Addition of Trans-Resveratrol-Loaded Highly Concentrated Double Emulsion to Yoghurts: Effect on Physicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
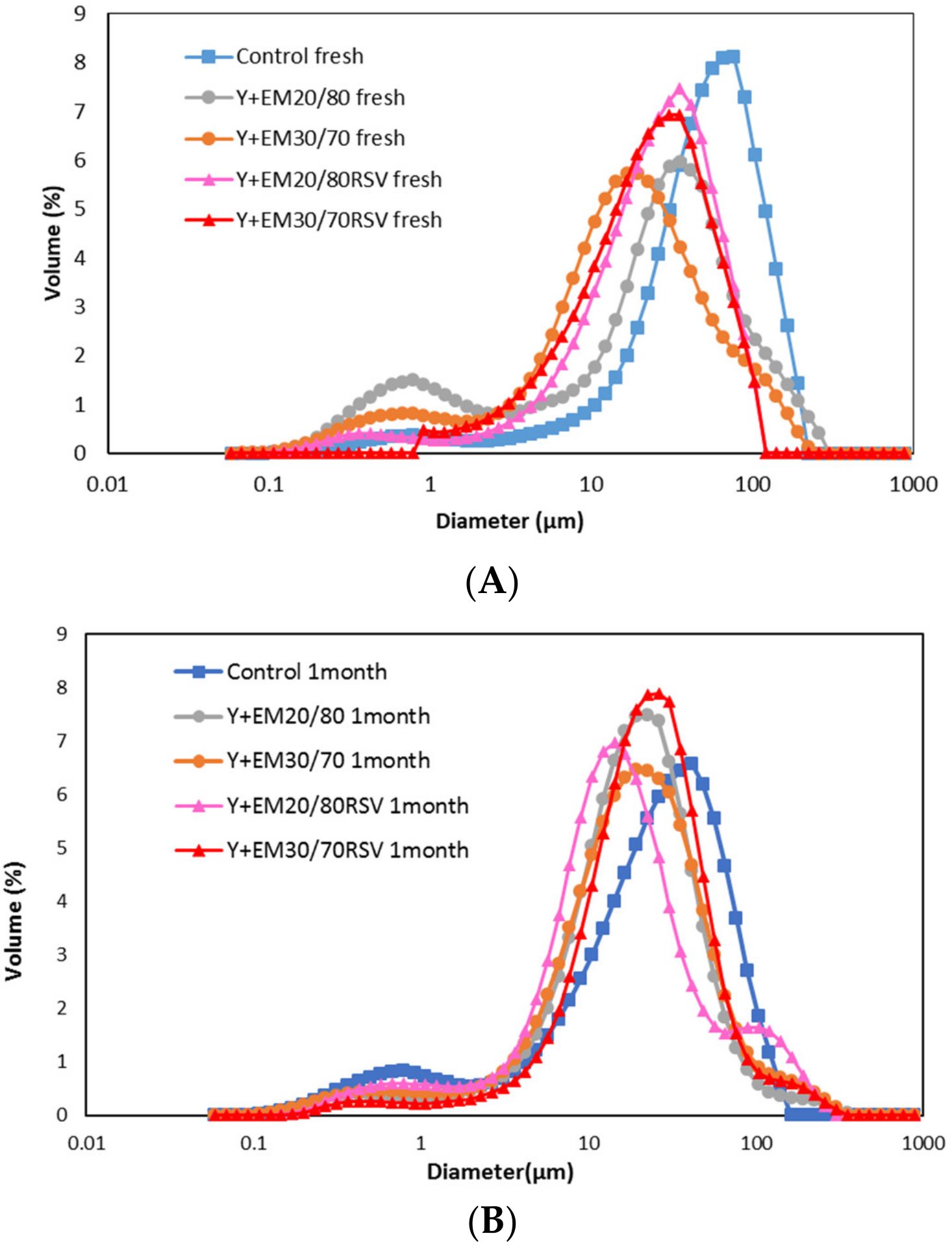
2.1. Droplet Size Distribution
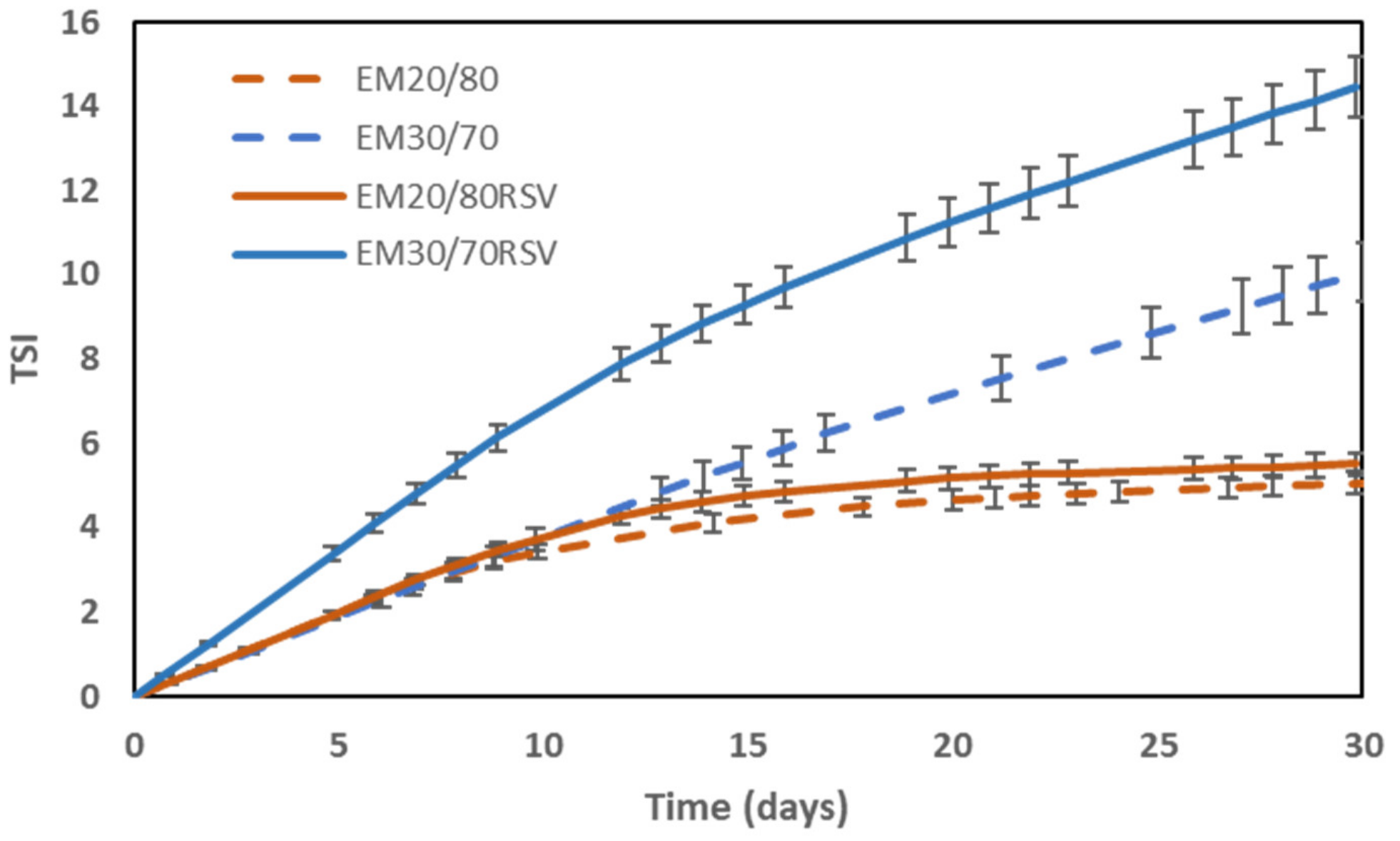
2.2. Colloidal Stability
2.3. Rheology
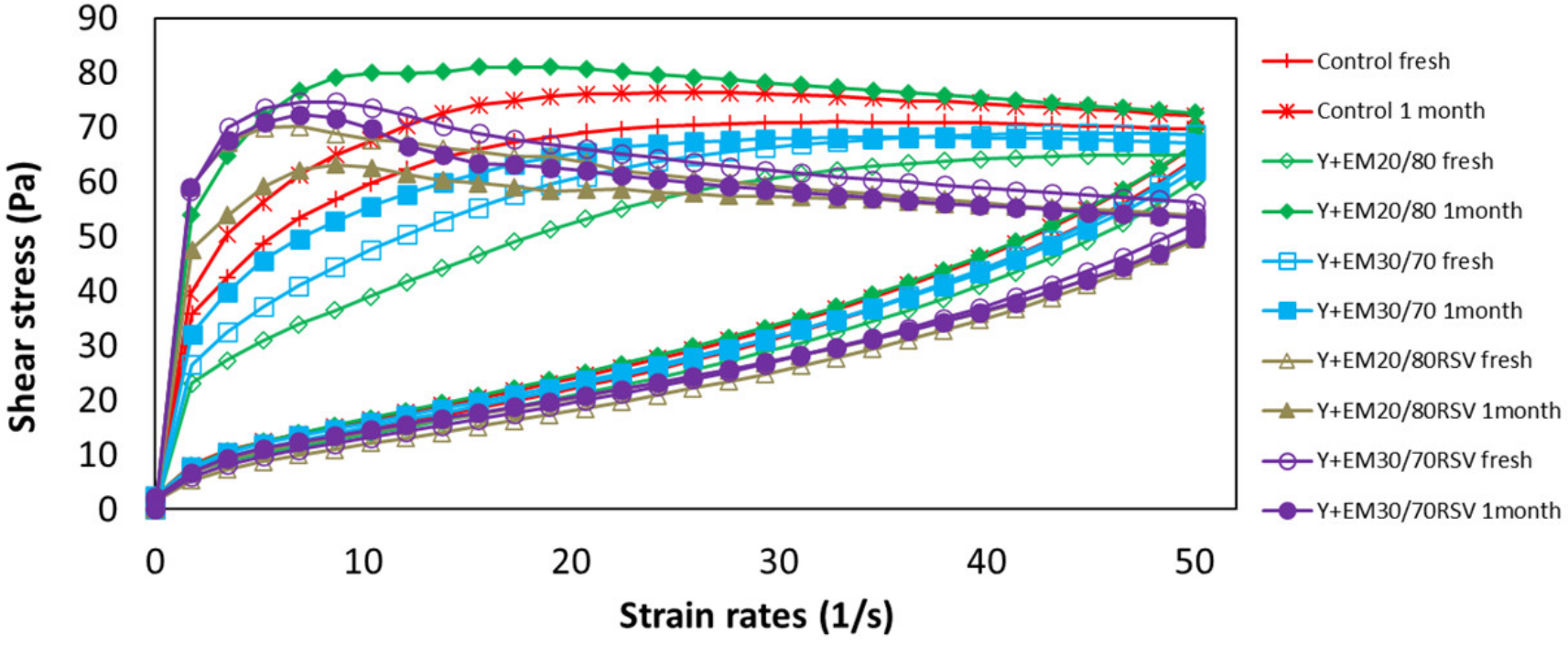
2.3.1. Flow Curves
2.3.2. Frequency Sweep
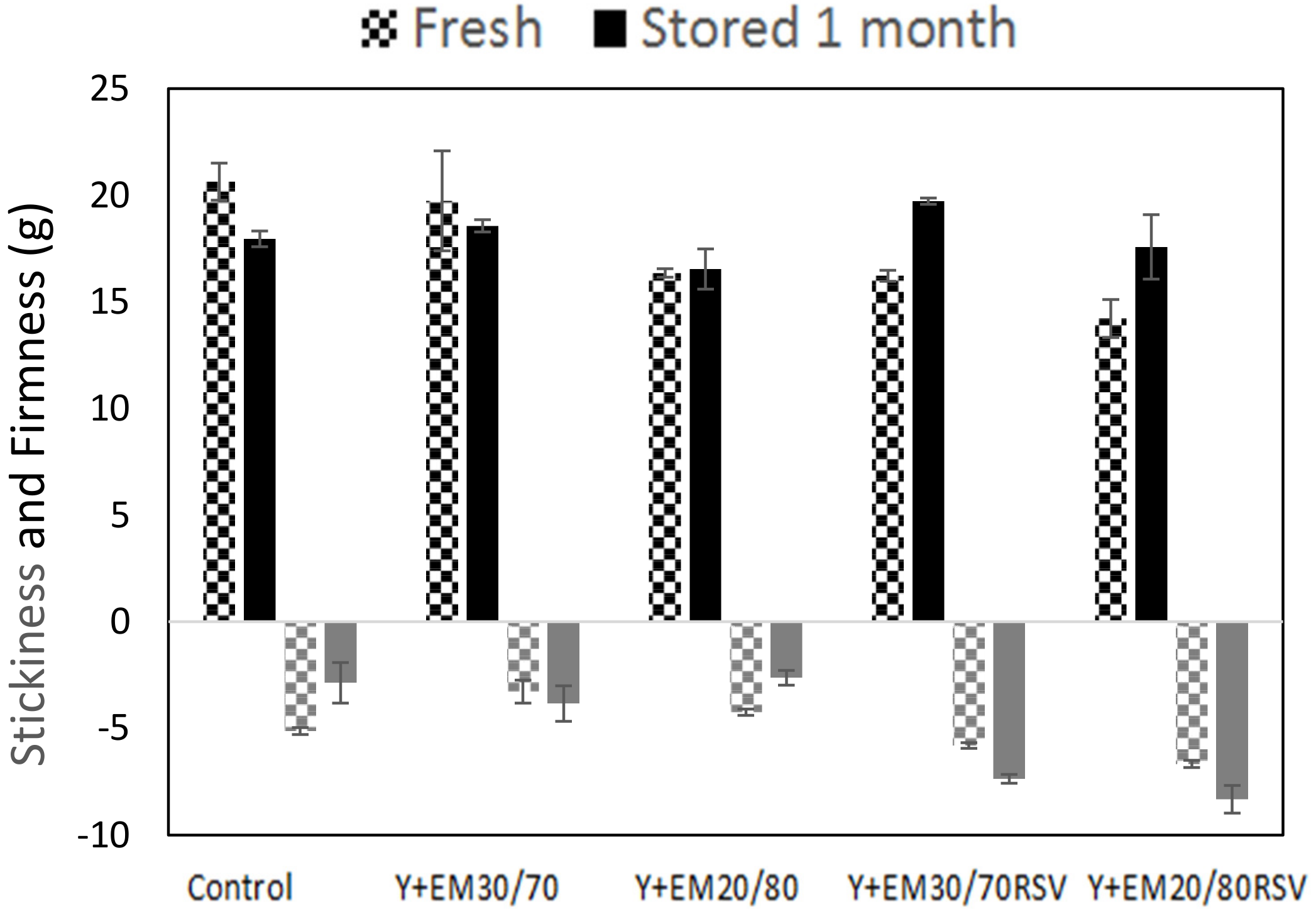
2.4. Texturometry
2.5. Colorimetry
2.6. Visual Inspection
2.7. Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Water-in-Oil (W1/O) Inner Emulsion Preparation
3.2.2. Water-in-Oil-in-Water (W1/O/W2) Double Emulsions Preparation
3.2.3. Yoghurts Preparation
3.2.4. Yoghurt Characterisation
Droplet Size Distribution
Colloidal Stability
Rheology
Texturometry
Colorimetry
Visual Inspection
Antioxidant Activity
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, S.; Ji, X.; Shen, X.; You, J.; Liang, X.; Yin, H.; Zhao, L. Current innovations in nutraceuticals and functional foods for intervention of non-alcoholic fatty liver disease. Pharmacol. Res. 2021, 166, 105517. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Health benefits, extraction and development of functional foods with curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; De Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Ye, X.; Li, R.; Chen, H.; Wang, Y.; Yong, H.; Pan, M.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and In fl ammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Vamanu, E. Polyphenolic Nutraceuticals to Combat Oxidative Stress Through Microbiota Modulation. Front. Pharmacol. 2019, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Embry, C.; Marsiske, M.; Lu, X.; Doss, H.; Leeuwenburgh, C.; Manini, T.M. Safety and metabolic outcomes of resveratrol supplementation in older adults: Results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014, 57, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Pando, D.; Beltrán, M.; Gerone, I.; Matos, M.; Pazos, C. Resveratrol entrapped niosomes as yoghurt additive. Food Chem. 2015, 170, 281–287. [Google Scholar] [CrossRef]
- Ruivo, J.; Francisco, C.; Oliveira, R.; Figueiras, A. The main potentialities of resveratrol for drug delivery systems. Braz. J. Pharm. Sci. 2015, 51, 499–514. [Google Scholar] [CrossRef] [Green Version]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [Green Version]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure–activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Muzzio, M.; Huang, Z.; Hu, S.-C.; Johnson, W.D.; McCormick, D.L.; Kapetanovic, I.M. Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC-MS/MS and their pharmacokinetics in dogs. J. Pharm. Biomed. Anal. 2012, 5, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New delivery systems to improve the bioavailability of resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Machado, N.; Fernández, M.; Díaz, D. Recent Strategies in Resveratrol Delivery Systems. Chempluschem 2019, 84, 951–973. [Google Scholar] [CrossRef]
- Vian, M.; Tomao, V.; Gallet, S.; Coulomb, P.; Lacombe, J. Simple and rapid method for cis- and trans-resveratrol and piceid isomers determination in wine by high-performance liquid chromatography using Chromolith columns. J. Chromatogr. A 2005, 1085, 224–229. [Google Scholar] [CrossRef]
- Silva, C.G.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martínez, C.; Canle, M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Pando, D.; Caddeo, C.; Manconi, M.; Fadda, A.; Pazos, C. Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J. Pharm. Pharmacol. 2013, 65, 1158–1167. [Google Scholar] [CrossRef]
- Pando, D.; Gutiérrez, G.; Pazos, C. Preparation and characterization of niosomes containing resveratrol. J. Food Eng. 2013, 117, 227–234. [Google Scholar] [CrossRef]
- Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Parekh, H.S.; Popat, A. Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J. Colloid Interface Sci. 2016, 462, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Hemar, Y.; Cheng, L.J.; Oliver, C.M.; Sanguansri, L.; Augustin, M. Encapsulation of resveratrol using Water-in-Oil-in-Water double emulsions. Food Biophys. 2010, 5, 120–127. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of resveratrol using food-grade concentrated double emulsions: Emulsion characterization and rheological behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 69–79. [Google Scholar] [CrossRef]
- Díaz-Ruiz, R.; Valdeón, I.; Álvarez, J.R.; Matos, M.; Gutiérrez, G. Simultaneously encapsulation of trans-resveratrol and vitamin D3 in highly concentrated double emulsions. J. Sci. Food Agric. 2020, 101, 3654–3664. [Google Scholar] [CrossRef]
- Matos, M.; Díaz-Ruiz, R.; Marefati, A.; Rayner, M.; Gutiérrez, G. Encapsulation of Antioxidants Using Double Emulsions. In Emulsion-Based Encapsulation of Antioxidants; Springer: Cham, Switzerland, 2020; pp. 249–286. ISBN 978-3-030-62051-6. [Google Scholar]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef] [PubMed]
- Kheynoor, N.; Hosseini, S.M.H.; Yousefi, G.H.; Hashemi Gahruie, H.; Mesbahi, G.R. Encapsulation of vitamin C in a rebaudioside-sweetened model beverage using water in oil in water double emulsions. Food Sci. Technol. 2018, 96, 419–425. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Campos-Montiel, R.G.; Lobato-Calleros, C.; Pedroza-Islas, R.; Vernon-Carter, E.J. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res. Int. 2009, 42, 292–297. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Iglesias, O.; Coca, J.; Pazos, C. Characterization, stability and rheology of highly concentrated monodisperse emulsions containing lutein. Food Hydrocoll. 2015, 49, 156–163. [Google Scholar] [CrossRef]
- Giroux, H.J.; Robitaille, G.; Britten, M. Controlled release of casein-derived peptides in the gastrointestinal environment by encapsulation in water-in-oil-in-water double emulsions. LWT-Food Sci. Technol. 2016, 69, 225–232. [Google Scholar] [CrossRef]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jiménez-Colmenero, F.; Bou, R. Comparison of simple, double and gelled double emulsions as hydroxytyrosol and n-3 fatty acid delivery systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef]
- O’Regan, J.; Mulvihill, D.M. Sodium caseinate-maltodextrin conjugate stabilized double emulsions: Encapsulation and stability. Food Res. Int. 2010, 43, 224–231. [Google Scholar] [CrossRef]
- Eisinaite, V.; Juraite, D.; Schroën, K.; Leskauskaite, D. Food-grade double emulsions as effective fat replacers in meat systems. J. Food Eng. 2017, 213, 54–59. [Google Scholar] [CrossRef]
- Jena, A.K.; Nayak, A.K.; De, A.; Mitra, D.; Samanta, A. Development of lamivudine containing multiple emulsions stabilized by gum odina. Future J. Pharm. Sci. 2018, 4, 71–79. [Google Scholar] [CrossRef]
- Evageliou, V.; Panagopoulou, E.; Mandala, I. Encapsulation of EGCG and esterified EGCG derivatives in double emulsions containing Whey Protein Isolate, Bacterial Cellulose and salt. Food Chem. 2019, 281, 171–177. [Google Scholar] [CrossRef]
- Giroux, H.J.; Constantineau, S.; Fustier, P.; Champagne, C.P.; St-Gelais, D.; Lacroix, M.; Britten, M. Cheese fortification using water-in-oil-in-water double emulsions as carrier for water soluble nutrients. Int. Dairy J. 2013, 29, 107–114. [Google Scholar] [CrossRef]
- Constantinides, P.P. Lipid Microemulsions for Improving Drug Dissolution and Oral Absorption: Physical and Biopharmaceutical Aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef]
- Constantinides, P.; Welzel, G.; Ellens, H.; Smith, P.; Sturgis, S.; Yiv, S.; Owen, A. Water-in-oil microemulsions containing medium-chain fatty acids/salts: Formulation and intestinal absorption enhancement evaluation. Pharm. Res. 1996, 13, 210–215. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley Interscience: Hoboken, NJ, USA, 2004; ISBN 978-0-471-67055-1. [Google Scholar]
- Aserin, A. Multiple Emulsion: Technology and Applications; Wiley Interscience: Hoboken, NJ, USA, 2008; ISBN 978-0-470-20925-7. [Google Scholar]
- Garti, N. Double emulsions—Scope, limitations and new achievements. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 233–246. [Google Scholar] [CrossRef]
- Conroy, D.M.; Gan, C.; Errmann, A.; Young, J. Fortifying wellbeing: How Chinese consumers and doctors navigate the role of functional foods. Appetite 2021, 164, 105296. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Díaz-Ruiz, R.; Martínez-Rey, L.; Laca, A.; Álvarez, J.R.; Gutiérrez, G.; Matos, M. Enhancing trans-Resveratrol loading capacity by forcing W1/O/W2 emulsions up to its colloidal stability limit. Colloids Surf. B Biointerfaces 2020, 193, 111130. [Google Scholar] [CrossRef]
- Östbring, K.; Matos, M.; Marefati, A.; Ahlström, C.; Gutiérrez, G. The Effect of pH and Storage Temperature on the Stability of Emulsions Stabilized by Rapeseed Proteins. Foods 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- El Kadri, H.; Lalou, S.; Mantzouridou, F.T.; Gkatzionis, K. Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Res. Int. 2018, 107, 325–336. [Google Scholar] [CrossRef]
- Lalou, S.; El Kadri, H.; Gkatzionis, K. Incorporation of water-in-oil-in-water (W1/O/W2) double emulsion in a set-type yogurt model. Food Res. Int. 2017, 100, 122–131. [Google Scholar] [CrossRef]
- Seth, D.; Mishra, H.N.; Deka, S.C. Effect of hydrocolloids on the physico-chemical and rheological properties of reconstituted sweetened yoghurt powder. J. Sci. Food Agric. 2017, 98, 1696–1702. [Google Scholar] [CrossRef]
- Domagala, J.; Sady, M.; Grega, T.; Bonczar, G. The influence of storage time on rheological properties and texture of yoghurts with the addition of oat-maltodextrin as the fat substitute. Int. J. Food Prop. 2005, 8, 439–448. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Mason, T.G. Advances and challenges in the rheology of concentrated emulsions and nanoemulsions. Adv. Colloid Interface Sci. 2017, 247, 397–412. [Google Scholar] [CrossRef]
- Cohen-Addad, S.; Hoehler, R. Rheology of foams and highly concentrated emulsions. Curr. Opin. Colloid Interface Sci. 2014, 19, 536–548. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Razavi, S.M.A. Influence of thermosonication treatment on the average size of fat globules, emulsion stability, rheological properties and color of camel milk cream. LWT-Food Sci. Technol. 2020, 132, 109852. [Google Scholar] [CrossRef]
- Toczek, K.; Glibowski, P.; Kordowska-Wiater, M.; Iłowiecka, K. Rheological and textural properties of emulsion spreads based on milk fat and inulin with the addition of probiotic bacteria. Int. Dairy J. 2022, 124, 105217. [Google Scholar] [CrossRef]
- Fu, R.; Li, J.; Zhang, T.; Zhu, T.; Cheng, R.; Wang, S.; Zhang, J. Salecan stabilizes the microstructure and improves the rheological performance of yogurt. Food Hydrocoll. 2018, 81, 474–480. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, R.; Luo, T.; Che, X.; Bansal, N.; Liu, X. Physiochemical properties of modified starch under yogurt manufacturing conditions and its relation to the properties of yogurt. J. Food Eng. 2019, 245, 11–17. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Matos, M.; Barrero, P.; Pando, D.; Iglesias, O.; Pazos, C. Iron-entrapped niosomes and their potential application for yogurt fortification. LWT-Food Sci. Technol. 2016, 74, 550–556. [Google Scholar] [CrossRef]
- Skryplonek, K.; Henriques, M.; Gomes, D.; Viegas, J.; Fonseca, C.; Pereira, C.; Dmytrów, I.; Mituniewicz-Małek, A. Characteristics of lactose-free frozen yogurt with κ-carrageenan and corn starch as stabilizers. J. Dairy Sci. 2019, 102, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT-Food Sci. Technol. 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Alqah, H.A.S.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT 2021, 137, 110389. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton, I.T. W1/O/W2 double emulsions stabilised by fat crystals—Formulation, stability and salt release. J. Colloid Interface Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef]
- Jiang, J.; Mei, Z.; Xu, J.; Sun, D. Effect of inorganic electrolytes on the formation and the stability of water-in-oil (W/O) emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 82–90. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Yan, W. Measurement and correlation of solubility of trans-resveratrol in 11 solvents at T = (278.2, 288.2, 298.2, 308.2, and 318.2) K. J. Chem. Thermodyn. 2008, 40, 735–738. [Google Scholar] [CrossRef]
- Wilson, R.; Van Schie, B.; Howes, D. Overview of the preparation, use and biological studies on polyglycerol polyricinoleate (PGPR). Food Chem. Toxicol. 1998, 36, 711–718. [Google Scholar] [CrossRef]
- Wolf, F.; Koehler, K.; Schuchmann, H. Stabilization of water droplets in oil with PGPR for use in oral and dermal applications. J. Food Process Eng. 2013, 36, 276–283. [Google Scholar] [CrossRef]
- Laca, A.; Paredes, B.; Díaz, M. Lipid-enriched egg yolk fraction as ingredient in cosmetic emulsions. J. Texture Stud. 2012, 43, 12–28. [Google Scholar] [CrossRef]
- Wei, S.-T.; Ou, L.-C.; Luo, M.R.; Hutchings, B.J.B. Optimization of food expectations using product colour and appearance. Food Qual. Prefer. 2012, 23, 49–62. [Google Scholar] [CrossRef]
- Laca, A.; Sáenz, M.C.; Paredes, B.; Díaz, M. Rheological properties, stability and sensory evaluation of low-cholesterol mayonnaises prepared using egg yolk granules as emulsifying agent. J. Food Eng. 2010, 97, 243–252. [Google Scholar] [CrossRef]
- Moussi, K.; Nayak, B.; Perkins, L.B.; Dahmoune, F.; Madani, K.; Chibane, M. HPLC-DAD profile of phenolic compounds and antioxidant activity of leaves extract of Rhamnus alaternus L. Ind. Crop. Prod. 2015, 74, 858–866. [Google Scholar] [CrossRef]


| Control | Y+EM20/80 | Y+EM30/70 | Y+EM20/80RSV | Y+EM30/70RSV | |
|---|---|---|---|---|---|
| ΔE | 0.081 ± 0.05 | 0.281 ± 0.02 | 0.420 ± 0.08 | 1.105 ± 0.08 | 0.350 ± 0.11 |
| Type of Yoghurt | Antioxidant Capacity (Fresh) | Antioxidant Capacity (After One Month) |
|---|---|---|
| Plain yoghurt | 31 ± 0.7% a,x | 31 ± 0.9% h,x |
| Yoghurts enriched with double emulsions (20/80) with RSV (0.5 mg/kg) | 32 ± 0.6% b,x | 32 ± 1.0% h,x |
| Yoghurts enriched with double emulsions (30/70) with RSV (0.8 mg/kg) | 35 ± 0.9% c,x | 36 ± 1.1% i,x |
| Yoghurts containing free RSV (0.5 mg/kg) | 30 ± 0.6% d,x | 30 ± 0.8% h,x |
| Yoghurts containing free RSV (0.8 mg/kg) | 35 ± 0.9% c,x | 31 ± 0.8% h,y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Ruiz, R.; Laca, A.; Sánchez, M.; Fernández, M.R.; Matos, M.; Gutiérrez, G. Addition of Trans-Resveratrol-Loaded Highly Concentrated Double Emulsion to Yoghurts: Effect on Physicochemical Properties. Int. J. Mol. Sci. 2022, 23, 85. https://doi.org/10.3390/ijms23010085
Díaz-Ruiz R, Laca A, Sánchez M, Fernández MR, Matos M, Gutiérrez G. Addition of Trans-Resveratrol-Loaded Highly Concentrated Double Emulsion to Yoghurts: Effect on Physicochemical Properties. International Journal of Molecular Sciences. 2022; 23(1):85. https://doi.org/10.3390/ijms23010085
Chicago/Turabian StyleDíaz-Ruiz, Rocío, Amanda Laca, Marta Sánchez, Manuel Ramón Fernández, María Matos, and Gemma Gutiérrez. 2022. "Addition of Trans-Resveratrol-Loaded Highly Concentrated Double Emulsion to Yoghurts: Effect on Physicochemical Properties" International Journal of Molecular Sciences 23, no. 1: 85. https://doi.org/10.3390/ijms23010085
APA StyleDíaz-Ruiz, R., Laca, A., Sánchez, M., Fernández, M. R., Matos, M., & Gutiérrez, G. (2022). Addition of Trans-Resveratrol-Loaded Highly Concentrated Double Emulsion to Yoghurts: Effect on Physicochemical Properties. International Journal of Molecular Sciences, 23(1), 85. https://doi.org/10.3390/ijms23010085








