Molecular Networking for Drug Toxicities Studies: The Case of Hydroxychloroquine in COVID-19 Patients
Abstract
:1. Introduction
2. Results
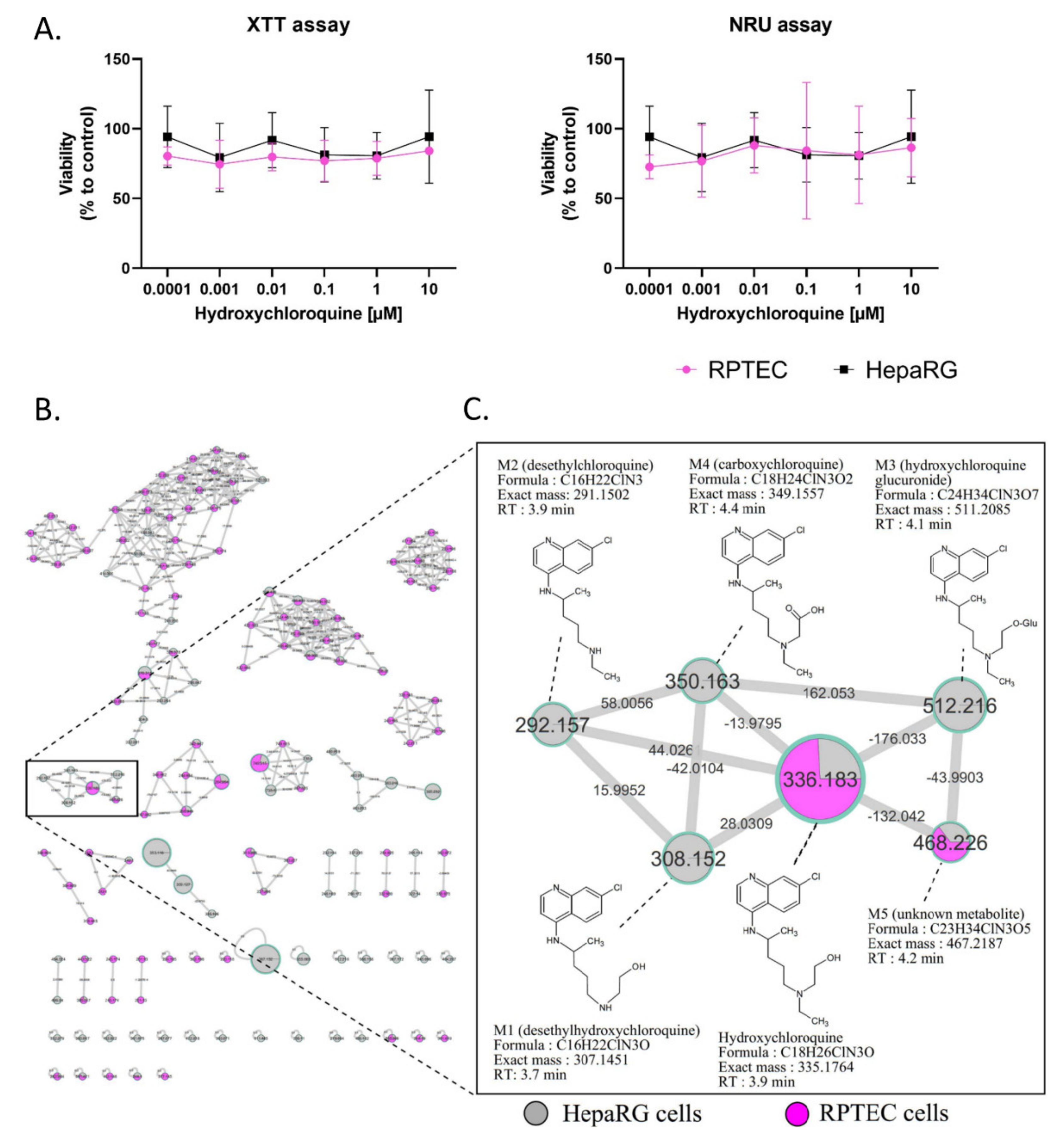
2.1. Cytotoxicity and Metabolism of Hydroxychloroquine
2.2. In Vivo Metabolism of Hydroxychloroquine
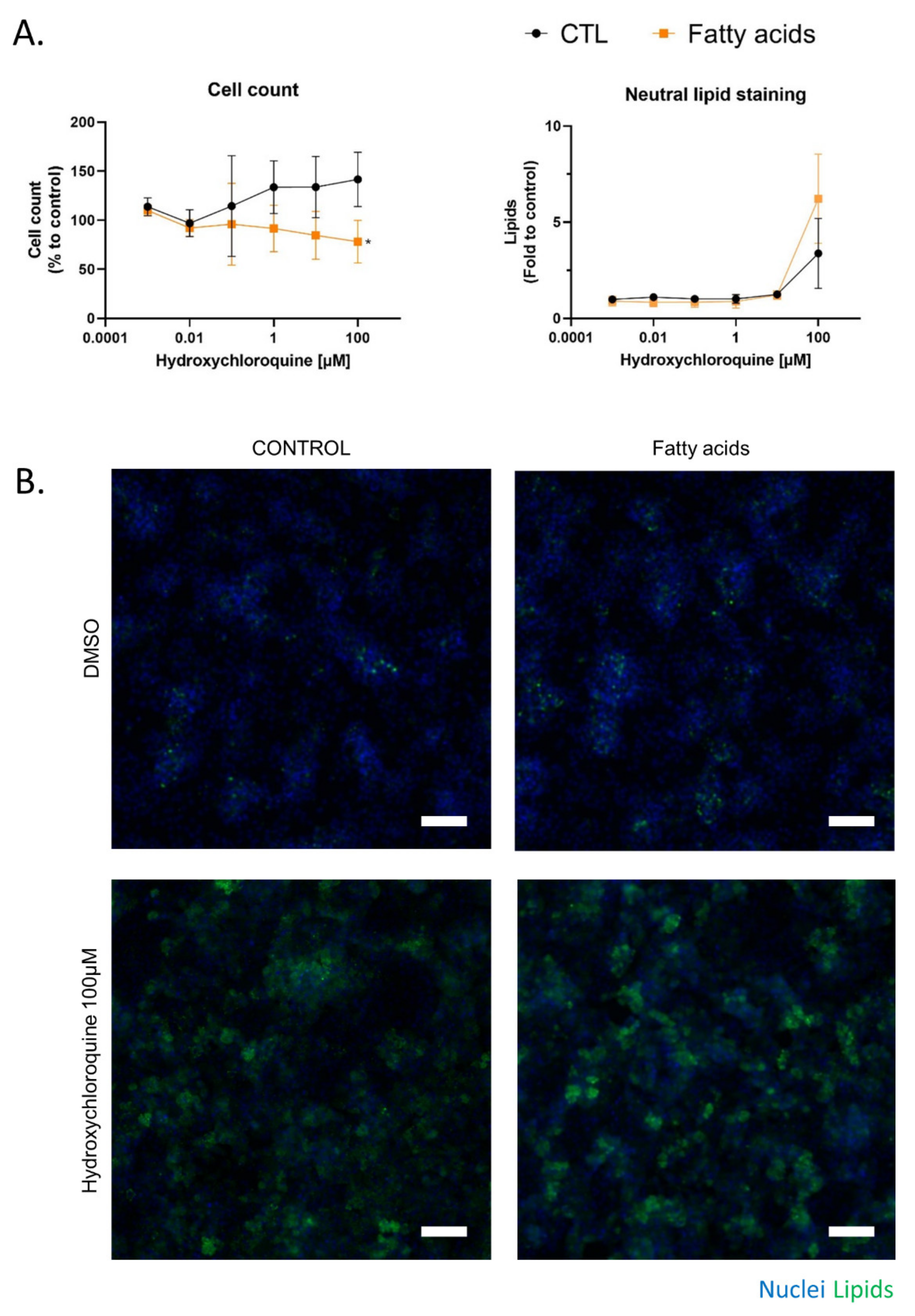
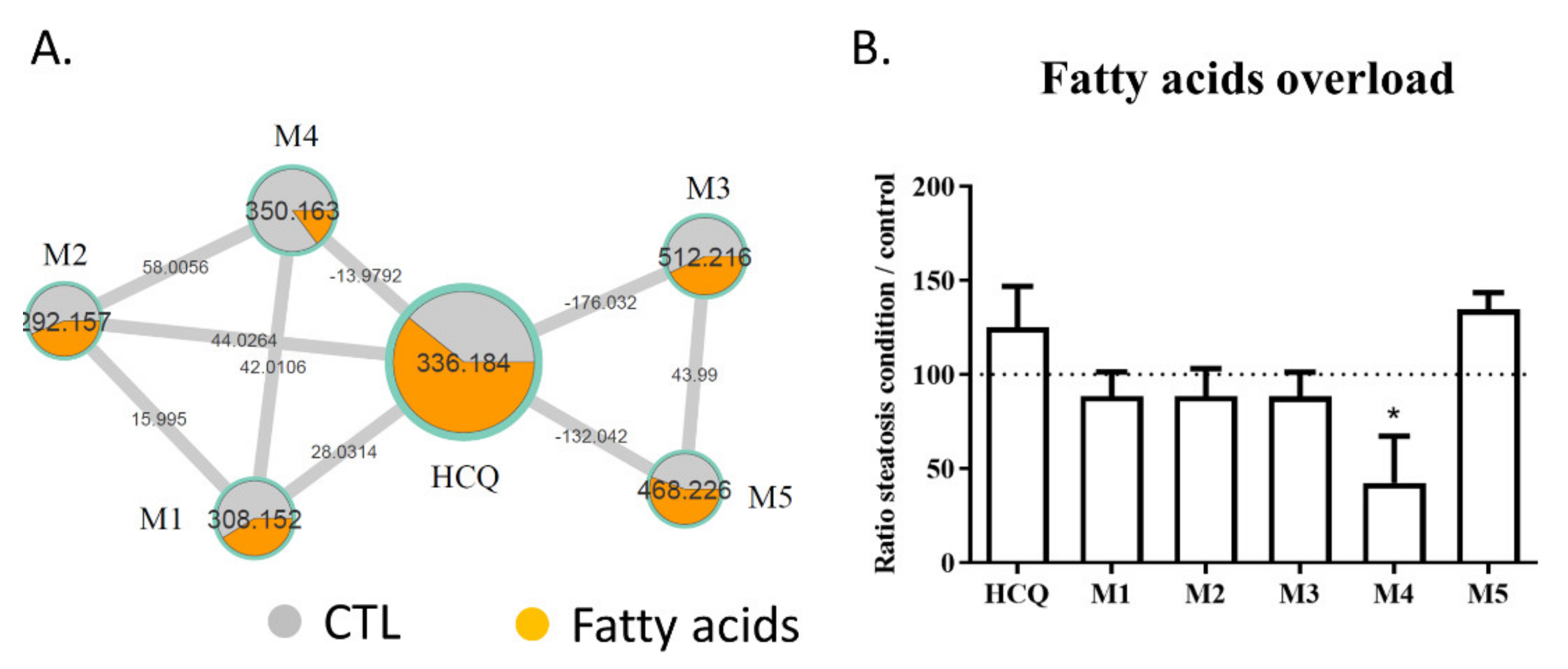
2.3. Fatty Acid Treatment Induces a Change in HCQ Metabolism and Toxicity
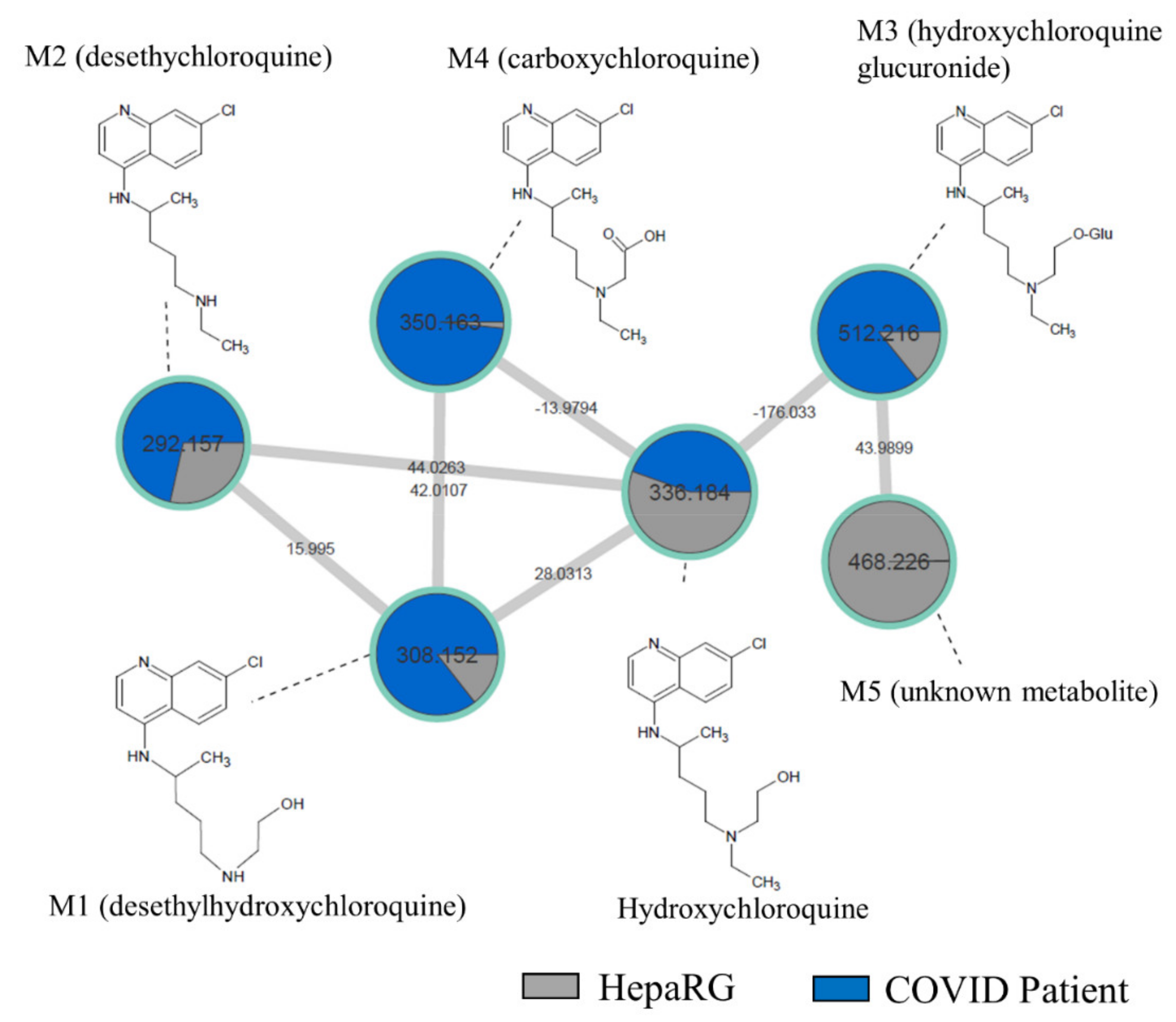
2.4. Comparison of HCQ Metabolization between In Vitro and Patients
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Cell Culture and Treatment
4.3. XTT Assay
4.4. Neutral Red Uptake (NRU) Assay
4.5. Immunofluorescence
4.6. High Content Analysis
4.7. Sample Extraction
4.8. LC-MS Settings
4.9. Molecular Networking Generation
4.10. Patients
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Deploying Biomolecules as Anti-COVID-19 Agents. Indian J. Microbiol. 2020, 60, 263–268. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Diet, Gut Microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 420–429. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney Disease Is Associated with In-Hospital Death of Patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Lütgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Nörz, D.; Heinrich, F.; Meißner, K.; Wichmann, D.; et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef]
- Shao, M.; Li, X.; Liu, F.; Tian, T.; Luo, J.; Yang, Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: A systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol. Res. 2020, 161, 105107. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Ghoneim, S.; Butt, M.U.; Hamid, O.; Shah, A.; Asaad, I. The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: A population-based study. Metab. Open 2020, 8, 100057. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.K.; Barbisan, C.C.; Cruz, I.R.; De Araújo, E.M.; Libânio, B.B.; Albuquerque, K.S.; Torres, U.S. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom. Radiol. 2020, 45, 2748–2754. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Lu, M.; Yang, D.; Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020, 40, 998–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferron, P.-J.; Gicquel, T.; Mégarbane, B.; Clément, B.; Fromenty, B. Treatments in COVID-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie 2020, 179, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; Prudon, B.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy). Available online: https://clinicaltrials.gov/ct2/show/NCT04315948 (accessed on 26 March 2020).
- Carrière, F.; Longhi, S.; Record, M. The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-CoV-2and other enveloped viruses hijacking the endocytic pathway. Biochimie 2020, 179, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.-S.; Im, J.-S.; Cho, J.-Y.; Bae, K.-S.; Klein, T.A.; Yeom, J.-S.; Kim, T.-S.; Choi, J.-S.; Jang, I.-J.; Park, J.-W. Pharmacokinetics of Hydroxychloroquine and Its Clinical Implications in Chemoprophylaxis against Malaria Caused by Plasmodium vivax. Antimicrob. Agents Chemother. 2009, 53, 1468–1475. [Google Scholar] [CrossRef] [Green Version]
- White, N.J.; Watson, J.A.; Hoglund, R.M.; Chan, X.H.S.; Cheah, P.Y.; Tarning, J. COVID-19 prevention and treatment: A critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020, 17, e1003252. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, Y.S.; Sleightholm, R.L.; Li, J.; Oupický, D.; Murry, D.J. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC–ESI–MS/MS: An application for pharmacokinetic studies. J. Chromatogr. B 2018, 1072, 320–327. [Google Scholar] [CrossRef]
- Collins, K.P.; Jackson, K.M.; Gustafson, D.L. Hydroxychloroquine: A Physiologically-Based Pharmacokinetic Model in the Context of Cancer-Related Autophagy Modulation. J. Pharmacol. Exp. Ther. 2018, 365, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, D.L.; Day, C.W.; Bailey, K.; Heiner, M.; Montgomery, R.; Lauridsen, L.; Chan, P.K.; Sidwell, R.W. Evaluation of Immunomodulators, Interferons and Known in Vitro SARS-CoV Inhibitors for Inhibition of SARS-CoV Replication in BALB/c Mice. Antivir. Chem. Chemother. 2006, 17, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagier, J.-C.; Million, M.; Gautret, P.; Colson, P.; Cortaredona, S.; Giraud-Gatineau, A.; Honoré, S.; Gaubert, J.-Y.; Fournier, P.-E.; Tissot-Dupont, H.; et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020, 36, 101791. [Google Scholar] [CrossRef] [PubMed]
- Painvin, B.; Guillot, P.; Verdier, M.; Gacouin, A.; Maamar, A. Pharmacocinétique de l’hydroxychloroquine chez les patients COVID-19 en réanimation: Étude de cohorte observationnelle. Méd. Malad. Infect. 2020, 50, S90–S91. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, S.; Allard, P.; Morel, I.; Gicquel, T. Application of a molecular networking approach for clinical and forensic toxicology exemplified in three cases involving 3-MeO-PCP, doxylamine, and chlormequat. Drug Test. Anal. 2019, 11, 669–677. [Google Scholar] [CrossRef]
- Le Daré, B.; Ferron, P.-J.; Allard, P.-M.; Clément, B.; Morel, I.; Gicquel, T. New insights into quetiapine metabolism using molecular networking. Sci. Rep. 2020, 10, 19921. [Google Scholar] [CrossRef]
- Allard, J.; Bucher, S.; Massart, J.; Ferron, P.-J.; Le Guillou, D.; Loyant, R.; Daniel, Y.; Launay, Y.; Buron, N.; Begriche, K.; et al. Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in HepaRG cells: Proof of multiple mechanism-based toxicity. Cell Biol. Toxicol. 2021, 37, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Aninat, C.; Piton, A.; Glaise, D.; Le Charpentier, T.; Langouët, S.; Morel, F.; Guguen-Guillouzo, C.; Guillouzo, A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab. Dispos. 2005, 34, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.X.; Kaeslin, G.; Ranall, M.V.; Blaskovich, M.A.; Becker, B.; Butler, M.S.; Little, M.H.; Lash, L.H.; Cooper, M.A. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: Comparison of HK -2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol. Res. Perspect. 2015, 3, e00148. [Google Scholar] [CrossRef]
- Michaut, A.; Le Guillou, D.; Moreau, C.; Bucher, S.; McGill, M.; Martinais, S.; Gicquel, T.; Morel, I.; Robin, M.-A.; Jaeschke, H.; et al. A cellular model to study drug-induced liver injury in nonalcoholic fatty liver disease: Application to acetaminophen. Toxicol. Appl. Pharmacol. 2016, 292, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Bucher, S.; Le Guillou, D.; Allard, J.; Pinon, G.; Begriche, K.; Tête, A.; Sergent, O.; Lagadic-Gossmann, D.; Fromenty, B. Possible Involvement of Mitochondrial Dysfunction and Oxidative Stress in a Cellular Model of NAFLD Progression Induced by Benzo[a]pyrene/Ethanol CoExposure. Oxidative Med. Cell. Longev. 2018, 2018, 4396403. [Google Scholar] [CrossRef] [Green Version]
- Le Guillou, D.; Bucher, S.; Begriche, K.; Hoët, D.; Lombès, A.; Labbe, G.; Fromenty, B. Drug-Induced Alterations of Mitochondrial DNA Homeostasis in Steatotic and Nonsteatotic HepaRG Cells. J. Pharmacol. Exp. Ther. 2018, 365, 711–726. [Google Scholar] [CrossRef]
- Teo, W.; Caprariello, A.V.; Morgan, M.L.; Luchicchi, A.; Schenk, G.J.; Joseph, J.T.; Geurts, J.J.G.; Stys, P.K. Nile Red fluorescence spectroscopy reports early physicochemical changes in myelin with high sensitivity. Proc. Natl. Acad. Sci. USA 2021, 118, e2016897118. [Google Scholar] [CrossRef] [PubMed]
- E Furst, D. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996, 5, 11–15. [Google Scholar] [CrossRef]
- Rendic, S.; Guengerich, S.P.R.A.F.P. Metabolism and Interactions of Chloroquine and Hydroxychloroquine with Human Cytochrome P450 Enzymes and Drug Transporters. Curr. Drug Metab. 2020, 21, 1127–1135. [Google Scholar] [CrossRef]
- Le Daré, B.; Allard, S.; Bouvet, R.; Baert, A.; Allard, P.-M.; Morel, I.; Gicquel, T. A case of fatal acebutolol poisoning: An illustration of the potential of molecular networking. Int. J. Leg. Med. 2019, 134, 251–256. [Google Scholar] [CrossRef]
- Pelletier, R.; Le Daré, B.; Grandin, L.; Couette, A.; Ferron, P.-J.; Morel, I.; Gicquel, T. New psychoactive substance cocktail in an intensive care intoxication case elucidated by molecular networking. Clin. Toxicol. 2021, 59, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Paniri, A.; Hosseini, M.M.; Rasoulinejad, A.; Akhavan-Niaki, H. Molecular effects and retinopathy induced by hydroxychloroquine during SARS-CoV-2 therapy: Role of CYP450 isoforms and epigenetic modulations. Eur. J. Pharmacol. 2020, 886, 173454. [Google Scholar] [CrossRef]
- Bucher, S.; Jalili, P.; Le Guillou, D.; Begriche, K.; Rondel, K.; Martinais, S.; Zalko, D.; Corlu, A.; Robin, M.-A.; Fromenty, B. Bisphenol a induces steatosis in HepaRG cells using a model of perinatal exposure. Environ. Toxicol. 2016, 32, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Tummino, T.A.; Rezelj, V.V.; Fischer, B.; Fischer, A.; O’Meara, M.J.; Monel, B.; Vallet, T.; Zhang, Z.; Alon, A.; O’Donnell, H.R.; et al. Phospholipidosis is a shared mechanism underlying the in vitro antiviral activity of many repurposed drugs against SARS-CoV-2. bioRxiv 2021. [Google Scholar] [CrossRef]
- Allard, J.; LeGuillou, D.; Begriche, K.; Fromenty, B. Drug-Induced Liver Injury in Obesity and Nonalcoholic Fatty Liver Disease. Adv. Pharmacol. 2019, 85, 75–107. [Google Scholar]
- Boya, P.; Polo, R.-A.G.; Poncet, D.; Andreau, K.; Vieira, H.; Roumier, T.; Perfettini, J.-L.; Kroemer, G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in non-alcoholic fatty liver disease. Int. J. Biochem. Cell Biol. 2018, 95, 93–99. [Google Scholar] [CrossRef]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines Down-Regulate Expression of Major Cytochrome P-450 Enzymes in Adult Human Hepatocytes in Primary Culture. Mol. Pharm. 1993, 44, 707–715. [Google Scholar]
- Kihara, T.; Toda, A.; Umesue, I.; Ono, N.; Shigematsu, H.; Soeda, S.; Shimeno, H. Effect of interleukin 1? -induced fever on hepatic drug metabolism in rat. Xenobiotica 1998, 28, 559–569. [Google Scholar] [CrossRef]
- Legendre, C.; Hori, T.; Loyer, P.; Aninat, C.; Ishida, S.; Glaise, D.; Lucas-Clerc, C.; Boudjema, K.; Guguen-Guillouzo, C.; Corlu, A.; et al. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF-1α involvement in CYP3A4 repression. Eur. J. Cancer 2009, 45, 2882–2892. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.-A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Cerec, V.; Glaise, D.; Garnier, D.; Morosan, S.; Turlin, B.; Drenou, B.; Gripon, P.; Kremsdorf, D.; Guguen-Guillouzo, C.; Corlu, A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 2007, 45, 957–967. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

| Characteristics | |
|---|---|
| Patients D0/D7 n | 17/17 |
| Age, median IQR | 57 (54–67) |
| Male, n (%) | 12 (70) |
| ICU, Clinical Ward, n | 17/17 |
| Length of stay in ICU (days), median IQR | 14 (6.5–21.5) |
| Length of stay of hospital (days) | 19 (10–23) |
| Comorbidities | |
| BMI (kg/m2), median IQR | 29 (27.0–32.5) |
| Diabetes, n (%) | 3 (17.6) |
| Cirrhosis, n (%) | 0 (0) |
| Chronic kidney disease, n (%) | 2 (11.8) |
| Severity criteria and events occurring during follow up | |
| PaO2/FiO2 at D4, median (IQR) | 200 (174–264) |
| Renal failure, n (%) | 8 (47) |
| Death, n (%) | 2 (11.8) |
| Ratio M1 | Ratio M2 | Ratio M3 | Ratio M4 | Ratio M5 | |
|---|---|---|---|---|---|
| Age | 0.25 | −0.18 | 0.03 | 0.21 | 0.06 |
| BMI | 0.03 | 0.03 | 0.11 | 0.02 | 0.02 |
| Temperature | −0.56 | −0.59 | 0.32 | 0.41 | 0.15 |
| P/F | 0.21 | 0.08 | −0.24 | −0.44 | −0.44 |
| Cortisol | −0.11 | −0.10 | 0.19 | 0.30 | −0.01 |
| PaO2 | −0.25 | −0.15 | 0.57 | 0.38 | 0.90 |
| Lactate | 0.50 | 0.14 | 0.02 | 0.28 | 0.51 |
| Urea | −0.30 | −0.10 | 0.67 | 0.80 | −0.04 |
| Creatinine | −0.21 | −0.06 | 0.69 | 0.95 | 0.07 |
| CRP | −0.27 | −0.34 | −0.04 | 0.11 | −0.27 |
| PCT | −0.16 | −0.24 | 0.51 | 0.37 | 0.71 |
| Bilirubin | −0.32 | −0.24 | 0.07 | 0.51 | −0.23 |
| AST | 0.01 | 0.50 | 0.03 | −0.10 | −0.13 |
| ALT | 0.11 | 0.61 | 0.06 | −0.16 | −0.04 |
| ALP | −0.10 | 0.36 | 0.22 | 0.05 | −0.07 |
| GGT | −0.22 | 0.18 | 0.19 | −0.16 | −0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferron, P.-J.; Le Daré, B.; Bronsard, J.; Steichen, C.; Babina, E.; Pelletier, R.; Hauet, T.; Morel, I.; Tarte, K.; Reizine, F.; et al. Molecular Networking for Drug Toxicities Studies: The Case of Hydroxychloroquine in COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 82. https://doi.org/10.3390/ijms23010082
Ferron P-J, Le Daré B, Bronsard J, Steichen C, Babina E, Pelletier R, Hauet T, Morel I, Tarte K, Reizine F, et al. Molecular Networking for Drug Toxicities Studies: The Case of Hydroxychloroquine in COVID-19 Patients. International Journal of Molecular Sciences. 2022; 23(1):82. https://doi.org/10.3390/ijms23010082
Chicago/Turabian StyleFerron, Pierre-Jean, Brendan Le Daré, Julie Bronsard, Clara Steichen, Elodie Babina, Romain Pelletier, Thierry Hauet, Isabelle Morel, Karin Tarte, Florian Reizine, and et al. 2022. "Molecular Networking for Drug Toxicities Studies: The Case of Hydroxychloroquine in COVID-19 Patients" International Journal of Molecular Sciences 23, no. 1: 82. https://doi.org/10.3390/ijms23010082
APA StyleFerron, P.-J., Le Daré, B., Bronsard, J., Steichen, C., Babina, E., Pelletier, R., Hauet, T., Morel, I., Tarte, K., Reizine, F., Clément, B., Fromenty, B., & Gicquel, T. (2022). Molecular Networking for Drug Toxicities Studies: The Case of Hydroxychloroquine in COVID-19 Patients. International Journal of Molecular Sciences, 23(1), 82. https://doi.org/10.3390/ijms23010082







