On the Effect of pH, Temperature, and Surfactant Structure on Bovine Serum Albumin–Cationic/Anionic/Nonionic Surfactants Interactions in Cacodylate Buffer–Fluorescence Quenching Studies Supported by UV Spectrophotometry and CD Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CPC | Hexadecylpyridinium chloride |
| CTAB | Hexadecyltrimethylammonium bromide |
| SDS | Sodium dodecyl sulfate |
| TWEEN 20 | Polyethylene glycol sorbitan monolaurate |
| TWEEN 40 | Polyethylene glycol sorbitan monopalmitate |
| TWEEN 80 | Polyethylene glycol sorbitan monooleate |
| CACO | Cacodylate buffer |
| CD | Circular dichroism |
References
- Menger, F.M.; Rhee, J.U.; Rhee, H.K. Applications of surfactants to synthetic organic chemistry. J Org. Chem. 1975, 40, 3803–3805. [Google Scholar] [CrossRef]
- Abe, M. Synthesis and applications of surfactants containing fluorine. Curr. Opin. Colloid Interface Sci. 1999, 4, 354–356. [Google Scholar] [CrossRef]
- Luk, Y.Y.; Abbott, N.L. Applications of functional surfactants. Curr. Opin. Colloid Interface Sci. 2002, 7, 267–275. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Singh, A.; Van Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121. [Google Scholar] [CrossRef]
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial applications of dimeric surfactants: A review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R.D.C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural surfactants and their applications for heavy oil removal in industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Vasilescu, M.; Angelescu, D.; Almgren, M.; Valstar, A. Interactions of globular proteins with surfactants studied with fluorescence probe methods. Langmuir 1999, 15, 2635–2643. [Google Scholar] [CrossRef]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Jones, M.N. Surfactant interactions with biomembranes and proteins. Chem. Soc. Rev. 1992, 21, 127–136. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta 2017, 1859, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, O.; Kogut, M.; Żamojć, K.; Samsonov, S.; Makowska, J.; Tesmar, A.; Chmur, K.; Wyrzykowski, D.; Chmurzyński, L. Effect of tetraphenylborate on physicochemical properties of bovine serum albumin. Molecules 2021, 26, 6565. [Google Scholar] [CrossRef]
- Nielsen, A.D.; Borch, K.; Westh, P. Thermochemistry of the specific binding of C12 surfactants to bovine serum albumin. Biochim. Biophys. Acta 2000, 1479, 321–331. [Google Scholar] [CrossRef]
- Sharma, V.; Yañez, O.; Zúñiga, C.; Kumar, A.; Singh, G.; Cantero-López, P. Protein-surfactant interactions: A multitechnique approach on the effect of Co-solvents over bovine serum albumin (BSA)-cetyl pyridinium chloride (CPC) system. Chem. Phys. Lett. 2020, 747, 137349. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation behavior of gemini surfactants and their interaction with macromolecules in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef]
- Morris, S.A.; Thompson, R.T.; Glenn, R.W.; Ananthapadmanabhan, K.P.; Kasting, G.B. Mechanisms of anionic surfactant penetration into human skin: Investigating monomer, micelle and submicellar aggregate penetration theories. Int. J. Cosmet. Sci. 2019, 41, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, P.; Lu, H.; Yan, S.; Lu, Z. Influences of cationic, anionic, and nonionic surfactants on alkaline-induced intermediate of bovine serum albumin. Int. J. Biol. Macromol. 2010, 46, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ao, M.; Xu, G.; Liu, T.; Zhang, J. Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2013, 389, 175–181. [Google Scholar] [CrossRef]
- Yakimova, L.; Padnya, P.; Tereshina, D.; Kunafina, A.; Nugmanova, A.; Osin, Y.; Evtugyn, V.; Stoikov, I. Interpolyelectrolyte mixed nanoparticles from anionic and cationic thiacalix [4] arenes for selective recognition of model biopolymers. J. Mol. Liq. 2019, 279, 9–17. [Google Scholar] [CrossRef]
- Faustino, C.M.; Calado, A.R.; Garcia-Rio, L. Gemini surfactant−protein interactions: Effect of pH, temperature, and surfactant stereochemistry. Biomacromolecules 2009, 10, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Alam, M.S. Effect of pH and surfactant on the protein: A perspective from theory and experiments. Int. J. Biol. Macromol. 2018, 107, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D. Protein–surfactant interactions: A tale of many states. Biochim. Biophys. Acta 2011, 1814, 562–591. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Alam, M.S. Influence of micelles on protein’s denaturation. Int. J. Biol. Macromol. 2020, 145, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Alam, M.S. Role of (single/double chain surfactant) micelles on the protein aggregation. Int. J. Biol. Macromol. 2019, 122, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Alam, M.S. Spectroscopic studies of the aggregation behavior of Human Serum Albumin and cetyltrimethylammonium bromide. Int. J. Biol. Macromol. 2020, 158, 394–400. [Google Scholar] [CrossRef]
- Arsiccio, A.; McCarty, J.; Pisano, R.; Shea, J.E. Effect of surfactants on surface-induced denaturation of proteins: Evidence of an orientation-dependent mechanism. J. Phys. Chem. B 2018, 122, 11390–11399. [Google Scholar] [CrossRef]
- Kelley, D.J.M.D.; McClements, D.J. Interactions of bovine serum albumin with ionic surfactants in aqueous solutions. Food Hydrocoll. 2003, 17, 73–85. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.H.T.P.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modelling. Biochim. Biophys. Acta 2002, 1594, 84–99. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Tabak, M. Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim. Acta A 2000, 56, 2255–2271. [Google Scholar] [CrossRef]
- Bordbar, A.K.; Saboury, A.A.; Housaindokht, M.R.; Moosavi-Movahedi, A.A. Statistical effects of the binding of ionic surfactant to protein. J. Colloid Interface Sci. 1997, 192, 415–419. [Google Scholar] [CrossRef]
- Erfani, A.; Khosharay, S.; Flynn, N.H.; Ramsey, J.D.; Aichele, C.P. Effect of zwitterionic betaine surfactant on interfacial behavior of bovine serum albumin (BSA). J. Mol. Liq. 2020, 318, 114067. [Google Scholar] [CrossRef]
- Ruiz-Peña, M.; Oropesa-Nuñez, R.; Pons, T.; Louro, S.R.W.; Pérez-Gramatges, A. Physico-chemical studies of molecular interactions between non-ionic surfactants and bovine serum albumin. Colloids Surf. B Biointerfaces 2010, 75, 282–289. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chakraborty, I.; Moulik, S.P.; Ghosh, S. Physicochemical and conformational studies on BSA− surfactant interaction in aqueous medium. Langmuir 2009, 25, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Soroka, K.; Vithanage, R.S.; Phillips, D.A.; Walker, B.; Dasgupta, P.K. Fluorescence properties of metal complexes of 8-hydroxyquinoline-5-sulfonic acid and chromatographic applications. Anal. Chem. 1987, 59, 629–636. [Google Scholar] [CrossRef]
- Yorozu, T.; Hoshino, M.; Imamura, M. Fluorescence studies of pyrene inclusion complexes with. alpha.-, beta.-, and. gamma.-cyclodextrins in aqueous solutions. Evidence for formation of pyrene dimer in. gamma.-cyclodextrin cavity. J. Phys. Chem. 1982, 86, 4426–4429. [Google Scholar] [CrossRef]
- Żamojć, K.; Jacewicz, D.; Zdrowowicz, M.; Chmurzyński, L. Kinetics of the reaction between 1,3-diphenylisobenzofuran and nitrogen dioxide studied by steady-state fluorescence. Res. Chem. Intermed. 2013, 39, 3023–3031. [Google Scholar] [CrossRef]
- Yi, L.; Li, H.; Sun, L.; Liu, L.; Zhang, C.; Xi, Z. A highly sensitive fluorescence probe for fast thiol-quantification assay of glutathione reductase. Angew. Chem. Int. Ed. 2009, 48, 4034–4037. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Wiczk, W.; Jacewicz, D.; Chmurzyński, L. Dihydroxycoumarins as highly selective fluorescent probes for the fast detection of 4-hydroxy-TEMPO in aqueous solution. RSC Adv. 2015, 5, 63807–63812. [Google Scholar] [CrossRef]
- Cohen, B.E.; McAnaney, T.B.; Park, E.S.; Jan, Y.N.; Boxer, S.G.; Jan, L.Y. Probing protein electrostatics with a synthetic fluorescent amino acid. Science 2002, 296, 1700–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.M.Y.; Katz, A. Steady-state fluorescence-based investigation of the interaction between protected thiols and gold nanoparticles. Langmuir 2002, 18, 2413–2420. [Google Scholar] [CrossRef]
- De, S.; Girigoswami, A.; Das, S. Fluorescence probing of albumin–surfactant interaction. J. Colloid Interface Sci. 2005, 285, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, A. Interactions between surfactants and the skin–Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Akram, M.; Ansari, F.; Bhat, I.A. Probing interaction of bovine serum albumin (BSA) with the biodegradable version of cationic gemini surfactants. J. Mol. Liq. 2019, 276, 519–528. [Google Scholar] [CrossRef]
- Sharma, V.; Yañez, O.; Alegría-Arcos, M.; Kumar, A.; Thakur, R.C.; Cantero-López, P. A physicochemical and conformational study of co-solvent effect on the molecular interactions between similarly charged protein surfactant (BSA-SDBS) system. J. Chem. Thermodyn. 2020, 142, 106022. [Google Scholar] [CrossRef]
- Aslam, J.; Lone, I.H.; Ansari, F.; Aslam, A.; Aslam, R.; Akram, M. Molecular binding interaction of pyridinium based gemini surfactants with bovine serum albumin: Insights from physicochemical, multispectroscopic, and computational analysis. Spectrochim. Acta A 2021, 250, 119350. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.U.H.; Maurya, J.K.; Ali, S.; Ubaid-Ullah, S.; Khan, A.B.; Patel, R. Molecular interaction of cationic gemini surfactant with bovine serum albumin: A spectroscopic and molecular docking study. Process Biochem. 2014, 49, 623–630. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Żmudzińska, W.; Uber, D.; Wierzbicka, M.; Wiczk, W.; Chmurzyński, L. Probing the binding of Cu2+ ions to a fragment of the Aβ(1–42) polypeptide using fluorescence spectroscopy, isothermal titration calorimetry and molecular dynamics simulations. Biophys. Chem. 2016, 216, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Y.; Liu, X.; Zhao, S. A steady-state and time-resolved fluorescence, circular dichroism study on the binding of myricetin to bovine serum albumin. Luminescence 2009, 24, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Zhou, B.; Liu, Y.X.; Zhou, C.X.; Ding, X.L.; Liu, Y. Fluorescence study on the interaction of bovine serum albumin with p-aminoazobenzene. J. Fluoresc. 2008, 18, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gorodnichev, E.S.; Kuleshova, A.A.; Volkova, O.I.; Saletsky, A.M. The binding of bovine serum albumin with dye molecules at different pH values. Fluorescence lifetime studies. Laser Phys. 2021, 31, 065601. [Google Scholar] [CrossRef]
- Cui, F.L.; Fan, J.; Ma, D.L.; Liu, M.C.; Chen, X.G.; Hu, Z.D. A study of the interaction between a new reagent and serum albumin by fluorescence spectroscopy. Anal. Lett. 2003, 36, 2151–2166. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Copper(II) complexation by fragment of central part of FBP28 protein from Mus musculus. Biophys. Chem. 2018, 241, 55–60. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Feng, Z.; Jia, L. Spectroscopic investigation of the interaction of pyridinium surfactant with bovine serum albumin. J. Solut. Chem. 2015, 44, 293–306. [Google Scholar] [CrossRef]
- Żamojć, K.; Wiczk, W.; Chmurzyński, L. The influence of the type of substituents and the solvent on the interactions between different coumarins and selected TEMPO analogues–Fluorescence quenching studies. Chem. Phys. 2018, 513, 188–194. [Google Scholar] [CrossRef]
- Gauthier, T.D.; Shane, E.C.; Guerin, W.F.; Seitz, W.R.; Grant, C.L. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ. Sci. Technol. 1986, 20, 1162–1166. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry 1973, 12, 4171–4179. [Google Scholar] [CrossRef]
- Eftink, M.R.; Ghiron, C.A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry 1976, 15, 672–680. [Google Scholar] [CrossRef]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching of indole and model micelle systems. J. Phys. Chem. 1976, 80, 486–493. [Google Scholar] [CrossRef]
- Arık, M.; Çelebi, N.; Onganer, Y. Fluorescence quenching of fluorescein with molecular oxygen in solution. J. Photochem. Photobiol. A 2005, 170, 105–111. [Google Scholar] [CrossRef]
- Żamojć, K.; Bylińska, I.; Wiczk, W.; Chmurzyński, L. Fluorescence quenching studies on the interactions between chosen fluoroquinolones and selected stable TEMPO and PROXYL nitroxides. Int. J. Mol. Sci. 2021, 22, 885. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Liu, Y.; Zhang, L.X.; Zhao, R.M.; Qu, S.S. Studies of interaction between colchicine and bovine serum albumin by fluorescence quenching method. J. Mol. Struct. 2005, 750, 174–178. [Google Scholar] [CrossRef]
- Geng, F.; Zheng, L.; Yu, L.; Li, G.; Tung, C. Interaction of bovine serum albumin and long-chain imidazolium ionic liquid measured by fluorescence spectra and surface tension. Process Biochem. 2010, 45, 306–311. [Google Scholar] [CrossRef]
- Anand, U.; Jash, C.; Mukherjee, S. Spectroscopic probing of the microenvironment in a protein−surfactant assembly. J. Phys. Chem. B 2010, 114, 15839–15845. [Google Scholar] [CrossRef] [PubMed]
- Kandagal, P.B.; Shaikh, S.M.T.; Manjunatha, D.H.; Seetharamappa, J.; Nagaralli, B.S. Spectroscopic studies on the binding of bioactive phenothiazine compounds to human serum albumin. J. Photochem. Photobiol. A 2007, 189, 121–127. [Google Scholar] [CrossRef]
- Khan, A.B.; Khan, J.M.; Ali, M.S.; Khan, R.H. Interaction of amphiphilic drugs with human and bovine serum albumins. Spectrochim. Acta A 2012, 97, 119–124. [Google Scholar] [CrossRef]
- Butowska, K.; Żamojć, K.; Kogut, M.; Kozak, W.; Wyrzykowski, D.; Wiczk, W.; Czub, J.; Piosik, J.; Rak, J. The product of matrix metalloproteinase cleavage of doxorubicin conjugate for anticancer drug delivery: Calorimetric, spectroscopic, and molecular dynamics studies on peptide–doxorubicin binding to DNA. Int. J. Mol. Sci. 2020, 21, 6923. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, J.S.; Froehlich, E.; Tajmir-Riahi, H.A. Study of curcumin and genistein interactions with human serum albumin. J. Pharm. Biomed. Anal. 2009, 49, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Maurya, J.K.; Singh, U.K.; Khan, A.B.; Ali, M.; Singh, P.; Patel, R. Spectroscopic and docking studies on the interaction between pyrrolidinium based ionic liquid and bovine serum albumin. Spectrochim. Acta A 2014, 124, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.; Zhou, L.; Yang, L.; Xia, G.; Chen, Z.; Duan, M. Synthesis and binding with BSA of a new gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1159–1169. [Google Scholar] [CrossRef]
- Khan, A.B.; Khan, J.M.; Ali, M.S.; Khan, R.H.; Din, K.U. Spectroscopic approach of the interaction study of amphiphilic drugs with the serum albumins. Colloids Surf. B Biointerfaces 2011, 87, 447–453. [Google Scholar] [CrossRef]
- Mehta, S.K.; Bhasin, K.K.; Kumar, A. An insight into the micellization of dodecyldimethylethylammonium bromide (DDAB) in the presence of bovine serum albumin (BSA). J. Colloid Interface Sci. 2008, 323, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wang, Y. Comparative studies on interactions of bovine serum albumin with cationic gemini and single-chain surfactants. J. Phys. Chem. B 2006, 110, 8499–8505. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, Q.; Li, J.L.; Jiang, L.; Gu, W.; Liu, X.; Tian, J.L.; Yan, S.P. Two water-soluble copper(II) complexes: Synthesis, characterization, DNA cleavage, protein binding activities and in vitro anticancer activity studies. J. Inorg. Biochem. 2014, 137, 46–56. [Google Scholar] [PubMed]
- Wang, Y.Q.; Zhang, H.M.; Zhang, G.C.; Tao, W.H.; Fei, Z.H.; Liu, Z.T. Spectroscopic studies on the interaction between silicotungstic acid and bovine serum albumin. J. Pharm. Biomed. 2007, 43, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Green, R.J.; Su, T.J.; Joy, H.; Lu, J.R. Interaction of lysozyme and sodium dodecyl sulfate at the air−liquid interface. Langmuir 2000, 16, 5797–5805. [Google Scholar] [CrossRef]
- Hazra, P.; Chakrabarty, D.; Chakraborty, A.; Sarkar, N. Probing protein-surfactant interaction by steady state and time-resolved fluorescence spectroscopy. Biochem. Biophys. Res. Commun. 2004, 314, 543–549. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Kasemo, B.; Brzezinski, P. Structural changes in hemoglobin during adsorption to solid surfaces: Effects of pH, ionic strength, and ligand binding. Proc. Natl. Acad. Sci. USA 1998, 95, 12271–12276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.N.; Liu, Y.; Niu, L.Y.; Zhao, C.P. Spectroscopic studies on the interaction of bovine serum albumin with surfactants and apigenin. Spectrochim. Acta A 2012, 94, 357–364. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, R.; Xi, J. Comparative studies of interactions of hemoglobin with single-chain and with gemini surfactants. J. Colloid Interface Sci. 2009, 331, 470–475. [Google Scholar] [CrossRef]
- Dıaz, X.; Abuin, E.; Lissi, E. Quenching of BSA intrinsic fluorescence by alkylpyridinium cations: Its relationship to surfactant-protein association. J. Photochem. Photobiol. A 2003, 155, 157–162. [Google Scholar] [CrossRef]
- Tang, J.; Luan, F.; Chen, X. Binding analysis of glycyrrhetinic acid to human serum albumin: Fluorescence spectroscopy, FTIR, and molecular modeling. Bioorg. Med. Chem. 2006, 14, 3210–3217. [Google Scholar] [CrossRef]
- Han, X.L.; Mei, P.; Liu, Y.; Xiao, Q.; Jiang, F.L.; Li, R. Binding interaction of quinclorac with bovine serum albumin: A biophysical study. Spectrochim. Acta A 2009, 74, 781–787. [Google Scholar] [CrossRef]
- Mote, U.S.; Bhattar, S.L.; Patil, S.R.; Kolekar, G.B. Interaction between felodipine and bovine serum albumin: Fluorescence quenching study. Luminescence 2010, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, R.; Teng, Y.; Liu, X. The interaction between Ag+ and bovine serum albumin: A spectroscopic investigation. Sci. Total Environ. 2011, 409, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Callis, P.R. Binding phenomena and fluorescence quenching. I: Descriptive quantum principles of fluorescence quenching using a supermolecule approach. J. Mol. Struct. 2014, 1077, 14–21. [Google Scholar] [CrossRef]
- Aprodu, I.; Dumitras, L.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Spectroscopic and molecular modeling investigation on the interaction between folic acid and bovine lactoferrin from encapsulation perspectives. Foods 2020, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N. Circular dichroism of peptides and proteins. In Circular Dichroism: Principles and Applications, 2nd ed.; Berova, N., Nakanishi, K., Woody, R.W., Eds.; Wiley: New York, NY, USA, 2000; pp. 601–620. [Google Scholar]
- Tesmar, A.; Kogut, M.M.; Żamojć, K.; Grabowska, O.; Chmur, K.; Samsonov, S.A.; Makowska, J.; Wyrzykowski, D.; Chmurzyński, L. Physicochemical nature of sodium dodecyl sulfate interactions with bovine serum albumin revealed by interdisciplinary approaches. J. Mol. Liq. 2021, 340, 117185. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Compton, L.A.; Johnson Jr, W.C. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 1986, 155, 155–167. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]

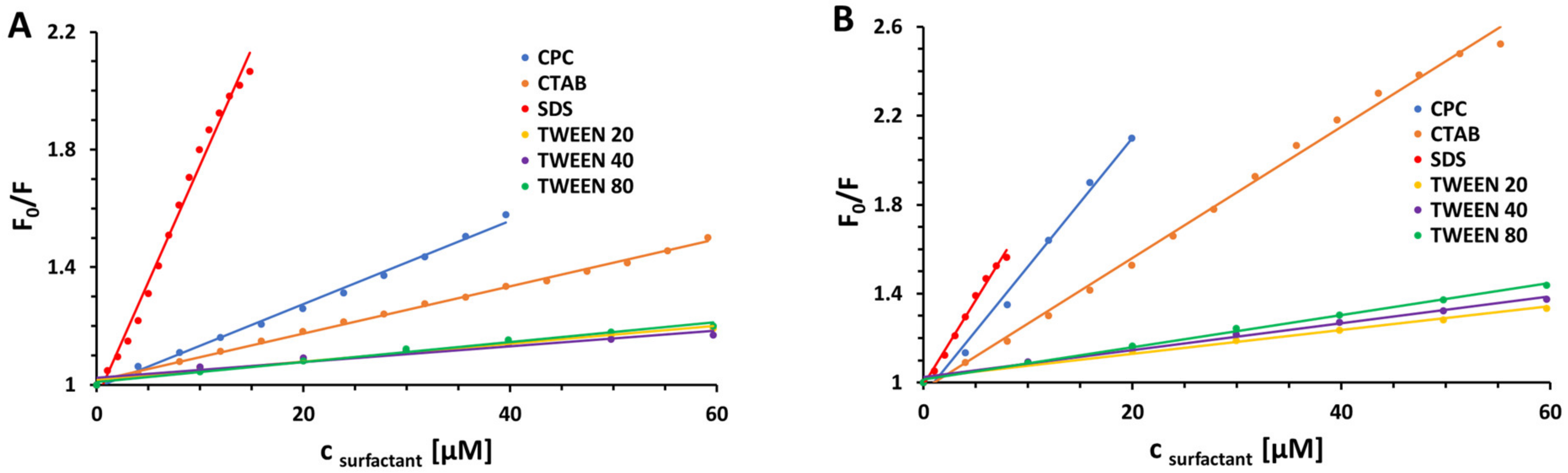
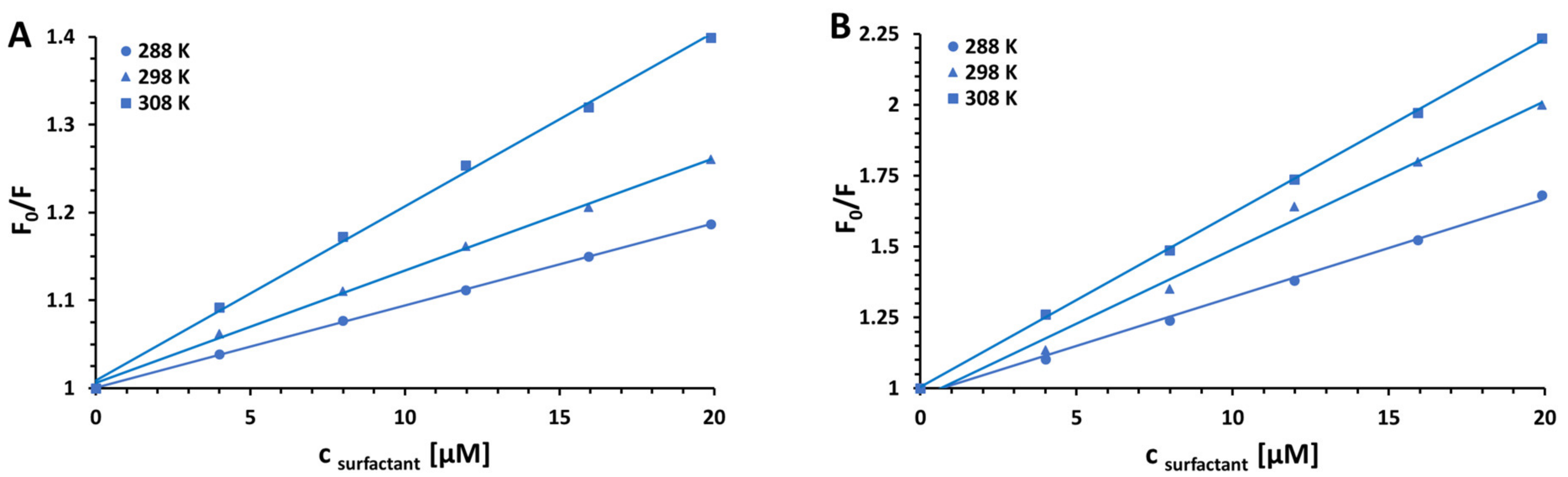
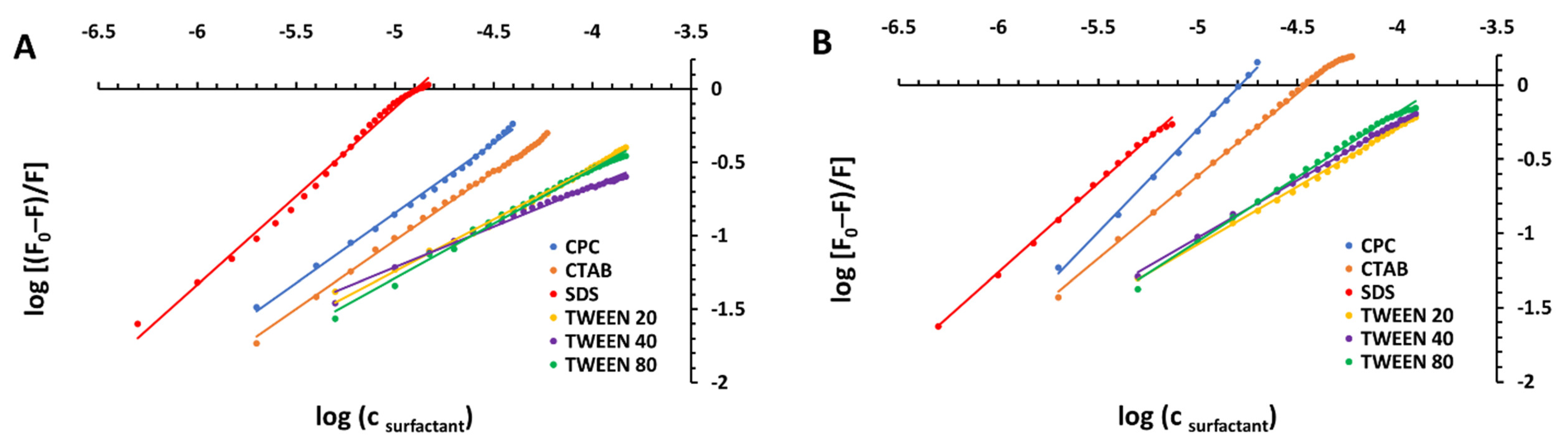
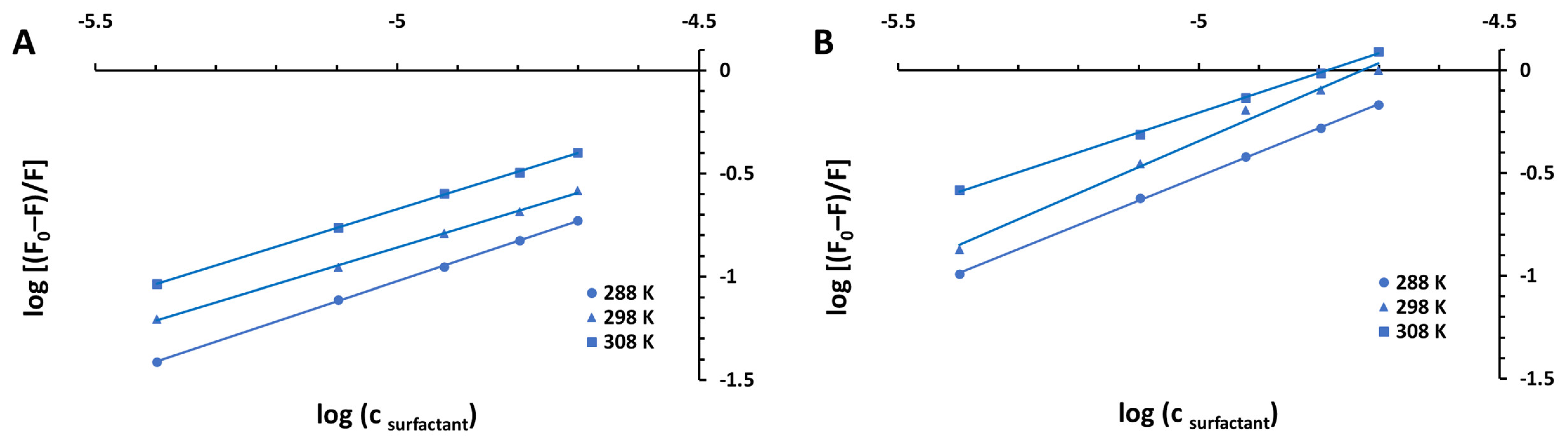

| Surfactant | pH | KSV [M−1] | R2 | kq [M−1 s−1] | |
|---|---|---|---|---|---|
| CPC | 5.0 | 288 K | 0.94 × 104 | 1.000 | 1.64 × 1012 |
| 298 K | 1.28 × 104 | 0.998 | 2.25 × 1012 | ||
| 308 K | 1.98 × 104 | 0.998 | 3.47 × 1012 | ||
| 7.0 | 288 K | 3.45 × 104 | 0.996 | 5.75 × 1012 | |
| 298 K | 5.23 × 104 | 0.992 | 8.72 × 1012 | ||
| 308 K | 6.14 × 104 | 1.000 | 10.2 × 1012 | ||
| CTAB | 5.0 | 0.81 × 104 | 0.997 | 1.41 × 1012 | |
| 7.0 | 2.95 × 104 | 0.996 | 4.92 × 1012 | ||
| SDS | 5.0 | 7.97 × 104 | 0.989 | 14.0 × 1012 | |
| 7.0 | 7.65 × 104 | 0.992 | 12.8 × 1012 | ||
| TWEEN 20 | 5.0 | 0.31 × 104 | 0.978 | 0.54 × 1012 | |
| 7.0 | 0.53 × 104 | 0.987 | 0.89 × 1012 | ||
| TWEEN 40 | 5.0 | 0.27 × 104 | 0.938 | 0.47 × 1012 | |
| 7.0 | 0.61 × 104 | 0.988 | 1.01 × 1012 | ||
| TWEEN 80 | 5.0 | 0.34 × 104 | 0.987 | 0.60 × 1012 | |
| 7.0 | 0.73 × 104 | 0.997 | 1.21 × 1012 | ||
| Surfactant | pH | Ka [M−1] | R2 | n | |
|---|---|---|---|---|---|
| CPC | 5.0 | 288 K | 0.73 × 104 | 1.000 | 0.98 |
| 298 K | 0.37 × 104 | 0.999 | 0.89 | ||
| 308 K | 0.74 × 104 | 1.000 | 0.91 | ||
| 7.0 | 288 K | 23.8 × 104 | 1.000 | 1.18 | |
| 298 K | 98.2 × 104 | 0.990 | 1.27 | ||
| 308 K | 4.22 × 104 | 0.999 | 0.97 | ||
| CTAB | 5.0 | 0.40 × 104 | 0.998 | 0.93 | |
| 7.0 | 9.12 × 104 | 0.998 | 1.11 | ||
| SDS | 5.0 | 77.6 × 104 | 0.992 | 1.20 | |
| 7.0 | 85.1 × 104 | 0.998 | 1.20 | ||
| TWEEN 20 | 5.0 | 0.02 × 104 | 0.993 | 0.70 | |
| 7.0 | 0.07 × 104 | 0.998 | 0.78 | ||
| TWEEN 40 | 5.0 | 0.04 × 104 | 0.989 | 0.55 | |
| 7.0 | 0.07 × 104 | 0.999 | 0.78 | ||
| TWEEN 80 | 5.0 | 0.03 × 104 | 0.992 | 0.74 | |
| 7.0 | 0.19 × 104 | 0.994 | 0.87 | ||
| System | pH | α-Helix [%] | Strand [%] | Turns [%] | Unordered [%] |
|---|---|---|---|---|---|
| BSA | 5.0 | 61 | 7 | 12 | 19 |
| 7.0 | 63 | 15 | 8 | 14 | |
| BSA + SDS | 5.0 | 56 | 12 | 13 | 18 |
| 7.0 | 59 | 15 | 10 | 17 | |
| BSA + CPC | 5.0 | 58 | 9 | 12 | 20 |
| 7.0 | 54 | 20 | 11 | 14 | |
| BSA + CTAB | 5.0 | 60 | 9 | 10 | 21 |
| 7.0 | 55 | 18 | 10 | 16 | |
| BSA + TWEEN 20 | 5.0 | 61 | 9 | 10 | 20 |
| 7.0 | 60 | 15 | 10 | 16 | |
| BSA + TWEEN 40 | 5.0 | 62 | 10 | 11 | 18 |
| 7.0 | 60 | 16 | 6 | 18 | |
| BSA + TWEEN 80 | 5.0 | 61 | 14 | 10 | 14 |
| 7.0 | 54 | 20 | 11 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żamojć, K.; Wyrzykowski, D.; Chmurzyński, L. On the Effect of pH, Temperature, and Surfactant Structure on Bovine Serum Albumin–Cationic/Anionic/Nonionic Surfactants Interactions in Cacodylate Buffer–Fluorescence Quenching Studies Supported by UV Spectrophotometry and CD Spectroscopy. Int. J. Mol. Sci. 2022, 23, 41. https://doi.org/10.3390/ijms23010041
Żamojć K, Wyrzykowski D, Chmurzyński L. On the Effect of pH, Temperature, and Surfactant Structure on Bovine Serum Albumin–Cationic/Anionic/Nonionic Surfactants Interactions in Cacodylate Buffer–Fluorescence Quenching Studies Supported by UV Spectrophotometry and CD Spectroscopy. International Journal of Molecular Sciences. 2022; 23(1):41. https://doi.org/10.3390/ijms23010041
Chicago/Turabian StyleŻamojć, Krzysztof, Dariusz Wyrzykowski, and Lech Chmurzyński. 2022. "On the Effect of pH, Temperature, and Surfactant Structure on Bovine Serum Albumin–Cationic/Anionic/Nonionic Surfactants Interactions in Cacodylate Buffer–Fluorescence Quenching Studies Supported by UV Spectrophotometry and CD Spectroscopy" International Journal of Molecular Sciences 23, no. 1: 41. https://doi.org/10.3390/ijms23010041
APA StyleŻamojć, K., Wyrzykowski, D., & Chmurzyński, L. (2022). On the Effect of pH, Temperature, and Surfactant Structure on Bovine Serum Albumin–Cationic/Anionic/Nonionic Surfactants Interactions in Cacodylate Buffer–Fluorescence Quenching Studies Supported by UV Spectrophotometry and CD Spectroscopy. International Journal of Molecular Sciences, 23(1), 41. https://doi.org/10.3390/ijms23010041






