A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition
Abstract
:1. Introduction
2. Results
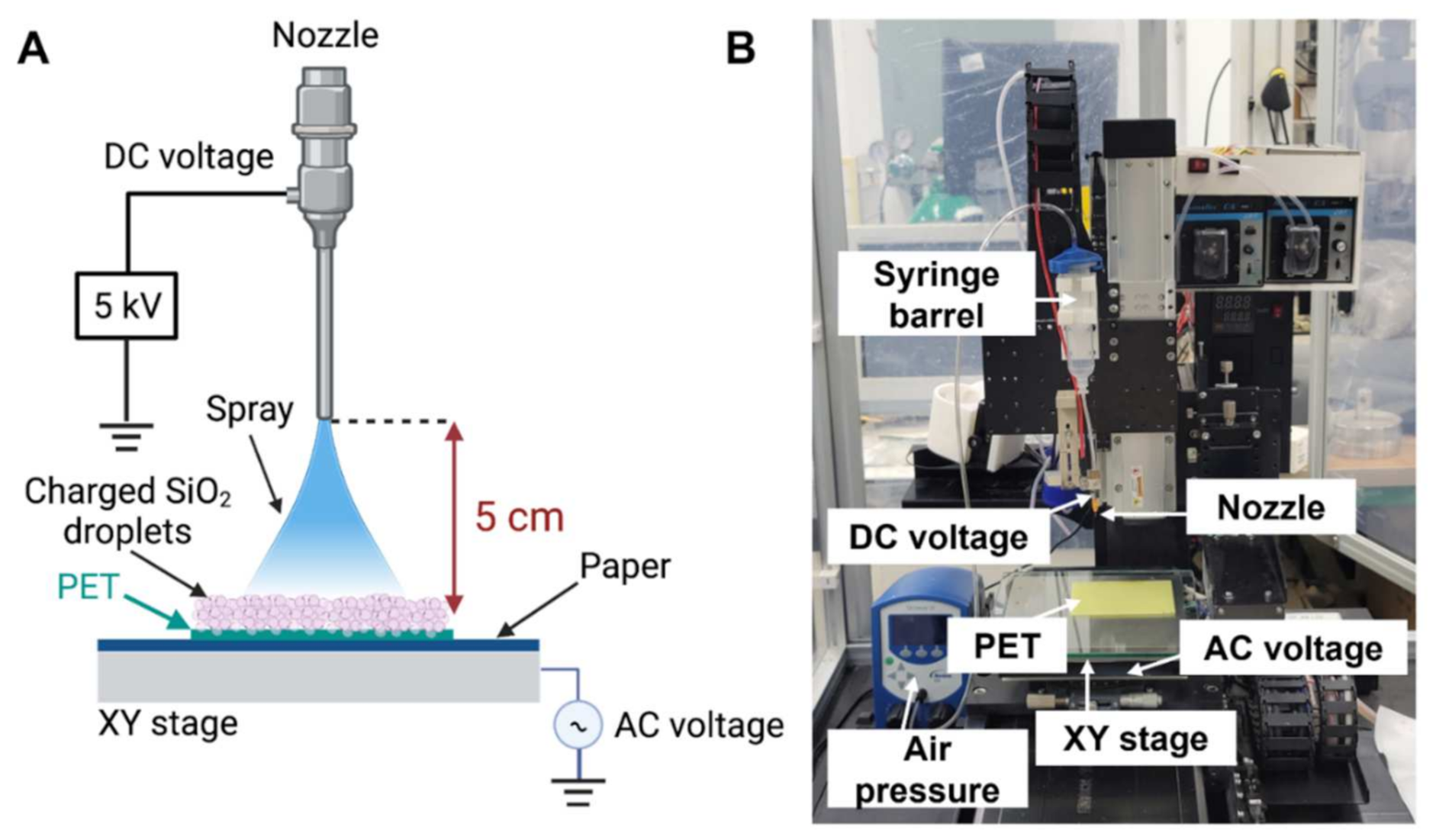
2.1. Fabrication of a SiO2-Deposited Surface Using an Electrospray Technique
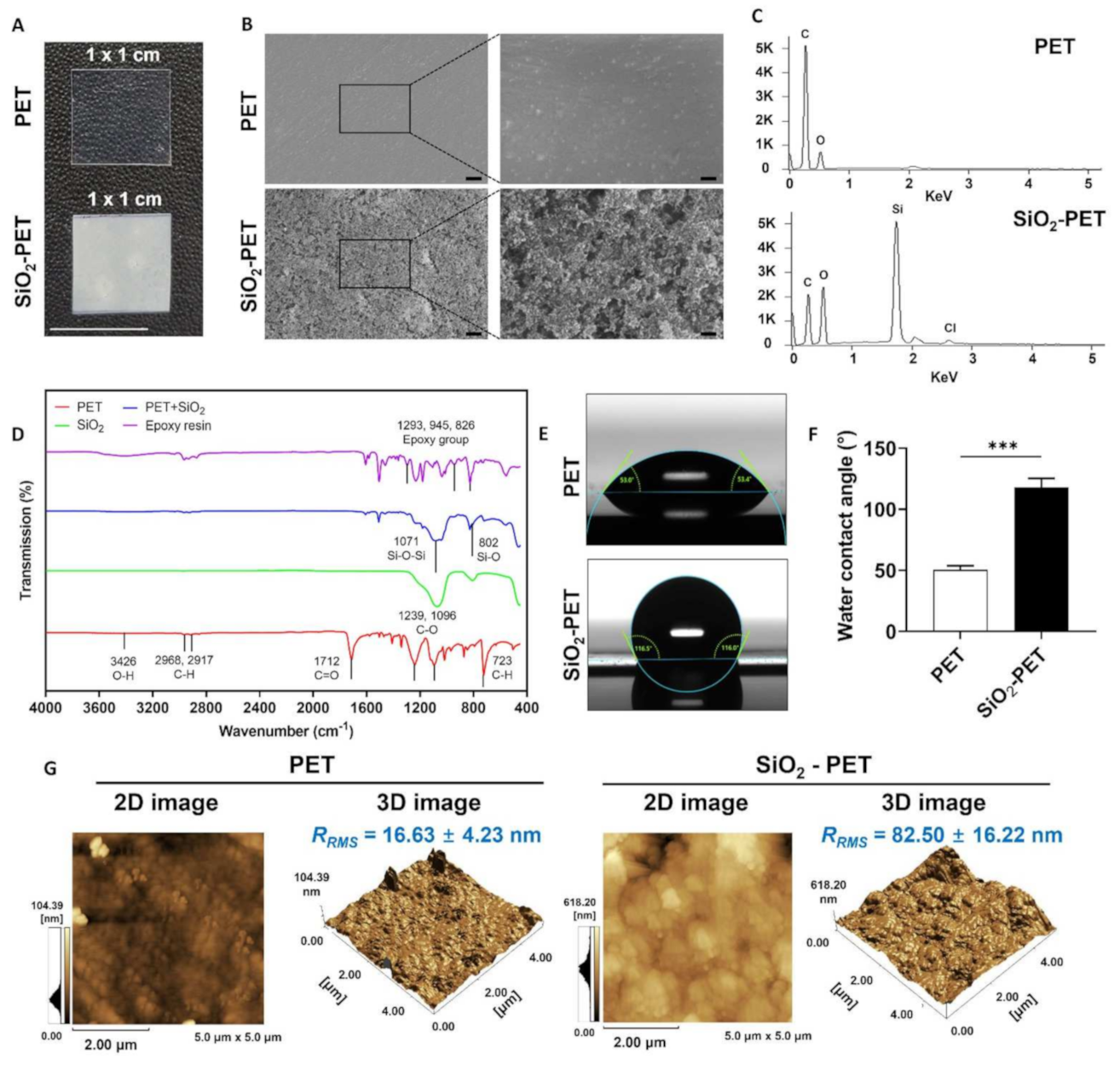
2.2. Characterization of the SiO2-Deposited Surface by SEM, FTIR, and AFM
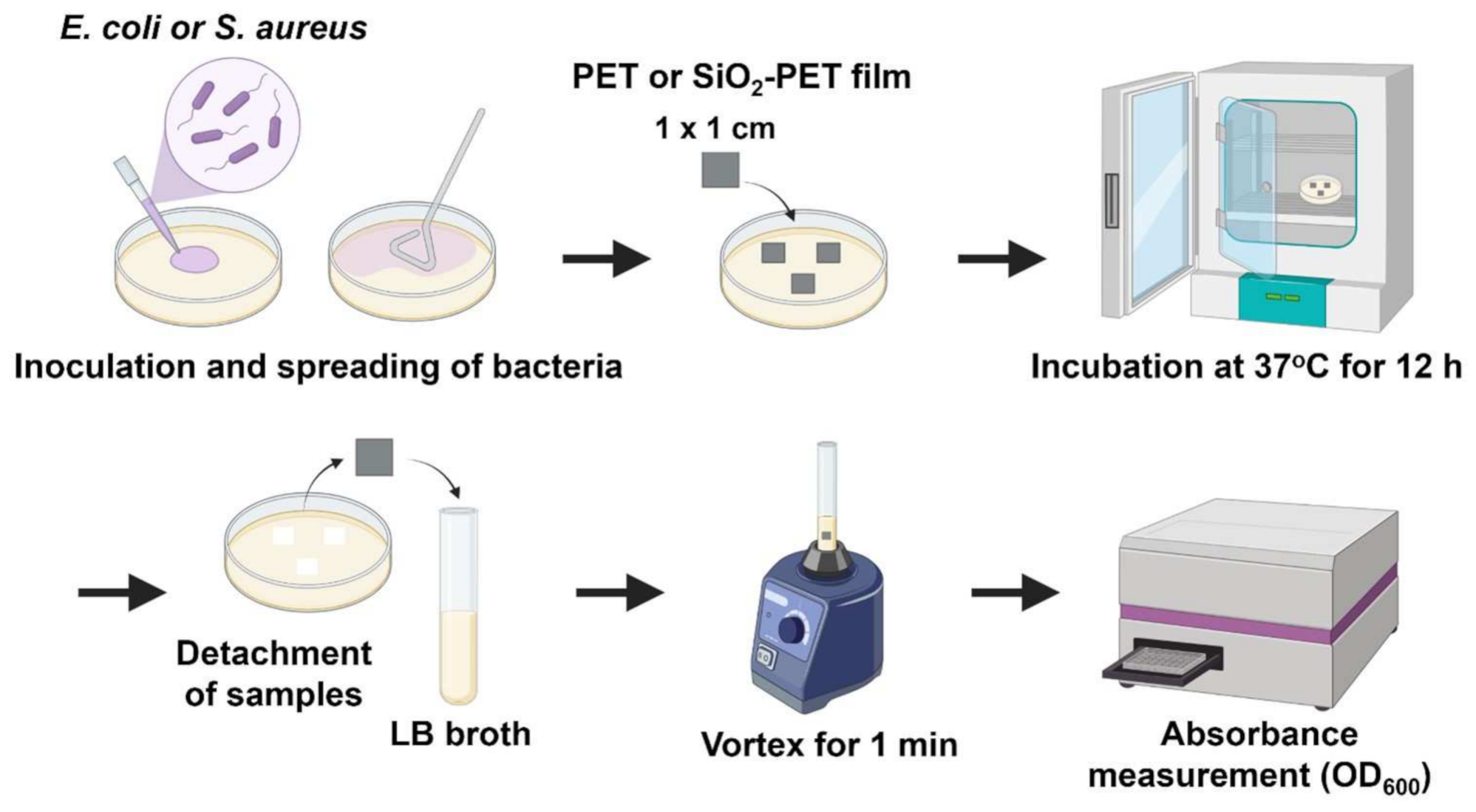
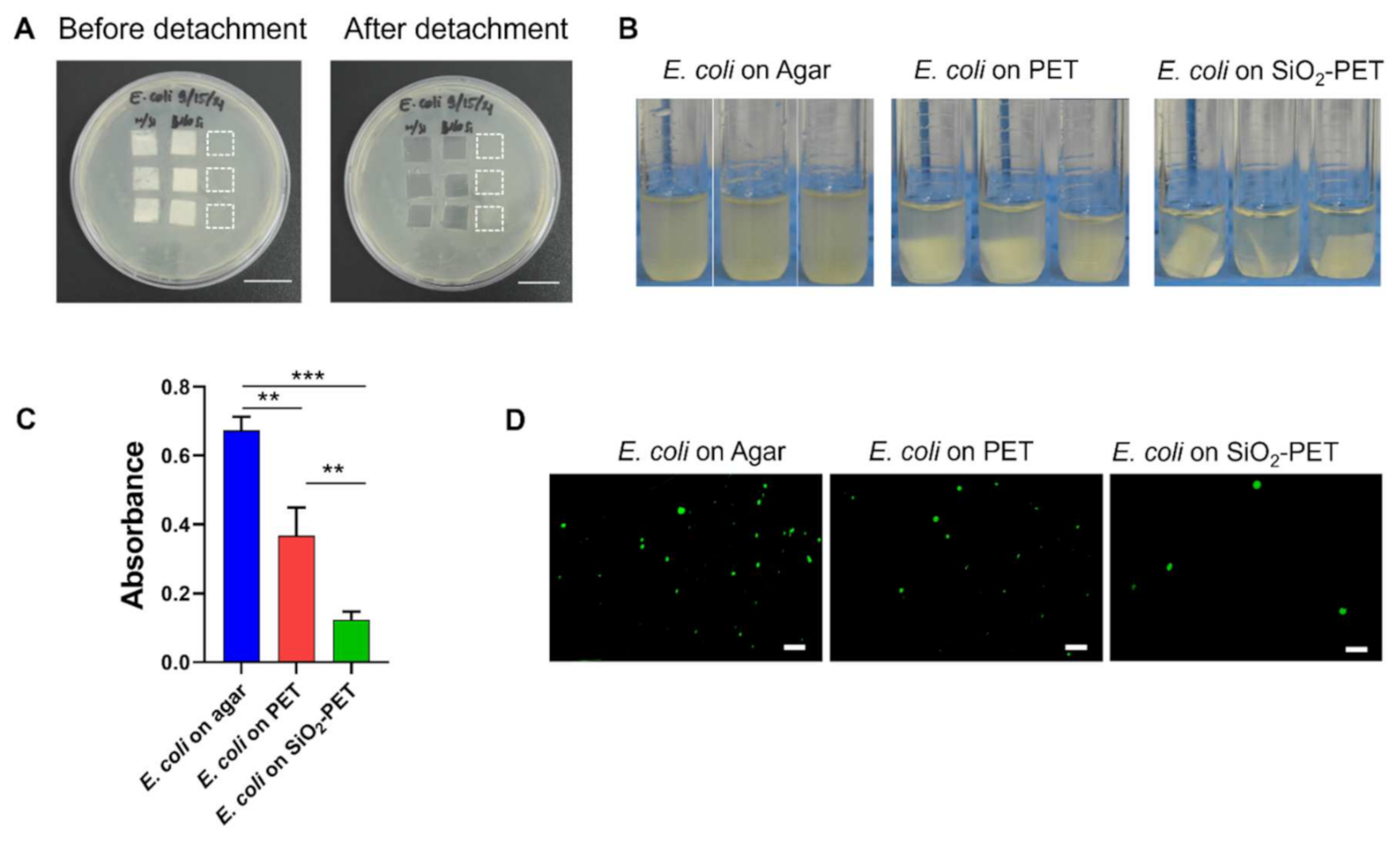
2.3. Antibacterial Properties of SiO2-Deposited Surfaces
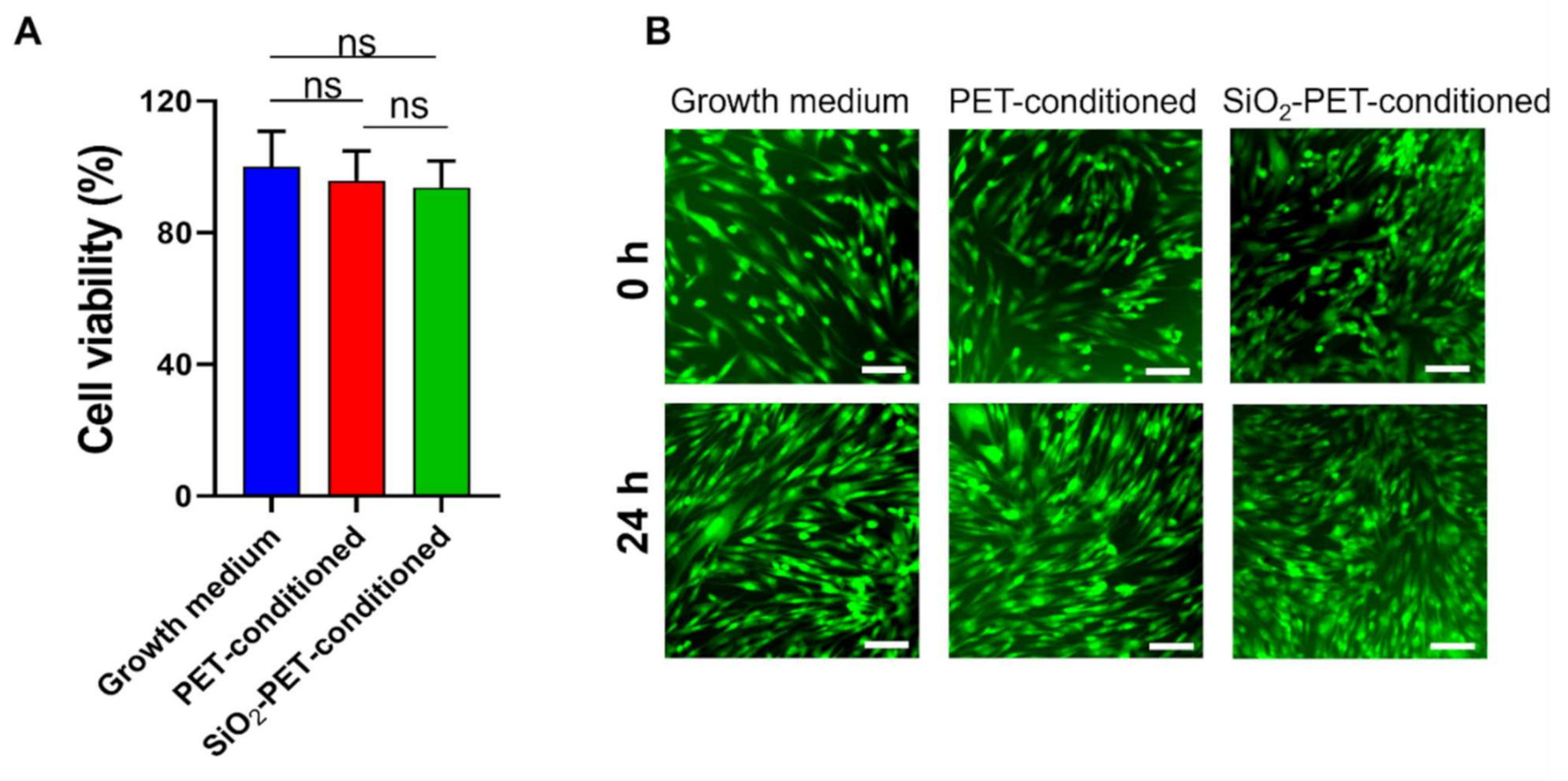
2.4. Cytotoxicity Test for In Vitro Cell Culture
3. Discussion
4. Materials and Methods
4.1. Preparation of Deposition Materials
4.2. Electrospray-Based Silicon Deposition onto PET Films
4.3. Surface Characterization by FTIR, SEM, and AFM
4.4. Contact Angle Measurement and Roll-Off Angle Test
4.5. Bacterial Strains and Culture Conditions
4.6. Assessment of Antibacterial Activity or Bacterial Adhesion
4.7. Live/Dead Assays for Bacteria
4.8. Cell Culture
4.9. In Vitro Cytotoxicity Tests and Cell Viability Analysis Using HDFs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Alternating current |
| AFM | Atomic force microscopy |
| CFU | colony forming unit |
| DC | direct current |
| DMEM | Dulbecco’s modified eagle’s medium |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| EPS | exopolysaccharide |
| FBS | fetal bovine serum |
| FTIR | Fourier-transform infrared spectroscopy |
| GFP | green fluorescent protein |
| HDF | human dermal fibroblast |
| LB | Luria-Bertani |
| LPS | lipopolysachharide |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| OD | optical density |
| PBS | phosphate-buffered saline |
| PET | polyethylene terephthalate |
| PI | polyimide |
| P/S | penicillin-streptomycin |
| Ra | average roughness |
| RRMS | root-mean-square roughness |
| SEM | scanning electron microscope |
| SiO2 | silicon dioxide |
| SR | silicone rubber |
| TiO2 | titanium dioxide |
| WCA | water contact angle |
| ZnO | zinc oxide |
References
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [Green Version]
- Dertli, E.; Mayer, M.J.; Narbad, A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Fila, G.; Grinholc, M.; Nakonieczna, J. Innovative strategies to overcome biofilm resistance. Biomed. Res. Int. 2013, 2013, 150653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Zhan, W.; Cao, L.; Hu, C.; Qu, Y.; Yu, Q.; Chen, H. Multifunctional and Regenerable Antibacterial Surfaces Fabricated by a Universal Strategy. ACS Appl. Mater. Interfaces 2016, 8, 30048–30057. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef]
- Klemm, P.; Vejborg, R.M.; Hancock, V. Prevention of bacterial adhesion. Appl. Microbiol. Biotechnol. 2010, 88, 451–459. [Google Scholar] [CrossRef]
- Ga, D.H.; Lim, C.M.; Jang, Y.; Son, T.I.; Han, D.K.; Joung, Y.K. Surface-Modifying Effect of Zwitterionic Polyurethane Oligomers Complexed with Metal Ions on Blood Compatibility. Tissue Eng. Regen. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Elzaabalawy, A.; Verberne, P.; Meguid, S.A. Multifunctional Silica-Silicone Nanocomposite with Regenerative Superhydrophobic Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 42827–42837. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antibacterial surfaces prepared by electrospray coating of photocatalytic nanoparticles. Chem. Eng. J. 2018, 334, 1108–1118. [Google Scholar] [CrossRef]
- Yoon, H.; Joshi, B.; Na, S.H.; Yoon, S.S. Antibacterial Activity and Photocatalysis of Electrosprayed Titania Films. J. Electrochem. Soc. 2012, 159, H823–H827. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.F.; Liu, X.W.; Sun, G.Y.; Li, D.H.; Wei, H.B. A decomposable silica-based antibacterial coating for percutaneous titanium implant. Int. J. Nanomed. 2017, 12, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Vladkova, T.; Angelov, O.; Stoyanova, D.; Gospodinova, D.; Gomes, L.; Soares, A.; Mergulhao, F.; Ivanova, I. Magnetron co-sputtered TiO2/SiO2/Ag nanocomposite thin coatings inhibiting bacterial adhesion and biofilm formation. Surf. Coat. Technol. 2020, 384. [Google Scholar] [CrossRef]
- Swar, S.; Zajicova, V.; Rysova, M.; Lovetinska-Slamborova, I.; Volesky, L.; Stibor, I. Biocompatible surface modification of poly(ethylene terephthalate) focused on pathogenic bacteria: Promising prospects in biomedical applications. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Caykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.K.; Phung, T.H.; Oh, S.; Kim, S.H.; Ng, T.N.; Kwon, K.S. High-Efficiency Electrospray Deposition Method for Nonconductive Substrates: Applications of Superhydrophobic Coatings. ACS Appl. Mater. Interfaces 2021, 13, 18227–18236. [Google Scholar] [CrossRef]
- Zhi, D.F.; Wang, H.H.; Jiang, D.; Parkin, I.P.; Zhang, X. Reactive silica nanoparticles turn epoxy coating from hydrophilic to super-robust superhydrophobic. RSC Adv. 2019, 9, 12547–12554. [Google Scholar] [CrossRef] [Green Version]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnology 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and application of Fe3O4@SiO2@TiO2 for photocatalytic decomposition of organic matrix simultaneously with magnetic solid phase extraction of heavy metals prior to ICP-MS analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Handke, M.; Sitarz, M.; Dlugon, E. Amorphous SiCxOy coatings from ladder-like polysilsesquioxanes. J. Mol. Struct. 2011, 993, 193–197. [Google Scholar] [CrossRef]
- Nunes, C.S.; da Silva, M.J.V.; da Silva, D.C.; Freitas, A.R.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. PET depolymerisation in supercritical ethanol catalysed by [Bmim][BF4]. RSC Adv. 2014, 4, 20308–20316. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Irska, I.; Taraghi, I.; Pilawka, R.; Gu, J.L.; Li, X.H.; Tu, Y.F.; Piesowicz, E. Synthesis and characterization of poly(ethylene terephthalate-co-1,4-cyclohexanedimethylene terephtlatate)-block-poly(tetramethylene oxide) copolymers. RSC Adv. 2017, 7, 41745–41754. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotech. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Startek, K.; Szczurek, A.; Tran, T.N.L.; Krzak, J.; Bachmatiuk, A.; Lukowiak, A. Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil. Coatings 2021, 11, 573. [Google Scholar] [CrossRef]
- Ozkan, E.; Crick, C.C.; Taylor, A.; Allan, E.; Parkin, I.P. Copper-based water repellent and antibacterial coatings by aerosol assisted chemical vapour deposition. Chem. Sci. 2016, 7, 5126–5131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svirinovsky, A.; Perelshtein, I.; Natan, M.; Banin, E.; Gedanken, A. Imparting superhydrophobic and biocidal functionalities to a polymeric substrate by the sonochemical method. Ultrason. Sonochem. 2018, 44, 398–403. [Google Scholar] [CrossRef]
- Park, K.; Jeong, H.; Tanum, J.; Yoo, J.C.; Hong, J. Developing regulatory property of gelatin-tannic acid multilayer films for coating-based nitric oxide gas delivery system. Sci. Rep. 2019, 9, 8308. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Poojari, Y. Silicones for Encapsulation of Medical Device Implants. Silicon-Neth. 2017, 9, 645–649. [Google Scholar] [CrossRef]
- Shah, K.W. Nanosynthesis Techniques of Silica-Coated Nanostructures. In Novel Nanomaterials-Synthesis and Applications; Kyzas, G.Z., Mitropoulos, A.C., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Lei, L.; Gamboa, A.R.; Kuznetsova, C.; Littlecreek, S.; Wang, J.; Zou, Q.; Zahn, J.D.; Singer, J.P. Self-limiting electrospray deposition on polymer templates. Sci. Rep. 2020, 10, 17290. [Google Scholar] [CrossRef]
- Tang, J.; Gomez, A. Controlled mesoporous film formation from the deposition of electrosprayed nanoparticles. Aerosol. Sci. Tech. 2017, 51, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Parhizkar, M.; Reardon, P.J.T.; Knowles, J.C.; Browning, R.J.; Stride, E.; Pedley, R.B.; Grego, T.; Edirisinghe, M. Performance of novel high throughput multi electrospray systems for forming of polymeric micro/nanoparticles. Mater. Des. 2017, 126, 73–84. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [Green Version]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protsak, I.S.; Morozov, Y.M.; Zhang, D.; Gun’ko, V.M. Surface Chemistry of Nanohybrids with Fumed Silica Functionalized by Polydimethylsiloxane/Dimethyl Carbonate Studied Using (1)H, (13)C, and (29)Si Solid-State NMR Spectroscopy. Molecules 2021, 26, 5974. [Google Scholar] [CrossRef]
- Koubali, H.; El Louali, M.; Zahir, H.; Soufiani, S.; Mabrouki, M.; Latrache, H. Physicochemical characterization of glass and polyethylene surfaces treated with different surfactants and their effects on bacterial adhesion. Int. J. Adhes. Adhes. 2021, 104, 102754. [Google Scholar] [CrossRef]
- Li, A.L.; Wang, G.F.; Ma, Y.W.; Zhao, C.Y.; Zhang, F.Y.; He, Q.; Zhang, F.W. Study on preparation and properties of superhydrophobic surface of RTV silicone rubber. J. Mater. Res. Technol. 2021, 11, 135–143. [Google Scholar] [CrossRef]
- Schneider, L.; Laustsen, M.; Mandsberg, N.; Taboryski, R. The Influence of Structure Heights and Opening Angles of Micro- and Nanocones on the Macroscopic Surface Wetting Properties. Sci. Rep. 2016, 6, 21400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzel Kaya, G.; Medaglia, S.; Candela-Noguera, V.; Tormo-Mas, M.A.; Marcos, M.D.; Aznar, E.; Deveci, H.; Martinez-Manez, R. Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of epsilon-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier. Pharmaceutics 2020, 12, 1126. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Padmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater. Des. 2018, 140, 332–344. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, L.; Levanen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Duvernoy, M.C.; Mora, T.; Ardre, M.; Croquette, V.; Bensimon, D.; Quilliet, C.; Ghigo, J.M.; Balland, M.; Beloin, C.; Lecuyer, S.; et al. Asymmetric adhesion of rod-shaped bacteria controls microcolony morphogenesis. Nat. Commun. 2018, 9, 1120. [Google Scholar] [CrossRef] [Green Version]
- Dorr, T.; Moynihan, P.J.; Mayer, C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaussart, A.; El-Kirat-Chatel, S. Microbial adhesion and ultrastructure from the single-molecule to the single-cell levels by Atomic Force Microscopy. Cell Surf. 2019, 5, 100031. [Google Scholar] [CrossRef]
- Lima, M.; Teixeira-Santos, R.; Gomes, L.C.; Faria, S.I.; Valcarcel, J.; Vazquez, J.A.; Cerqueira, M.A.; Pastrana, L.; Bourbon, A.I.; Mergulhao, F.J. Development of Chitosan-Based Surfaces to Prevent Single- and Dual-Species Biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. Molecules 2021, 26, 4378. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Surel, U.; Wilczewska, A.Z.; Mystkowska, J.; Piktel, E.; Gu, X.; Namiot, Z.; Akowska, A.K.L.; Savage, P.B.; Bucki, R. Bactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticles. J. Nanobiotechnol. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakade, K.; Jindai, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Adhesion and Bactericidal Properties of a Wettability-Controlled Artificial Nanostructure. ACS Appl. Nano. Mater. 2018, 1, 5736–5741. [Google Scholar] [CrossRef]
- Pillai, S.K.R.; Reghu, S.; Vikhe, Y.; Zheng, H.; Koh, C.H.; Chan-Park, M.B. Novel Antimicrobial Coating on Silicone Contact Lens Using Glycidyl Methacrylate and Polyethyleneimine Based Polymers. Macromol. Rapid Comm. 2020, 41, 2000175. [Google Scholar] [CrossRef] [PubMed]
- Dulski, M.; Gawecki, R.; Sulowicz, S.; Cichomski, M.; Kazek-Kesik, A.; Wala, M.; Lesniak-Ziolkowska, K.; Simka, W.; Mrozek-Wilczkiewicz, A.; Gaweda, M.; et al. Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 507. [Google Scholar] [CrossRef]
- Chi, F.; Zeng, Y.; Liu, C.; Liang, D.; Li, Y.; Xie, R.; Pan, N.; Ding, C. Enhancing mechanical stability of sol-gel silica antireflection coatings via ammonia treatment at low temperature. Results Phys. 2020, 18, 103315. [Google Scholar] [CrossRef]
- Navarro-Palomares, E.; Gonzalez-Saiz, P.; Renero-Lecuna, C.; Martin-Rodriguez, R.; Aguado, F.; Gonzalez-Alonso, D.; Barquin, L.; Gonzalez, J.; Banobre-Lopez, M.; Fanarraga, M.L.; et al. Dye-doped biodegradable nanoparticle SiO2 coating on zinc- and iron-oxide nanoparticles to improve biocompatibility and for in vivo imaging studies. Nanoscale 2020, 12, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Fritsch-Decker, S.; An, Z.; Yan, J.; Hansjosten, I.; Al-Rawi, M.; Peravali, R.; Diabate, S.; Weiss, C. Silica Nanoparticles Provoke Cell Death Independent of p53 and BAX in Human Colon Cancer Cells. Nanomaterials 2019, 9, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remzova, M.; Zouzelka, R.; Brzicova, T.; Vrbova, K.; Pinkas, D.; Rossner, P.; Topinka, J.; Rathousky, J. Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials 2019, 9, 968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N. Dynamics of a water droplet on a hydrophobic inclined surface: Influence of droplet size and surface inclination angle on droplet rolling. RSC Adv. 2017, 7, 48806–48818. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levana, O.; Hong, S.; Kim, S.H.; Jeong, J.H.; Hur, S.S.; Lee, J.W.; Kwon, K.-S.; Hwang, Y. A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition. Int. J. Mol. Sci. 2022, 23, 513. https://doi.org/10.3390/ijms23010513
Levana O, Hong S, Kim SH, Jeong JH, Hur SS, Lee JW, Kwon K-S, Hwang Y. A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition. International Journal of Molecular Sciences. 2022; 23(1):513. https://doi.org/10.3390/ijms23010513
Chicago/Turabian StyleLevana, Odelia, Soonkook Hong, Se Hyun Kim, Ji Hoon Jeong, Sung Sik Hur, Jin Woo Lee, Kye-Si Kwon, and Yongsung Hwang. 2022. "A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition" International Journal of Molecular Sciences 23, no. 1: 513. https://doi.org/10.3390/ijms23010513
APA StyleLevana, O., Hong, S., Kim, S. H., Jeong, J. H., Hur, S. S., Lee, J. W., Kwon, K.-S., & Hwang, Y. (2022). A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition. International Journal of Molecular Sciences, 23(1), 513. https://doi.org/10.3390/ijms23010513







