Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation
Abstract
:1. Introduction
2. Results
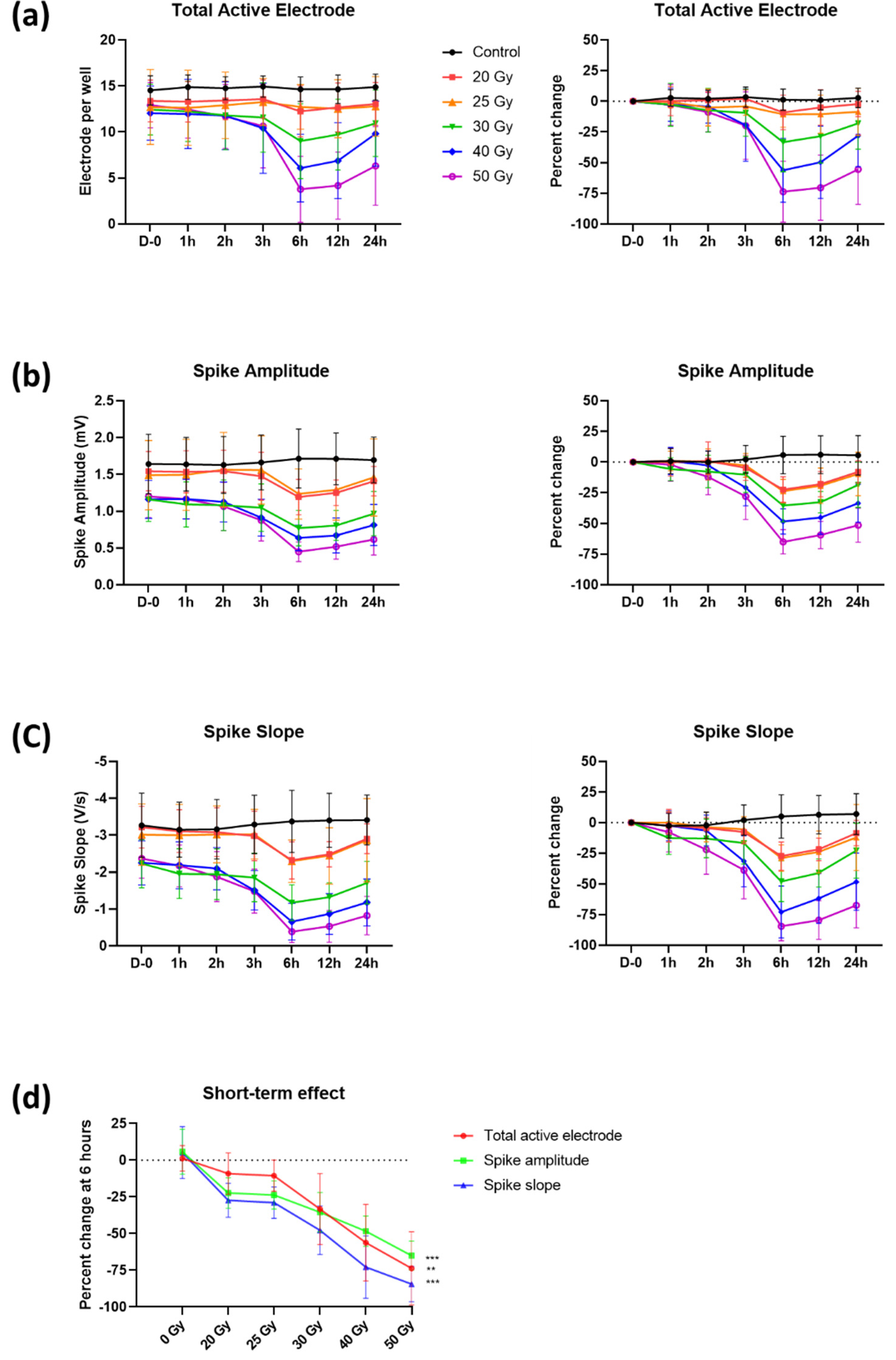
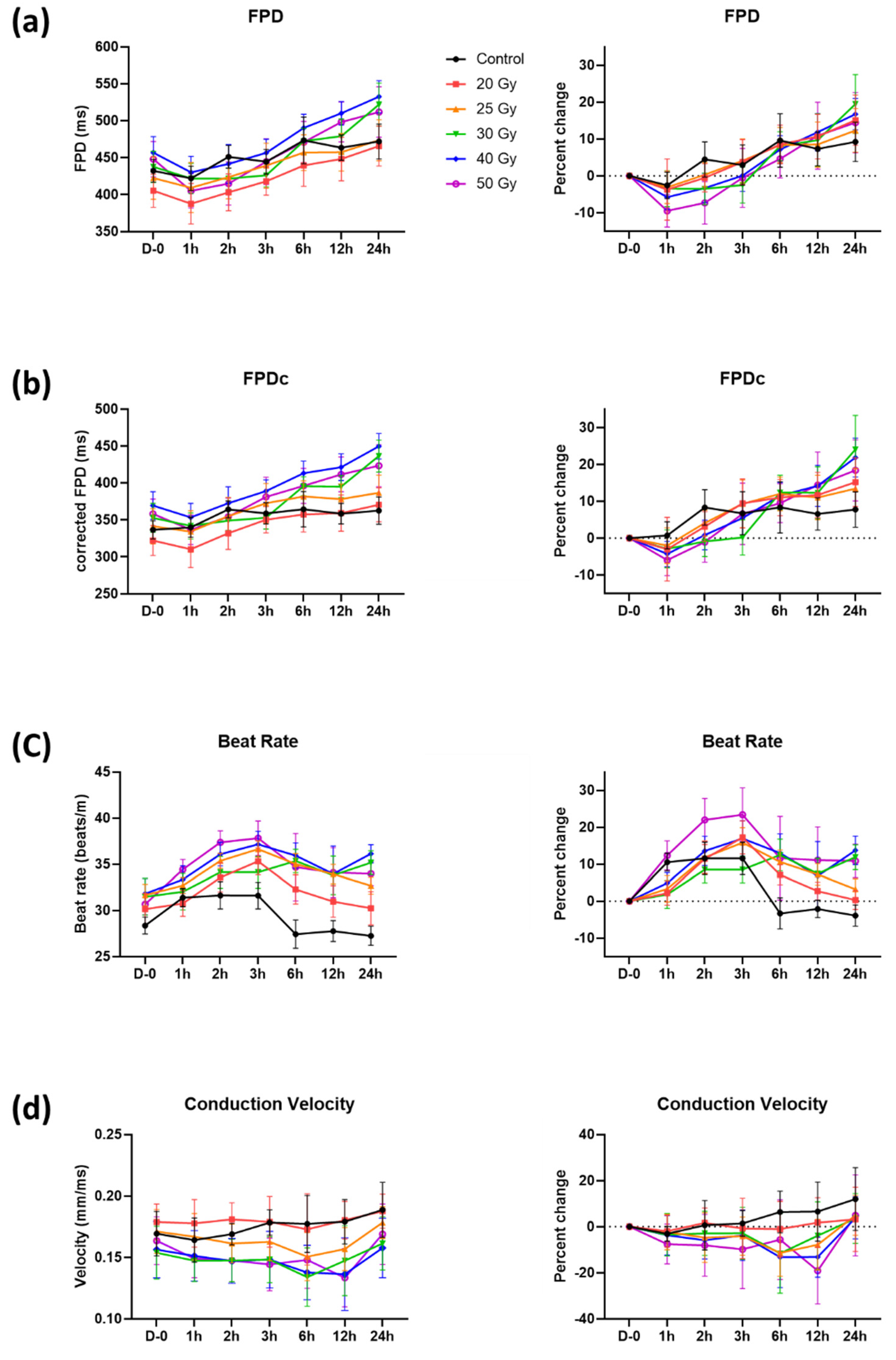
2.1. Immediate Electrophysiological Effects of High-Dose Irradiation on Human iPSC-CMs
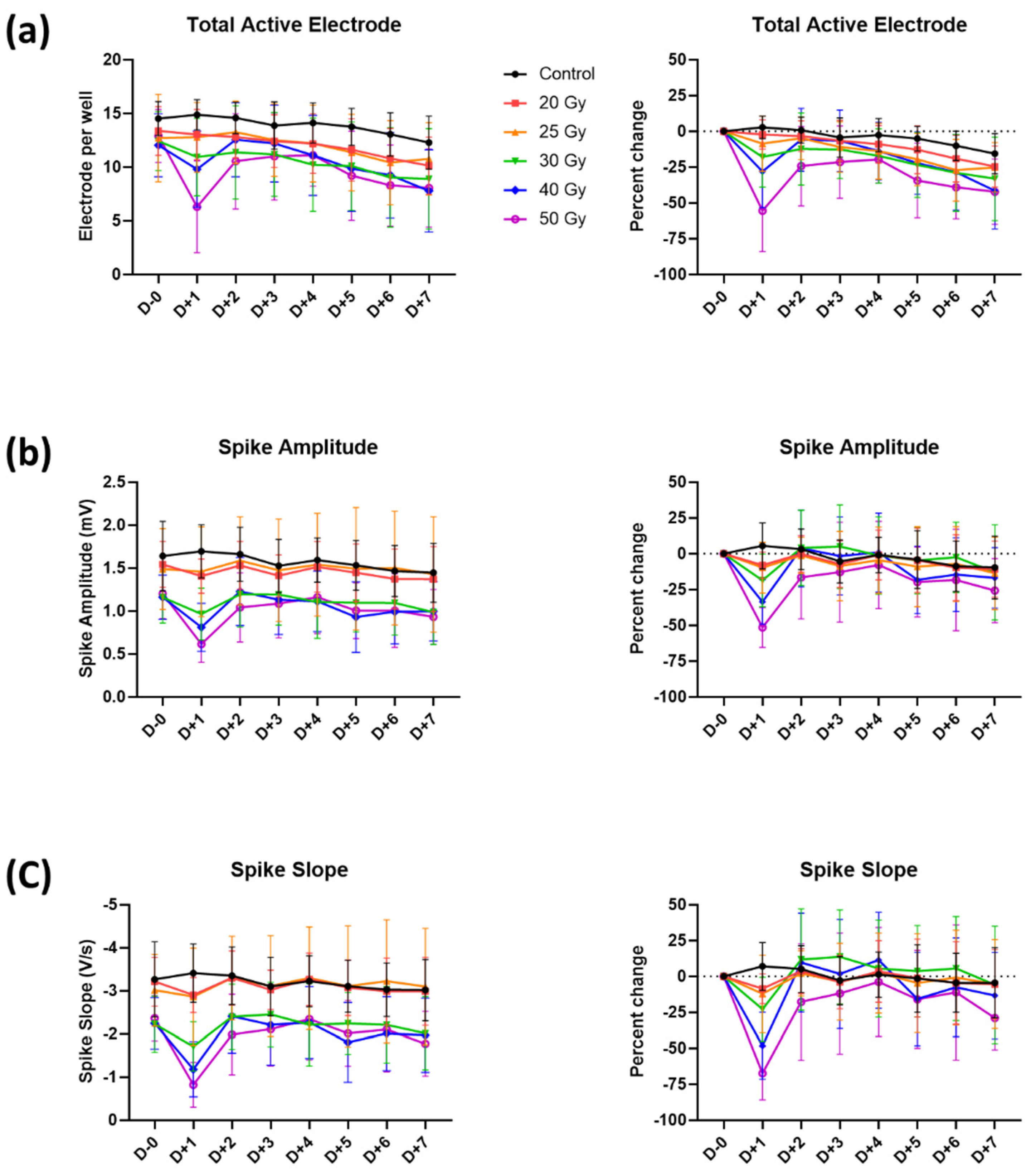
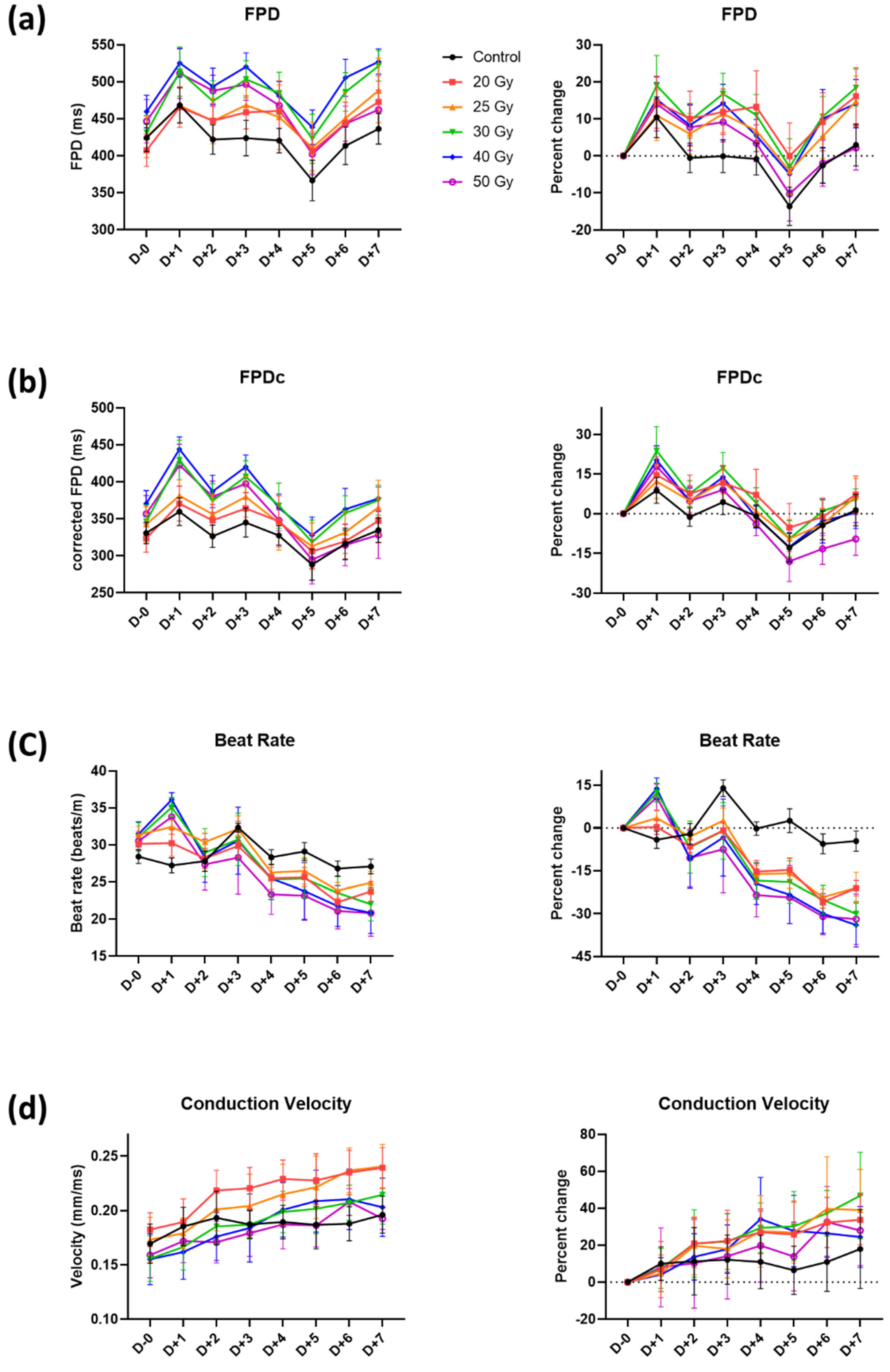
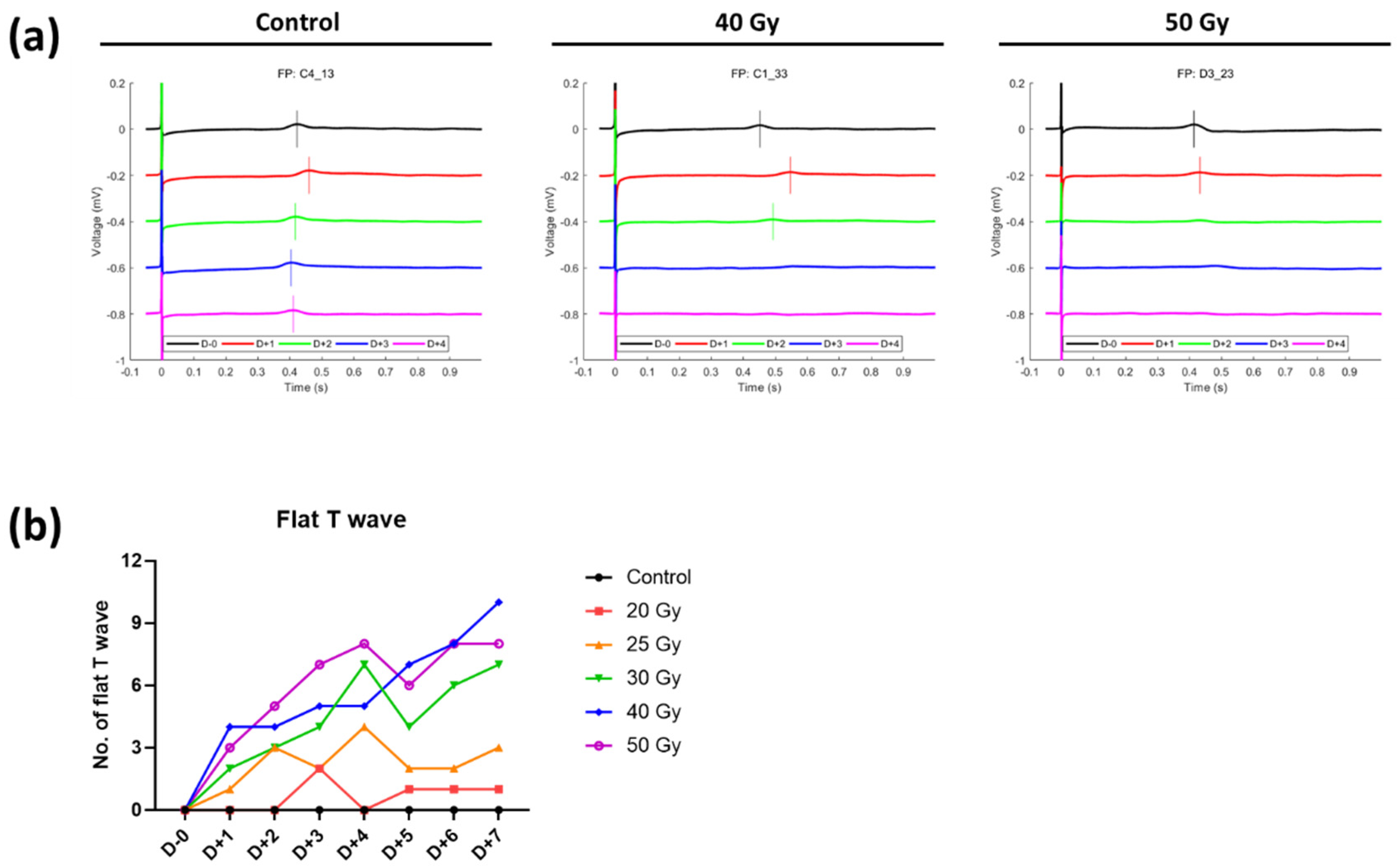
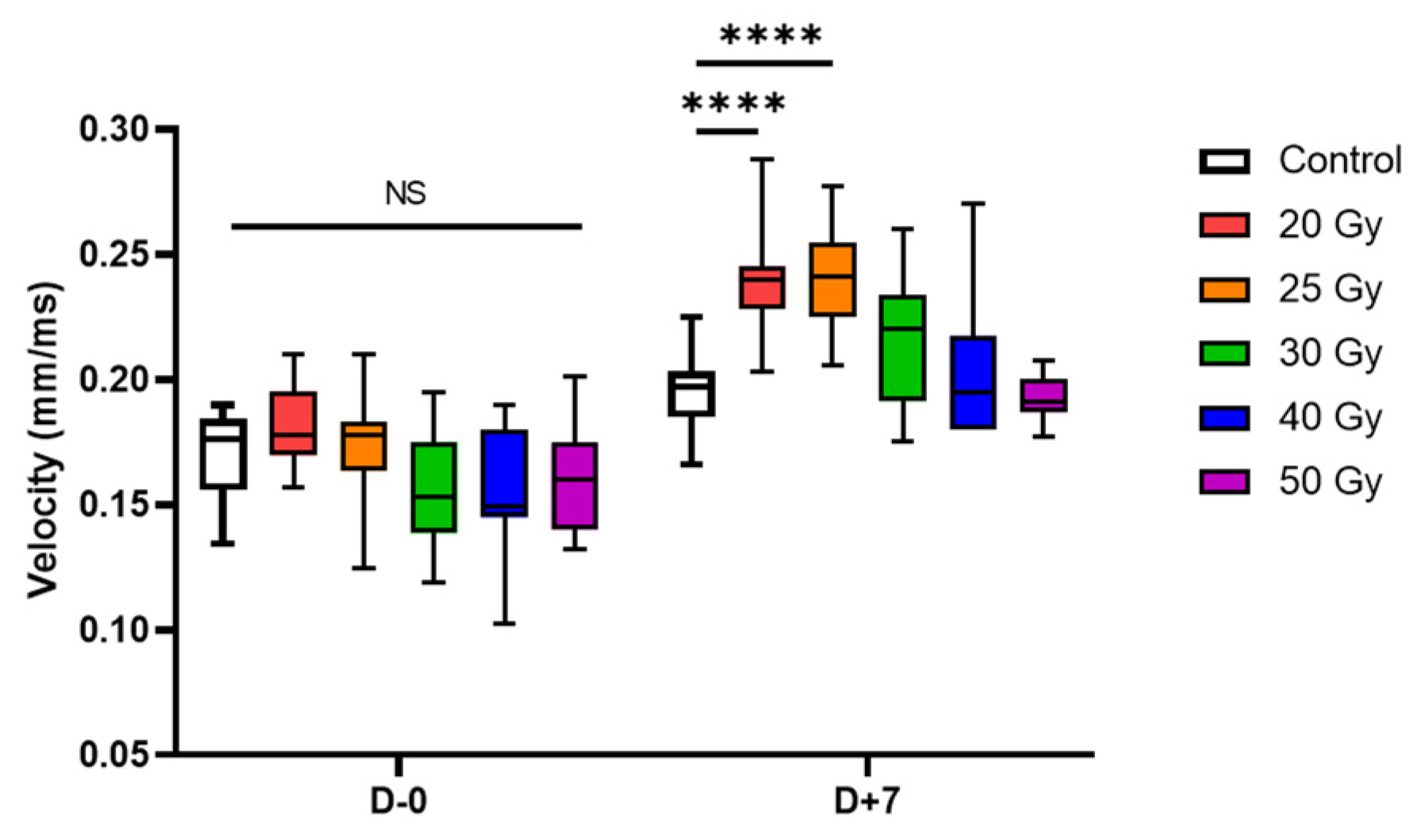
2.2. Recovery of Human iPSC-CMs from Immediate Electrophysiological Alterations Induced by High-Dose Irradiation
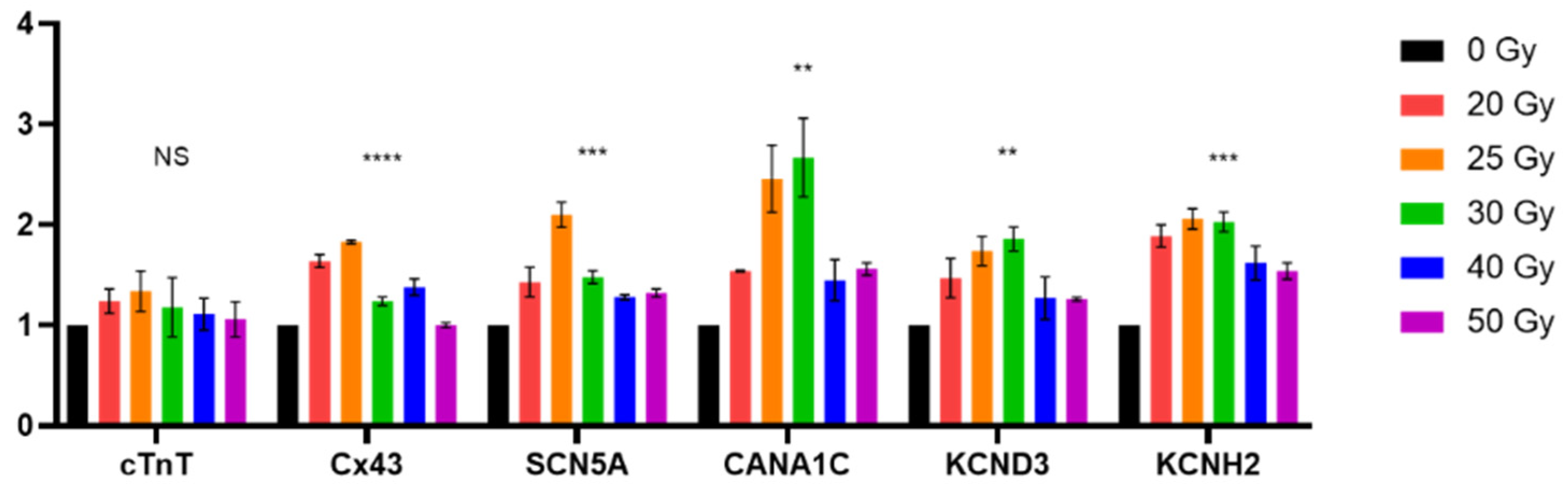
2.3. High-Dose Irradiation Promotes Increased Expression of Human iPSC-CM Genes
3. Discussion
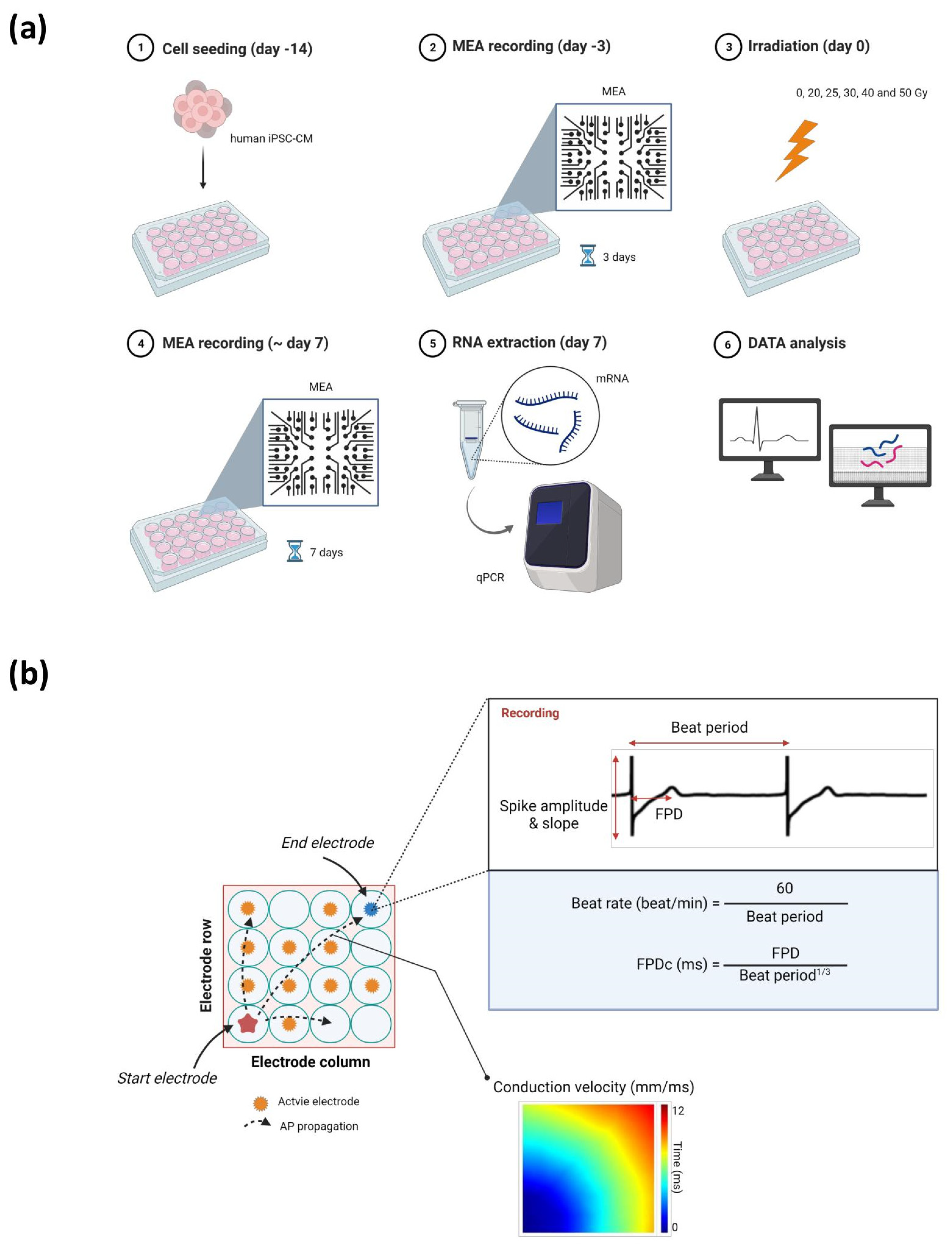
4. Materials and Methods
4.1. Preparation of Human iPSC-CMs
4.2. Irradiation
4.3. MEA Recording Analysis
4.4. Real-Time qPCR
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden cardiac death: Epidemiology and risk factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markman, T.M.; Nazarian, S. Treatment of ventricular arrhythmias: What’s new? Trends Cardiovasc. Med. 2019, 29, 249–261. [Google Scholar] [CrossRef]
- Moss, A.J.; Greenberg, H.; Case, R.B.; Zareba, W.; Hall, W.J.; Brown, M.W.; Daubert, J.P.; McNitt, S.; Andrews, M.L.; Elkin, A.D. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004, 110, 3760–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, W.G.; Wilber, D.J.; Natale, A.; Jackman, W.M.; Marchlinski, F.E.; Talbert, T.; Gonzalez, M.D.; Worley, S.J.; Daoud, E.G.; Hwang, C.; et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction. Circulation 2008, 118, 2773–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM task group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [Green Version]
- Dickfeld, T.; Tian, J.; Ahmad, G.; Jimenez, A.; Turgeman, A.; Kuk, R.; Peters, M.; Saliaris, A.; Saba, M.; Shorofsky, S.; et al. MRI—Guided ventricular tachycardia ablation: Integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ. Arrhythmia Electrophysiol. 2011, 4, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Seo, J.; Kim, H.J.; Kim, M.; Yoon, H.; Jo, S.W.; Oh, S.; Chang, J.H. Early changes in rat heart after high-dose irradiation: Implications for antiarrhythmic effects of cardiac radioablation. J. Am. Heart Assoc. 2021, 10, e019072. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Garg, V.; Shrestha, R.; Sanguinetti, M.C.; Kamp, T.J.; Wu, J.C. Human Induced pluripotent stem cell–derived cardiomyocytes as models for cardiac channelopathies. Circ. Res. 2018, 123, 224–243. [Google Scholar] [CrossRef]
- Hayes, H.B.; Nicolini, A.M.; Arrowood, C.A.; Chvatal, S.A.; Wolfson, D.W.; Cho, H.C.; Sullivan, D.D.; Chal, J.; Fermini, B.; Clements, M.; et al. Novel method for action potential measurements from intact cardiac monolayers with multiwell microelectrode array technology. Sci. Rep. 2019, 9, 11893. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T.; Boven, K.-H.; Günther, E.; Fejtl, M. Micro-electrode arrays in cardiac safety pharmacology: A novel tool to study QT interval prolongation. Drug Saf. 2004, 27, 763–772. [Google Scholar] [CrossRef]
- Zwartsen, A.; de Korte, T.; Nacken, P.; de Lange, D.W.; Westerink, R.H.S.; Hondebrink, L. Cardiotoxicity screening of illicit drugs and new psychoactive substances (NPS) in human iPSC-derived cardiomyocytes using microelectrode array (MEA) recordings. J. Mol. Cell. Cardiol. 2019, 136, 102–112. [Google Scholar] [CrossRef]
- Ipsen, S.; Blanck, O.; Oborn, B.; Bode, F.; Liney, G.; Hunold, P.; Rades, D.; Schweikard, A.; Keall, P.J. Radiotherapy beyond cancer: Target localization in real-time MRI and treatment planning for cardiac radiosurgery. Med. Phys. 2014, 41, 120702. [Google Scholar] [CrossRef]
- Rapp, F.; Simoniello, P.; Wiedemann, J.; Bahrami, K.; Grünebaum, V.; Ktitareva, S.; Durante, M.; Lugenbiel, P.; Thomas, D.; Lehmann, H.I.; et al. Biological cardiac tissue effects of high-energy heavy ions—Investigation for Myocardial ablation. Sci. Rep. 2019, 9, 5000. [Google Scholar] [CrossRef]
- Zhang, D.M.; Navara, R.; Yin, T.; Szymanski, J.; Goldsztejn, U.; Kenkel, C.; Lang, A.; Mpoy, C.; Lipovsky, C.E.; Qiao, Y.; et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat. Commun. 2021, 12, 5558. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ning, F.; Du, Y.; Song, B.; Yang, D.; Salvage, S.C.; Wang, Y.; Fraser, J.A.; Zhang, S.; Ma, A.; et al. Calcium-dependent Nedd4-2 upregulation mediates degradation of the cardiac sodium channel Nav1.5: Implications for heart failure. Acta Physichologica 2017, 221, 44–58. [Google Scholar] [CrossRef]
- Zicha, S.; Maltsev, V.A.; Nattel, S.; Sabbah, H.N.; Undrovinas, A.I. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure. J. Mol. Cell. Cardiol. 2004, 37, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, P.A.; van Rijsingen, I.A.W.; Asimaki, A.; Jongbloed, J.D.H.; van Veldhuisen, D.J.; Wiesfeld, A.C.P.; Cox, M.G.P.J.; van Lochem, L.T.; de Boer, R.A.; Hofstra, R.M.W.; et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: Evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur. J. Heart Fail. 2012, 14, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Ednie, A.R.; Horton, K.-K.; Wu, J.; Bennett, E.S. Expression of the sialyltransferase, ST3Gal4, impacts cardiac voltage-gated sodium channel activity, refractory period and ventricular conduction. J. Mol. Cell. Cardiol. 2013, 59, 117–127. [Google Scholar] [CrossRef]
- DeMarco, K.R.; Clancy, C.E. Cardiac Na channels: Structure to function. Curr. Top. Membr. 2016, 78, 287–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Tien, N.T.; de Haan, L.; Louisse, J.; Rietjens, I.M.C.M.; Bouwmeester, H. Evaluation of in vitro models of stem cell-derived cardiomyocytes to screen for potential cardiotoxicity of chemicals. Toxicol. Vitr. 2020, 67, 104891. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Peng, Y.; Lang, H.; Xinyong, C.; Zhiyi, Z.; Xiaocheng, W.; Hong, Z.; Liang, S. Oxidative stress in radiation-induced cardiotoxicity. Oxid. Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Davidson, M.M.; Zhou, H.; Wang, C.; Walker, W.F.; Hei, T.K. Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1-dependent mitochondrial fission. Cancer Res. 2013, 73, 6700–6710. [Google Scholar] [CrossRef] [Green Version]
- Abdullaev, S.; Gubina, N.; Bulanova, T.; Gaziev, A. Assessment of nuclear and mitochondrial DNA, expression of mitochondria-related genes in different brain regions in rats after whole-body x-ray irradiation. Int. J. Mol. Sci. 2020, 21, 1196. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, H.; Dudley, S.C. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ. Res. 2010, 107, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Dudley, S.C. The role of the unfolded protein response in arrhythmias. Channels 2018, 12, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Kobayashi, T.; Nishioka, A.; Kariya, S.; Hamasato, S.; Seguchi, H.; Yoshida, S. Radiation-induced reactive oxygen species formation prior to oxidative DNA damage in human peripheral T cells. Int. J. Mol. Med. 2003, 11, 149–152. [Google Scholar] [CrossRef]
- Claro, S.; Teixeira Ferreira, A.; Etsuko Miyamoto Oshiro, M. Radiation-generated ros induce apoptosis via mitochondrial. In Free Radical Medicine and Biology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Lee, J.K.; Nam, J.H.; Ha, T.Y.; Lim, Y.H.; Kil, S.H. The Effect of ionizing radiation on the ultrastructural changes and mechanism on the cytoplasmic organelles. J. Life Sci. 2017, 27, 708–725. [Google Scholar]
- Sovari, A.A. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol. Res. Pract. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, F.O.; Whitaker, J.; Neji, R.; Roujol, S.; O’Neill, M.; Plank, G.; Bishop, M.J. Factors Promoting conduction slowing as substrates for block and reentry in infarcted hearts. Biophys. J. 2019, 117, 2361–2374. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, P.A.; Schuessler, R.B.; Davis, L.M.; Beyer, E.C.; Johnson, C.M.; Yamada, K.A.; Saffitz, J.E. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J. Clin. Investig. 1997, 99, 1991–1998. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Tanabe, T.; Tanaka, E.; Mori, H.; Furusawa, Y.; Zareba, W.; Yamazaki, M.; Nakagawa, H.; Honjo, H. Heavy ion radiation up-regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc. Res. 2006, 72, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Amino, M.; Yoshioka, K.; Fujibayashi, D.; Hashida, T.; Furusawa, Y.; Zareba, W.; Ikari, Y.; Tanaka, E.; Mori, H.; Inokuchi, S.; et al. Year-long upregulation of connexin43 in rabbit hearts by heavy ion irradiation. Am. J. Physiol. Circ. Physiol. 2010, 298, H1014–H1021. [Google Scholar] [CrossRef] [Green Version]
- Amino, M.; Yoshioka, K.; Furusawa, Y.; Tanaka, S.; Kawabe, N.; Hashida, T.; Tsukada, T.; Izumi, M.; Inokuchi, S.; Tanabe, T.; et al. Inducibility of ventricular arrhythmia 1 year following treatment with heavy ion irradiation in dogs with myocardial infarction. Pacing Clin. Electrophysiol. 2017, 40, 379–390. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiley, P.J.; Storz, G. Exploiting thiol modifications. PLoS Biol. 2004, 2, e400. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, M.; Qian, P.C.; Boeck, M.; Bredfeldt, J.; Seuntjens, J.; Hijal, T.; Bernier, M.L.; Zei, P. The rapidly-developing area of radiocardiology: Principles, complications and applications of radiotherapy on the heart. Can. J. Cardiol. 2021, 37, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.; Hayase, J.; Hu, P.; Cao, M.; Deng, J.; Ajijola, O.; Do, D.; Vaseghi, M.; Buch, E.; Khakpour, H.; et al. Non-invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: An institutional experience. J. Interv. Card. Electrophysiol. 2021, 61, 535–543. [Google Scholar] [CrossRef]
- Krug, D.; Blanck, O.; Andratschke, N.; Guckenberger, M.; Jumeau, R.; Mehrhof, F.; Boda-Heggemann, J.; Seidensaal, K.; Dunst, J.; Pruvot, E.; et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm 2021, 18, 2137–2145. [Google Scholar] [CrossRef]
- Van der Ree, M.H.; Blanck, O.; Limpens, J.; Lee, C.H.; Balgobind, B.V.; Dieleman, E.M.T.; Wilde, A.A.M.; Zei, P.C.; de Groot, J.R.; Slotman, B.J.; et al. Cardiac radioablation—A systematic review. Heart Rhythm 2020, 17, 1381–1392. [Google Scholar] [CrossRef]
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac Toxicity after radiotherapy for stage III non–small-cell lung cancer: Pooled Analysis of dose-escalation trials delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Choi, S.W.; Park, Y.-G.; Kim, S.J.; Choi, C.H.; Cha, M.-J.; Chang, J.H. Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation. Int. J. Mol. Sci. 2022, 23, 351. https://doi.org/10.3390/ijms23010351
Kim JS, Choi SW, Park Y-G, Kim SJ, Choi CH, Cha M-J, Chang JH. Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation. International Journal of Molecular Sciences. 2022; 23(1):351. https://doi.org/10.3390/ijms23010351
Chicago/Turabian StyleKim, Jae Sik, Seong Woo Choi, Yun-Gwi Park, Sung Joon Kim, Chang Heon Choi, Myung-Jin Cha, and Ji Hyun Chang. 2022. "Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation" International Journal of Molecular Sciences 23, no. 1: 351. https://doi.org/10.3390/ijms23010351
APA StyleKim, J. S., Choi, S. W., Park, Y.-G., Kim, S. J., Choi, C. H., Cha, M.-J., & Chang, J. H. (2022). Impact of High-Dose Irradiation on Human iPSC-Derived Cardiomyocytes Using Multi-Electrode Arrays: Implications for the Antiarrhythmic Effects of Cardiac Radioablation. International Journal of Molecular Sciences, 23(1), 351. https://doi.org/10.3390/ijms23010351





