7, e1001302. [Google Scholar] [CrossRef]
- Tokpah, D.P.; Li, H.W.; Wang, L.Y.; Liu, X.Y.; Mulbah, Q.S.; Liu, H.X. An Assessment System for Screening Effective Bacteria as Biological Control Agents against Magnaporthe grisea on Rice. Biol. Control 2016, 103, 21–29. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhao, Q.; Liu, K.Y.; Zhang, Z.G.; Wang, Y.C.; Zheng, X.B. Mgcrz1, a Transcription Factor of Magnaporthe grisea, Controls Growth, Development and is Involved in Full Virulence. FEMS Microbiol. Lett. 2009, 293, 160–169. [Google Scholar] [CrossRef]
- Sellem, I.; Triki, M.A.; Elleuch, L.; Cheffi, M.; Chakchouk, A.; Smaoui, S. The Use of Newly Isolated Streptomyces Strain TN258 as a Potential Biocontrol Agent of Potato Tubers Leak Caused by Pythium Ultimum. J. Basic Microbiol. 2017, 57, 393. [Google Scholar] [CrossRef]
- Han, T.; You, C.; Zhang, L.; Feng, C.; Zhang, C.; Wang, J. Biocontrol Potential of Antagonist Bacillus subtilis Tpb55 Against Tobacco Black Shank. BiolControl 2016, 61, 195–205. [Google Scholar] [CrossRef]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M.E. Potential of A New Strain of Bacillus amyloliquefaciens BUZ-14 As A Biocontrol Agent of Postharvest Fruit Diseases. Food Microbiol. 2013, 63, 101–110. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, C.; Li, Y.; Xu, J.R. Germination and Infectivity of Microconidia in the Rice Blast Fungus Magnaporthe oryzae. Nat. Commun. 2014, 5, 4518. [Google Scholar] [CrossRef]
- Leelasuphakul, W.; Sivanunsakul, P.; Phongpaichit, S. Purification, Characterization and Synergistic Activity of Beta-1,3-Glucanase and Antibiotic Extract from An Antagonistic Bacillus subtilis NSRS 89-24 Against Rice Blast and Sheath Blight. Enzyme Microb. Technol. 2006, 38, 990–997. [Google Scholar] [CrossRef]
- Chernin, L.; Chet, I. Microbial Enzymes in the Biocontrol of Plant Pathogens and Pests. In Enzymes in the Environment: Activity, Ecology, and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 171–226. [Google Scholar]
- Li, R.Y.; Wu, X.M.; Yin, X.H.; Liang, J.N.; Li, M. The Natural Product Citral can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.R.; Hamer, J.E. MAP Kinase and Camp Signalling Regulate Infection Structure Formation and Pathogenic Growth in The Rice Blast Fungus Magnaporthe grisea. Genes Dev. 1996, 10, 2696–2706. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under Pressure: Investigating the biology of Plant Infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.-Y.; Kim, K.S.; Rho, H.-S.; Chi, M.-H.; Choi, J.; Park, J.; Kong, S.; Park, J.; Goh, J.; et al. Homeobox Transcription Factors are Required for Conidiation and Appressorium Development in The Rice Blast Fungus Magnaporthe oryzae. PLoS Genet. 2009, 5, e1000757. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Lin, C.; He, C. Conidiophore Stalk-Less1 Encodes a Putative Zinc-Finger Protein Involved in The Early Stage of Conidiation and Mycelial Infection in Magnaporthe oryzae. Mol. Plant Microbe Interact. 2009, 22, 402–410. [Google Scholar] [CrossRef]
- Kong, L.A.; Li, G.T.; Liu, Y.; Liu, M.G.; Zhang, S.J.; Yang, J.; Zhou, X.Y.; Peng, Y.L.; Xu, J.R. Differences Between Appressoria Formed by Germ Tubes and Appressorium-Like Structures Developed by Hyphal Tips in Magnaporthe oryzae. Fungal Genet. Biol. 2013, 56, 33–41. [Google Scholar] [CrossRef]
- Howard, R.J.; Valent, B. Breaking and Entering: Host Penetration by the Fungal Rice Blast Pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Magnaporthe grisea Cutinase2 Mediates Appressorium Differentiation and Host Penetration and is required for Full Virulence. Plant Cell 2007, 19, 2674–2689. [Google Scholar] [CrossRef]
- Jeon, J.; Goh, J.; Yoo, S.; Chi, M.H.; Choi, J.; Rho, H.S.; Park, J.; Han, S.S.; Kim, B.R.; Park, S.Y.; et al. A Putative MAP Kinase, MCK1, is required for Cell Wall Integrity and Pathogenicity of the Rice Blast Fungus, Magnaporthe oryzae. Mol. Plant Microbe Interact. 2008, 21, 525–534. [Google Scholar] [CrossRef] [PubMed]




| Involved in Response to Oxidative Stress | ||
|---|---|---|
| sod1 | GCGGCTTCCACATCCACACCTTTG | GGTCCAGCGTTGCCAGTCTTGAG |
| sod2 | CCAGTGTTTGGCATTGACATG | TCAGCCGTCTTCCAGTTGATG |
| sod3 | TCTCCGGCAAGATTATGGAGC | TTGGCGTCATTCTTGGCCT |
| cat1 | CCTCTGACGTTGGCGGCCCTTTC | CCGTGTCCGTGCTGCCTCGTG |
| cat2 | CCGTCTGGGCATCAACTGGGAAG | GCTGGGCGTGGTCGTGGTAG |
| cat3 | TCAAGTCGGTTCAGGAGATGGAG | TTGTTGCGTCTTCAATCGGAGTG |
| cat4 | GAGGAGCCCAGCAACGCACAAGAG | CTGAGGACGACAAGGCCGCCATTC |
| cat5 | GCTGGGCTGATCTGCTGGTCCTTG | TCCTTGCTGTAACGGTGGCTGTCG |
| cat6 | CGGCTGCGGTGTCTTGTCCATAC | CCTTGTCGGCGTTCTGGCGAAG |
| Involved in Response to Osmotic Stress | ||
| sho1 | AGCGTCAACAACCTCGTCTAC | AGGCGTGGAGTTCTCAAAG |
| sln1 | TCGGTGCCATCACTCCTTCCAACG | GCGGACGAGCAATGCCAACAGC |
| ssk1 | AGAACTTTAGCACCGAGCCCTTTC | AGAAGCAGCAGCAGACGATTGG |
| ypd1 | TGACCAGGTCGAGGAGACGTTTGC | TCGGCGTCGGGTTCTGATGAGC |
| msn2 | GCCCGCCACGCCCATCTAC | ACCGAGGTCTCAACCGAGTCAAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgosi, V.T.; Tingting, B.; Ying, Z.; Liu, H. Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 5314. https://doi.org/10.3390/ijms23105314
Kgosi VT, Tingting B, Ying Z, Liu H. Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. International Journal of Molecular Sciences. 2022; 23(10):5314. https://doi.org/10.3390/ijms23105314
Chicago/Turabian StyleKgosi, Veronica Tshogofatso, Bao Tingting, Zhao Ying, and Hongxia Liu. 2022. "Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae" International Journal of Molecular Sciences 23, no. 10: 5314. https://doi.org/10.3390/ijms23105314
APA StyleKgosi, V. T., Tingting, B., Ying, Z., & Liu, H. (2022). Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. International Journal of Molecular Sciences, 23(10), 5314. https://doi.org/10.3390/ijms23105314






