Inhibition of Bruton Tyrosine Kinase Reduces Neuroimmune Cascade and Promotes Recovery after Spinal Cord Injury
Abstract
1. Introduction
2. Results
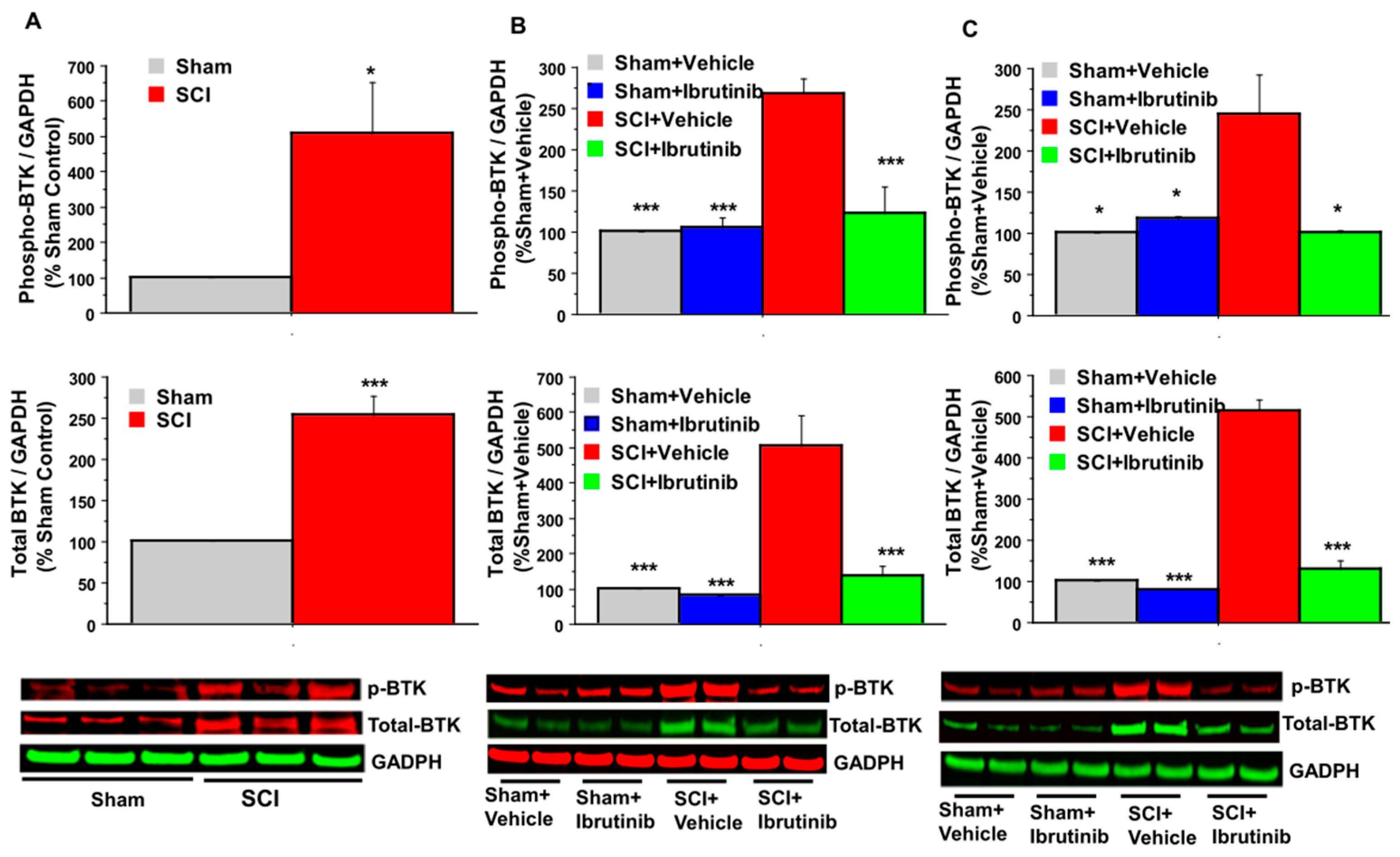
2.1. BTK Upregulation and Phosphorylation (Activation) following SCI, and Inhibition by Ibrutinib
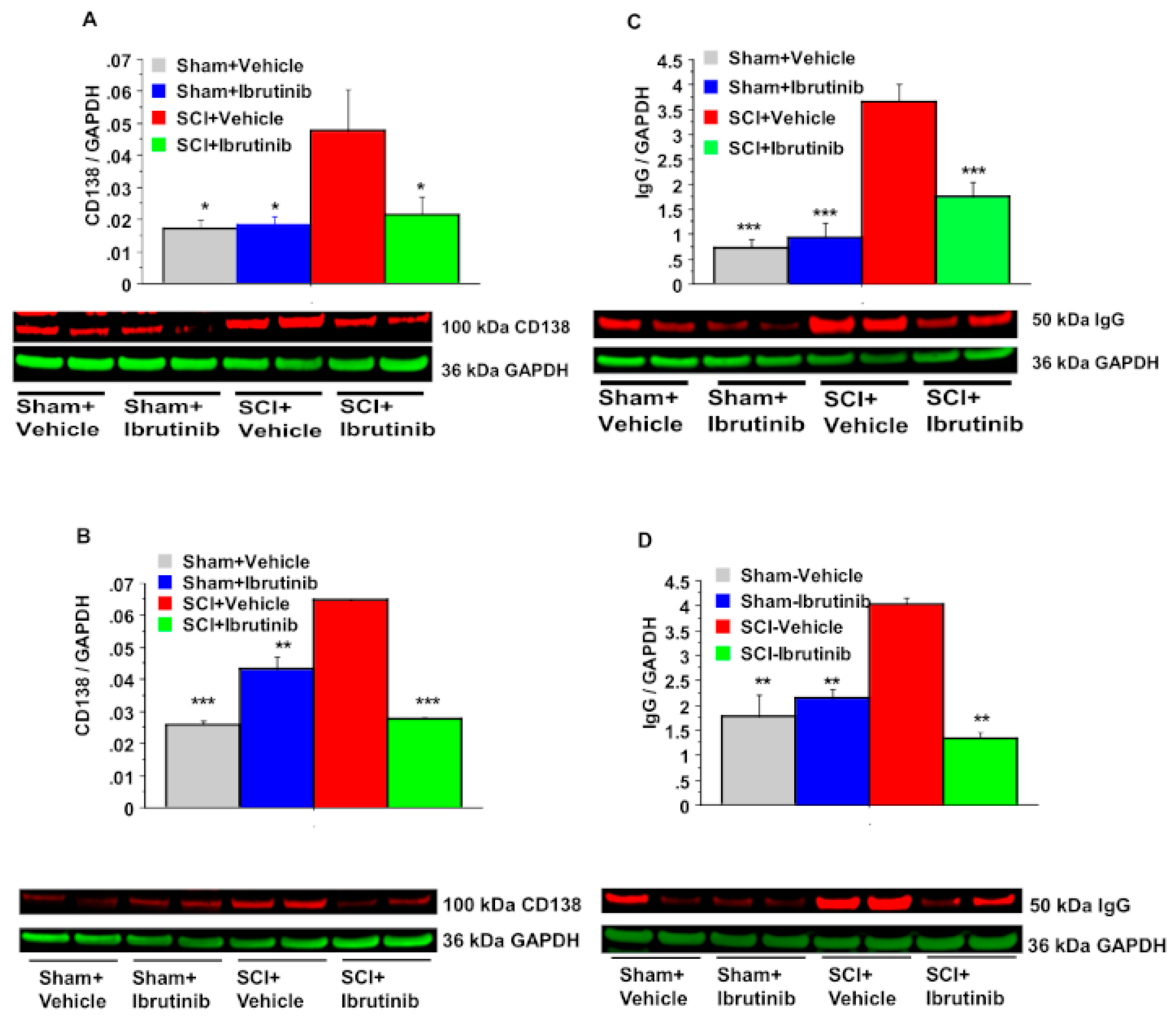
2.2. BTK Inhibition with Ibrutinib Treatment Reduces Plasma Cell Formation and Antibody Production in the Injured Spinal Cord
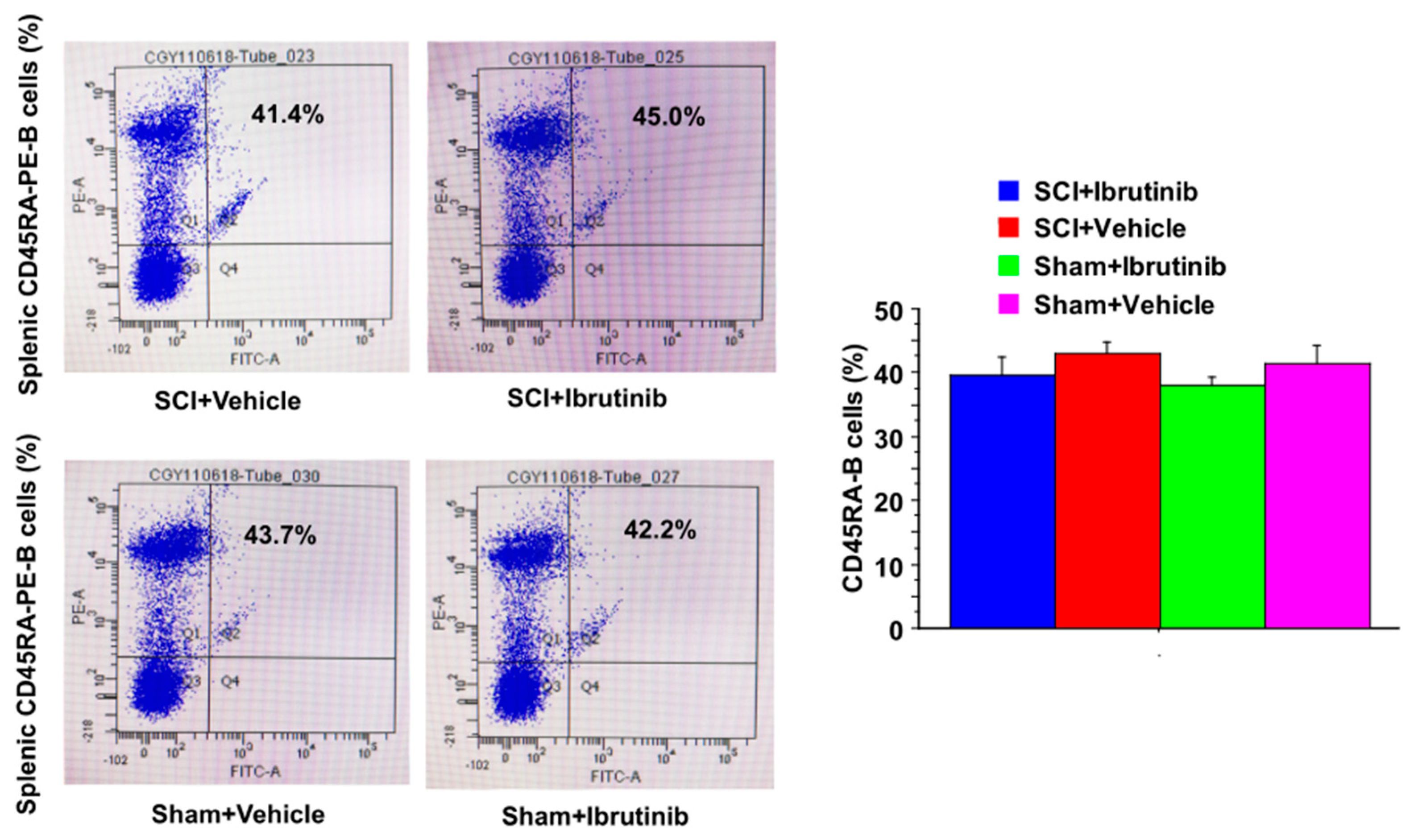
2.3. Ibrutinib Did Not Result in Reduced Levels of Splenic B Cells
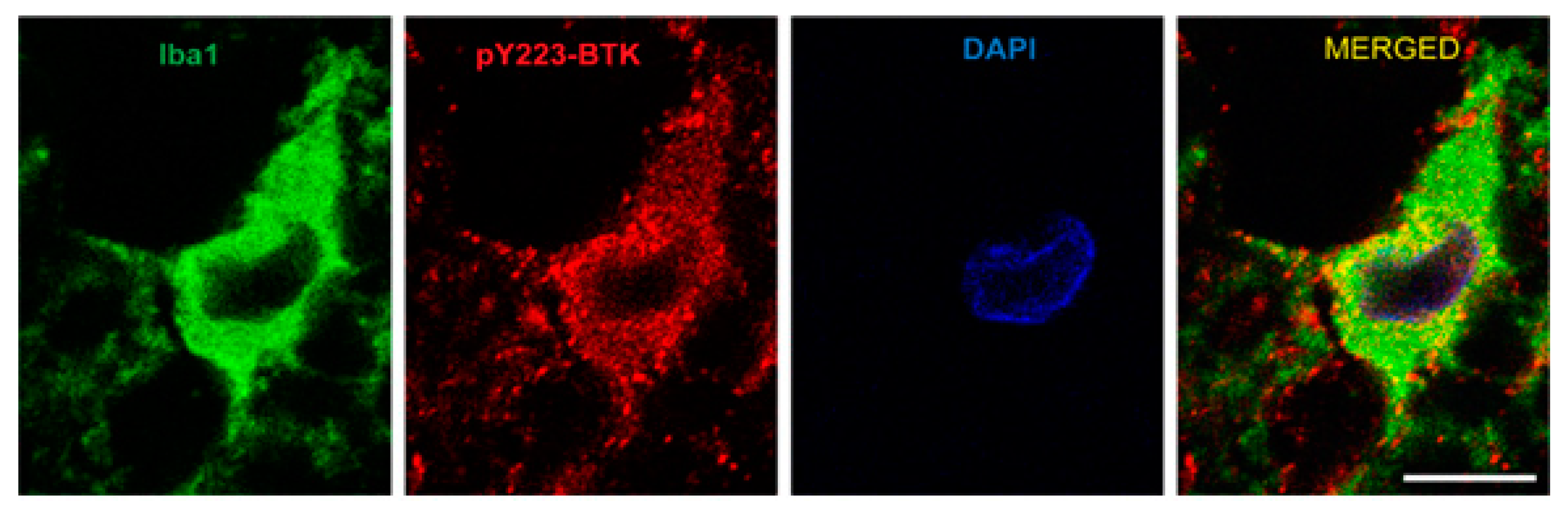
2.4. Active Microglia/Macrophages or Astrocytes Express Phospho-BTK following Acute SCI in Rats
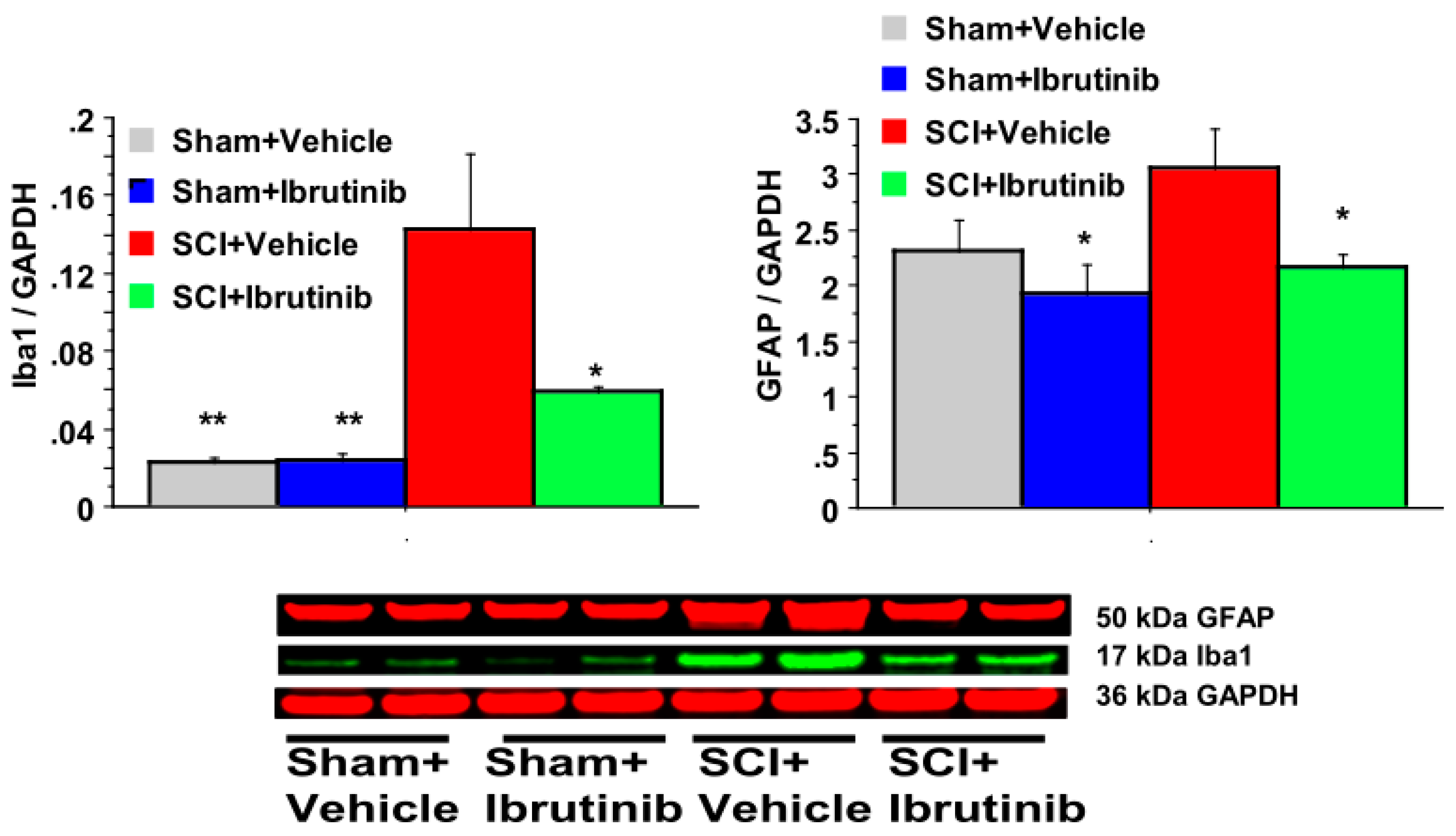
2.5. BTK Inhibition with Ibrutinib Treatment Reduces Activation of Microglia/Macrophages and Astrocytes following SCI in Rats
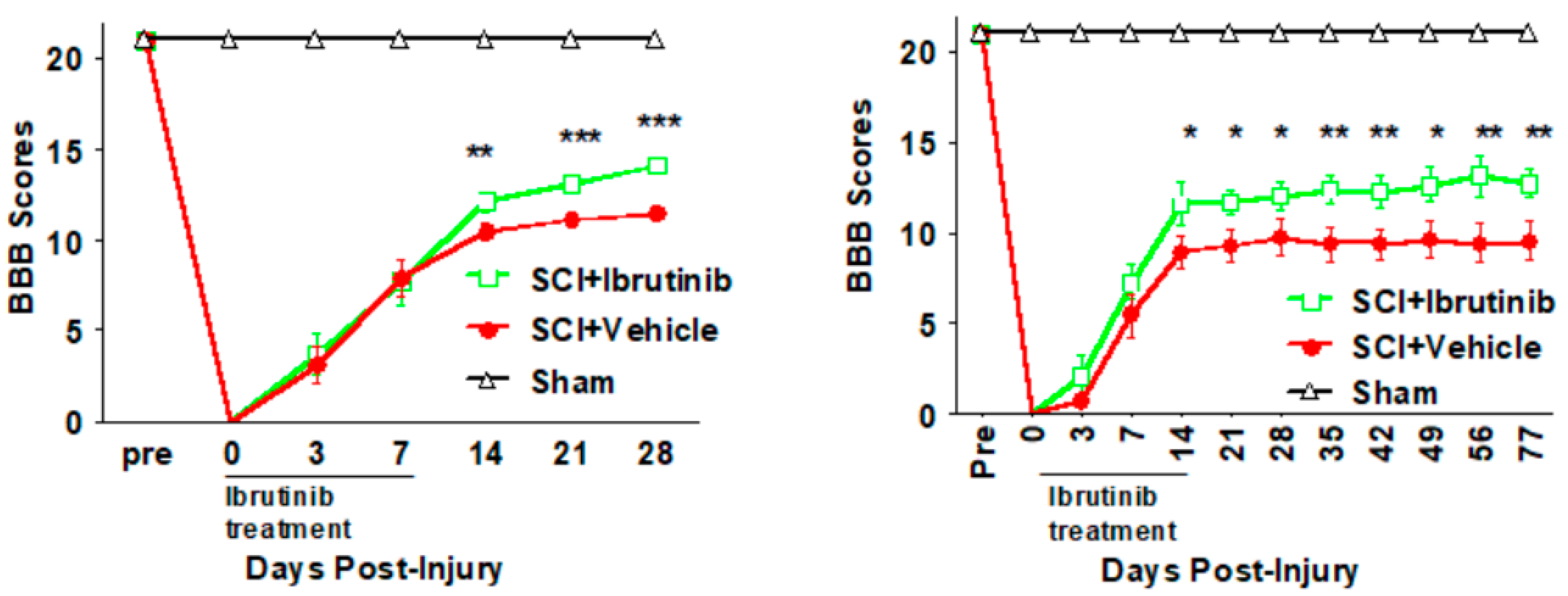
2.6. BTK Inhibition with Ibrutinib Treatment Improves Functional Outcomes
3. Discussion
4. Materials and Methods
4.1. Rigorous Experimental Design
4.2. Animals
4.3. Antibodies and Chemicals
4.4. Contusional SCI
4.5. Ibrutinib Intraperitoneal (IP) Administration
4.6. Assessment of Locomotor Function
4.7. Monitoring Bladder Infection and Bleeding
4.8. Spinal Cord Tissue Processing
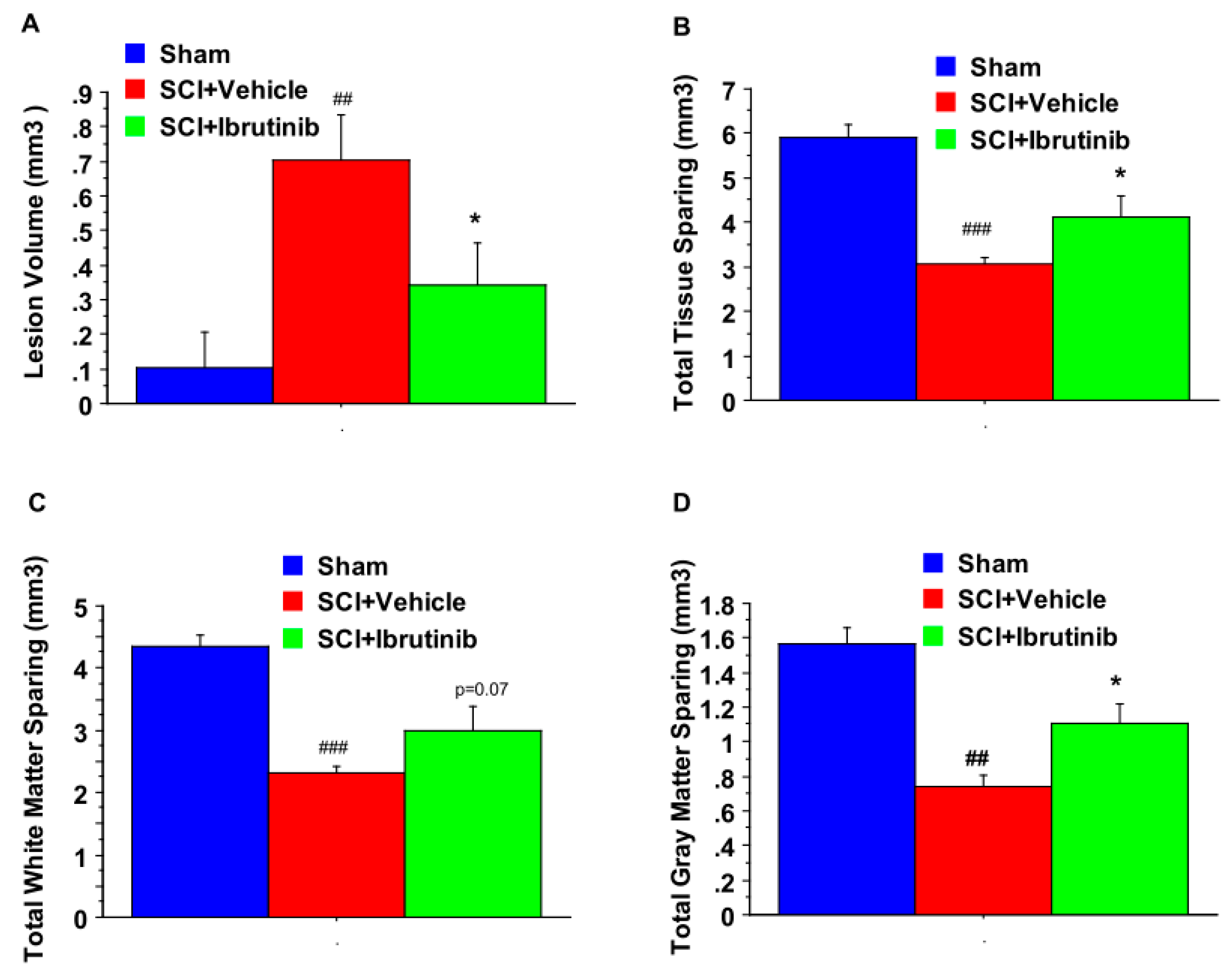
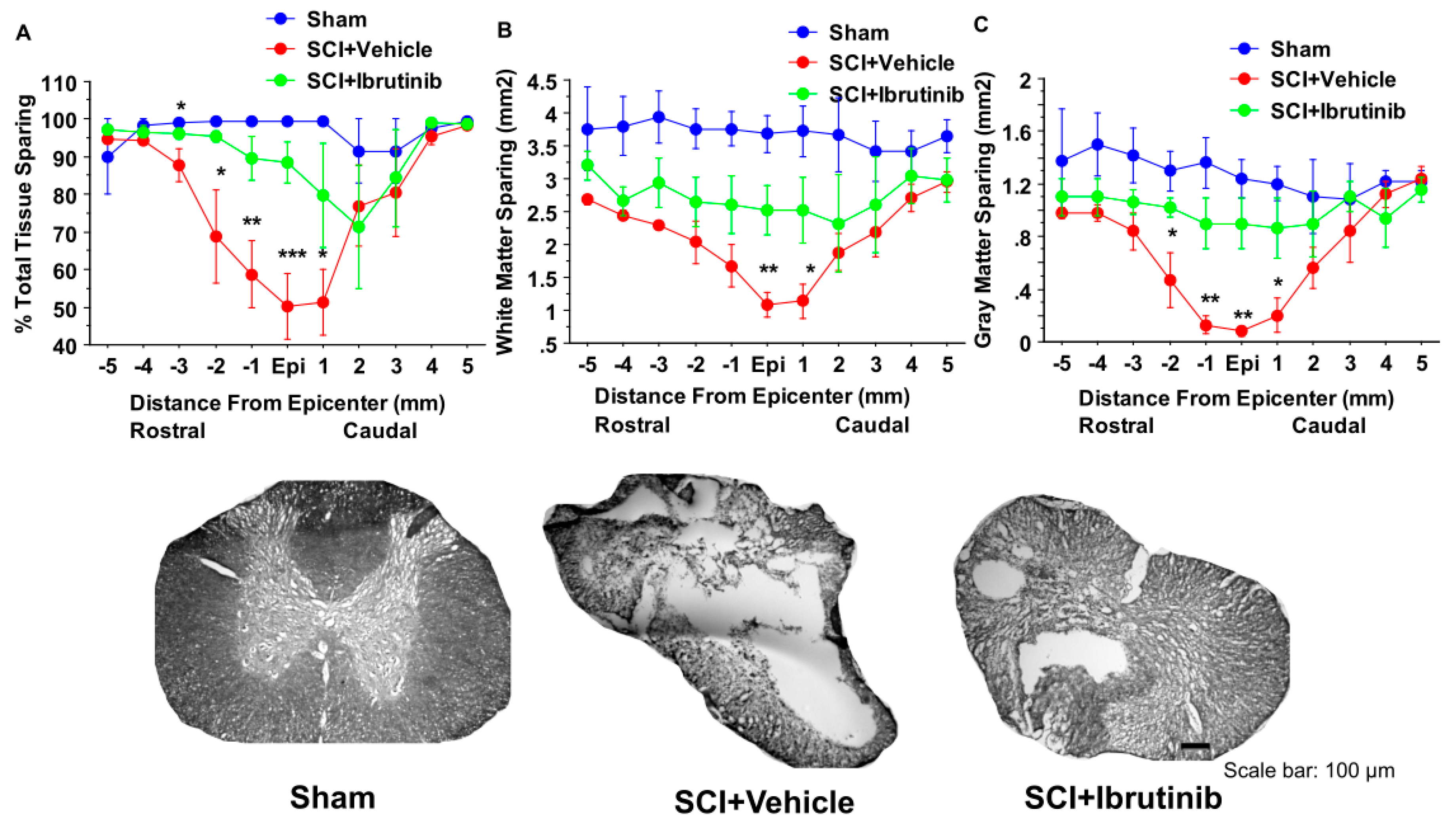
4.9. Assessment of Lesion Volume, Total Tissue Sparing, White Matter Sparing, and Gray Matter Sparing
4.10. Western Blotting
4.11. Double Immunofluorescence Confocal Imaging Analysis
4.12. Flow Cytometry Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Den Hauwe, L.; Sundgren, P.C.; Flanders, A.E. Spinal Trauma and Spinal Cord Injury (SCI). In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Germany, 2020; pp. 231–240. [Google Scholar]
- Merritt, C.H.; Taylor, M.A.; Yelton, C.J.; Ray, S.K. Economic impact of traumatic spinal cord injuries in the United States. Neuroimmunol. Neuroinflamm. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Lasfargues, J.E.; Custis, D.; Morrone, F.; Carswell, J.; Nguyen, T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia 1995, 33, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain J. Neurol. 2006, 129, 3249–3269. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Anderson, A.J. Mechanisms and pathways of inflammatory responses in CNS trauma: Spinal cord injury. J. Spinal. Cord Med. 2002, 25, 70–79. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Lucin, K.M.; Sanders, V.M.; McGaughy, V.M.; Popovich, P.G. Spinal cord injury triggers systemic autoimmunity: Evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006, 99, 1073–1087. [Google Scholar] [CrossRef]
- Dekaban, G.A.; Thawer, S. Pathogenic antibodies are active participants in spinal cord injury. J. Clin. Investig. 2009, 119, 2881–2884. [Google Scholar] [CrossRef]
- Jones, T.B. Lymphocytes and autoimmunity after spinal cord injury. Exp. Neurol. 2014, 258, 78–90. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal. Cord 2015, 53, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Popovich, P.G. B cells and autoantibodies: Complex roles in CNS injury. Trends Immunol. 2010, 31, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef]
- Hawthorne, A.L.; Popovich, P.G. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics 2011, 8, 252–261. [Google Scholar] [CrossRef]
- Yu, C.G.; Bondada, V.; Ghoshal, S.; Singh, R.; Pistilli, C.K.; Dayaram, K.; Iqbal, H.; Sands, M.; Davis, K.; Bondada, S.; et al. Repositioning Flubendazole for Spinal Cord Injury. J. Neurotrauma 2019, 36, 2618–2630. [Google Scholar] [CrossRef]
- Sabirzhanov, B.; Li, Y.; Coll-Miro, M.; Matyas, J.J.; He, J.; Kumar, A.; Ward, N.; Yu, J.; Faden, A.I.; Wu, J. Inhibition of NOX2 signaling limits pain-related behavior and improves motor function in male mice after spinal cord injury: Participation of IL-10/miR-155 pathways. Brain Behav. Immun. 2019, 80, 73–87. [Google Scholar] [CrossRef]
- Bowes, A.L.; Yip, P.K. Modulating inflammatory cell responses to spinal cord injury: All in good time. J. Neurotrauma 2014, 31, 1753–1766. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Guan, Z.; Popovich, P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Investig. 2009, 119, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, J.W.; Battaglino, R.A.; Salles, L.; Jha, P.; Sudhakar, S.; Garshick, E.; Stott, H.L.; Zafonte, R.; Morse, L.R. B-cell maturation antigen, a proliferation-inducing ligand, and B-cell activating factor are candidate mediators of spinal cord injury-induced autoimmunity. J. Neurotrauma 2013, 30, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Hampe, C.S. B Cell in Autoimmune Diseases. Scientifica 2012, 2012, 215308. [Google Scholar] [CrossRef]
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef]
- Viau, M.; Zouali, M. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 2005, 114, 17–26. [Google Scholar] [CrossRef]
- Cargill, T.; Culver, E.L. The Role of B Cells and B Cell Therapies in Immune-Mediated Liver Diseases. Front. Immunol. 2021, 12, 661196. [Google Scholar] [CrossRef]
- Stensland, Z.C.; Cambier, J.C.; Smith, M.J. Therapeutic Targeting of Autoreactive B Cells: Why, How, and When? Biomedicines 2021, 9, 83. [Google Scholar] [CrossRef]
- Herrera, J.J.; Haywood-Watson, R.J.; Grill, R.J. Acute and chronic deficits in the urinary bladder after spinal contusion injury in the adult rat. J. Neurotrauma 2010, 27, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kjell, J.; Finn, A.; Hao, J.; Wellfelt, K.; Josephson, A.; Svensson, C.I.; Wiesenfeld-Hallin, Z.; Eriksson, U.; Abrams, M.; Olson, L. Delayed Imatinib Treatment for Acute Spinal Cord Injury: Functional Recovery and Serum Biomarkers. J. Neurotrauma 2015, 32, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Kadekawa, K.; Kashyap, M.; Pore, S.; Yoshimura, N. Spontaneous Recovery of Reflex Voiding Following Spinal Cord Injury Mediated by Anti-inflammatory and Neuroprotective Factors. Urology 2016, 88, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Chow, W.N.; Sato-Bigbee, C.; Graf, M.R.; Graham, R.S.; Colello, R.J.; Young, H.F.; Mathern, B.E. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J. Neurotrauma 2009, 26, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Singh, R.; Crowdus, C.; Raza, K.; Kincer, J.; Geddes, J.W. Fenbendazole improves pathological and functional recovery following traumatic spinal cord injury. Neuroscience 2014, 256, 163–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rip, J.; Van Der Ploeg, E.K.; Hendriks, R.W.; Corneth, O.B.J. The Role of Bruton’s Tyrosine Kinase in Immune Cell Signaling and Systemic Autoimmunity. Crit. Rev. Immunol. 2018, 38, 17–62. [Google Scholar] [CrossRef]
- Hutcheson, J.; Vanarsa, K.; Bashmakov, A.; Grewal, S.; Sajitharan, D.; Chang, B.Y.; Buggy, J.J.; Zhou, X.J.; Du, Y.; Satterthwaite, A.B.; et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res. Ther. 2012, 14, R243. [Google Scholar] [CrossRef]
- Corneth, O.B.J.; Klein Wolterink, R.G.J.; Hendriks, R.W. BTK Signaling in B Cell Differentiation and Autoimmunity. Curr. Top. Microbiol. Immunol. 2016, 393, 67–105. [Google Scholar]
- Crofford, L.J.; Nyhoff, L.E.; Sheehan, J.H.; Kendall, P.L. The role of Bruton’s tyrosine kinase in autoimmunity and implications for therapy. Expert. Rev. Clin. Immunol. 2016, 12, 763–773. [Google Scholar] [CrossRef]
- Vetrie, D.; Vorechovsky, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarstrom, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The gene involved in X-linked agammaglobulinaemia is a member of the Src family of protein-tyrosine kinases 1993. J. Immunol. 2012, 188, 2948–2955. [Google Scholar]
- Mohamed, A.J.; Nore, B.F.; Christensson, B.; Smith, C.I. Signalling of Bruton’s tyrosine kinase, Btk. Scand. J. Immunol. 1999, 49, 113–118. [Google Scholar] [CrossRef]
- Burger, J.A.; Buggy, J.J. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk. Lymphoma 2013, 54, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Muller, B.; Wirth, T. Bruton’s Tyrosine Kinase is involved in innate and adaptive immunity. Histol. Histopathol. 2005, 20, 945–955. [Google Scholar] [PubMed]
- Waisman, A.; Liblau, R.S.; Becher, B. Innate and adaptive immune responses in the CNS. Lancet Neurol. 2015, 14, 945–955. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.R.; Bittner, Z.; Liu, X.; Dang, T.M.; Radsak, M.P.; Brunner, C. Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front. Immunol. 2017, 8, 1454. [Google Scholar] [CrossRef] [PubMed]
- Whang, J.A.; Chang, B.Y. Bruton’s tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. Drug Discov. Today 2014, 19, 1200–1204. [Google Scholar] [CrossRef]
- Hartkamp, L.M.; Fine, J.S.; van Es, I.E.; Tang, M.W.; Smith, M.; Woods, J.; Narula, S.; DeMartino, J.; Tak, P.P.; Reedquist, K.A. Btk inhibition suppresses agonist-induced human macrophage activation and inflammatory gene expression in RA synovial tissue explants. Ann. Rheum. Dis. 2015, 74, 1603–1611. [Google Scholar] [CrossRef]
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A.; et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5, eabd0110. [Google Scholar] [CrossRef]
- Ito, M.; Shichita, T.; Okada, M.; Komine, R.; Noguchi, Y.; Yoshimura, A.; Morita, R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015, 6, 7360. [Google Scholar] [CrossRef]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef]
- Estupinan, H.Y.; Berglof, A.; Zain, R.; Smith, C.I.E. Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front. Cell Dev. Biol. 2021, 9, 630942. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2001, 32, 1208–1215. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000, 113, 3073–3084. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Martin, A.; Grassner, L.; Garcia-Ovejero, D.; Paniagua-Torija, B.; Barroso-Garcia, G.; Arandilla, A.G.; Mach, O.; Turrero, A.; Vargas, E.; Alcobendas, M.; et al. Elevated Autoantibodies in Subacute Human Spinal Cord Injury Are Naturally Occurring Antibodies. Front. Immunol. 2018, 9, 2365. [Google Scholar] [CrossRef]
- Hayes, K.C.; Hull, T.C.; Delaney, G.A.; Potter, P.J.; Sequeira, K.A.; Campbell, K.; Popovich, P.G. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma 2002, 19, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Basso, D.M.; Sodhi, A.; Pan, J.Z.; Hart, R.P.; MacCallum, R.C.; Lee, S.; Whitacre, C.C.; Popovich, P.G. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: Implications for autoimmune vaccine therapy. J. Neurosci. 2002, 22, 2690–2700. [Google Scholar] [CrossRef]
- O’Connell, F.P.; Pinkus, J.L.; Pinkus, G.S. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 2004, 121, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Torke, S.; Pretzsch, R.; Hausler, D.; Haselmayer, P.; Grenningloh, R.; Boschert, U.; Bruck, W.; Weber, M.S. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 2020, 140, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ulndreaj, A.; Tzekou, A.; Mothe, A.J.; Siddiqui, A.M.; Dragas, R.; Tator, C.H.; Torlakovic, E.E.; Fehlings, M.G. Characterization of the Antibody Response after Cervical Spinal Cord Injury. J. Neurotrauma 2017, 34, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Sanders, V.M.; Jones, T.B.; Malarkey, W.B.; Popovich, P.G. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 2007, 207, 75–84. [Google Scholar] [CrossRef]
- Bonasia, C.G.; Abdulahad, W.H.; Rutgers, A.; Heeringa, P.; Bos, N.A. B Cell Activation and Escape of Tolerance Checkpoints: Recent Insights from Studying Autoreactive B Cells. Cells 2021, 10, 1190. [Google Scholar] [CrossRef]
- Kil, L.P.; de Bruijn, M.J.; van Nimwegen, M.; Corneth, O.B.; van Hamburg, J.P.; Dingjan, G.M.; Thaiss, F.; Rimmelzwaan, G.F.; Elewaut, D.; Delsing, D.; et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 2012, 119, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.J.; Metzler, G.; Wray-Dutra, M.; Jackson, S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017, 17, 421–436. [Google Scholar] [CrossRef]
- Chang, B.Y.; Huang, M.M.; Francesco, M.; Chen, J.; Sokolove, J.; Magadala, P.; Robinson, W.H.; Buggy, J.J. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res. Ther. 2011, 13, R115. [Google Scholar] [CrossRef]
- Whyburn, L.R.; Halcomb, K.E.; Contreras, C.M.; Lowell, C.A.; Witte, O.N.; Satterthwaite, A.B. Reduced dosage of Bruton’s tyrosine kinase uncouples B cell hyperresponsiveness from autoimmunity in lyn−/− mice. J. Immunol. 2003, 171, 1850–1858. [Google Scholar] [CrossRef]
- Casili, G.; Impellizzeri, D.; Cordaro, M.; Esposito, E.; Cuzzocrea, S. B-Cell Depletion with CD20 Antibodies as New Approach in the Treatment of Inflammatory and Immunological Events Associated with Spinal Cord Injury. Neurotherapeutics 2016, 13, 880–894. [Google Scholar] [CrossRef]
- Menekse, G.; Daglioglu, E.; Nacar, O.A.; Polat, E.; Ozdol, C.; Dalgic, A.; Take, G.; Okten, A.I.; Belen, A.D. The neuroprotective effects of rituximab in rat spinal cord injury model: An immunohistochemical study. Turk. Neurosurg. 2013, 23, 783–790. [Google Scholar]
- Dale, R.C.; Brilot, F.; Duffy, L.V.; Twilt, M.; Waldman, A.T.; Narula, S.; Muscal, E.; Deiva, K.; Andersen, E.; Eyre, M.R.; et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 2014, 83, 142–150. [Google Scholar] [CrossRef]
- Mangla, A.; Khare, A.; Vineeth, V.; Panday, N.N.; Mukhopadhyay, A.; Ravindran, B.; Bal, V.; George, A.; Rath, S. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 2004, 104, 1191–1197. [Google Scholar] [CrossRef]
- Keaney, J.; Gasser, J.; Gillet, G.; Scholz, D.; Kadiu, I. Inhibition of Bruton’s Tyrosine Kinase Modulates Microglial Phagocytosis: Therapeutic Implications for Alzheimer’s Disease. J. Neuroimmune Pharmacol. 2019, 14, 448–461. [Google Scholar] [CrossRef]
- Torabi, S.; Anjamrooz, S.H.; Zeraatpisheh, Z.; Aligholi, H.; Azari, H. Ibrutinib reduces neutrophil infiltration, preserves neural tissue and enhances locomotor recovery in mouse contusion model of spinal cord injury. Anat Cell Biol. 2021, 54, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Ni Gabhann, J.; Hams, E.; Smith, S.; Wynne, C.; Byrne, J.C.; Brennan, K.; Spence, S.; Kissenpfennig, A.; Johnston, J.A.; Fallon, P.G.; et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS ONE 2014, 9, e85834. [Google Scholar]
- Rothhammer, V.; Quintana, F.J. Control of autoimmune CNS inflammation by astrocytes. Semin. Immunopathol. 2015, 37, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.R.; Lin, Y.; Langan, T.J.; Iwakura, Y.; Chou, R.C. Innate Immune Functions of Astrocytes are Dependent Upon Tumor Necrosis Factor-Alpha. Sci. Rep. 2020, 10, 7047. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Begolka, W.S.; Olson, J.K.; Elhofy, A.; Karpus, W.J.; Miller, S.D. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 2005, 49, 360–374. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Siess, W. Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease. Cancers 2021, 13, 1103. [Google Scholar] [CrossRef]
- Dmitrieva, E.A.; Nikitin, E.A.; Ignatova, A.A.; Vorobyev, V.I.; Poletaev, A.V.; Seregina, E.A.; Voronin, K.A.; Polokhov, D.M.; Maschan, A.A.; Novichkova, G.A.; et al. Platelet function and bleeding in chronic lymphocytic leukemia and mantle cell lymphoma patients on ibrutinib. J. Thromb. Haemost. 2020, 18, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Maffei, R.; Audrito, V.; Martinelli, S.; Ten Hacken, E.; Zucchini, P.; Grisendi, G.; Potenza, L.; Luppi, M.; Burger, J.A.; et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016, 7, 65968–65981. [Google Scholar] [CrossRef]
- Shaw, M.L. Second-generation BTK inhibitors hit the treatment bullseye with fewer off-target effects. Am. J. Manag. Care 2020, 26, SP226–SP227. [Google Scholar] [PubMed]
- Francesco, M.R.; Wong, M.; LaStant, J.; Finkle, D.; Loewenstein, N.; Macsata, R.; Lindstrom, M.M.; Shu, J.; Ton, T.; Zhu, J.; et al. PRN2246, a potent and selective blood brain barrier penetrating BTK inhibitor, exhibits efficacy in central nervous system immunity (abstract). Mult. Scler. 2017, 23, P989. [Google Scholar]
- Dolgin, E. BTK blockers make headway in multiple sclerosis. Nat. Biotechnol. 2021, 39, 3–5. [Google Scholar] [CrossRef]
- Smith, P.F.; Owens, T.D.; Langrish, C.L.; Xing, Y.; Francesco, M.R.; Shu, J.; Hartmann, S.; Karr, D.; Burns, R.; Quesenberry, R.; et al. Phase 1 Clinical Trial of PRN2246 (SAR442168), a Covalent BTK Inhibitor Demonstrates Safety, CNS Exposure and Therapeutic Levels of BTK Occupancy (Abstract). Mult. Scler. J. 2019, 25 (Suppl. 1), 52. [Google Scholar]
- Kwon, B.K.; Okon, E.B.; Tsai, E.; Beattie, M.S.; Bresnahan, J.C.; Magnuson, D.K.; Reier, P.J.; McTigue, D.M.; Popovich, P.G.; Blight, A.R.; et al. A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J. Neurotrauma 2011, 28, 1525–1543. [Google Scholar] [CrossRef]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef]
- Chen, L.S.; Bose, P.; Cruz, N.D.; Jiang, Y.; Wu, Q.; Thompson, P.A.; Feng, S.; Kroll, M.H.; Qiao, W.; Huang, X.; et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood 2018, 132, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Li, Y.; Raza, K.; Yu, X.X.; Ghoshal, S.; Geddes, J.W. Calpain 1 knockdown improves tissue sparing and functional outcomes after spinal cord injury in rats. J. Neurotrauma 2013, 30, 427–433. [Google Scholar] [CrossRef] [PubMed]
| Treatment Group | Actual Force (kdyn) | Displacement (μm) | Velocity (mm/s) |
|---|---|---|---|
| SCI + Ibrutinib | 183.00 ± 0.95 | 1095.00 ± 54.6 | 123.10 ± 1.69 |
| SCI + Vehicle | 184.20 ± 0.96 | 1241.30 ± 88.19 | 121.60 ± 1.43 |
| Groups | 1 W | 2 W | 3 W | 4 W | 5 W | 6 W | 7 W | 8 W | 11 W |
|---|---|---|---|---|---|---|---|---|---|
| Ibr * | 266 ± 2.9 | 266 ± 2.8 | 272.3 ± 2.5 | 278 ± 2.3 | 290 ± 1.8 * | 301 ± 3.1 * | 312 ± 4.8 * | 321 ± 6.1 | 340 ± 9.9 |
| Veh | 259 ± 5.3 | 261 ± 6.9 | 263.3 ± 5.6 | 268 ± 5.2 | 276 ± 4.8 | 286 ± 5.5 | 294 ± 6.6 | 305 ± 6.9 | 317 ± 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.G.; Bondada, V.; Iqbal, H.; Moore, K.L.; Gensel, J.C.; Bondada, S.; Geddes, J.W. Inhibition of Bruton Tyrosine Kinase Reduces Neuroimmune Cascade and Promotes Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 355. https://doi.org/10.3390/ijms23010355
Yu CG, Bondada V, Iqbal H, Moore KL, Gensel JC, Bondada S, Geddes JW. Inhibition of Bruton Tyrosine Kinase Reduces Neuroimmune Cascade and Promotes Recovery after Spinal Cord Injury. International Journal of Molecular Sciences. 2022; 23(1):355. https://doi.org/10.3390/ijms23010355
Chicago/Turabian StyleYu, Chen Guang, Vimala Bondada, Hina Iqbal, Kate L. Moore, John C. Gensel, Subbarao Bondada, and James W. Geddes. 2022. "Inhibition of Bruton Tyrosine Kinase Reduces Neuroimmune Cascade and Promotes Recovery after Spinal Cord Injury" International Journal of Molecular Sciences 23, no. 1: 355. https://doi.org/10.3390/ijms23010355
APA StyleYu, C. G., Bondada, V., Iqbal, H., Moore, K. L., Gensel, J. C., Bondada, S., & Geddes, J. W. (2022). Inhibition of Bruton Tyrosine Kinase Reduces Neuroimmune Cascade and Promotes Recovery after Spinal Cord Injury. International Journal of Molecular Sciences, 23(1), 355. https://doi.org/10.3390/ijms23010355





