New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling
Abstract
1. Introduction
1.1. Pulmonary Hypertension (PH)
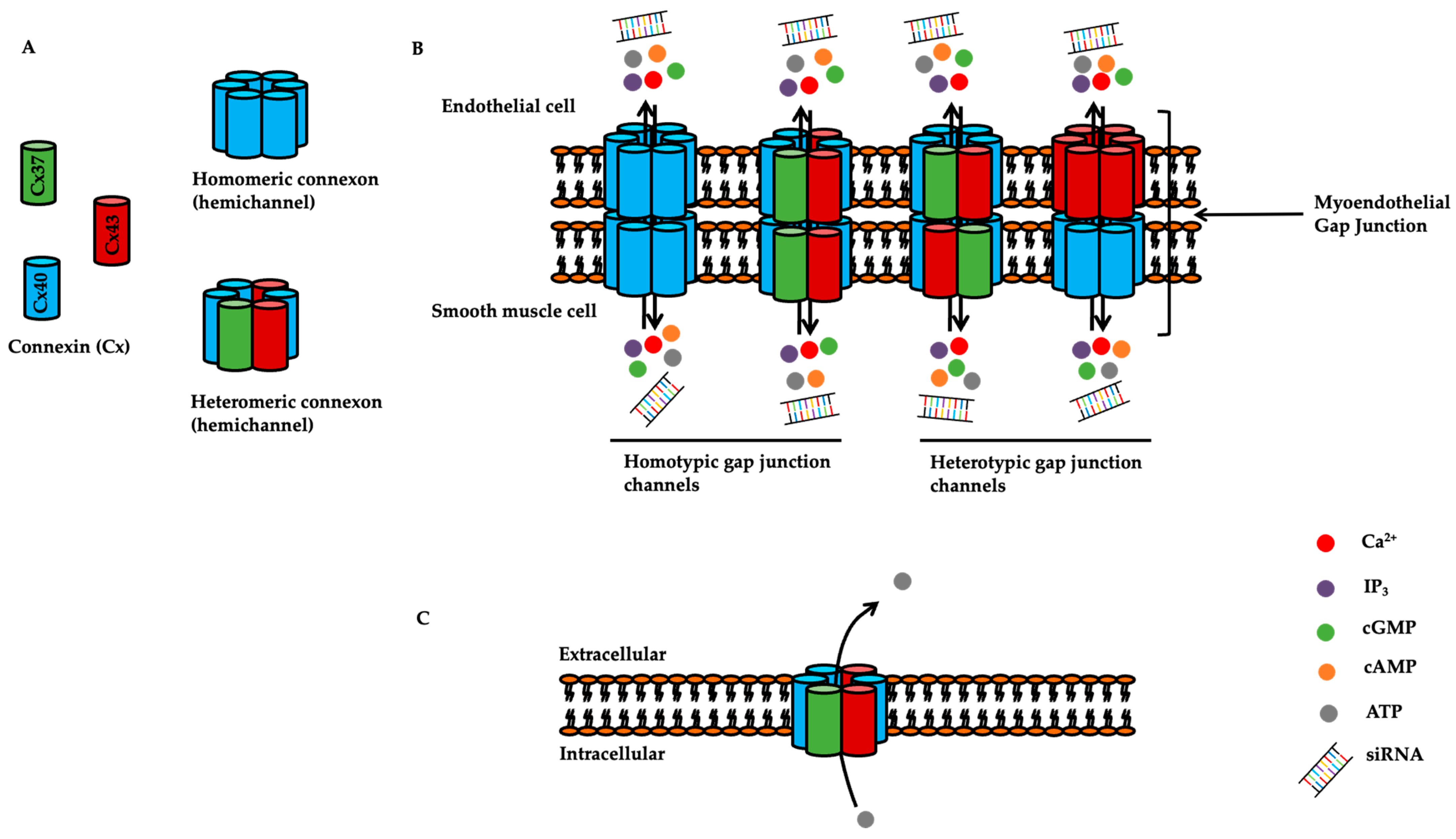
1.2. Overview of Connexins
2. Expression and Function of Connexins in the Pulmonary Circulation
2.1. Expression of Connexins in the Pulmonary Vasculature
2.2. Altered Connexin Expression in the Pulmonary Vasculature of Patients with PH and Animal Models of PH
2.3. Oestrogen-Induced Regulation of Connexin Expression
2.4. Connexin-Mediated Signalling in Pulmonary Vascular Reactivity
2.5. Connexin-Mediated Signalling in Pulmonary Vascular Remodelling and Development of PH
2.6. Connexin-Mediated Signalling in the Right Ventricle in Animal Models of PH
| Connexins | Protein Expression in PH/PAH Patients | Protein Expression in Animal and Cellular Models of PH | Role in Development of PH in Animal Models |
|---|---|---|---|
| Cx37 | ↓ in PAECs from PAH patients [29] | ↔ in rat PASMCs exposed to acute hypoxia [24] ↑ in rat PAFs exposed to acute hypoxia [24] ↓ in human lung tissue section from PAH patients [29] | Unknown |
| Cx40 | ↓in PAECs from PAH patients [29] | ↓ protein expression in PAECs from mouse with chronic hypoxia-induced PH [32] ↓ protein expression in rat PASMCs exposed to acute hypoxia [24] ↔ protein expression in rat PAFs exposed to acute hypoxia [24] ↓ protein expression in lung tissues from the rat monocrotaline model [33] | Hypoxic pulmonary vasoconstriction reduced in Cx40−/− mice and by pharmacological inhibition of Cx40 [53] Cx40−/− mice are protected against hypoxia-induced PH [53] |
| Cx43 | ↑in PAs from patients with chronic hypoxic PH [28] ↓in PASMCs in patients with idiopathic PAH [28] ↔ in PAECs in patients with idiopathic PAH [28] | ↓ in whole lung tissue from chronic hypoxia mouse [35] ↑ in whole lung tissue from sugen /hypoxic rat [24] ↑ in rat PAFs exposed to acute hypoxia [24] ↔ in rat PASMCs exposed to acute hypoxia (5% O2) [24] ↓ in rat PASMCs exposed to acute hypoxia (3% O2) [36] ↓ in right ventricle of rat monocrotaline model [64,65,66,69] Internalization and lateralization of Cx43 in the right ventricle of the rat monocrotaline model [67,68] | Cx43+/− mice are protected against hypoxia-induced pulmonary vascular remodelling and lung inflammation [28] |
| Cx45 | Unknown | ↔ in response to acute hypoxia in rat PASMCs and rat PAFs [24] | Unknown |
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Tuder, R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017, 367, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, D.; Li, M.; Plecitá-Hlavatá, L.; D’Alessandro, A.; Tauber, J.; Riddle, S.; Kumar, S.; Flockton, A.; McKeon, B.A.; et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 2017, 136, 2468–2485. [Google Scholar] [CrossRef] [PubMed]
- Carlin, C.M.; Celnik, D.F.; Pak, O.; Wadsworth, R.; Peacock, A.J.; Welsh, D.J. Low-dose fluvastatin reverses the hypoxic pulmonary adventitial fibroblast phenotype in experimental pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2012, 47, 140–148. [Google Scholar] [CrossRef]
- Wilson, K.S.; Buist, H.; Suveizdyte, K.; Liles, J.T.; Budas, G.R.; Hughes, C.; MacLean, M.R.; Johnson, M.; Church, A.C.; Peacock, A.J.; et al. Apoptosis signal-regulating kinase 1 inhibition in in vivo and in vitro models of pulmonary hypertension. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef]
- Sysol, J.R.; Machado, R.F. Classification and pathophysiology of pulmonary hypertension. Contin. Cardiol. Educ. 2018, 4, 2–12. [Google Scholar] [CrossRef]
- Fallon, R.F.; Goodenough, D.A. Five-hour half-life of mouse liver gap-junction protein. J. Cell Biol. 1981, 90, 521–526. [Google Scholar] [CrossRef]
- Beardslee, M.A.; Laing, J.G.; Beyer, E.C.; Saffitz, J.E. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 1998, 83, 629–635. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R. Evolutionary analyses of gap junction protein families. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 4–14. [Google Scholar] [CrossRef]
- Martin, P.E.; Evans, W.H. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 2004, 62, 378–387. [Google Scholar] [CrossRef]
- Bai, D.; Yue, B.; Aoyama, H. Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 9–21. [Google Scholar] [CrossRef]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function. Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Meşe, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V.; Polosina, Y.Y.; Miller, H.; Potapova, I.A.; Valiuniene, L.; Doronin, S.; Mathias, R.T.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 2005, 568, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Anjo, S.I.; Manadas, B.; Sluijter, J.P.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 1–4. [Google Scholar]
- Billaud, M.; Marthan, R.; Savineau, J.P.; Guibert, C. Vascular smooth muscle modulates endothelial control of vasoreactivity via reactive oxygen species production through myoendothelial communications. PLoS ONE. 2009, 4, e6432. [Google Scholar] [CrossRef]
- Billaud, M.; Dahan, D.; Marthan, R.; Savineau, J.P.; Guibert, C. Role of the gap junctions in the contractile response to agonists in pulmonary artery from two rat models of pulmonary hypertension. Respir. Res. 2011, 12, 1–3. [Google Scholar] [CrossRef]
- Nakamura, K.; INAI, T.; Nakamura, K.; Shibata, Y. Distribution of gap junction protein connexin 37 in smooth muscle cells of the rat trachea and pulmonary artery. Arch. Histol. Cytol. 1999, 62, 27–37. [Google Scholar] [CrossRef]
- Li, X.; Simard, J.M. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1890–H1898. [Google Scholar] [CrossRef]
- McNair, A.J.; Wilson, K.S.; Martin, P.E.; Welsh, D.J.; Dempsie, Y. Connexin 43 plays a role in proliferation and migration of pulmonary arterial fibroblasts in response to hypoxia. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Yeh, H.I.; Rothery, S.; Dupont, E.; Coppen, S.R.; Severs, N.J. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ. Res. 1998, 83, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Yeh, H.I.; Rothery, S.; Dupont, E.; Coppen, S.R.; Severs, N.J. Connexin make-up of endothelial gap junctions in the rat pulmonary artery as revealed by immunoconfocal microscopy and triple-label immunogold electron microscopy. J. Histochem. Cytochem. 1999, 47, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gairhe, S.; Bauer, N.N.; Gebb, S.A.; McMurtry, I.F. Myoendothelial gap junctional signaling induces differentiation of pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L527–L535. [Google Scholar] [CrossRef]
- Bouvard, C.; Genet, N.; Phan, C.; Rode, B.; Thuillet, R.; Tu, L.; Robillard, P.; Campagnac, M.; Soleti, R.; De La Roque, E.D.; et al. Connexin-43 is a promising target for pulmonary hypertension due to hypoxaemic lung disease. Eur. Respir. J. 2020, 55, 1900169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwangbo, C.; Hu, X.; Kang, Y.; Papangeli, I.; Mehrotra, D.; Park, H.; Ju, H.; McLean, D.L.; Comhair, S.A.; et al. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation 2015, 131, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, J.; Anderson, J.P.; Wu, J.; Gleim, S.R.; Kundu, R.K.; McLean, D.L.; Kim, J.D.; Park, H.; Jin, S.W.; et al. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circ. Res. 2013, 113, 22–31. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, C.N.; Hajji, N.; Oliver, E.; Cotroneo, E.; Wharton, J.; Wang, D.; Li, M.; McKinsey, T.A.; Stenmark, K.R.; et al. Histone deacetylation inhibition in pulmonary hypertension: Therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 2012, 126, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Zhang, Q.; Cabrera, J.T.; Zheng, Q.; Tsuji-Hosokawa, A.; Watanabe, M.; Hosokawa, S.; Xiong, M.; Jain, P.P.; Ashton, A.W.; et al. Chronic Hypoxia Decreases Endothelial Connexin 40, Attenuates Endothelium-Dependent Hyperpolarization–Mediated Relaxation in Small Distal Pulmonary Arteries, and Leads to Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e018327. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yin, N.; Hu, L.; Fan, H.; Yu, D.; Zhang, W.; Wang, S.; Feng, Y.; Fan, C.; Cao, F.; et al. Sildenefil increases connexin 40 in smooth muscle cells through activation of BMP pathways in pulmonary arterial hypertension. Int. J. Clin. Exp. Pathol. 2014, 7, 4674. [Google Scholar]
- Welsh, D.J.; Scott, P.H.; Peacock, A.J. p38 MAP kinase isoform activity and cell cycle regulators in the proliferative response of pulmonary and systemic artery fibroblasts to acute hypoxia. Pulm. Pharmacol. Ther. 2006, 19, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Htet, M.; Nally, J.E.; Shaw, A.; Foote, B.E.; Martin, P.E.; Dempsie, Y. Connexin 43 plays a role in pulmonary vascular reactivity in mice. Int. J. Mol. Sci. 2018, 19, 1891. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Yi, D.; Wei, L.; Li, Y.; Zhang, L. Tanshinone IIA promotes pulmonary artery smooth muscle cell apoptosis in vitro by inhibiting the JAK2/STAT3 signaling pathway. Cell. Physiol. Biochem. 2014, 33, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Pausch, C.; Grünig, E.; Klose, H.; Staehler, G.; Huscher, D.; Pittrow, D.; Olsson, K.M.; Vizza, C.D.; Gall, H.; et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020, 39, 1435–1444. [Google Scholar] [CrossRef]
- Morris, H.; Denver, N.; Gaw, R.; Labazi, H.; Mair, K.; MacLean, M.R. Sex differences in pulmonary hypertension. Clin. Chest Med. 2021, 42, 217–228. [Google Scholar] [CrossRef]
- Firestone, G.L.; Kapadia, B.J. Minireview: Regulation of gap junction dynamics by nuclear hormone receptors and their ligands. Mol. Endocrinol. 2012, 26, 1798–1807. [Google Scholar] [CrossRef]
- Geimonen, E.; Jiang, W.; Ali, M.; Fishman, G.I.; Garfield, R.E.; Andersen, J. Activation of protein kinase C in human uterine smooth muscle induces connexin-43 gene transcription through an AP-1 site in the promoter sequence. J. Biol. Chem. 1996, 271, 23667–23674. [Google Scholar] [CrossRef]
- Geimonen, E.; Boylston, E.; Royek, A.; Andersen, J. Elevated connexin-43 expression in term human myometrium correlates with elevated c-Jun expression and is independent of myometrial estrogen receptors. J. Clin. Endocrinol. Metab. 1998, 83, 1177–1185. [Google Scholar]
- Ren, J.; Wang, X.H.; Wang, G.C.; Wu, J.H. 17β Estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone 2013, 53, 587–596. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.C.; Lee, T.M. 17β-estradiol decreases vulnerability to ventricular arrhythmias by preserving Connexin43 protein in infarcted rats. Eur. J. Pharmacol. 2010, 629, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Cheng, Y.K.; Lu, D.Y.; Wang, S.L.; Chang, C.N.; Chang, P.C.; Yeh, W.L. Inhibition of estrogen receptor reduces connexin 43 expression in breast cancers. Toxicol. Appl. Pharmacol. 2018, 338, 182–190. [Google Scholar] [CrossRef]
- Dempsie, Y.; Morecroft, I.; Welsh, D.J.; MacRitchie, N.A.; Herold, N.; Loughlin, L.; Nilsen, M.; Peacock, A.J.; Harmar, A.; Bader, M.; et al. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation 2008, 117, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 29. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreño, G.; Callejo, M.; Barreira, B.; Morales-Cano, D.; Esquivel-Ruiz, S.; Filice, M.; Moreno, L.; Cogolludo, A.; Perez-Vizcaino, F. miR-1 induces endothelial dysfunction in rat pulmonary arteries. J. Physiol. Biochem. 2019, 75, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreño, G.; Callejo, M.; Barreira, B.; Morales-Cano, D.; Esquivel-Ruiz, S.; Moreno, L.; Cogolludo, A.; Perez-Vizcaino, F. miR-1 is increased in pulmonary hypertension and downregulates Kv1. 5 channels in rat pulmonary arteries. J. Physiol. 2019, 597, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, J.; Zuo, X.; Cao, Q.; Xie, W.; Wang, H. Inhibiting miR-1 attenuates pulmonary arterial hypertension in rats. Mol. Med. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Aaronson, P.I.; Robertson, T.P.; Knock, G.A.; Becker, S.; Lewis, T.H.; Snetkov, V.; Ward, J.P. Hypoxic pulmonary vasoconstriction: Mechanisms and controversies. J. Physiol. 2006, 570, 53–58. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Thébaud, B.; Weir, E.K.; Archer, S.L. Hypoxic pulmonary vasoconstriction: Redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J. Mol. Cell. Cardiol. 2004, 37, 1119–1136. [Google Scholar] [CrossRef]
- Weissmann, N.; Sommer, N.; Schermuly, R.T.; Ghofrani, H.A.; Seeger, W.; Grimminger, F. Oxygen sensors in hypoxic pulmonary vasoconstriction. Cardiovasc. Res. 2006, 71, 620–629. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Nickles, H.T.; Ranke, H.; Tabuchi, A.; Hoffmann, J.; Tabeling, C.; Barbosa-Sicard, E.; Chanson, M.; Kwak, B.R.; et al. Hypoxic pulmonary vasoconstriction requires connexin 40–mediated endothelial signal conduction. J. Clin. Investig. 2012, 122, 4218–4230. [Google Scholar] [CrossRef]
- Kizub, I.V.; Strielkov, I.V.; Shaifta, Y.; Becker, S.; Prieto-Lloret, J.; Snetkov, V.A.; Soloviev, A.I.; Aaronson, P.I.; Ward, J.P. Gap junctions support the sustained phase of hypoxic pulmonary vasoconstriction by facilitating calcium sensitization. Cardiovasc. Res. 2013, 99, 404–411. [Google Scholar] [CrossRef]
- Dempsie, Y.; MacLean, M.R. Role of the serotonin transporter in pulmonary arterial hypertension. Expert Rev. Clin. Pharmacol. 2008, 1, 749–757. [Google Scholar] [CrossRef]
- Gairhe, S.; Bauer, N.N.; Gebb, S.A.; McMurtry, I.F. Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L767–L777. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Johnstone, S.; Vidal-Brime, L.; Lynn, K.S.; Koval, M. Connexins: Synthesis, post-translational modifications, and trafficking in health and disease. Int. J. Mol. Sci. 2018, 19, 1296. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.R.; Kroncke, B.M.; Straub, A.C.; Best, A.K.; Dunn, C.A.; Mitchell, L.A.; Peskova, Y.; Nakamoto, R.K.; Koval, M.; Lo, C.W.; et al. MAPK phosphorylation of connexin 43 promotes binding of cyclin E and smooth muscle cell proliferation. Circ. Res. 2012, 111, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Good, M.E.; Nelson, T.K.; Simon, A.M.; Burt, J.M. A functional channel is necessary for growth suppression by Cx37. J. Cell Sci. 2011, 124, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Good, M.E.; Ek-Vitorín, J.F.; Burt, J.M. Structural determinants and proliferative consequences of connexin 37 hemichannel function in insulinoma cells. J. Biol. Chem. 2014, 289, 30379–30386. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Burnier, L.; Roatti, A.; Chassot, A.; Roth, I.; Sutter, E.; Galan, K.; Pfenniger, A.; Chanson, M.; Kwak, B.R. Unexpected role for the human Cx37 C1019T polymorphism in tumour cell proliferation. Carcinogenesis 2010, 31, 1922–1931. [Google Scholar] [CrossRef]
- Han, X.J.; Zhang, W.F.; Wang, Q.; Li, M.; Zhang, C.B.; Yang, Z.J.; Tan, R.; Gan, L.; Zhang, L.; Lan, X.; et al. HIF-1α promotes the proliferation and migration of pulmonary arterial smooth muscle cells via activation of Cx43. J. Cell Mol. Med. 2021, 25, 10663–10673. [Google Scholar] [CrossRef]
- van der Velden, H.M.; Jongsma, H.J. Cardiac gap junctions and connexins: Their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc. Res. 2002, 54, 270–279. [Google Scholar] [CrossRef]
- Chang, L.T.; Sun, C.K.; Sheu, J.J.; Chiang, C.H.; Youssef, A.A.; Lee, F.Y.; Wu, C.J.; Yip, H.K. Cilostazol therapy attenuates monocrotaline-induced pulmonary arterial hypertension in rat model. Circ. J. 2008, 72, 825–831. [Google Scholar] [CrossRef]
- Sun, C.K.; Lin, Y.C.; Yuen, C.M.; Chua, S.; Chang, L.T.; Sheu, J.J.; Lee, F.Y.; Fu, M.; Leu, S.; Yip, H.K. Enhanced protection against pulmonary hypertension with sildenafil and endothelial progenitor cell in rats. Int. J. Cardiol. 2012, 162, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Lu, H.I.; Zhen, Y.Y.; Leu, S.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Sheu, J.J.; Hsu, S.Y.; et al. Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur. J. Pharm. Sci. 2013, 50, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Sasano, C.; Honjo, H.; Takagishi, Y.; Uzzaman, M.; Emdad, L.; Shimizu, A.; Murata, Y.; Kamiya, K.; Kodama, I. Internalization and dephosphorylation of connexin43 in hypertrophied right ventricles of rats with pulmonary hypertension. Circ. J. 2007, 71, 382–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, X.Y.; He, J.G. The remodeling of connexin in the hypertrophied right ventricular in pulmonary arterial hypertension and the effect of a dual ET receptor antagonist (bosentan). Pathol. Res. Pract. 2009, 205, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Strauss, B.; Sassi, Y.; Bueno-Beti, C.; Ilkan, Z.; Raad, N.; Cacheux, M.; Bisserier, M.; Turnbull, I.C.; Kohlbrenner, E.; Hajjar, R.J.; et al. Intra-tracheal gene delivery of aerosolized SERCA2a to the lung suppresses ventricular arrhythmias in a model of pulmonary arterial hypertension. J. Mol. Cell. Cardiol. 2019, 127, 20–30. [Google Scholar] [CrossRef]
- Marsh, S.R.; Williams, Z.J.; Pridham, K.J.; Gourdie, R.G. Peptidic Connexin43 Therapeutics in Cardiac Reparative Medicine. J. Cardiovasc. Dev. Dis. 2021, 8, 52. [Google Scholar] [CrossRef]
- Naus, C.C.; Giaume, C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Transl. Med. 2016, 14, 330. [Google Scholar] [CrossRef] [PubMed]
| Group 1 | Pulmonary arterial hypertension (PAH)
|
| Group 2 | PH due to left heart disease |
| Group 3 | PH due to lung disease and/or hypoxia |
| Group 4 | Chronic thromboembolic PH (CTEPH) |
| Group 5 | PH with unclear or multifactorial mechanisms |
| Cell Type | Connexins | References |
|---|---|---|
| Pulmonary artery endothelial cells (PAECs) | Cx43, Cx40, and Cx37 | [20,21,22,25,26,27] |
| Pulmonary artery smooth muscle cells (PASMCs) | Cx43, Cx40, Cx37, and Cx45 | [20,21,22,23,25,26,27] |
| Pulmonary artery fibroblasts (PAFs) | Cx43, Cx40, Cx37, and Cx45 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Htet, M.; Nally, J.E.; Martin, P.E.; Dempsie, Y. New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling. Int. J. Mol. Sci. 2022, 23, 379. https://doi.org/10.3390/ijms23010379
Htet M, Nally JE, Martin PE, Dempsie Y. New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling. International Journal of Molecular Sciences. 2022; 23(1):379. https://doi.org/10.3390/ijms23010379
Chicago/Turabian StyleHtet, Myo, Jane. E. Nally, Patricia. E. Martin, and Yvonne Dempsie. 2022. "New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling" International Journal of Molecular Sciences 23, no. 1: 379. https://doi.org/10.3390/ijms23010379
APA StyleHtet, M., Nally, J. E., Martin, P. E., & Dempsie, Y. (2022). New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling. International Journal of Molecular Sciences, 23(1), 379. https://doi.org/10.3390/ijms23010379







