Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis
Abstract
:1. Introduction
2. Results
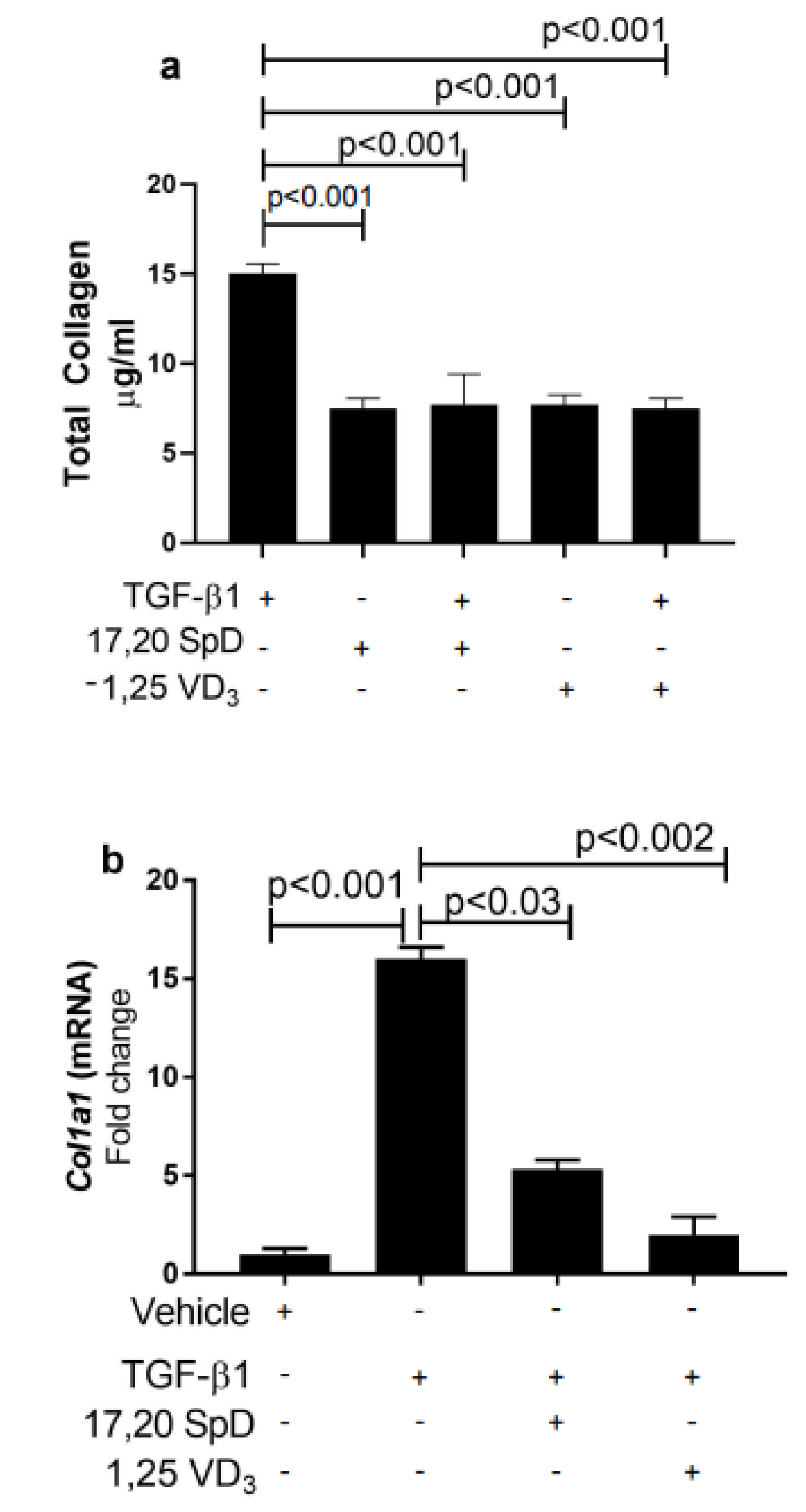
2.1. 17,20S(OH)2pD Suppressed COL1A1 Expression and Total Collagen Production in Healthy Normal and SSc Fibroblasts
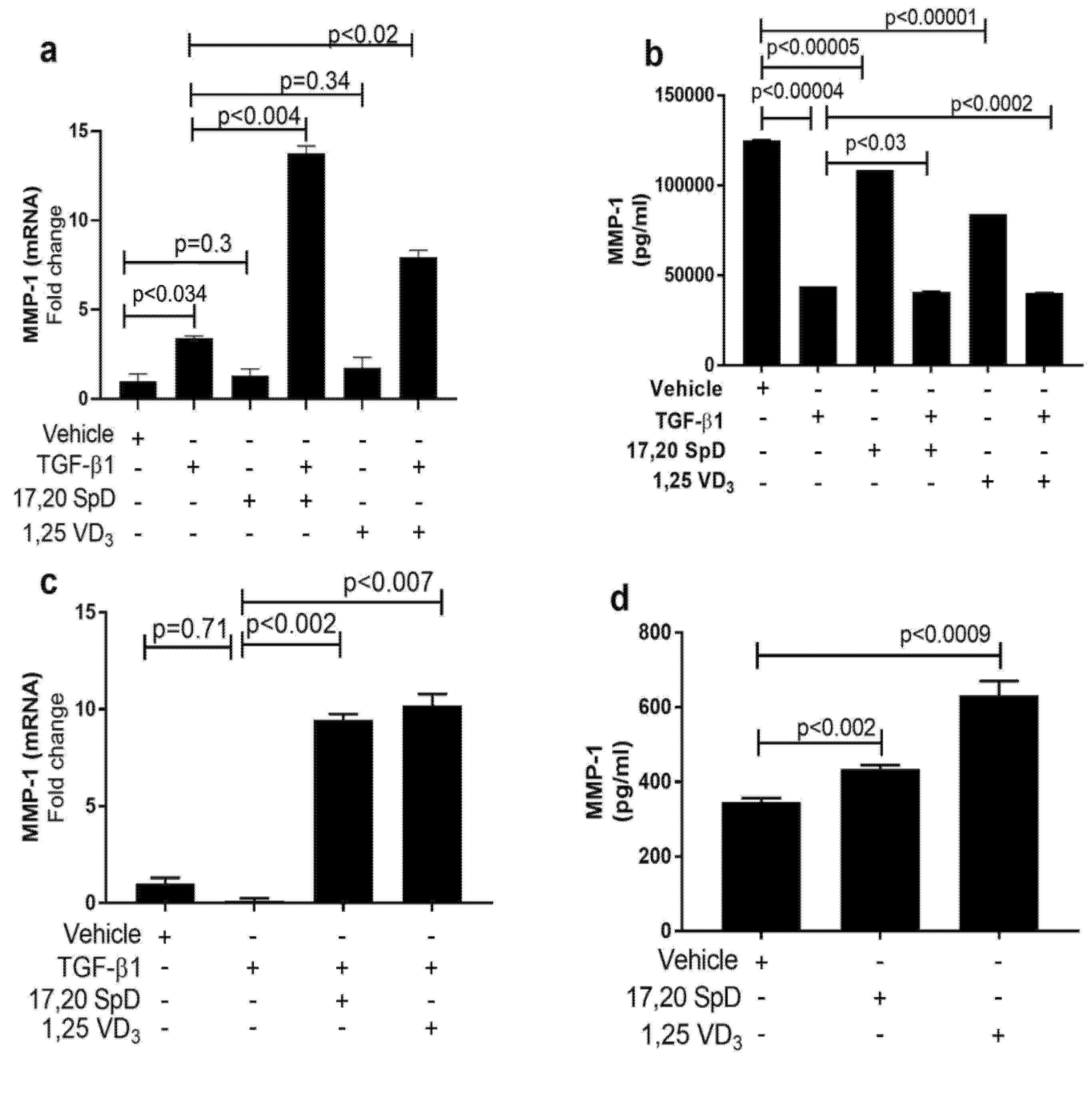
2.2. 17,20S(OH)2pD Increases MMP-1 Expression in SSc Fibroblasts
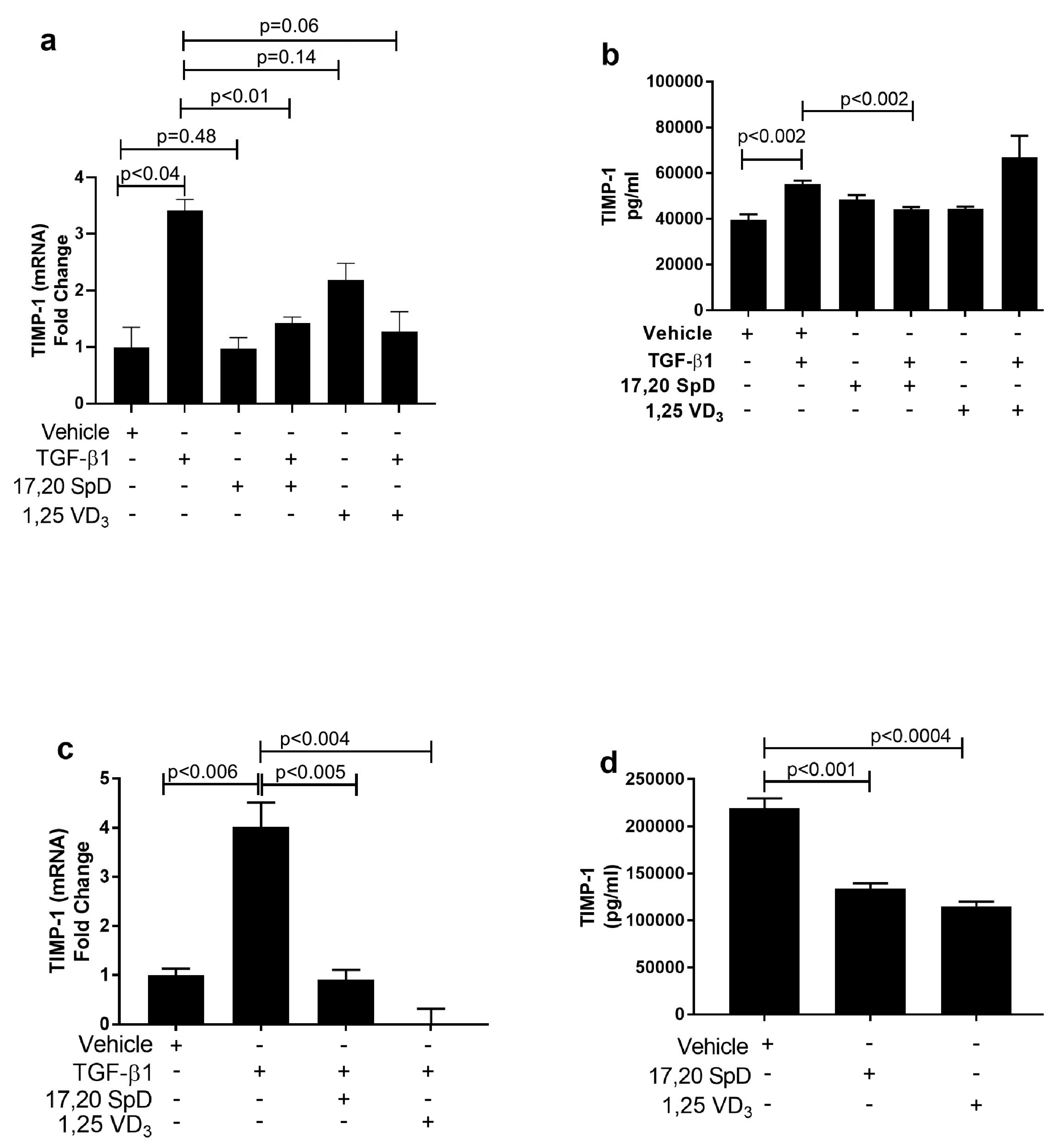
2.3. 17,20S(OH)2pD Decreases TIMP-1 Expression in SSc Fibroblasts
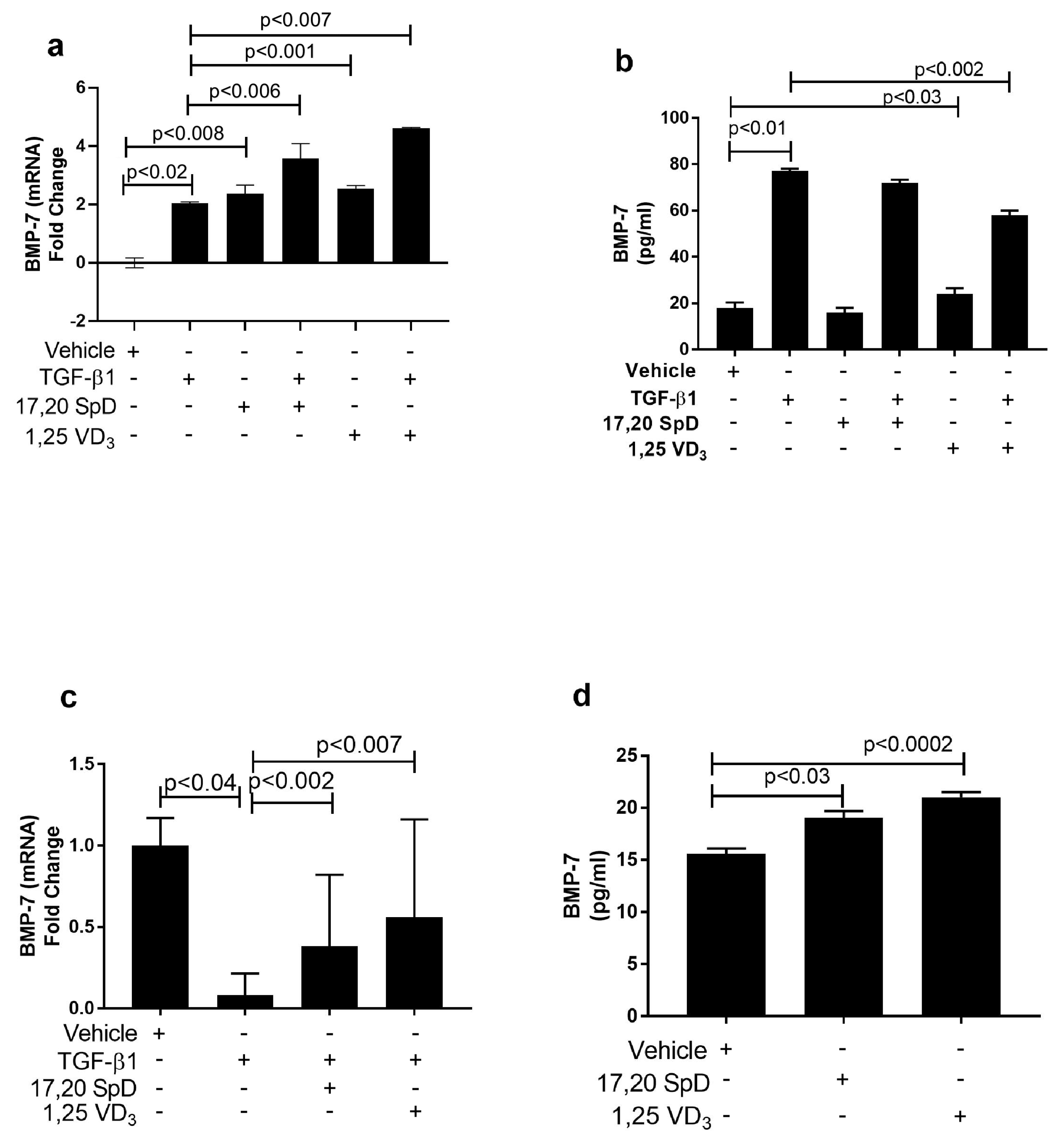
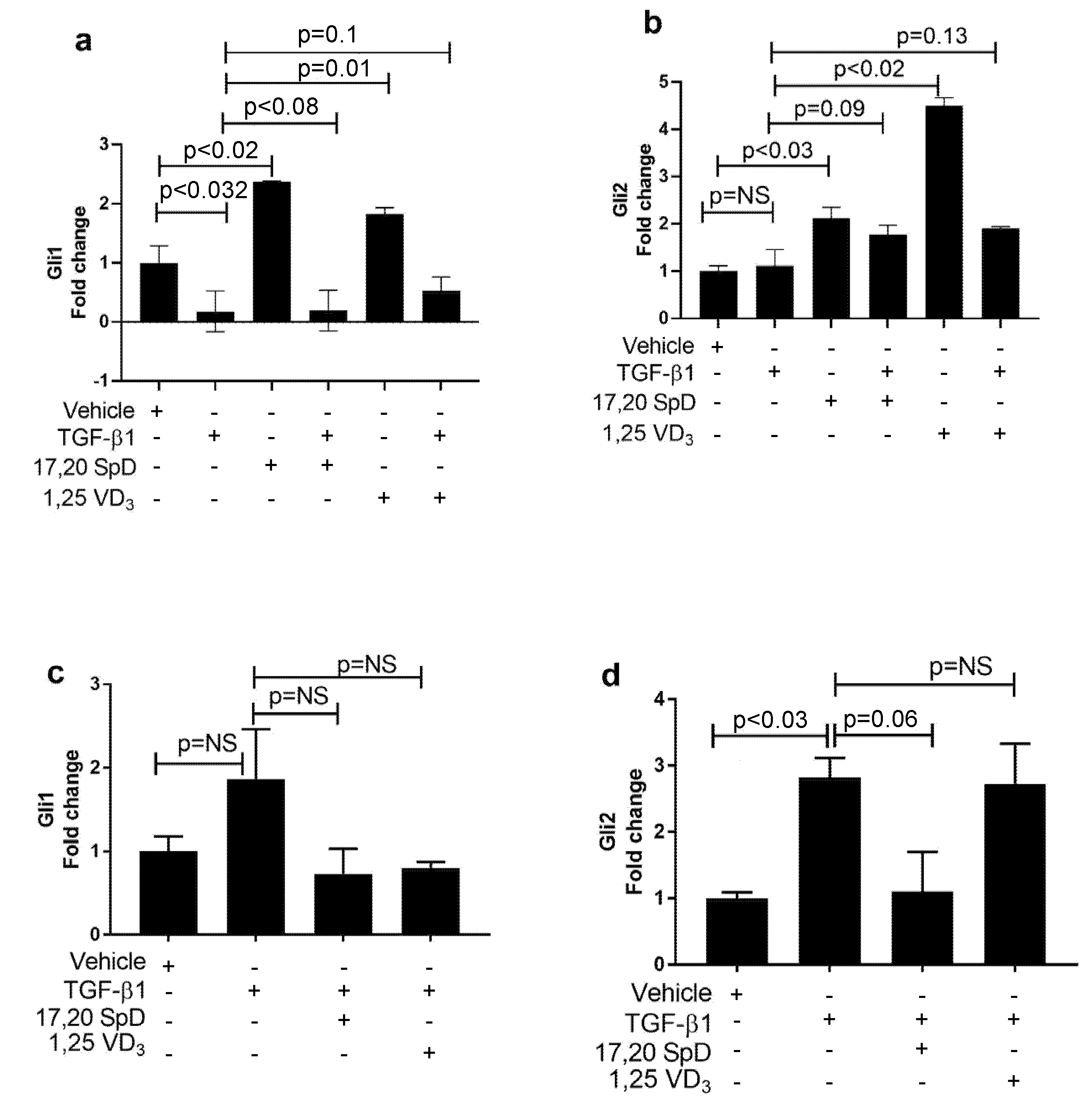
2.4. 17,20S(OH)2pD Modulates Other Mediators of Fibrosis In Vitro
3. Discussion
4. Materials and Methods
4.1. Secosteroids
4.2. Cell Lines and Culture
4.3. Quantitative Real-Time PCR
4.4. Protein Analysis
4.5. Analysis of Total Collagen
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1:25(OH)2D3 | 1:25-dihydroxyvitamin D3 |
| 17,20(OH)2pD | 17,20-dihydroxypregnacalciferol |
| BMP-7 | Bone morphogenic protein 7 |
| Gli1 | Glioma-associated oncogene homolog 1 |
| Gli2 | Glioma-associated oncogene homolog 2 |
| MMP-1 | Metalloproteinase-1 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PGES | Prostaglandin E2 synthetase |
| TIMP-1 | Tissue inhibitor of metalloproteinase-1 |
| TGF-β1 | Transforming growth factor-β1 |
References
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of systemic sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.A.; Trojanowska, M. Fibrosis in systemic sclerosis. Rheum. Dis. Clin. N. Am. 2008, 34, 115–143. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postlethwaite, A.E.; Shigemitsu, H.; Kanangat, S. Cellular origins of fibroblasts: Possible implications for organ fibrosis in systemic sclerosis. Curr. Opin. Rheumatol. 2004, 16, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Raghow, R.; Postlethwaite, A.E.; Keski-Oja, J.; Moses, H.L.; Kang, A.H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J. Clin. Investig. 1987, 79, 1285–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postlethwaite, A.E.; Keski-Oja, J.; Moses, H.L.; Kang, A.H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J. Exp. Med. 1987, 165, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Keski-Oja, J.; Raghow, R.; Sawdey, M.; Loskutoff, D.J.; Postlethwaite, A.E.; Kang, A.H.; Moses, H.L. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J. Biol. Chem. 1988, 263, 3111–3115. [Google Scholar] [CrossRef]
- Yuan, W.; Varga, J. Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J. Biol. Chem. 2001, 276, 38502–38510. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.S.; Chang, H.H.; Yeh, C.Y.; Chang, M.C.; Chan, C.P.; Kuo, H.Y.; Liu, H.C.; Liao, W.C.; Jeng, P.Y.; Yeung, S.Y.; et al. Transforming growth factor beta 1 increases collagen content, and stimulates procollagen I and tissue inhibitor of metalloproteinase-1 production of dental pulp cells: Role of MEK/ERK and activin receptor-like kinase-5/Smad signaling. J. Formos. Med. Assoc. 2017, 116, 351–358. [Google Scholar] [CrossRef]
- Khan, I.; Agarwal, P.; Thangjam, G.S.; Radhesh, R.; Rao, S.G.; Kondaiah, P. Role of TGF-beta and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors 2011, 29, 119–127. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, M.; Shah, A.A.; Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 2005, 280, 8094–8100. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.R.; Monemdjou, R.; Ghassemi-Kakroodi, P.; Fahmi, H.; Perez, G.; Liu, S.; Shi-Wen, X.; Parapuram, S.K.; Kojima, F.; Denton, C.P.; et al. mPGES-1 null mice are resistant to bleomycin-induced skin fibrosis. Arthritis Res. Ther. 2011, 13, R6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanekura, T.; Higashi, Y.; Kanzaki, T. Cyclooxygenase-2 expression and prostaglandin E2 biosynthesis are enhanced in scleroderma fibroblasts and inhibited by UVA irradiation. J. Rheumatol. 2001, 28, 1568–1572. [Google Scholar]
- Li, N.N.; Xu, Y.Y.; Chen, X.L.; Fan, Y.P.; Wu, J.H. Role of the prostaglandin E2/E-prostanoid 2 receptor signalling pathway in TGFbeta-induced mice mesangial cell damage. Biosci. Rep. 2014, 34, e00159. [Google Scholar] [CrossRef]
- Li, Y.J.; Kanaji, N.; Wang, X.Q.; Sato, T.; Nakanishi, M.; Kim, M.; Michalski, J.; Nelson, A.J.; Farid, M.; Basma, H.; et al. Prostaglandin E2 switches from a stimulator to an inhibitor of cell migration after epithelial-to-mesenchymal transition. Prostaglandins Other Lipid Mediat. 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Liu, J.; Wu, Z.; Liu, T.; Ullenbruch, M.R.; Ding, L.; Henke, C.A.; Bitterman, P.B.; Phan, S.H. Reemergence of hedgehog mediates epithelial-mesenchymal crosstalk in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Dennler, S.; Andre, J.; Alexaki, I.; Li, A.; Magnaldo, T.; ten Dijke, P.; Wang, X.J.; Verrecchia, F.; Mauviel, A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007, 67, 6981–6986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bhakhri, B.K.; Debata, P.K. Nutritional rickets presenting with myelofibrosis. Indian J. Pediatr. 2010, 77, 1437–1439. [Google Scholar] [CrossRef]
- Pelajo, C.F.; Lopez-Benitez, J.M.; Miller, L.C. Vitamin D and autoimmune rheumatologic disorders. Autoimmun. Rev. 2010, 9, 507–510. [Google Scholar] [CrossRef]
- Vacca, A.; Cormier, C.; Piras, M.; Mathieu, A.; Kahan, A.; Allanore, Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J. Rheumatol. 2009, 36, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, G.; Data, V.; Carignola, R.; Angeli, A. Hypovitaminosis D in systemic sclerosis. J. Rheumatol. 2009, 36, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambichler, T.; Chrobok, I.; Hoxtermann, S.; Kreuter, A. Significantly decreased serum 25-hydroxyvitamin d levels in a large german systemic sclerosis cohort. J. Rheumatol. 2011, 38, 2494. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Amital, H.; Agmon-Levin, N.; Alon, D.; Sanchez-Castanon, M.; Lopez-Hoyos, M.; Matucci-Cerinic, M.; Szucs, G.; Shapira, Y.; Szekanecz, Z.; et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: A retrospective cohort study and review of the literature. Autoimmun. Rev. 2011, 10, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Ozuguz, U.; Tutuncu, Y.A.; Erden, G.; Berker, D.; Acar, K.; Aydin, Y.; Akbaba, G.; Helvaci, N.; Guler, S. Serum transforming growth factor-beta levels in patients with vitamin D deficiency. Eur. J. Intern. Med. 2012, 23, 93–97. [Google Scholar] [CrossRef]
- Humbert, P.; Dupond, J.L.; Agache, P.; Laurent, R.; Rochefort, A.; Drobacheff, C.; de Wazieres, B.; Aubin, F. Treatment of scleroderma with oral 1,25-dihydroxyvitamin D3: Evaluation of skin involvement using non-invasive techniques. Results of an open prospective trial. Acta Derm. Venereol. 1993, 73, 449–451. [Google Scholar]
- Elst, E.F.; Van Suijlekom-Smit, L.W.; Oranje, A.P. Treatment of linear scleroderma with oral 1,25-dihydroxyvitamin D3 (calcitriol) in seven children. Pediatr. Dermatol. 1999, 16, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Caca-Biljanovska, N.G.; Vlckova-Laskoska, M.T.; Dervendi, D.V.; Pesic, N.P.; Laskoski, D.S. Treatment of generalized morphea with oral 1,25-dihydroxyvitamin D3. Adv. Exp. Med. Biol. 1999, 455, 299–304. [Google Scholar]
- Oblak, M.; Mlinsek, G.; Kandus, A.; Buturovic-Ponikvar, J.; Arnol, M. Effects of paricalcitol on biomarkers of inflammation and fibrosis in kidney transplant recipients: Results of a randomized controlled trial. Clin. Nephrol. 2017, 88, 119–125. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Yan, Y.; Liu, H.; Huang, Q.; Xiao, Y.; Gong, Z.; Du, J. Vitamin D protects against diabetic nephropathy: Evidence-based effectiveness and mechanism. Eur. J. Pharmacol. 2019, 845, 91–98. [Google Scholar] [CrossRef]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Marcus, J.F.; Shalev, S.M.; Harris, C.A.; Goodin, D.S.; Josephson, S.A. Severe hypercalcemia following vitamin d supplementation in a patient with multiple sclerosis: A note of caution. Arch. Neurol. 2012, 69, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Bhattacharya, K.G.; Wang, J.; Li, W.; Jiao, Y.; Gu, W.; Brown, M.; et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013, 98, E298–E303. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Li, W.; Bhattacharya, S.K.; Smith, R.A.; Johnson, P.L.; Chen, J.; Nelson, K.E.; Tuckey, R.C.; Miller, D.; Jiao, Y.; et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Investig. Dermatol. 2011, 131, 1167–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol 2013, 5, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Wang, J.; Janjetovic, Z.; Chen, J.; Tuckey, R.C.; Nguyen, M.N.; Tang, E.K.; Miller, D.; Li, W.; Slominski, A.T. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell. Endocrinol. 2012, 361, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Higley, H.; Persichitte, K.; Chu, S.; Waegell, W.; Vancheeswaran, R.; Black, C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud’s phenomenon. Arthritis Rheum. 1994, 37, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Q.; Wang, L.; Tu, W.; Chu, H.; Ding, W.; Jiang, S.; Ma, Y.; Shi, X.; Pu, W.; et al. Increased expression of latent TGF-beta-binding protein 4 affects the fibrotic process in scleroderma by TGF-beta/SMAD signaling. Lab. Investig. 2017, 97, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Nikitorowicz-Buniak, J.; Denton, C.P.; Abraham, D.; Stratton, R. Partially evoked epithelial-mesenchymal transition (EMT) is associated with increased tgfbeta signaling within lesional scleroderma skin. PLoS ONE 2015, 10, e0134092. [Google Scholar]
- Usategui, A.; Criado, G.; Del Rey, M.J.; Fare, R.; Pablos, J.L. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch. Dermatol. Res. 2014, 306, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Wongtrakool, C.; Welch, T.; Steinmeyer, A.; Zugel, U.; Roman, J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, J.J.; Liu, X.; Koteish, A.; Mezey, E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human alpha1 (I) collagen expression and type I collagen formation. Liver Int. 2013, 33, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, H.; Wang, Z.; Jinnin, M.; Nakayama, W.; Inoue, K.; Honda, N.; Nakashima, T.; Kajihara, I.; Makino, K.; Makino, T.; et al. EBI3 downregulation contributes to Type I collagen overexpression in scleroderma skin. J. Immunol. 2015, 195, 3565–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.; Whitfield, M.L. Transforming growth factor-beta in systemic sclerosis (scleroderma). Front. Biosci. 2009, 1, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.C.; Markle, L.S.; Vickers, J.L.; Petitt, M.S.; Raimer, S.S.; McNeese, C. The imbalanced expression of matrix metalloproteinases in nephrogenic systemic fibrosis. J. Am. Acad. Dermatol. 2010, 63, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Postlethwaite, A.E.; Myers, L.K.; Hasty, K.A. Supernatants from culture of type I collagen-stimulated PBMC from patients with cutaneous systemic sclerosis versus localized scleroderma demonstrate suppression of MMP-1 by fibroblasts. Clin. Rheumatol. 2012, 31, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Cai, W.M.; Weng, H.L.; Hu, Z.R.; Lu, J.; Zheng, M.; Liu, R.H. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J. Gastroenterol. 2003, 9, 2490–2496. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Sun, J.; Lemaitre, V.; D’Armiento, J.M. Transgenic expression of matrix metalloproteinase-1 inhibits myocardial fibrosis and prevents the transition to heart failure in a pressure overload mouse model. Hypertens. Res. 2008, 31, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef] [Green Version]
- Kohyama, T.; Ertl, R.F.; Valenti, V.; Spurzem, J.; Kawamoto, M.; Nakamura, Y.; Veys, T.; Allegra, L.; Romberger, D.; Rennard, S.I. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L1257–L1263. [Google Scholar] [CrossRef] [Green Version]
- Kolodsick, J.E.; Peters-Golden, M.; Larios, J.; Toews, G.B.; Thannickal, V.J.; Moore, B.B. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am. J. Respir. Cell Mol. Biol. 2003, 29, 537–544. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Kowal, K.; Distler, O.; Rojewska, J.; Bodzenta-Lukaszyk, A.; Michel, B.A.; Gay, R.E.; Gay, S.; Sierakowski, S. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: An imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis Rheum. 2005, 52, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, A.L.; Milla, C.M.; Lira, J.C.; Ramirez, R.; Checa, M.; Barrera, L.; Garcia-Alvarez, J.; Carbajal, V.; Becerril, C.; Gaxiola, M.; et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L978–L990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brozyna, A.A.; Zmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and overlapping effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on gene expression in human epidermal keratinocytes: Identification of ahr as an alternative receptor for 20,23(OH)(2)D3. Int. J. Mol. Sci. 2018, 19, 3072. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Kim, T.K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmijewski, M.A.; Li, W.; Zjawiony, J.K.; Sweatman, T.W.; Chen, J.; Miller, D.D.; Slominski, A.T. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids 2009, 74, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postlethwaite, A.E.; Smith, G.N., Jr.; Lachman, L.B.; Endres, R.O.; Poppleton, H.M.; Hasty, K.A.; Seyer, J.M.; Kang, A.H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163–171. J. Clin. Investig. 1989, 83, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown Lobbins, M.L.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.-K.; Janjetovic, Z.; Tuckey, R.C.; Scott, I.-S.O.; et al. Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis. Int. J. Mol. Sci. 2022, 23, 367. https://doi.org/10.3390/ijms23010367
Brown Lobbins ML, Slominski AT, Hasty KA, Zhang S, Miller DD, Li W, Kim T-K, Janjetovic Z, Tuckey RC, Scott I-SO, et al. Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis. International Journal of Molecular Sciences. 2022; 23(1):367. https://doi.org/10.3390/ijms23010367
Chicago/Turabian StyleBrown Lobbins, Monica L., Andrzej T. Slominski, Karen A. Hasty, Sicheng Zhang, Duane D. Miller, Wei Li, Tae-Kang Kim, Zorica Janjetovic, Robert C. Tuckey, Imara-Safi O. Scott, and et al. 2022. "Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis" International Journal of Molecular Sciences 23, no. 1: 367. https://doi.org/10.3390/ijms23010367






