Abstract
Long-term cardiovascular complications of cancer therapy are becoming ever more prevalent due to increased numbers of cancer survivors. Cancer therapy-induced cardiotoxicity (CTIC) is an incompletely understood consequence of various chemotherapies, targeted anti-cancer agents and radiation therapy. It is typically detected clinically by a reduction in cardiac left ventricular ejection fraction, assessed by echocardiography. However, once cardiac functional decline is apparent, this indicates irreversible cardiac damage, highlighting a need for the development of diagnostics which can detect CTIC prior to the onset of functional decline. There is increasing evidence to suggest that pathological alterations to cardiac metabolism play a crucial role in the development of CTIC. This review discusses the metabolic alterations and mechanisms which occur in the development of CTIC, with a focus on doxorubicin, trastuzumab, imatinib, ponatinib, sunitinib and radiotherapy. Potential methods to diagnose and predict CTIC prior to functional cardiac decline in the clinic are evaluated, with a view to both biomarker and imaging-based approaches. Finally, the therapeutic potential of therapies which manipulate cardiac metabolism in the context of adjuvant cardioprotection against CTIC is examined. Together, an integrated view of the role of metabolism in pathogenesis, diagnosis and treatment is presented.
1. Introduction
Globally, the incidence of cancer is on the rise, and this trend is expected to continue, in part due to population aging [1]. Yet, thanks to large steps forward in cancer therapy, in many countries mortality rates are now falling [1]. This has created a growing population of cancer survivors, who face the long-term complications of cancer therapy. Cardiotoxicity, referring to cardiac damage and dysfunction, is a severe cause of co-morbidity and mortality in cancer therapies, with the range of therapies associated with cardiotoxicity including: doxorubicin, trastuzumab, sunitinib, imatinib, ponatinib and radiotherapy [2]. Despite extensive research, the pathophysiology of cancer therapy-induced cardiotoxicity (CTIC) with these agents remains unclear. Further, there remains a clinically unmet need to identify which patients are at risk of developing CTIC, especially heart failure. In this regard, imaging techniques and biomarkers are needed to detect at-risk patients before cardiac functional decline becomes apparent. Finally, there is a requirement for more targeted therapeutic options in these patients, which are currently limited to the standard of care heart failure medication such as angiotensin-converting enzyme inhibitors and beta blockers [3].
As a mechanical pump, the heart must constantly generate ATP (adenosine triphosphate) to sustain continuous contraction [4]. This ATP is rapidly hydrolysed to fuel both contractile shortening and ion pumps, such as sarcoplasmic reticulum Ca2+-ATPase, amongst others. Normally, the main metabolic substrates of the heart are fatty acids (60–90%) and carbohydrates (10–40%), with more than 95% of the ATP in the heart being generated through oxidative phosphorylation, and a small proportion through glycolysis, yielding lactate. With a high turnover of ATP, there is fine matching of ATP hydrolysis and ATP generation in order to maintain contractile function, requiring exquisite metabolic control to meet cardiac demand [4]. A loss of this cardiac metabolic control can be seen in ischaemic heart failure [5], and alterations to cardiac metabolism likely play a role in the pathophysiology of CTIC [6,7]. In this review, we will focus on the long-term consequences of several cancer therapies, namely, their role in causing heart failure. We will discuss their effects on cardiac metabolism and explore how dysregulated metabolism can be used both for detection and as drug targets in CTIC. We shall explore doxorubicin, an anthracycline chemotherapeutic; trastuzumab, a HER2 inhibitor; the tyrosine kinase inhibitor sunitinib, imatinib and ponatinib, as well as radiotherapy.
Clinically, should metabolic changes occur prior to functional deficits within the myocardium, an early detection of metabolic shifts could provide a sensitive, predictive test of CTIC. There has been rapid development in this field, and here we shall explore the feasibility of using blood biomarkers, 18F-fluorodeoxyglucose PET/CT (18F-FDG PET/CT) and hyperpolarised 13C magnetic resonance imaging (MRI) to detect early alterations in metabolism, preceding CTIC. Whilst early detection of CTIC could allow for rapid cessation of cancer therapy in affected patients to minimise further damage, this would affect cancer outcomes. Instead, the development of adjuvant cardioprotective therapies for delivery to CTIC-vulnerable patients could revolutionise their healthcare, meaning effective cancer therapies could continue to be used, whilst also preventing myocardial damage. As altered cardiac metabolism may represent a pathogenic cause of CTIC, agents which act upon myocardial metabolism could prevent these changes and provide a cardioprotective solution. In this review, we shall examine the roles of metformin, the SGLT2 inhibitors and resveratrol to determine their potential as future adjuvant therapies for cancer patients on cardiotoxic chemo- or radiotherapy.
2. Metabolic Dysfunction Leading to Cardiotoxicity of Specific Chemotherapies
2.1. Doxorubicin
Doxorubicin is a chemotherapeutic antibiotic of the anthracycline group first isolated in 1969 from Streptomyces peucetius [8]. It is regularly used to treat solid and haematological malignancies, including breast cancer, ovarian cancer and acute myeloid leukaemia [9]. Though efficacious, doxorubicin usage clinically is restricted by its cardiac side-effects. Though doxorubicin is associated with a number of acute cardiovascular complications, these are usually minor and transient [10]. However, longer term CTIC is a serious complication of treatment, with doxorubicin-induced heart failure (HF) carrying a mortality rate of 60% once diagnosed [11]. The total dose of doxorubicin is a major risk factor in CTIC development [11,12,13], with an incidence of 3–5% at 400 mg/m2 rising to 18–48% at a 700 mg/m2 cumulative dose [11,14]. Currently, the primary defence against doxorubicin-HF is limiting the cumulative dose to less than 450 mg/m2, but this also limits the therapeutic benefit and does not prevent all cases of CTIC [15]. There are several proposed mechanisms to account for doxorubicin cardiotoxicity, likely distinct from its anti-tumour inhibition of topoisomerase II causing disruption of DNA synthesis [15]. Oxidative stress [16], altered cardiac gene expression [15] and mitochondrial dysfunction [17] have been suggested as some mechanisms of doxorubicin-induced cardiotoxicity [18,19]. Indeed cardiomyocyte death may be a multifactorial process [20] (Figure 1).
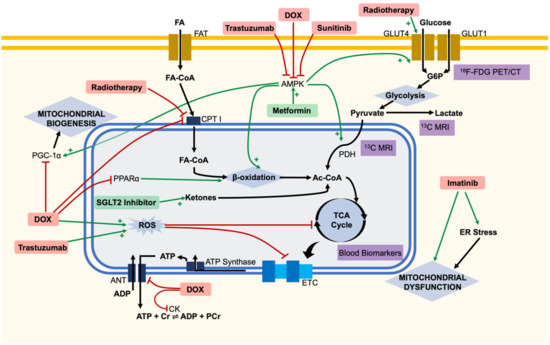
Figure 1.
Overview of metabolic changes within the cardiomyocyte in the pathogenesis of cancer therapy-induced cardiotoxicity. Cartoon representation of some explored targets of cancer therapies, with potential diagnostic techniques and therapeutics highlighted. Doxorubicin (DOX) has been linked to widespread metabolic dysfunction throughout the cell. Doxorubicin, trastuzumab and sunitinib have all been linked to the inhibition of adenosine monophosphate-activated protein kinase (AMPK), leading to downstream metabolic dysfunction. Glucose uptake into cells can be detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) imaging clinically. Circulating TCA cycle metabolites have been detected in patients and proposed as blood biomarkers. 13C hyperpolarised magnetic resonance imaging (MRI) has been used to measure the flux through PDH, both preclinically and in patients. Metformin may provide cardioprotection through activation of AMPK. The sodium-glucose cotransporter 2 (SGLT2) inhibitors may provide an alternative cardiac substrate of ketones to avoid bioenergetic failure. Ac-CoA, acetyl-CoA; ANT, adenine nucleotide translocator; CK, creatine kinase; CPT I, carnitine palmitoyltransferase I; Cr, creatine; ER, endoplasmic reticulum; ETC, electron transport chain; FA, fatty acid; FA-CoA, acyl-CoA; FAT, fatty acid transporters; G6P, glucose 6-phosphate; GLUT, glucose transporter; PDH, pyruvate dehydrogenase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivater-1α; PPARα, peroxisome proliferator-activated receptor alpha; ROS, reactive oxygen species.
The oxidative stress hypothesis remains the most popular theory of cardiotoxicity and has been reviewed extensively elsewhere [16]. As doxorubicin contains a quinone moiety, it can undergo redox cycling, resulting in the production of reactive oxygen species (ROS) and placing oxidative stress upon the cell. This leads to numerous downstream effects such as mitochondrial dysfunction, inflammation and apoptotic pathway activation [16]. The mitochondrion is hypothesised to be both a source and a target of ROS, with subsequent mitochondrial dysfunction associated with CTIC. As oxidative phosphorylation within the mitochondrion is responsible for more than 95% of the ATP used within normal cardiomyocytes [4], this dysfunction is hypothesised to alter cellular energetics. Indeed, doxorubicin has been associated with a decrease in the oxidative phosphorylation capacity of mitochondria and with changes to substrate usage and energy transfer, which may be responsible for the energetic failure and death of cardiomyocytes [21]. Redox cycling by doxorubicin has been linked to inhibition of complex I of the electron transport chain (ETC), with further direct inhibition of succinate dehydrogenase, succinate oxidase and cytochrome C oxidase due to doxorubicin’s affinity for cardiolipin, an inner mitochondrial membrane phospholipid [22] (Figure 1). Recently, in silico modelling has proposed that a dysfunctional positive feedback loop develops in the mitochondrion, where ROS production inhibits the ETC, both directly and through damage of mitochondrial DNA, resulting in the production of further ROS and increasing mitochondrial dysfunction until bioenergetic failure occurs [23]. However, there is conflicting evidence regarding the oxidative stress hypothesis, with anti-oxidant therapies providing partial efficacy at best [16] and it has been suggested that oxidative stress may not be responsible for mitochondrial dysfunction and loss, nor any downstream metabolic effects of doxorubicin [24]. However, human-induced pluripotent stem cell (iPSC)-derived mesenchymal stem cell (MSC) administration to murine models of doxorubicin cardiotoxicity demonstrated an attenuation of CTIC through functional mitochondrial transfer to cardiac tissue [25], demonstrating the vital role of mitochondria in the development of CTIC.
Doxorubicin has been hypothesised to directly affect cardiac substrate usage. In particular, radiotracer and metabolomics studies in rats have suggested a decrease in both glucose and fatty acid utilisation preceding the onset of cardiac functional decline [26,27]. Some rat studies have suggested doxorubicin inhibits fatty acid oxidation by preventing mitochondrial entry of long-chain fatty acids through direct inhibition of carnitine palmitoyltransferase I (CPT I) [28], whilst others have suggested this to be due to transcriptional alterations in the fatty acid oxidation pathway within mitochondria [29]. Furthermore, altered cellular gene expression has been hypothesised to be the root cause, as doxorubicin has been found to decrease cardiac expression of peroxisome proliferator-activated receptor alpha (PPARα) in murine models, a key regulator of β-oxidation [30]. In addition, doxorubicin was found to also inhibit the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), potentially favouring a glycolytic state over fatty acid oxidation, and indicating a loss of mitochondrial biogenesis [30]. A loss of fatty acid oxidation capacity is a pathological event also seen in ischaemic heart disease [4] and may be key to CTIC.
However, the bioenergetic failure of cardiomyocytes may also be partly driven by a loss of cellular energetic buffering and availability of cytoplasmic ATP. In models of cardiotoxicity, doxorubicin has been shown to inhibit the adenine nucleotide translocator (ANT), which is responsible for exchanging free ATP for ADP across the mitochondrial inner membrane, compromising the availability of cytosolic ATP, despite mitochondrial production [30]. Moreover, doxorubicin has been shown to impair the creatine kinase reaction in cardiomyocytes [31]. Creatine kinase is the key enzyme which facilitates the reaction converting creatine to phosphocreatine (using a phosphate group from ATP), providing a vital rapid energy buffer. The reaction is reversed during high energy usage, providing an immediate source of ATP—the loss of this system constitutes the loss of an important metabolic safety net [31]. In vivo animal magnetic resonance spectroscopy studies have shown that there is a decrease in the phosphocreatine:ATP ratio early on with doxorubicin administration, prior to cardiac decline, suggesting the loss of high-energy phosphate buffering could constitute an important role in energetic failure of the cell [32] (Figure 1).
More recently, perturbed AMP-activated protein kinase (AMPK) signalling has been proposed as another potential mechanism of doxorubicin cardiotoxicity [33]. AMPK is a heterotrimeric protein complex, with a catalytic α subunit and regulatory β and γ subunits [34]. AMPK is an important regulator of cellular energy homeostasis due to AMP allosterically activating the γ subunit [35]. Further activation occurs at phosphorylation sites on both the α and β subunits, through the action of AMPK kinases [36,37]. Once activated, cardiac AMPK phosphorylates several downstream effectors, such as phosphofructokinase-2 (PFK2) and glucose transporter 4 (GLUT4), resulting in increased glucose uptake and metabolism. Similarly, fatty acid uptake and metabolism is also upregulated both directly and indirectly by AMPK [37]. AMPK activation furthermore promotes mitochondrial biogenesis through increased expression of genes such as PGC-1α [38]. As well as upregulating ATP production, AMPK downregulates anabolic pathways such as protein synthesis and cellular proliferation, primarily thought to be via inhibition of the mechanistic target of rapamycin (mTOR) [33]. Doxorubicin has repeatedly been shown to inhibit cardiac AMPK in a variety of in vivo experimental models, though the precise mechanism of inhibition remains unclear [33,39], and AMPK’s role in doxorubicin cardiotoxicity has been reviewed previously [36]. Therefore, aberrant AMPK inhibition may result in a loss of normal cellular energy signalling and impaired mitochondrial biogenesis, leading to the altered metabolic state seen in doxorubicin cardiotoxicity (Figure 1). Together, these theories all place metabolic alterations centrally in the pathogenesis of doxorubicin cardiomyopathy (Table 1).

Table 1.
Cardiac metabolic alterations of cancer therapies implicated in the pathogenesis of cardiotoxicity. In vivo and iPSC studies have been selected for clinically relevant mechanistic insight. AMPK, AMP-activated protein kinase; CPT I, carnitine palmitoyltransferase I; GLUT4, glucose transporter type 4; mTOR, mechanistic target of rapamycin; PCr, phosphocreatine; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PPARα, peroxisome proliferator-activated receptor alpha.
2.2. Trastuzumab
Trastuzumab is a monoclonal antibody that targets the extracellular region of the human epidermal growth factor receptor 2 (HER2), causing inhibition of the pathway. HER2, an oncogene, is overexpressed in around 20–30% of breast cancers. Therefore, the use of trastuzumab adjuvant oncotherapy has proved clinically successful for treatment of these cancers [50]. However, trastuzumab therapy is associated with cardiotoxicity in 20–45% of patients [51,52,53] and heart failure in 2–3% of patients [51,52]. Though some of this damage is thought to be reversible upon trastuzumab cessation, there is a clear element of long-term, irreversible damage to the myocardium [54], and cardiotoxicity is strongly associated with treatment interruption [53], highlighting the severity of the issue. Moreover, trastuzumab is often used as an adjuvant therapy with doxorubicin, further potentiating cardiotoxicity [54]. Mechanisms underlying trastuzumab’s cardiotoxic effect are thought to be linked to the direct inhibition of the HER2 pathway, which is expressed in adult human cardiomyocytes [55]. Metabolically, these include the production of ROS, altered gene expression, and changes to ATP production [7,56]. This metabolic angle shall be explored here (Table 1).
Mitochondrial dysfunction may lead to cardiomyocyte death in trastuzumab CTIC. Recently, mitochondrial dysfunction has been demonstrated in rabbits administered subcutaneous trastuzumab, with disorganised cristae and disruption to both the inner and outer membranes of the mitochondria evident. Oral antioxidant administration was able to prevent some of these effects, suggesting that ROS at the level of the mitochondrion may play a role in dysfunction, though this purely descriptive study is limited in nature [41]. Supporting this, in vitro studies have found that erbB2 (the non-human equivalent of HER2) blockade increases ROS production, likely at the site of the mitochondrion, which may lead to cell death [57]. Indeed, erbB2 has been shown to translocate to the mitochondria and regulate cellular metabolism in other cell lines [58]. Together, one could suggest that trastuzumab’s direct inhibition of HER2 may lead to pathological alterations in mitochondrial function, creating energetic deficits alongside ROS production.
Trastuzumab may also act upon AMPK, the master cellular energy regulator, to place cardiomyocytes in a pathological energetic state. In embryonic human primary cardiomyocytes, it was first demonstrated that trastuzumab was unable to activate AMPK, though another HER2 inhibitor, GW2974, was able to activate AMPK. AMPK activation was demonstrated to stimulate fatty acid oxidation, providing a metabolic stress response deemed protective to cardiomyocytes in the face of a TNFα challenge [59]. Recently, two studies in human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) have shown trastuzumab to dysregulate metabolism [42,43], likely at the level of AMPK [42]. iPSC-CMs treated with trastuzumab demonstrate a marked contractile dysfunction, mimicking the clinical picture of CTIC. iPSC-CMs treated with trastuzumab were shown to have lower levels of AMPK phosphorylation (corresponding to activation), glucose uptake, cellular ATP, oxidative phosphorylation complexes and mitochondrial respiratory capacity [42,43], indicating a widespread energetic impairment. Transcriptome pathway analysis confirmed significant alterations in the expression of genes relating to metabolic pathways [42,43]. In this model, AICAR (an AMPK activator), alongside AMPK potentiators such as metformin, rosiglitazone, resveratrol and lipoic acid were able to avert these defective metabolic effects and showed a rescue of cardiotoxic symptoms and cardiomyocyte contraction [42], suggesting potential promises in treatment centred on AMPK modulation. Thus, AMPK inhibition may lie at the heart of trastuzumab-related cardiotoxicity, resulting in metabolic alterations which lead to cellular death (Figure 1).
2.3. Sunitinib
Sunitinib is a small-molecule, multi-targeted receptor tyrosine kinase inhibitor, which inhibits a wide variety of targets including platelet-derived growth factor receptors (PDGFRs) and vascular endothelial growth factor receptors (VEGFRs) amongst others. It is used in the treatment of advanced renal cell carcinoma, gastrointestinal stromal tumours, pancreatic cancer and neuroendocrine tumours [60]. As with other tyrosine kinase inhibitors, sunitinib is associated with a severe risk of cardiotoxicity, affecting up to 30% of patients, with severe cardiovascular events seen in up to 10% [61]. Approximately half of this cardiac dysfunction is thought to be reversible, leaving a significant cardiovascular burden of risk associated with the drug [62].
The mechanisms underlying sunitinib cardiotoxicity remain relatively unexplored, though it is thought to include coronary microvascular dysfunction due to effects on VEGFR and PDGFR [60,63]. However, off-target inhibition of AMPK has also been suggested as a key element in toxicity. One such study found that sunitinib causes a loss of the mitochondrial membrane potential and bioenergetic failure within cardiomyocytes. In mice, sunitinib was found to reduce the activity of cardiac AMPK, thought to be due to direct inhibition. Indeed, adenoviral transfer of a constitutively active form of AMPK decreased sunitinib induced cell death, suggesting AMPK to be a key target in sunitinib cardiotoxicity [45]. In agreement, a recent study in a mouse model found that trimetazidine (an anti-angina drug) was able to ameliorate sunitinib CTIC through AMPK activation [46] (Table 1). Overall, sunitinib may in part cause a bioenergetic failure within cardiomyocytes through inhibition of AMPK, amongst other effects (Figure 1).
2.4. Imatinib
Imatinib is a small molecule inhibitor of the constitutively active fusion BCR-ABL kinase which is present in more than 90% of chronic myeloid leukaemia (CML) cases and 30% of B-cell acute lymphoblastic leukaemia cases [64]. Imatinib’s effective inhibition has revolutionised the treatment of these diseases, but concerns have been raised about cardiotoxicity associated with usage [65]. Despite initial fears, CTIC is thought to be a rare event with imatinib, with just eight cases of heart failure attributed to imatinib in a retrospective analysis of 1276 patients [66]. Nevertheless, an understanding of cardiotoxicity may prove important for the treatment of these rare cases and provide insight into the mechanism of similar cardiotoxic drugs. Initial investigations in neonatal rat ventricular cardiomyocytes have suggested imatinib may induce stress within the endoplasmic reticulum, leading to mitochondrial failure and cellular death [65]. However, a more recent study on H9c [2] cells has suggested oxidative stress to be the initial factor leading to mitochondrial dysfunction and eventual apoptosis, dependent on the mitochondrial scaffold protein Sab [67]. Whilst far from conclusive, mitochondrial dysfunction may play a role in imatinib-mediated cardiotoxicity (Figure 1).
2.5. Ponatinib
Ponatinib is a third generation BCR-ABL kinase inhibitor which was created to counter the T315I gatekeeper mutation which causes imatinib resistance in patients with CML [68]. Despite being clinically efficacious, ponatinib has been associated with a high rate of cardiovascular adverse events, with a recent retrospective study finding 5% of patients with HF and more than 20% to experience an adverse cardiovascular event with ponatinib therapy [68]. Analysis of the FDA adverse event reporting system database has found ponatinib to carry the greatest risk of cardiotoxicity amongst all kinase inhibitors [69], with disproportionally high reporting of cardiac failure, ischaemic heart disease and hypertension compared to other anticancer therapies for CML [70].
Ponatinib’s cardiotoxic effects may be due to both vascular effects on the coronary microvasculature and direct effects on the myocardium itself, linked to the promiscuity of its kinase inhibition [71]. Indeed, ponatinib has been found to be antiangiogenic in vitro [72], and mouse studies have found some evidence of a global microvascular angiopathy in the coronary vessels [73]. In cardiomyocytes, ponatinib has been shown to inhibit the AKT and ERK signalling pathways, inducing cardiomyocyte apoptosis, which can be ameliorated by activation of the pathways with neuregulin-1β [74]. The PI3K-AKT signalling pathway is involved in many downstream effects on cellular metabolism, including glucose uptake and glycolysis. An inhibition of the pathway may negatively affect the cellular energetic state, favouring cardiomyocyte apoptosis [75]. Indeed, ponatinib has been shown to strongly impair oxidative metabolism and weakly impair glycolysis in vitro within hepatocytes, albeit at higher concentrations that are reached in plasma, though potentially at those seen in the heart/liver [76].
2.6. Radiotherapy
Radiotherapy is an important treatment option as either a monotherapy or adjuvant for a wide variety of cancers. However, adverse cardiovascular events, labelled under radiation-induced heart disease (RIHD), have caused significant morbidity and mortality in patients. Though the incidence varies depending on the specific malignancy and dose of radiotherapy used, RIHD has been widely reported in Hodgkin’s lymphoma [77] and breast cancer [78,79]. In a recent study examining breast cancer patients, the cumulative incidence of cardiac events was found to increase proportionally by 16.5% per Gray of radiation patients were exposed to [79].
Endothelial injury, inflammation, oxidative stress and ER stress have all been implicated in the mechanism of RIHD [80]. Despite endothelial cell injury gaining prominence as the primary cause of RIHD [80], clinical radiotracer studies have shown myocardial alterations in metabolic uptake evident in irradiated segments of the heart, which was not linked to the vascular territories of coronary arteries [48]. Further, a recent in vivo study in beagles has suggested that much of the sequalae of RIHD is not associated with an increase in pro-inflammatory cytokines. Instead, the team identified an increase in GLUT4 expression and a decrease in CPT1 expression in irradiated myocardium, alongside mitochondrial damage and increased glucose uptake, suggesting a switch from fatty acid oxidation to glycolysis [47] (Figure 1), a metabolic shift seen in the pathogenesis of ischaemic heart failure [4]. These findings extend upon a clinical study of patients with oesophageal cancer, who were treated with chemoradiotherapy. It was found that 20% of these patients exhibited increased glucose uptake (through 18F-FDG PET-metabolic imaging) in the irradiated myocardium, though this was not linked to any RIHD, and the adjuvant chemotherapy presents a significant confounding variable [49]. Nevertheless, taken together, these studies suggest that myocardial metabolic alterations may play an important role in the pathogenesis of RIHD.
3. Predicting Cardiotoxicity Using Metabolic Markers
Though our understanding of the mechanisms behind CTIC is improving, clinically a major unmet need is to be able to predict which patients are likely to suffer from CTIC. The alterations in myocardial metabolism associated with CTIC present a potential solution to this issue. As myocardial metabolism is within the pathophysiology of disease, early identification of metabolic shifts could be predictive of cardiotoxicity and allow for appropriate alterations to therapy to be taken. There are a variety of techniques which can be conducted to do this, including measuring blood biomarkers, radiolabelled PET-CT imaging and hyperpolarised MR imaging (Figure 1). Here, we shall examine how these techniques have been applied to identify early signs of CTIC, as well as a wider exploration of their potential use based on studies from other conditions (human studies summarised in Table 2).

Table 2.
Clinical studies investigating the correlation of plasma metabolites and metabolic imaging with the onset of cardiotoxicity. 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography; HER2, human epidermal growth factor receptor 2; LC-MS, liquid chromatography–mass spectrometry; SUV, standardized uptake value.
3.1. Blood Biomarkers
Blood biomarkers are easily obtainable and tested for, and if found to provide a high sensitivity and specificity, could be rapidly put into clinical practice. Traditional biomarkers of cardiac injury may present too late for predictive therapy, for example, an increase in troponin-I levels after anthracycline administration has been associated with cardiotoxicity, but this is an indication that cardiac injury has already occurred [83]. Instead, an examination of plasma metabolites can provide insight into changes in cellular metabolism, potentially identifying early changes in bioenergetics before the onset of cardiac damage. Recently, this was examined in a small study of patients with HER2+ breast cancer treated with adjuvant therapy of anthracyclines, taxanes and trastuzumab. Using liquid chromatography mass spectroscopy (LC-MS), the team analysed fasting blood plasma samples of patients at various timepoints in therapy. The group found that patients who went on to develop cardiotoxicity exhibited significantly lower levels of citric acid and aconitic acid prior to functional changes detected by echocardiography. Indeed, the magnitude of the decrease of citric acid/aconitic acid was correlated with the change in the left ventricular ejection fraction (LVEF) at a later time point [40]. Though this study did not examine the predictive value of these metabolites, it highlights early changes in the citric acid cycle as a potential source of important plasma biomarkers for cardiotoxicity.
Though there are few studies directly looking at CTIC, other heart failure studies suggest that plasma biomarkers of metabolism could prove promising. In one such recent approach, baseline plasma samples were taken from patients in a randomised controlled trial examining the role of spironolactone in heart failure with preserved ejection fraction. Elevated levels of fatty acid binding protein-4, a protein correlated to the rate of fatty acid metabolism, were shown to be strongly linked to the later development of heart failure, independent of other risk factors [84]. This highlights how baseline plasma studies could provide a very early prediction of cardiac toxicity if applied to CTIC.
However, understanding the meaning of these plasma biomarker studies can be difficult as they may not directly correlate to cardiac metabolism. In a recent study of heart failure patients, a metabolomics approach was used, taking blood samples from the femoral artery, coronary sinus and femoral vein in an attempt to directly assess cardiac metabolism. Heart failure was shown to increase ketone body and lactate usage within the heart itself, but this was only apparent through a comparison of arterial and coronary sinus samples, not femoral vein samples, demonstrating that peripheral blood samples may not be representative of the picture in the heart [85]. Nevertheless, advances in biomarker-based techniques could rapidly revolutionise our predictive capabilities in cardiotoxicity.
3.2. 12F-FDG PET/CT
Cancer therapy-induced metabolic changes in part can alter substrate use within the myocardium. The use of radiotracers for metabolic substrate uptake, combined with anatomical imaging, could provide a valuable technique to visualise changes in cardiac glucose uptake, which could predict future changes to cardiac function. 18F-fluorodeoxyglucose (18F-FDG) is a radiolabelled analogue of glucose, which is taken up by cellular glucose transporters and is phosphorylated by hexokinases, where it becomes trapped within the cell and cannot undergo further metabolism. This accumulation can be seen using positron emission tomography (PET) alongside computerised tomography (CT) for signal co-localisation with tissue anatomy [86]. 18F-FDG PET/CT is a technique widely used to identify metabolically active cancers, but also has potential to detect alterations to glucose uptake in the myocardium.
A recent animal model of RIHD has suggested 18F-FDG PET/CT can be used to identify metabolic changes prior to any detectable changes in cardiac function. Cardiac 18F-FDG standardised uptake values were found to be significantly increased in beagles who had undergone myocardial irradiation, compared to controls, prior to any functional changes being seen, suggesting this may be a valuable technique for the early detection of CTIC [47] and highlighting a shift towards glucose usage. However, studies in humans have been less clear. A small study of lymphoma patients treated with doxorubicin found it to have mixed effects upon 18F-FDG uptake, though cardiac function parameters were unavailable [81]. On the other hand, a recent retrospective study of breast cancer patients who had anthracycline- or trastuzumab-based oncotherapy found an association between 18F-FDG uptake in the right ventricular myocardium immediately post-therapy and the later development of cardiotoxicity after controlling for age, radiotherapy and treatment type. Though not early enough to change treatment choices, this may still be of prognostic value [44]. However, these results must be treated with caution—these retrospective 18F-FDG PET/CT scans are conducted primarily for tumour visualisation, with a differing protocol to myocardial specific 18F-FDG PET/CT, which may lead to spurious results. Nevertheless, the technique shows some promise and warrants further clinical exploration. As such, a case-control trial is currently recruiting lymphoma patients to assess the value of 18F-FDG PET/CT as a predictive tool for chemo- or immunotherapy induced cardiotoxicity (NCT04555642) [87].
3.3. Hyperpolarised 13C MRI
Though 18F-FDG PET/CT has been proven to be a useful imaging technique, it is limited to demonstrating the cellular uptake of glucose and cannot show any alterations to the metabolic fate of substrates. 13C magnetic resonance spectroscopy allows for the direct evaluation of the metabolic fates of the 13C-containing compound. The low sensitivity of this technique has been addressed by hyperpolarised magnetic resonance, which increases the 13C signal by several orders of magnitude [88]. This makes the technique viable for imaging metabolic fluxes in patients, and hyperpolarised 13C MRI is well-tolerated by patients [89]. The technique has been successfully used in a porcine model of heart failure, demonstrating a decrease in pyruvate dehydrogenase flux (representing a move to anaerobic glycolysis) prior to any functional changes seen using echocardiography [90]. Further, the technique has been used to demonstrate cardiac metabolic alterations in diabetic patients [91].
Looking at CTIC, there are several studies that support the use of hyperpolarised 13C MRI to predict functional decline. In a clinically relevant rat model of doxorubicin-induced cardiotoxicity, one such study utilised hyperpolarised MRI with [1-13C]pyruvate and [2-13C]pyruvate to assess changes to pyruvate dehydrogenase flux and TCA cycle flux, respectively. Doxorubicin administration was associated with a decrease in the [13C]bicarbonate:[13C] pyruvate ratio compared to controls prior to the detection of cardiac dysfunction. This reflects a decreased flux through pyruvate dehydrogenase, in line with expected decreases in mitochondrial oxidative phosphorylation [24]. This represents an important detection of an early sign of CTIC. Furthermore, a pilot study in 10 breast cancer patients treated with adjuvant doxorubicin found similar results of a decrease in the [13C]bicarbonate/total 13C signal post doxorubicin administration using hyperpolarized [1-13C]pyruvate [82]. As a result of these promising studies, there are clinical trials currently recruiting breast cancer [92] and thoracic cancer [93] patients to evaluate the feasibility of [1-13C]pyruvate hyperpolarised MRI to detect radiation-induced cardiotoxicity (NCT03685175, NCT04044872). Overall, there have been rapid advances in the utility of hyperpolarized 13C MRI, and there is great promise for its diagnostic potential, despite its currently limited availability and high cost [94].
4. Adjuvant Cardiotherapy
Being able to identify which patients are likely to experience CTIC would allow for early decision making about therapeutic options, not just limited to halting or changing cancer therapies. Instead, this could allow for the early administration of adjuvant cardioprotective therapies, allowing for effective chemotherapy regimens to be continued, whilst protecting the heart of vulnerable patients. Though this is a field in its infancy, as many of the pathological metabolic alterations in CTIC are similar to those seen in heart failure of other origins, we can look to recent developments in ischaemic heart failure to envision some potential cardioprotective therapies which could be applied more widely to CTIC. Here, we explore two potential therapies—the sodium-glucose transport protein 2 (SGLT2) inhibitors, and metformin; both may provide a cardioprotective effect through metabolic alterations, representing a small pool of potential therapies in this rapidly expanding field (Figure 1).
4.1. Metformin
Firstly, metformin is a widely used first-line drug in the treatment of type 2 diabetes mellitus, with a strong safety profile. In addition to its glucoregulatory effects, primarily thought to be due to suppression of hepatic gluconeogenesis, metformin is thought to alter energy metabolism in part through an activation of AMPK, though this is a hotly debated topic [95]. As a side effect of therapy, metformin has been shown to be cardioprotective in diabetes, going beyond its role in glucose control [96]. Beyond diabetes, metformin has been demonstrated to be effective at reducing mortality in heart failure patients with preserved ejection fraction in a recent meta-analysis [97]. A cardioprotective effect in heart failure has been hypothesised to act in two ways: by stimulating the insulin-mediated uptake of glucose in the myocardium and by activating AMPK and restoring pathological cardiac energetics [96]. These positive findings all suggest metformin may be a strong candidate as an adjuvant therapy to prevent CTIC.
Indeed, a recent retrospective database study of patients with early stage breast cancer who received adjuvant breast radiotherapy found metformin use to significantly decrease the risk of major cardiac events (HR = 0.79), suggesting metformin could reduce the risk of RIHD [98]. Though a limited retrospective study, it is an early sign that metformin could provide value if given to patients likely to develop CTIC. Looking to preclinical models, metformin has been shown to provide a cardioprotective effect against doxorubicin in rats [99]. In vitro, metformin’s cardioprotection against doxorubicin has been demonstrated to be directly due through AMPK activation, with AMPK inhibitors able to abolish any protective effect [100]. This AMPK activation directly reverses the inhibition seen with doxorubicin administration and may also reduce oxidative stress within the cell through its role as an energy stress sensor [101]. In human iPSCs, metformin has also been shown to ameliorate the trastuzumab-induced dysfunction, put down to its activating effects on AMPK [42]. Interestingly, metformin may play a dual-role in cancer therapy, as it has been associated with a decrease in cancer mortality in patients with type II diabetes [102]. This was recently investigated by the METTEN study, which examined whether metformin was able to increase the pathological complete response (clearance of cancer) in HER2-positive breast cancers treated with traditional chemotherapeutics + trastuzumab [103]. Though the study was inconclusive due to a lack of power, this highlights the research interest into the potential for metformin as a cancer therapy adjuvant. With further research, metformin can be envisioned to potentially both improve cancer therapy responsiveness as well as providing protection against CTIC.
4.2. SGLT2 Inhibitors
SGLT2 inhibitors are a modern class of drugs which were originally introduced as a treatment for type 2 diabetes mellitus, as they block glucose reabsorption in the proximal convoluted tubule of the kidney, increasing glycosuria and reducing blood glucose levels [104]. The EMPA-REG OUTCOME trial examined how the SGLT2 inhibitor empagliflozin (EMPA) affected cardiovascular outcomes in type 2 diabetes mellitus patients. It found EMPA to significantly reduce the risk of adverse cardiovascular events, with post-hoc analysis finding the effect to be independent of glycaemic control [105]. Moreover, the DAPA-HF trial showed similar outcomes with another SGLT2 inhibitor, dapagliflozin, in heart failure patients with reduced ejection fraction, regardless of type 2 diabetes mellitus status [106]. This suggests SGLT2 inhibitors may have an important cardioprotective role, which cannot solely be explained through its diuretic effect, suggesting it to be a strong candidate against CTIC.
Indeed, one widely proposed mechanism of cardioprotection with SGLT2 inhbitors has been the ‘thrifty substrate’ hypothesis, suggesting that a decrease in available glucose due to SGLT2 inhibitors forces metabolism to favour the oxidation of free fatty acids and places the body into a ketogenic state, creating a mild, persistant hyperketonemia. The preferential oxidation of ketones within the heart is more oxygen efficient, reducing the stress placed upon the heart [107], and removes a reliance upon anaerobic glycolysis. A similar mechanism may confer cardioprotection against cancer therapies. A number of murine models have found EMPA to prevent doxorubicin induced cardiotoxicity [108,109,110,111,112], with an improvement in LV function that is not seen with pure reductions in circulating volume, such as furosemide treatment [108]. One such recent study attributes this cardioprotection due to elevations in the ketone β-hydroxybutyrate which were seen upon EMPA administration. Direct β-hydroxybutyrate treatment alongside doxorubicin was sufficient to reduce symptoms of cardiotoxicity, suggesting the production of a hyperketonic state may be protective. Within cardiomyocytes, β-hydroxybutyrate administration, alongside doxorubicin, resulted in a decrease in mitochondrial dysfunction and ROS levels [112], recaptiulating effects of ketone administration seen in mouse models of ischaemia-reperfusion [113]. Despite this attractive hypothesis, other studies have suggested a variety of cardioprotective mechanisms of EMPA cardioprotection including upregulated PGC-1α [109], altered autophagy [110] and decreases in pro-inflammatory cytokines [111]. This is an actively expanding field, and there are likely to be rapid further advances on the topic. Though to the best of our knowledge in vivo studies of EMPA cardioprotection with other cancer therapies have not been performed to date, early cardiomyocyte studies with dapagliflozin suggest it may also provide benefit with trastuzumab treatment [114].
4.3. Resveratrol
Resveratrol ([3,5],4′-trihydroxy-trans-stilbene) is a naturally occurring polyphenol, found in a number of plants, notably in high concentrations in the skin and seeds of grapes [115]. Available as a dietary supplement, resveratrol has attracted particular interest within the scientific community due its antioxidant effect, usually tested at far higher concentrations than present naturally [115]. Though not yet in clinical use, resveratrol’s potential anti-inflammatory, anti-cancer and antimicrobial effects may provide therapeutic benefit for a variety of disaeses. In the cardiac sphere, a randomised controlled trial of 40 patients demonstrated that resveratrol administration in stable coronary artery disease patients (who had a history of myocardial infarction) significantly impoved LV diastolic function and resulted in a decrease in circulating LDL cholesterol [116]. Previous in vivo studies in murine postinfarction heart failure models have suggested this may be due, at least in part, to an antioxidant effect decreasing oxidative stress. Signalling pathway alterations have also been implicated in the cardioprotective role of resveratrol, including the AKT pathway, which is involved in cellular survival signalling, but also glucose uptake and metabolism control [117]. As a safe, well-tolerated compound, these findings may extend to a potential protective effect against CTIC.
In the context of CTIC, resveratrol’s role has previously been expertly reviewed [118], with a range of potential cardioprotective effects. Here, we shall focus upon the possible metabolic effects of the drug as a potential cancer therapy adjuvant. In vivo, resveratrol has been demonstrated in murine models to offer cardioprotection against doxorubicin administration [119,120] and may potentiate the anticancer effect of doxorubicin as well [121]. Some of this cardioprotection may be due to a restoration of mitochondrial dysfunction, perhaps due to AMPK activation [122], SIRT1 activation [123], or directly increased expression of mitochondrial ETC complexes [120]. The direct AMPK effect has also been explored in human-iPSC models of trastuzumab CTIC, where resveratrol was able to overcome contractile dysfunction induced by trastuzumab, thought to be due to its role in activating AMPK [42]. Looking to radiotherapy, black grape juice (containing high concentrations of resveratrol) administration to rats was found to prevent an increase in lactate dehydrogenase levels after radiation administration, suggesting it may have a protective effect at the myocardium, though this study is of limited quality [124]. Recently, a metabolomics-based approach analysing cardiac tissue in murine models of RIHD demonstrated that resveratrol can prevent the radiation-induced decrease in choline-containing metabolites and unsaturated fatty acid metabolites, implying alterations to cardiac metabolism may play a role in its cardioprotective effect [125]. Though our understanding of resveratrol is more limited than other potential adjuvant therapies, more research could yield exciting insights into a possible therapeutic role for this compound in CTIC patients.
5. Conclusions
In summary, CTIC represents a severe limitation of cancer therapies, likely in part due to metabolic disturbances at the level of the cardiomyocyte. It appears that both mitochondria and the AMPK signalling pathway may be important mediators of this metabolic dysfunction, both resulting in potential bioenergetic failure of the cardiomyocyte. Despite a need for more clinical research, it appears more novel targeted chemotherapies such as sunitinib and ponatinib may also exert cardiotoxic effects through these pathways. It is evident that changes in myocardial metabolism, preceding functional changes in CTIC can be detected through a variety of techniques, including plasma metabolites (biomarkers), 18F-FDG PET/CT and hyperpolarised 13C MRI, with rapid advances in diagnostic potential. A refinement of these techniques will make them permissible for routine clinical use, allowing for the detection and prediction of patients susceptible to CTIC, at an early enough stage to prevent functional decline from occurring. An innovative approach to drugs which can alter metabolism, applied in this context, could provide a cardioprotective effect as an adjuvant therapy in targeted vulnerable patients. Though more translational research is needed, exploring myocardial metabolism for early diagnosis and treatment of CTIC is an exciting and emerging avenue.
Author Contributions
Conceptualization, K.N.T. and A.C.; resources, K.N.T.; writing—original draft preparation, A.C.; writing—review and editing, K.N.T.; visualization, A.C.; supervision, K.N.T.; project administration, K.N.T.; funding acquisition, K.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was supported by an Oxford BHF Centre of Research Excellence (grant code RE/18/3/34214) to K.N.T. The APC was funded by the British Heart Foundation.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Li, D.L.; Hill, J.A. Heart failure and loss of metabolic control. J. Cardiovasc. Pharmacol. 2014, 63, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Della Sala, A.; Tocchetti, C.G.; Porporato, P.E.; Ghigo, A. Metabolic Aspects of Anthracycline Cardiotoxicity. Curr. Treat. Options Oncol. 2021, 22, 18. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-mediated cardiotoxicity: Current understanding, challenges, and frontiers. Antib. Ther. 2018, 1, 13–17. [Google Scholar] [CrossRef]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Kilickap, S.; Akgul, E.; Aksoy, S.; Aytemir, K.; Barista, I. Doxorubicin-induced second degree and complete atrioventricular block. Europace 2005, 7, 227–230. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefrak, E.A.; Piťha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Takemura, G.; Fujiwara, H. Doxorubicin-Induced Cardiomyopathy. From the Cardiotoxic Mechanisms to Management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Šimůnek, T.; Štěrba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Gerši, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Bhuiyan, M.A.N.; Panchatcharam, M.; Pattillo, C.B.; Orr, A.W.; Sadoshima, J.; Hill, J.A.; et al. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; Tirupathi, T.P.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; dos Santos Goldenberg, R.C. Doxorubicin-Induced Cardiotoxicity: From Mechanisms to Development of Efficient Therapy. In Cardiotoxicity; InTech: London, UK, 2018. [Google Scholar]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; Zuppinger, C.; Wallimann, T.; Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006, 41, 389–405. [Google Scholar] [CrossRef]
- Marcillat, O.; Zhang, Y.; Davies, K.J.A. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem. J. 1989, 259, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, B.L.; Niederer, S. A Biophysical Systems Approach to Identifying the Pathways of Acute and Chronic Doxorubicin Mitochondrial Cardiotoxicity. PLoS Comput. Biol. 2016, 12, e1005214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, K.N.; Perera, C.; Ball, V.; Henry, J.A.; Miller, J.J.; Kerr, M.; West, J.A.; Sharma, E.; Broxholme, J.; Logan, A.; et al. Early detection of doxorubicin-induced cardiotoxicity in rats by its cardiac metabolic signature assessed with hyperpolarized MRI. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Z.; Jiang, D.; Liang, X.; Liao, S.; Zhang, Z.; Yue, W.; Li, X.; Chiu, S.M.; Chai, Y.H.; et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Rep. 2016, 7, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakasugi, S.; Fischman, A.J.; Babich, J.W.; Callahan, R.J.; Elmaleh, D.R.; Wilkinson, R.; Strauss, H.W. Myocardial substrate utilization and left ventricular function in adriamycin cardiomyopathy. J. Nucl. Med. 1993, 34, 1529–1535. [Google Scholar] [PubMed]
- Yuan, Y.; Fan, S.; Shu, L.; Huang, W.; Xie, L.; Bi, C.; Yu, H.; Wang, Y.; Li, Y. Exploration the Mechanism of Doxorubicin-Induced Heart Failure in Rats by Integration of Proteomics and Metabolomics Data. Front. Pharmacol. 2020, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, S.; El-Merzabani, M.M.; Sayed-Ahmed, M.; Taylor, D.A.; Lowe, J.E. Acute and chronic effects of adriamycin on fatty acid oxidation in isolated cardiac myocytes. J. Mol. Cell. Cardiol. 1997, 29, 789–797. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Sousa, R.P.B.; Cadete, V.J.J.; Lopaschuk, G.D.; Palmeira, C.M.M.; Bjork, J.A.; Wallace, K.B. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology 2010, 270, 92–98. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Li, X.; Yang, T.; Jiang, Q. Effects of PPARα/PGC-1α on the myocardial energy metabolism during heart failure in the doxorubicin induced dilated cardiomyopathy in mice. Int. J. Clin. Exp. Med. 2014, 7, 2435. [Google Scholar]
- Tokarska-Schlattner, M.; Wallimann, T.; Schlattner, U. Multiple interference of anthracyclines with mitochondrial creatine kinases: Preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol. Pharmacol. 2002, 61, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Maslov, M.Y.; Chacko, V.P.; Hirsch, G.A.; Akki, A.; Leppo, M.K.; Steenbergen, C.; Weiss, R.G. Reduced in vivo high-energy phosphates precede adriamycin-induced cardiac dysfunction. Am. J. Physiol. - Hear. Circ. Physiol. 2010, 299, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Gratia, S.; Kay, L.; Potenza, L.; Seffouh, A.; Novel-Chate, V.; Schnebelen, C.; Sestili, P.; Schlattner, U.; Tokarska-Schlattner, M. Inhibition of AMPK signalling by doxorubicin: At the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress. Cardiovasc. Res. 2012, 95, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R.B. The role of AMPK in cardiomyocyte health and survival. Biochim. Biophys. Acta—Mol. Basis Dis. 2016, 1862, 2199–2210. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Timm, K.N.; Tyler, D.J. The Role of AMPK Activation for Cardioprotection in Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Drugs Ther. 2020, 34, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyck, J.R.B.; Lopaschuk, G.D. AMPK alterations in cardiac physiology and pathology: Enemy or ally? J. Physiol. 2006, 574, 95–112. [Google Scholar] [CrossRef]
- Marin, T.L.; Gongol, B.; Zhang, F.; Martin, M.; Johnson, D.A.; Xiao, H.; Wang, Y.; Subramaniam, S.; Chien, S.; Shyy, J.Y.J. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci. Signal. 2017, 10, eaaf7478. [Google Scholar] [CrossRef] [Green Version]
- Konishi, M.; Haraguchi, G.; Ohigashi, H.; Ishihara, T.; Saito, K.; Nakano, Y.; Isobe, M. Adiponectin protects against doxorubicin-induced cardiomyopathy by anti-apoptotic effects through AMPK up-regulation. Cardiovasc. Res. 2011, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Shi, X.; Farrell, L.; Lall, R.; Sebag, I.A.; Plana, J.C.; Gerszten, R.E.; Scherrer-Crosbie, M. Changes in Citric Acid Cycle and Nucleoside Metabolism Are Associated with Anthracycline Cardiotoxicity in Patients with Breast Cancer. J. Cardiovasc. Transl. Res. 2020, 13, 349–356. [Google Scholar] [CrossRef]
- Laird-Fick, H.S.; Tokala, H.; Kandola, S.; Kehdi, M.; Pelosi, A.; Wang, L.; Grondahl, B. Early morphological changes in cardiac mitochondria after subcutaneous administration of trastuzumab in rabbits: Possible prevention with oral selenium supplementation. Cardiovasc. Pathol. 2020, 44, 107159. [Google Scholar] [CrossRef]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients with Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef]
- Necela, B.M.; Axenfeld, B.C.; Serie, D.J.; Kachergus, J.M.; Perez, E.A.; Thompson, E.A.; Norton, N. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin. Transl. Med. 2017, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Cho, S.G.; Kang, S.R.; Yoo, S.W.; Kwon, S.Y.; Min, J.J.; Bom, H.S.; Song, H.C. Association between FDG uptake in the right ventricular myocardium and cancer therapy-induced cardiotoxicity. J. Nucl. Cardiol. 2020, 27, 2154–2163. [Google Scholar] [CrossRef]
- Kerkela, R.; Woulfe, K.C.; Durand, J.B.; Vagnozzi, R.; Kramer, D.; Chu, T.F.; Beahm, C.; Chen, M.H.; Force, T. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2009, 2, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Liu, L.; Qi, Y.; Bu, P. Trimetazidine ameliorates sunitinib-induced cardiotoxicity in mice via the AMPK/mTOR/autophagy pathway. Pharm. Biol. 2019, 57, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Li, X.; Song, J.; Guo, M.; Cai, H.; Wu, Z.; Wu, P.; Li, L.; Yang, M.; Wang, Y.; et al. Metabolic changes precede radiation-induced cardiac remodeling in beagles: Using noninvasive18f-fdg (18f-fludeoxyglucose) and13n-ammonia positron emission tomography/computed tomography scans. J. Am. Heart Assoc. 2020, 9, e016875. [Google Scholar] [CrossRef] [PubMed]
- Unal, K.; Mustafa, U.; Akdemir, O.; Akmansu, M. 18F-FDG PET/CT findings of radiotherapy-related myocardial changes in patients with thoracic malignancies. Nucl. Med. Commun. 2013, 34, 855–859. [Google Scholar] [CrossRef]
- Jingu, K.; Kaneta, T.; Nemoto, K.; Ichinose, A.; Oikawa, M.; Takai, Y.; Ogawa, Y.; Nakata, E.; Sakayauchi, T.; Takai, K.; et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 845–851. [Google Scholar] [CrossRef]
- Nahta, R.; Esteva, F.J. Trastuzumab: Triumphs and tribulations. Oncogene 2007, 26, 3637–3643. [Google Scholar] [CrossRef] [Green Version]
- Farolfi, A.; Melegari, E.; Aquilina, M.; Scarpi, E.; Ibrahim, T.; Maltoni, R.; Sarti, S.; Cecconetto, L.; Pietri, E.; Ferrario, C.; et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: A retrospective study of possible risk and protective factors. Heart 2013, 99, 634–639. [Google Scholar] [CrossRef]
- Tarantini, L.; Cioffi, G.; Gori, S.; Tuccia, F.; Boccardi, L.; Bovelli, D.; Lestuzzi, C.; Maurea, N.; Oliva, S.; Russo, G.; et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J. Card. Fail. 2012, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Suntheralingam, S.; Michalowska, M.; Amir, E.; Thavendiranathan, P. Trastuzumab-Related Cardiotoxicity and Cardiac Care in Patients With HER2 Positive Metastatic Breast Cancer. Am. J. Cardiol. 2020, 125, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Bouras, S.; Sawaya, H.; Sebag, I.A.; Cohen, V.; Picard, M.H.; Passeri, J.; Kuter, I.; Scherrer-Crosbie, M. Time trends of left ventricular ejection fraction and myocardial deformation indices in a cohort of women with breast cancer treated with anthracyclines, taxanes, and trastuzumab. J. Am. Soc. Echocardiogr. 2015, 28, 509–514. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Doggen, K.; Lemmens, K. The vulnerability of the heart as a pluricellular paracrine organ: Lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef] [Green Version]
- Gordon, L.I.; Burke, M.A.; Singh, A.T.K.; Prachand, S.; Lieberman, E.D.; Sun, L.; Naik, T.J.; Naga Prasad, S.V.; Ardehali, H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009, 284, 2080–2087. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Liu, Z.; Desai, S.; Zhao, Y.; Liu, H.; Pannell, L.K.; Yi, H.; Wright, E.R.; Owen, L.B.; Dean-Colomb, W.; et al. Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat. Commun. 2012, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Spector, N.L.; Yarden, Y.; Smith, B.; Lyass, L.; Trusk, P.; Pry, K.; Hill, J.E.; Xia, W.; Seger, R.; Bacus, S.S. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10607–10612. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Bu, P. Progress on the cardiotoxicity of sunitinib: Prognostic significance, mechanism and protective therapies. Chem. Biol. Interact. 2016, 257, 125–131. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Ewer, M.S.; Suter, T.M.; Lenihan, D.J.; Niculescu, L.; Breazna, A.; Demetri, G.D.; Motzer, R.J. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: A comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur. J. Cancer 2014, 50, 2162–2170. [Google Scholar] [CrossRef]
- Greineder, C.F.; Kohnstamm, S.; Ky, B. Heart failure associated with sunitinib: Lessons learned from animal models. Curr. Hypertens. Rep. 2011, 13, 436–441. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Kerkelä, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atallah, E.; Durand, J.-B.; Kantarjian, H.; Cortes, J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood 2007, 110, 1233–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, T.P.; Santiesteban, L.; Gomez, D.; Chambers, J.W. Sab mediates mitochondrial dysfunction involved in imatinib mesylate-induced cardiotoxicity. Toxicology 2017, 382, 24–35. [Google Scholar] [CrossRef]
- Chan, O.; Talati, C.; Isenalumhe, L.; Shams, S.; Nodzon, L.; Fradley, M.; Sweet, K.; Pinilla-Ibarz, J. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv. 2020, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Van Hasselt, J.G.C.; Rahman, R.; Hansen, J.; Stern, A.; Shim, J.V.; Xiong, Y.; Pickard, A.; Jayaraman, G.; Hu, B.; Mahajan, M.; et al. Transcriptomic profiling of human cardiac cells predicts protein kinase inhibitor-associated cardiotoxicity. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Cirmi, S.; El Abd, A.; Letinier, L.; Navarra, M.; Salvo, F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: An analysis of the FDA adverse event reporting system database (FAERS). Cancers 2020, 12, 826. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gover-Proaktor, A.; Granot, G.; Shapira, S.; Raz, O.; Pasvolsky, O.; Nagler, A.; Lev, D.L.; Inbal, A.; Lubin, I.; Raanani, P.; et al. Ponatinib reduces viability, migration, and functionality of human endothelial cells. Leuk. Lymphoma 2017, 58, 1455–1467. [Google Scholar] [CrossRef]
- Latifi, Y.; Moccetti, F.; Wu, M.; Xie, A.; Packwood, W.; Qi, Y.; Ozawa, K.; Shentu, W.; Brown, E.; Shirai, T.; et al. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood 2019, 133, 1597–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.P.; Glennon, M.S.; Umbarkar, P.; Gupte, M.; Galindo, C.L.; Zhang, Q.; Force, T.; Becker, J.R.; Lal, H. Ponatinib-induced cardiotoxicity: Delineating the signalling mechanisms and potential rescue strategies. Cardiovasc. Res. 2019, 115, 966–977. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Mingard, C.; Paech, F.; Bouitbir, J.; Krähenbühl, S. Mechanisms of toxicity associated with six tyrosine kinase inhibitors in human hepatocyte cell lines. J. Appl. Toxicol. 2018, 38, 418–431. [Google Scholar] [CrossRef]
- Hancock, S.L.; Hoppe, R.T.; Tucker, M.A. Factors Affecting Late Mortality From Heart Disease After Treatment of Hodgkin’s Disease. JAMA J. Am. Med. Assoc. 1993, 270, 1949–1955. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Bogaard, V.A.B.; Ta, B.D.P.; Van Der Schaaf, A.; Bouma, A.B.; Middag, A.M.H.; Bantema-Joppe, E.J.; Van Dijk, L.V.; Van Dijk-Peters, F.B.J.; Marteijn, L.A.W.; De Bock, G.H.; et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borde, C. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J. Radiol. 2012, 4, 220. [Google Scholar] [CrossRef]
- Park, J.M.; Reed, G.D.; Liticker, J.; Putnam, W.C.; Chandra, A.; Yaros, K.; Afzal, A.; MacNamara, J.; Raza, J.; Hall, R.G.; et al. Effect of Doxorubicin on Myocardial Bicarbonate Production from Pyruvate Dehydrogenase in Women with Breast Cancer. Circ. Res. 2020, 127, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple Plasma Biomarkers for Risk Stratification in Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef]
- Kawada, K.; Iwamoto, M.; Sakai, Y. Mechanisms underlying 18 F-fluorodeoxyglucose accumulation in colorectal cancer. World J. Radiol. 2016, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Early Diagnosis of Therapy-associated Cardiotoxicity Basing on Multi-tracer Multimodality PET/MRI—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04555642 (accessed on 26 October 2021).
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.H.; Lau, J.Y.C.; Chen, A.P.; Geraghty, B.J.; Perks, W.J.; Roifman, I.; Wright, G.A.; Connelly, K.A. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. 2016, 119, 1177–1182. [Google Scholar] [CrossRef]
- Agger, P.; Hyldebrandt, J.A.; Hansen, E.S.S.; Omann, C.; Bøgh, N.; Waziri, F.; Nielsen, P.M.; Laustsen, C. Magnetic resonance hyperpolarization imaging detects early myocardial dysfunction in a porcine model of right ventricular heart failure. Eur. Hear. J.-Cardiovasc. Imaging 2020, 21, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Rider, O.J.; Apps, A.; Miller, J.J.J.J.; Lau, J.Y.C.; Lewis, A.J.M.; Peterzan, M.A.; Dodd, M.S.; Lau, A.Z.; Trumper, C.; Gallagher, F.A.; et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ. Res. 2020, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Effect of Cardiotoxic Anticancer Chemotherapy on the Metabolism of [1-13C]Pyruvate in Cardiac Mitochondria—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03685175 (accessed on 26 October 2021).
- Hyperpolarized Carbon 13-Based Metabolic Imaging to Detect Radiation-Induced Cardiotoxicity—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04044872 (accessed on 26 October 2021).
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the art and future directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Inzucchi, S.E. Metformin and heart failure: Innocent until proven guilty. Diabetes Care 2005, 28, 2585–2587. [Google Scholar] [CrossRef] [Green Version]
- Halabi, A.; Sen, J.; Huynh, Q.; Marwick, T.H. Metformin treatment in heart failure with preserved ejection fraction: A systematic review and meta-regression analysis. Cardiovasc. Diabetol. 2020, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Hsieh, M.-C.; Qin, L.; Zhang, J.; Wu, S.-Y. Metformin reduces radiation-induced cardiac toxicity risk in patients having breast cancer. Am. J. Cancer Res. 2019, 9, 1017–1026. [Google Scholar] [PubMed]
- Argun, M.; Üzüm, K.; Sönmez, M.F.; Özyurt, A.; Karabulut, D.; Soyersarıca, Z.; Çilenk, K.T.; Unalmış, S.; Pamukcu, Ö.; Baykan, A.; et al. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol. J. Cardiol. 2016, 16, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, L.C.; Xu, Y.C.; Padbury, J.F.; Tseng, Y.T.; Yano, N. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: An in Vitro study. PLoS One 2014, 9, e104888. [Google Scholar] [CrossRef] [Green Version]
- Ajzashokouhi, A.H.; Bostan, H.B.; Jomezadeh, V.; Hayes, A.W.; Karimi, G. A review on the cardioprotective mechanisms of metformin against doxorubicin. Hum. Exp. Toxicol. 2020, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef] [Green Version]
- Martin-Castillo, B.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; Amillano, K.; Domínguez, S.; et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687–35704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Kosiborod, M.; Fitchett, D.; Wanner, C.; Hehnke, U.; Kaspers, S.; George, J.T.; Zinman, B. Improvement in cardiovascular outcomes with empaglifozin is independent of glycemic control. Circulation 2018, 138, 1904–1907. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatino, J.; De Rosa, S.; Tammè, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Barış, V.Ö.; Dinçsoy, A.B.; Gedikli, E.; Zırh, S.; Müftüoğlu, S.; Erdem, A. Empagliflozin Significantly Prevents the Doxorubicin-induced Acute Cardiotoxicity via Non-antioxidant Pathways. Cardiovasc. Toxicol. 2021, 21, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, C.C.; Lin, M.H.; Su, H.T.; Ho, M.Y.; Yeh, J.K.; Tsai, M.L.; Hsieh, I.C.; Wen, M.S. TLR9 binding to beclin 1 and mitochondrial SIRT3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology 2020, 9, 369. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Oh, C.M.; Cho, S.; Jang, J.Y.; Kim, H.; Chun, S.; Choi, M.; Park, S.; Ko, Y.G. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ. J. 2019, 49, 1183–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Yu, Y.; Zhang, Y.; Zhang, Z.; An, W.; Zhao, X. Treatment with D-β-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur. J. Pharmacol. 2018, 829, 121–128. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.; Botti, G.; Maurea, N. SGLT2 inhibitor dapagliflozin against anthracycline and trastuzumab-induced cardiotoxicity: The role of MYD88, NLRP3, Leukotrienes/Interleukin 6 axis and mTORC1 /Fox01/3a mediated apoptosis. Eur. Heart J. 2020, 41, ehaa946.3253. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, I.; Grant, M.; Zordoky, B. Leveraging the Cardio-Protective and Anticancer Properties of Resveratrol in Cardio-Oncology. Nutrients 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, N.; Zordoky, B.N.; Robertson, I.M.; Hamza, S.M.; Parajuli, N.; Soltys, C.L.M.; Beker, D.L.; Grant, M.K.; Razzoli, M.; Bartolomucci, A.; et al. Co-administration of resveratrol with doxorubicin in youngmice attenuates detrimental late-occurring cardiovascular changes. Cardiovasc. Res. 2018, 114, 1350–1359. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Rogan, K.J.; Sung, M.M.; Zordoky, B.N.; Haykowsky, M.J.; Young, M.E.; Jones, L.W.; Dyck, J.R.B. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Lin, X.L.; Guo, D.M.; Zhang, Y.; Yuan, C.; Tan, T.P.; Chen, Y.D.; Wu, S.J.; Ye, Z.F.; He, J. Resveratrol protects cardiomyocytes from doxorubicin-induced apoptosis through the AMPK/P53 pathway. Mol. Med. Rep. 2016, 13, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Brookins Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef]
- De Freitas, R.B.; Boligon, A.A.; Rovani, B.T.; Piana, M.; De Brum, T.F.; Da Silva Jesus, R.; Rother, F.C.; Alves, N.M.; Da Rocha, J.B.T.; Athayde, M.L.; et al. Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules 2013, 18, 12154–12167. [Google Scholar] [CrossRef] [Green Version]
- Gramatyka, M.; Widłak, P.; Gabryś, D.; Kulik, R.; Sokół, M. Resveratrol administration prevents radiation-related changes in metabolic profles of hearts 20 weeks after irradiation of mice with a single 2 Gy dose. Acta Biochim. Pol. 2020, 67, 629–632. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).